Abstract
Perioperative neurocognitive disorder (PND), including postoperative delirium and postoperative cognitive dysfunction (POCD), is a common postoperative complication in elderly patients, who represent an expanding segment of our population. PND is a multifactorial disease resulting in higher morbidity and mortality. The precise mechanism of PND is yet to be fully delineated. Identifying the modifiable risk factors and mechanisms for PND would be an important step forward in preventing such adverse events and thus improving patients' outcomes. It is increasingly recognized that gut microbiota also manifest effects in the central nervous system via the microbiota‐gut‐brain axis, which has emerged as an important player in shaping aspects of behavior and cognitive function. Recent studies have found that patients with cognitive dysfunction after surgery and anesthesia have obvious gut microbiome disorders. These findings are paralleled by a growing body of preclinical investigations aimed at better understanding how surgery and anesthesia affect the central nervous system and possibly contribute to cognitive decline. Here, we present a broad topical review of the literature supporting the role of gut microbiota in PND. We provide an overview of the mechanisms underlying the pathogenesis of PND from pre‐clinical and human studies. Therefore, gut microbiota could be a putative therapeutic target for PND in the future.
Keywords: Perioperative neurocognitive disorder, Perioperative neurocognitive disorder, Gut microbiota, Probiotics
Introduction
With the rapid development of medical care in today's society, more and more peopleneed surgical treatment, and the proportion of large‐scale operations and elderlypatients is increasing (Needham MJ, et al. 2017; Gore‐Booth J, et al. 2019).Perioperative neurocognitive disorder (PND) refers to the changes in cognitive functionafter anesthesia and surgery (Eckenhoff RG, et al., 2020). The working group on ‘Consensusrecommendations for the nomenclature of cognitive change associated with anesthesiaand surgery’ aligned PND with terminology, which be used to describe the destructionor changes of cognitive function that occurs before and after surgery in 2018 (Evered L, et al., 2018). PND include acute postoperative delirium and postoperative cognitivedysfunction (POCD) (Eckenhoff RG, et al., 2020). Elderly patients have the highestincidence of PND, with rates 24%–40% of patients aged 60 years or older requiringhospitalization for surgery, and is associated with substantially increased morbidity,mortality, and cost of care (Wang W, et al. 2014; Belrose JC, et al. 2019; Decker J, et al. 2020). Studies have identified the risk factors for PND including the patient's factors,anesthesia factors, and surgical factors, such as: old age, type of surgery, method anddepth of anesthesia, etc., but the exact cause is still unknown (Schupf N, et al. 2005; Evered LA, et al. 2021). To date, studies on PND have primarily focused on direct influences of surgery andanesthesia on the central nervous system (Martin CR, et al. 2018; Olotu C, et al. 2020). It has beenrecently recognized that gut microbiota interacts with the brain, and it is termed asmicrobiota‐gut‐brain axis (Sekirov I, et al., 2010). Modulation of this axis has beenrecently reported to affect cognitive function (Mayer EA, et al. 2015; Cryan JF, et al. 2019). Growing evidence suggests a possible role for gut microbiota in the pathogenesis of PND, both patients and animal models of PND share common molecular mechanismswith other dementias (Jiang XL, et al. 2019; Meng F, et al. 2019; Zhan G, et al. 2019; Wang X, et al. 2021). The aim of this review is to discuss recent evidence for the involvement of gutmicrobiota in PND, and to highlight possible mechanisms of relevance to PND fromp reclinical and early clinical studies.
Gut microbiota and gut‐brain axis
There are about 100 trillion and more than 1,000 species of bacteria living in the human intestine, which mainly belong to phylum Firmicutes, Bacteroides and actinomycetes (Sekirov I, et al. 2010; Toole PW, et al. 2015). These collections of microorganisms, termed the microbiota, and the host are in a mutually beneficial symbiosis state. Emerging evidence over the past 10 years, enabled by new technologies such as next‐generation sequencing, has also suggested the influence of the gut microbiome on health and disease extends beyond the gastrointestinal tract (Mayer EA, et al., 2015). Gut microbiota plays an important role in regulating human immune homeostasis, maintaining host mental health, regulating brain function, behavior and metabolism, etc. (Dinan TG, et al. 2017; Tang WH, et al. 2017). Changes in gut microbiota composition and function have been associated with a wide range of neurological and neuropsychiatric disorders, including Alzheimer’s disease (AD), Parkinson’s disease, and other cognitive disorders/dementias (Quigley E, et al. 2017; Sun M, et al. 2020). Its exact mechanism of gut microbiota affecting neurocognition remains to be elucidated.
The gut‐brain axis refers to the bidirectional communication between the brain and the gut microbes, and is crucial in maintaining homeostasis of the gastrointestinal, central nervous and microbial systems of human being (Sekirov I, et al., 2010). These microbiota‐gut‐brain interaction pathways may help explain the correlation between the gastrointestinal tract and neuropsychiatric/neurodevelopmental diseases (Xu X, et al., 2020). Contemporary research is starting to appreciate how gut microbiota influence the brain through their ability to produce and modify many metabolic, immunological and neurochemical factors in the gut that ultimately impact the nervous system (Bauer KC, et al., 2016). Current studies have shown that the gut microbiota mainly affects cognitive function through various pathways such as the regulation of immunity, the vagus nerve, the enteroendocrine system and the metabolites of gut microbiota (Alam A, et al., 2018).
The intestinal mucosal immune barrier is an important component of the intestinal barrier, mainly composed of abundant lymphocytes, macrophages, etc. The gut microbiota with the host mucosal immune system prevents harmful bacteria from multiplying by competing with harmful bacteria for nutrients (Sharon G, et al., 2016). With increasing Gram‐negative intestinal bacteria by intestinal flora disturbance, lipopolysaccharide (LPS) can induce pro‐inflammatory factors release and cause memory deficits (Jang SE, et al., 2018). In addition, cytokines entering the brain can activate the hypothalamic‐pituitary‐adrenal (HPA) axis to release cortisol and act on the downstream pathways of glucocorticoid receptors in the hippocampus, triggering depression‐like behavior (McEwen BS, et al. 2003; Jašarević E, et al. 2018).
The vagus nerve is one of the important pathways between the gut and the brain. The gut microbiota can directly or indirectly activate the vagus nerve and affect the immune response, endocrine system and other activities (Diaz Heijtz R, et al., 2011). In animal models, mice transplanted with Campylobacter increased anxiety‐like behaviors and c‐fos expression in the sensory nucleus of the vagus nerve; while administration of Bifidobacterium longum and Lactobacillus plumose could significantly reduce anxiety and depression in mice (Goehler LE, et al., 2008). Cutting off the vagus nerve can reduce the expression of GABA (B1b) mRNA in the hippocampus and amygdala, suggesting that the integrity of the vagus nerve plays an important role in the microbiota‐gut‐brain axis (Breit S, et al., 2018).
A variety of hormones and neurotransmitters synthesized by the gut microbiome and enteroendocrine cells can pass through the intestinal wall into the circulatory system and enter the brain, or directly act on the ascending pathway of the vagus nerve to participate in the regulation of brain signal transmission (Mittal R, et al., 2017). Dopamine produced by Bacillus and Escherichia coli participates in cognitive‐related neuronal signal transduction through dopamine receptors acting on the midbrain cortex Pathway (Hsieh TH, et al., 2020). The 5‐hydroxytryptamine (5‐HT) produced by Escherichia coli and enterochromaffin cells can regulate intestinal movement, pain and cognitive processes (Yano JM, et al. 2015; Hsieh TM, et al. 2020). In addition, the gut microbiota is also involved in the synthesis of various neurotransmitters such as γ‐aminobutyric acid (GABA) and acetylcholine (Bravo JA, et al., 2011).
In addition, the metabolites of gut microbiota, such as short‐chain fatty acids (SCFA) are lipids produced by intestinal microbes through the fermentation of dietary fiber, which can act on the on the central nervous system by regulating neuroplasticity, epigenetics, gene expression, and the immune system (Erny D, et al., 2015). SCFA may affect disease and behavior. Studies found exogenous administration of SCFA sodium butyrate can alter the expression of brain‐derived neurotrophic factor (BDNF), a neuronal factor associated with depression (Schroeder FA, et al., 2007). In the same study, long‐term use of exogenous sodium butyrate for more than 28 days caused a significant reduction in depression‐like behavior in mice (Schroeder FA, et al., 2007).
Pathogenesis of PND
The pathogenesis underlying PND remains elusive. Both modifiable and non‐modifiable factors may contribute to PND. To date, neuroinflammation, cholinergic nerves, Aβ accumulation and abnormal phosphorylation of Tau protein have proved to be closely related to the occurrence and development of PND (Needham MJ, et al., 2017).
Neuroinflammation
To date, an increasing number of studies have demonstrated that neuroinflammation plays a crucial role in PND (Subramaniyan S, et al., 2019). Patients subjected to major surgery often exhibit an acute‐phase inflammatory response. Following surgical trauma, the pro‐inflammatory cytokines including interleukin‐6 (IL‐6), tumor necrosis factor (TNF)‐α, and interferon (IFN) increase in central nervous system, which can be used as a predictor of the incidence of PND (Safavynia SA, et al., 2018). The release of pro‐inflammatory cytokines during surgery‐induced neuroinflammation also results in the activation of microglia in rodent’s hippocampal, are closely related to the impairment of spatial learning and memory after surgery (Fan W, et al., 2020). Inflammatory factor antagonists can improve the occurrence of PND. In addition, both simple anesthesia or anesthesia combined with surgery can trigger an inflammatory response and increase the level of pro‐inflammatory factors and inflammatory cells in the brain (Bittner EA, et al., 2011). In particular, the increased level of inflammatory response in the hippocampus leads to neuroinflammation and increases the risk of PND. The inflammatory process mediated by interleukin‐1β (IL‐1β) in the hippocampus is related to the occurrence of POCD. Knockout of IL‐1β gene or use of IL‐1β receptor antagonists can significantly reduce neuroinflammation and attenuate surgery‐induced postoperative memory impairment in mice (Cibelli M, et al., 2010). In elderly mice anesthetized with isoflurane increased the level of IL‐1β and TNF‐α in the hippocampus with cognitive dysfunction (Wang HL, et al., 2015). Clinical studies also have found that theincidence of POCD in elderly patients undergoing isoflurane anesthesia surgery was significantly higher than that of elderly patients in the propofol group (Cottrell JE, et al., 2020). It has been hypothesized that these complications are due to a malignant systemic inflammatory response following surgery that ultimately results in neuroinflammation, synaptic impairment, widespread neurodegeneration, and ultimately cognitive dysfunction.
Abnormal protein function
A growing body of research has shown that Alzheimer’s disease and POCD share multiple similarities in pathogenesis and clinical manifestations (Askarova S, et al. 2020; Lin X, et al. 2020). The characteristic pathological changes of Alzheimer’s disease, namely β amyloid (Aβ) deposition and Tau protein phosphorylation, have also been found in POCD animal and patient (Le Freche H, et al., 2012). The serum Aβ level of POCD patients was significantly higher (Tomaszewski D, 2015). In addition, the level of Aβ in the cerebrospinal fluid of patients receiving isoflurane anesthesia increased significantly within 24 hours after the operation, and the level of Aβ in the cerebrospinal fluid of patients anesthetized with desflurane also increased significantly within 2 hours after the operation (Zhang J, et al., 2020). Tau protein can maintain the stability of microtubules. Hyperphosphorylated Tau protein can reduce its ability to bind to tubulin, promote the depolymerization of normal microtubules, and ultimately lead to neuronal degeneration. The phosphorylation of Tau protein mediates long‐term synaptic inhibition, and rapidly causes long‐term synaptic enhancement and memory damage, and can even cause cognitive impairment through synaptic dysfunction and neuron loss. Therefore, Tau protein phosphorylation may be the downstream target of isoflurane‐induced neuroinflammation (Luo X, et al., 2014). By inhibiting the specific signaling pathways in the process of Tau protein phosphorylation, it may be able to effectively treat POCD and other related neurodegenerative diseases.
Synaptic dysfunction and changes in neurotransmitters
It is increasingly accepted that early cognitive impairment in PND results in considerable part from synaptic impairment (Lin X, et al., 2020). Cognitive function and decline in PND is associated with loss of synapses and dysfunction. Surgical trauma can result in the pathological activation of astrocytes and the release of pro‐inflammatory cytokines, which contribute to the subsequent impairment of synaptic function (Lyman M, et al., 2014). After the release of cytokines, a vicious circle occurs, whereby the cytokine leads to a decrease of calcium ions in astrocytes induced by glutamate, which leads to harmful effects on astrocyte reactivity and subsequent abnormal synaptic activity (Rose CR, et al., 2017). Postsurgical trauma can ultimately result in neuroinflammation, which, in turn, may cause synaptic dysfunction and precipitate the onset of neurodegenerative disease (Lin X, et al., 2020).
The central cholinergic system plays a critical role in the regulation of cognitive functions such as learning, emotional memory, and attention (Haam J, et al. 2017; Maurer SV, et al. 2017). Studies have shown that the dysfunction of the cholinergic system is related to age and cognitive decline caused by neurodegenerative diseases (Ferreira‐Vieira TH, et al., 2016). Anesthetics have a vital effect on the cholinergic system through directly decreasing the expression of acetylcholine transferase in the hippocampus, thereby inhibiting the synthesis of acetylcholine, or increasing the deposition of Aβ protein in the brain produces neurotoxic reactions (Zhan DY, et al. 2013; Kong FJ, et al. 2015). Treated with cholinergic in older people with POCD show an improved result (Hambrecht‐Wiedbusch VS, et al., 2014).
Glutamate acting via N‐methyl‐D‐aspartate receptors (NMDAR) may also be important in hippocampal learning and memory formation (Kumar A, et al., 2019). The effect of glutamate excitotoxicity on neurodegenerative diseases and neurological injuries has been established. Isoflurane can increase the transmission of γ‐aminobutyric acid (GABA) neurotransmitter, cause changes in NMDAR and γ‐aminobutyric acid receptor (GABAR), and antagonize glutamate on NMDAR, thereby directly inhibiting formation of LTP, leading to impaired learning and memory function (Westphalen RI, et al. 2003; Garcia PS, et al. 2010).
Stress
Studies in humans and in animals have shown stress can affect cognition in many ways (Vogel S, et al., 2016). Patients may experience hypoxia, cerebral cortex hypoperfusion, and impaired cortical function during surgery and anesthesia. These stressors may disrupt the nervous system. The level of cellular stress becomes evident with the increase of cortisol. With level of adrenal cortex hormones increasing, it induces changes in glutamatergic activity and dendritic reorganization, damages hippocampal neurons, and leads to the occurrence of PND (Van Bodegom M, et al., 2017). The psychological stressors can also trigger the activation of neuronal circuits and peripheral process, for example the inflammation (McEwen BS, et al., 2016). In mouse models similar to human cognitive impairment, cellular processes such as inflammation, proliferation/death and oxidative stress have been shown. This damage translates to changes in behavior, loss of memory, inability to make decisions and problems with attention (Tangestani Fard M, et al., 2019).
Gut microbiota and PND
Many factors in the perioperative period can affect the gut microbiota (Liang W, et al., 2019). The operation, the use of antibiotics in the perioperative period, the lack of enteral nutrition after the operation, and the use of opioids and antacids in the perioperative period have all been shown to affect the gut microbiota. studies of PND induced by surgery and anesthesia indicates that whether it is intestinal surgery or non‐ intestinal surgery, it is more likely to cause changes in gut microbiota after major surgery (Liufu N, et al., 2020). It is known that the imbalance of gut microbiota is related to neuroinflammation and cognitive dysfunction (Haase S, et al., 2020). Although the relationship between the gut microbiome and human health is well known, Liang et al. have now proposed a new type of connection between the perioperative gut microbiome and postoperative cognitive dysfunction (Liang P, et al., 2018). Antibiotics are one of the most commonly used drugs to prevent infection during the perioperative period, and long‐term use of antibiotics will inevitably lead to gut microbiome disturbance. Bacterial imbalance caused by antibiotics may lead to perioperative cognitive changes. Numerous clinical trials have been conducted to investigate the effects of probiotics/prebiotics on PND. More research needs to be done to confirm this finding and expand how dysbacteriosis can lead to cognitive dysfunction.
A number of studies have suggested gut microbiota may play a role in PND development and progression (Xu X, et al., 2020). Yang and colleagues present the first functional evidence that feeding with prebiotic galacto‐oligosaccharide alleviated cognitive dysfunction, and downregulated activation of microglia and expressions of pro‐inflammatory IL‐6 in the hippocampus from the rats after operation (Yang XD, et al., 2018). Another research from Zhan group shown a total of 24 types of gut microbiota changed in the POCD mice. In the fecal samples of POCD mice, Firmicutes phylum and Tenericutes phylum was significantly decreased while the abundance of E. coli and Chlamydiae phylum increased (Zhan G, et al., 2019). Furthermore, they found that the pseudo‐germ‐free mice (induced by antibiotics) exhibited abnormal behaviors, and the gut microbiota of the non‐POCD group could improve the abnormal behaviors of pseudo‐germ‐free mice, while the gut microbiota of the POCD group did not do so. The possible mechanisms are (1) probiotics promote intestinal bacteria to produce more exogenous polyamines, which not only inhibit the production of inflammatory factors, but also have anti‐mutagenic and antioxidant effects; (2) probiotics can also reduce the activity of inflammatory factors and increase the level of tryptophan‐derived neurotrophic factor (Oelschlaeger TA, 2010; Hadizadeh M, et al. 2019).
Aging is the key risk factor for PND. Aging is accompanied by changes in the gut Microbiome (Nagpal R, et al., 2018). These alterations may contribute to the development of pathophysiological PND. Wen et al., reported that the combination of aging and antibiotics will increase the permeability of BBB and induce POCD, which can be reversed by the application of Lactobacillus (Wen J, et al., 2020). More specifically, Liufu et al., reported that a preexisting disturbance in the gut microbiome leads to increased POCD in aged mouse model. Anesthesia/surgery caused different alterations in gut microbiota between the 8‐ and 19‐months old mice (Liufu N, et al., 2020). The anesthesia/surgery induced greater postoperative delirium‐like behavior, increased hippocampal IL‐6 levels, decreased synaptophysin levels, and mitochondrial dysfunction in 19 months old mice. Treatments with Lactobacillus and probiotic mitigated the anesthesia/surgery‐induced changes.
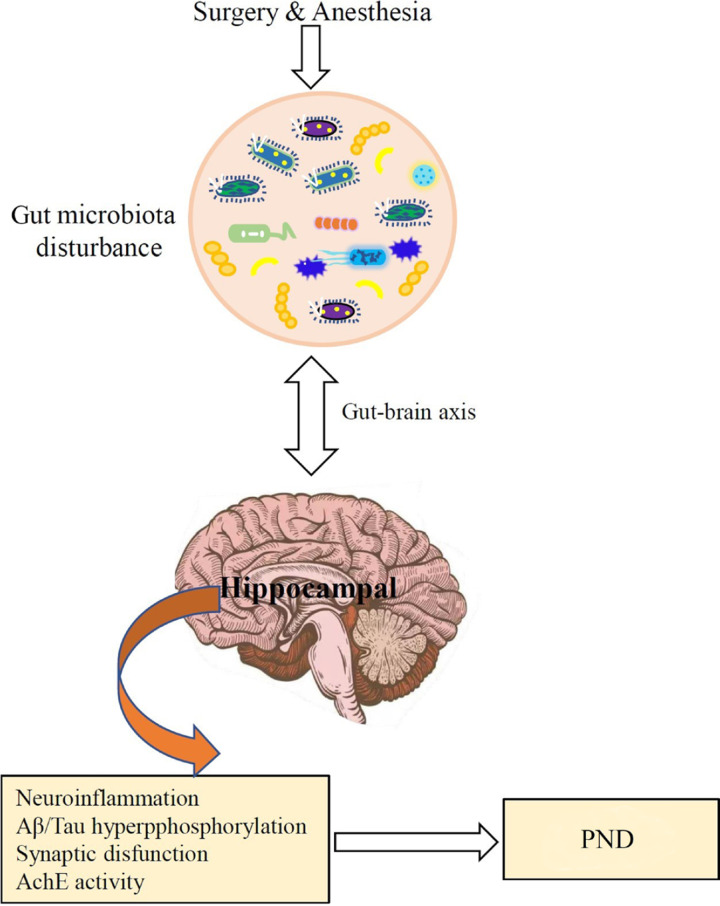
Although there are many studies that prove the correlation between intestinal flora and PND, the precise mechanisms involved in PND by gut microbiota are yet to be defined. The potential role of gut microbiota in the pathogenesis of PND is presented in Figure .
Figure .
The potential role of gut microbiota in the pathogenesis of perioperative neurocognitive disorder (PND).
Conclusions and future perspectives
It is well known that normal gut microbiota modulates brain development and behavior. As mentioned above, surgery and anesthesia can lead to intestinal flora disorders and changes of metabolites, which in turn can affect the neurological function and the range of brain damage through the gut‐brain axis and participate in the pathophysiological mechanism of PND. Notably, intestinal microflora approach yielded promising results in mouse models, there have been only a limited number of clinical trials in humans. Therefore, a more in‐depth understanding of the mechanism of the gut microbiota in the occurrence and development of PND will help the research and development of new therapeutic targets or new drugs for cognitive disorders, especially POCD.
Ethical statement
Not applicable.
Conflict of interest
There is no conflict of interest in this study.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 81760019, 81660193, 82060243, 81960214, and 82001604) and a joint fund from Zunyi Science and Technology Bureau and Zunyi Medical College (grant number 201724).
Transparency statement
All the authors affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Authors' contribution
Liang Dong and Juan Li conceived and designed the work that led to the submission; De‐Xing Liuand Chao Zhang revised the manuscript; Liang Dong approved the final version.
Acknowledgments
The author thanks Dr. Yong‐ping Liu for helpful suggestions.
References
- Alam A, Hana Z, Jin Z, Suen KC, Ma D. Surgery, neuroinflammation and cognitive impairment. EBioMedicine 2018. 37: 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askarova S, Umbayev B, Masoud AR. The Links Between the Gut Microbiome, Aging, Modern Lifestyle and Alzheimer's Disease. J. Front Cell Infect Microbiol 2020. 10: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer KC, Huus KE, Finlay BB. Microbes and the mind: emerging hallmarks of the gut microbiota‐brain axis. Cell Microbiol 2016. 18 (5): 632–644. [DOI] [PubMed] [Google Scholar]
- Belrose JC, Noppens RR. Anesthesiology and cognitive impairment: a narrative review of current clinical literature. BMC Anesthesiol 2019. 19 (1): 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner EA, Yue Y, Xie Z. Brief review: anesthetic neurotoxicity in the elderly, cognitive dysfunction and Alzheimer's disease. Can J Anaesth 2011. 58 (2): 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. J. Proc Natl Acad Sci USA 2011. 108 (38): 16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breit S, Kupferberg A, Rogler G, Hasler G. Vagus Nerve as Modulator of the Brain‐Gut Axis in Psychiatric and Inflammatory Disorders. Front Psychiatry 2018. 9: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibelli M, Fidalgo AR, Terrando N. Role of interleukin‐1beta in postoperative cognitive dysfunction. Ann Neurol 2010. 68 (3): 360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell JE, Hartung J. Anesthesia and Cognitive Outcome in Elderly Patients: A Narrative Viewpoint. J Neurosurg Anesthesiol 2020. 32 (1): 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, O', Riordan KJ . The Microbiota‐Gut‐Brain Axis. Physiol Rev 2019. 99 (4): 1877–2013. [DOI] [PubMed] [Google Scholar]
- Decker J, Kaloostian CL, Gurvich T. Beyond Cognitive Screening: Establishing an Interprofessional Perioperative Brain Health Initiative. J Am Geriatr Soc 2020. 68 (10): 2359–2364. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA 2011. 108 (7): 3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Cryan JF. The Microbiome‐Gut‐Brain Axis in Health and Disease. Gastroenterol Clin North Am 2017. 46 (1): 77–89. [DOI] [PubMed] [Google Scholar]
- Eckenhoff RG, Maze M, Xie Z. Perioperative Neurocognitive Disorder: State of the Preclinical Science. Anesthesiology 2020. 132 (1): 55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D, de Angelis Hrabě AL, Jaitin D. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015. 18 (7): 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evered L, Silbert B, Knopman DS. Recommendations for the Nomenclature of Cognitive Change Associated with Anaesthesia and Surgery‐2018. Anesthesiology 2018. 129 (5): 872–879. [DOI] [PubMed] [Google Scholar]
- Evered LA, Goldstein PA. Reducing Perioperative Neurocognitive Disorders (PND) Through Depth of Anesthesia Monitoring: A Critical Review. Int J Gen Med 2021. 14: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Mai L, Zhu X, Huang F, He H. The Role of Microglia in Perioperative Neurocognitive Disorders. Front Cell Neurosci 2020. 14: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira‐Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM. Alzheimer's disease: Targeting the Cholinergic System. Curr Neuropharmacol 2016. 14 (1): 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia PS, Kolesky SE, Jenkins A. General anesthetic actions on GABA(A) receptors. Curr Neuropharmacol 2010. 8 (1): 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler LE, Park SM, Opitz N, Lyte M, Gaykema RP. Campylobacter jejuni infection increases anxiety‐like behavior in the holeboard: possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav Immun 2008. 22 (3): 354–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore‐Booth J, Mellin‐Olsen J. Data matters: implications for surgery and anesthesia in achieving universal health coverage. Can J Anaesth 2019. 66 (2): 143–148. [DOI] [PubMed] [Google Scholar]
- Haam J, Yakel JL. Cholinergic modulation of the hippocampal region and memory function. J Neurochem 2017. 142 Suppl 2 (Suppl 2): 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase S, Wilck N, Haghikia A, Gold R, Mueller DN, Linker RA. The role of the gut microbiota and microbial metabolites in neuroinflammation. Eur J Immunol 2020. 50 (12): 1863–1870. [DOI] [PubMed] [Google Scholar]
- Hadizadeh M, Hamidi GA, Salami M. Probiotic supplementation improves the cognitive function and the anxiety‐like behaviors in the stressed rats. Iran J Basic Med Sci 2019. 22 (5): 506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambrecht‐Wiedbusch VS, Mitchell MF, Firn KA, Baghdoyan HA, Lydic R. Benzodiazepine site agonists differentially alter acetylcholine release in rat amygdala. Anesth Analg 2014. 118 (6): 1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh TH, Kuo CW, Hsieh KH. Probiotics Alleviate the Progressive Deterioration of Motor Functions in a Mouse Model of Parkinson's Disease. Brain Sci 2020. 10 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SE, Lim SM, Jeong JJ. Gastrointestinal inflammation by gut microbiota disturbance induces memory impairment in mice. Mucosal Immunol 2018. 11 (2): 369–379. [DOI] [PubMed] [Google Scholar]
- Jašarević E, Howard CD, Morrison K. The maternal vaginal microbiome partially mediates the effects of prenatal stress on offspring gut and hypothalamus. Nat Neurosci 2018. 21 (8): 1061–1071. [DOI] [PubMed] [Google Scholar]
- Jiang XL, Gu XY, Zhou XX. Intestinal dysbacteriosis mediates the reference memory deficit induced by anaesthesia/surgery in aged mice. Brain Behav Immun 2019. 80: 605–615. [DOI] [PubMed] [Google Scholar]
- Kong FJ, Ma LL, Zhang HH, Zhou JQ. Alpha 7 nicotinic acetylcholine receptor agonist GTS‐21 mitigates isoflurane‐induced cognitive impairment in aged rats. J Surg Res 2015. 194 (1): 255–261. [DOI] [PubMed] [Google Scholar]
- Kumar A, Foster TC. Alteration in NMDA Receptor Mediated Glutamatergic Neurotransmission in the Hippocampus During Senescence. Neurochem Res 2019. 44 (1): 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Freche H, Brouillette J, Fernandez‐Gomez FJ. Tau phosphorylation and sevoflurane anesthesia: an association to postoperative cognitive impairment. Anesthesiology 2012. 116 (4): 779–787. [DOI] [PubMed] [Google Scholar]
- Liang P, Shan W, Zuo Z. Perioperative use of cefazolin ameliorates postoperative cognitive dysfunction but induces gut inflammation in mice. J Neuroinflammation 2018. 15 (1): 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W, Yang Y, Wang H. Gut microbiota shifts in patients with gastric cancer in perioperative period. Medicine (Baltimore) 2019. 98 (35): e16626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Chen Y, Zhang P, Chen G, Zhou Y, Yu X. The potential mechanism of postoperative cognitive dysfunction in older people. Exp Gerontol 2020. 130: 1 10791. [DOI] [PubMed] [Google Scholar]
- Liufu N, Liu L, Shen S. Anesthesia and surgery induce age‐dependent changes in behaviors and microbiota. Aging (Albany NY) 2020. 12 (2): 1965–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Yang L, Chen X, Li S. Tau hyperphosphorylation: a downstream effector of isoflurane‐induced neuroinflammation in aged rodents. Med Hypotheses 2014. 82 (1): 94–96. [DOI] [PubMed] [Google Scholar]
- Lyman M, Lloyd DG, Ji X, Vizcaychipi MP, Ma D. Neuroinflammation: the role and consequences. Neurosci Res 2014. 79: 1–12. [DOI] [PubMed] [Google Scholar]
- Martin CR, Osadchiy V, Kalani A, Mayer EA. The Brain‐Gut‐Microbiome Axis. Cell Mol Gastroenterol Hepatol 2018. 6 (2): 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer SV, Williams CL. The Cholinergic System Modulates Memory and Hippocampal Plasticity via Its Interactions with Non‐Neuronal Cells. Front Immunol 2017. 8: 1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest 2015. 125 (3): 926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, Gray JD. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology 2016. 41 (1): 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav 2003. 43 (1): 2–15. [DOI] [PubMed] [Google Scholar]
- Meng F, Li N, Li D, Song B, Li L. The presence of elevated circulating trimethylamine N‐oxide exaggerates postoperative cognitive dysfunction in aged rats. Behav Brain Res 2019. 368: 11 1902. [DOI] [PubMed] [Google Scholar]
- Mittal R, Debs LH, Patel AP. Neurotransmitters: The Critical Modulators Regulating Gut‐Brain Axis. J Cell Physiol 2017. 232 (9): 2359–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal R, Mainali R, Ahmadi S. Gut microbiome and aging: Physiological and mechanistic insights. Nutr Healthy Aging 2018. 4 (4): 267–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham MJ, Webb CE, Bryden DC. Postoperative cognitive dysfunction and dementia: what we need to know and do. Br J Anaesth 2017. 119 (suppl_1): i115–i125. [DOI] [PubMed] [Google Scholar]
- Oelschlaeger TA. Mechanisms of probiotic actions ‐ A review. Int J Med Microbiol 2010. 300 (1): 57–62. [DOI] [PubMed] [Google Scholar]
- Olotu C. Postoperative neurocognitive disorders. Curr Opin Anaesthesiol 2020. 33 (1): 101–108. [DOI] [PubMed] [Google Scholar]
- Quigley E. Microbiota‐Brain‐Gut Axis and Neurodegenerative Diseases. Curr Neurol Neurosci Rep 2017. 17 (12): 94. [DOI] [PubMed] [Google Scholar]
- Rose CR, Felix L, Zeug A, Dietrich D, Reiner A, Henneberger C. Astroglial Glutamate Signaling and Uptake in the Hippocampus. Front Mol Neurosci 2017. 10: 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavynia SA, Goldstein PA The Role of Neuroinflammation in Postoperative Cognitive Dysfunction: Moving From Hypothesis to Treatment. Front Psychiatry 2018. 9: 752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant‐like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry 2007. 62 (1): 55–64. [DOI] [PubMed] [Google Scholar]
- Schupf N, Tang MX, Albert SM. Decline in cognitive and functional skills increases mortality risk in nondemented elderly. Neurology 2005. 65 (8): 1218–1226. [DOI] [PubMed] [Google Scholar]
- Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev 2010. 90 (3): 859–904. [DOI] [PubMed] [Google Scholar]
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK. The Central Nervous System and the Gut Microbiome. Cell 2016. 167 (4): 915–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniyan S, Terrando N. Neuroinflammation and Perioperative Neurocognitive Disorders. Anesth Analg 2019. 128 (4): 781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Ma K, Wen J. A Review of the Brain‐Gut‐Microbiome Axis and the Potential Role of Microbiota in Alzheimer's Disease. J Alzheimers Dis 2020. 73 (3): 849–865. [DOI] [PubMed] [Google Scholar]
- Tang WH, Kitai T, Hazen SL. Gut Microbiota in Cardiovascular Health and Disease. Circ Res 2017. 120 (7): 1183–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangestani Fard M, Stough C. A Review and Hypothesized Model of the Mechanisms That Underpin the Relationship Between Inflammation and Cognition in the Elderly. Front Aging Neurosci 2019. 11: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszewski D. Biomarkers of Brain Damage and Postoperative Cognitive Disorders in Orthopedic Patients: An Update. Biomed Res Int 2015: 40 2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole PW, Jeffery IB. Gut microbiota and aging. Science 2015. 350 (6265): 1214–1215. [DOI] [PubMed] [Google Scholar]
- Van Bodegom M, Homberg JR, Henckens M. Modulation of the Hypothalamic‐Pituitary‐Adrenal Axis by Early Life Stress Exposure. Front Cell Neurosci 2017. 11: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S, Schwabe L. Learning and memory under stress: implications for the classroom. NPJ Sci Learn 2016. 1: 16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Ma RH, Fang H, Xue ZG, Liao QW. Impaired Spatial Learning Memory after Isoflurane Anesthesia or Appendectomy in Aged Mice is Associated with Microglia Activation. J Cell Death 2015. 8: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wang Y, Wu H. Postoperative cognitive dysfunction: current developments in mechanism and prevention. Med Sci Monit 2014. 20: 1908–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen L, Xu Y. Gastrodin alleviates perioperative neurocognitive dysfunction of aged mice by suppressing neuroinflammation. Eur J Pharmacol 2021. 892: 173734. [DOI] [PubMed] [Google Scholar]
- Wen J, Ding Y, Wang L, Xiao Y. Gut microbiome improves postoperative cognitive function by decreasing permeability of the blood‐brain barrier in aged mice. Brain Res Bull 2020. 164: 249–256. [DOI] [PubMed] [Google Scholar]
- Westphalen RI, Hemmings HC. Effects of isoflurane and propofol on glutamate and GABA transporters in isolated cortical nerve terminals. Anesthesiology 2003. 98 (2): 364–372. [DOI] [PubMed] [Google Scholar]
- Xu X, Hu Y, Yan E, Zhan G, Liu C, Yang C. Perioperative neurocognitive dysfunction: thinking from the gut. Aging (Albany NY) 2020. 12 (15): 15797–15817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XD, Wang LK, Wu HY, Jiao L. Effects of prebiotic galacto‐oligosaccharide on postoperative cognitive dysfunction and neuroinflammation through targeting of the gut‐brain axis. BMC Anesthesiol 2018. 18 (1): 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson GP. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015. 161 (2): 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan DY, Du CK, Akiyama T. In vivo monitoring of acetylcholine release from cardiac vagal nerve endings in anesthetized mice. Auton Neurosci 2013. 176 (1–2): 91–94. [DOI] [PubMed] [Google Scholar]
- Zhan G, Hua D, Huang N. Anesthesia and surgery induce cognitive dysfunction in elderly male mice: the role of gut microbiota. Aging (Albany NY) 2019. 11 (6): 1778–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Tian M, Zheng H. Effects of anesthetic isoflurane and desflurane on human cerebrospinal fluid Aβ and τ level. Anesthesiology 2013. 119 (1): 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhu S, Jin P. Graphene oxide improves postoperative cognitive dysfunction by maximally alleviating amyloid beta burden in mice. Theranostics 2020. 10 (26): 11908–11920. [DOI] [PMC free article] [PubMed] [Google Scholar]