Abstract
Protoporphyrin IX (PPIX) is an intermediate in the heme biosynthesis pathway. Abnormal accumulation of PPIX due to certain pathological conditions such as erythropoietic protoporphyria and X-linked protoporphyria causes painful phototoxic reactions of the skin, which can significantly impact daily life. Endothelial cells in the skin have been proposed as the primary target for PPIX-induced phototoxicity through light-triggered generation of reactive oxygen species. Current approaches for the management of PPIX-induced phototoxicity include opaque clothing, sunscreens, phototherapy, blood therapy, antioxidants, bone marrow transplantation, and drugs that increase skin pigmentation. In this review, we discuss the present understanding of PPIX-induced phototoxicity including PPIX production and disposition, conditions that lead to PPIX accumulation, symptoms and individual differences, mechanisms, and therapeutics.
Keywords: Heme, Protoporphyrin IX, Erythropoietic protoporphyria, X-linked protoporphyria, Phototoxicity
1. Introduction
Under physiologic conditions, the production and disposition of protoporphyrin IX (PPIX), the immediate precursor of heme, are tightly regulated and maintained at low levels in the body (Brun and Sandberg, 1991). Whenever the intricate regulation of heme biosynthesis is perturbed due to certain disorders of enzymes or transporters, PPIX may accumulate in the liver and erythrocytes, potentially reaching a toxic level (Brun and Sandberg, 1991). It is well-recognized that over-accumulated PPIX leads to dermal phototoxicity due to its electron-rich structure (Lim, 1989). The severity of protoporphyria symptoms varies even among patients with the same mutation and degree of inherited enzyme deficiency, suggesting that additional factors contribute to PPIX accumulation and the severity of PPIX-induced phototoxicity (Fukuda et al., 2016; Ged et al., 2004; To-Figueras et al., 2007). Despite earlier reports of the clinically important pathophysiology of PPIX accumulation (Brun and Sandberg, 1991; Kosenow and Treibs, 1953; Magnus et al., 1961), the molecular mechanisms of PPIX-induced phototoxicity are not wellunderstood.
In the clinic, abnormal accumulation of PPIX occurs in patients with erythropoietic protoporphyria (EPP) and X-linked protoporphyria (XLP) (Balwani, 2019; Piomelli et al., 1975). EPP is the most common type of protoporphyria, affecting 1 in 50,000 to 200,000 individuals (Elder et al., 2013; Ramanujam and Anderson, 2015). XLP accounts for up to 10% of the patients diagnosed with protoporphyria in North America and 2% in Europe (Balwani, 2019). With light exposure, early or prodromal symptoms of protoporphyrias include itching and burning, followed by increasingly severe pain, erythema, and edema after more prolonged exposure, but usually without formation of blisters (Balwani, 2019). The time to first symptoms varies considerably among patients. Currently, options for the management of protoporphyrias include opaque clothing, sunscreens, phototherapy, blood therapy, antioxidants, bone marrow transplantation, and drugs that increase skin pigmentation (Dickey et al., 2022; Diffey and Farr, 1991; Heerfordt et al., 2023; Minder et al., 2009; Tintle et al., 2014). This review discusses our present knowledge regarding PPIX-induced phototoxicity from preclinical and clinical perspectives. PubMed® (https://pubmed.ncbi.nlm.nih.gov/) was used as the major database for the current review and supplemented with other databases such as Elsevier.
2. PPIX production and disposition
PPIX is mainly produced in the bone marrow (~85%) and secondarily in the liver (15%) through the heme biosynthesis pathway with the involvement of 8 enzymes (Fig. 1). It starts with the condensation of glycine and succinyl-CoA catalyzed by 5-aminolevulinic acid synthase (ALAS), the rate-limiting enzyme of the heme biosynthesis pathway, to form 5-aminolevulinic acid (ALA) in the mitochondria (Burch et al., 2018; Gibson et al., 1958; Phillips, 2019; Strand et al., 1970; Tschudy et al., 1975). Two isoforms of ALAS have been identified: ALAS1 is expressed in all tissues including the liver, and ALAS2 is expressed only in the bone marrow (Cable et al., 2000; Kolluri et al., 2005; Melefors et al., 1993). Next, ALA is transported out from mitochondria to cytoplasm and goes through four enzyme-dependent steps to form coproporphyrinogen III (Gibson et al., 1955). Coproporphyrinogen III is transported back into mitochondria and oxidized by coproporphyrinogen oxidase to produce protoporphyrinogen IX, which is further oxidized to produce PPIX by protoporphyrinogen oxidase (Bogorad, 1958a; b). The final step is the incorporation of iron into PPIX to produce heme, catalyzed by the enzyme ferrochelatase (FECH) (Riethmueller and Tuppy, 1964).
Fig. 1.
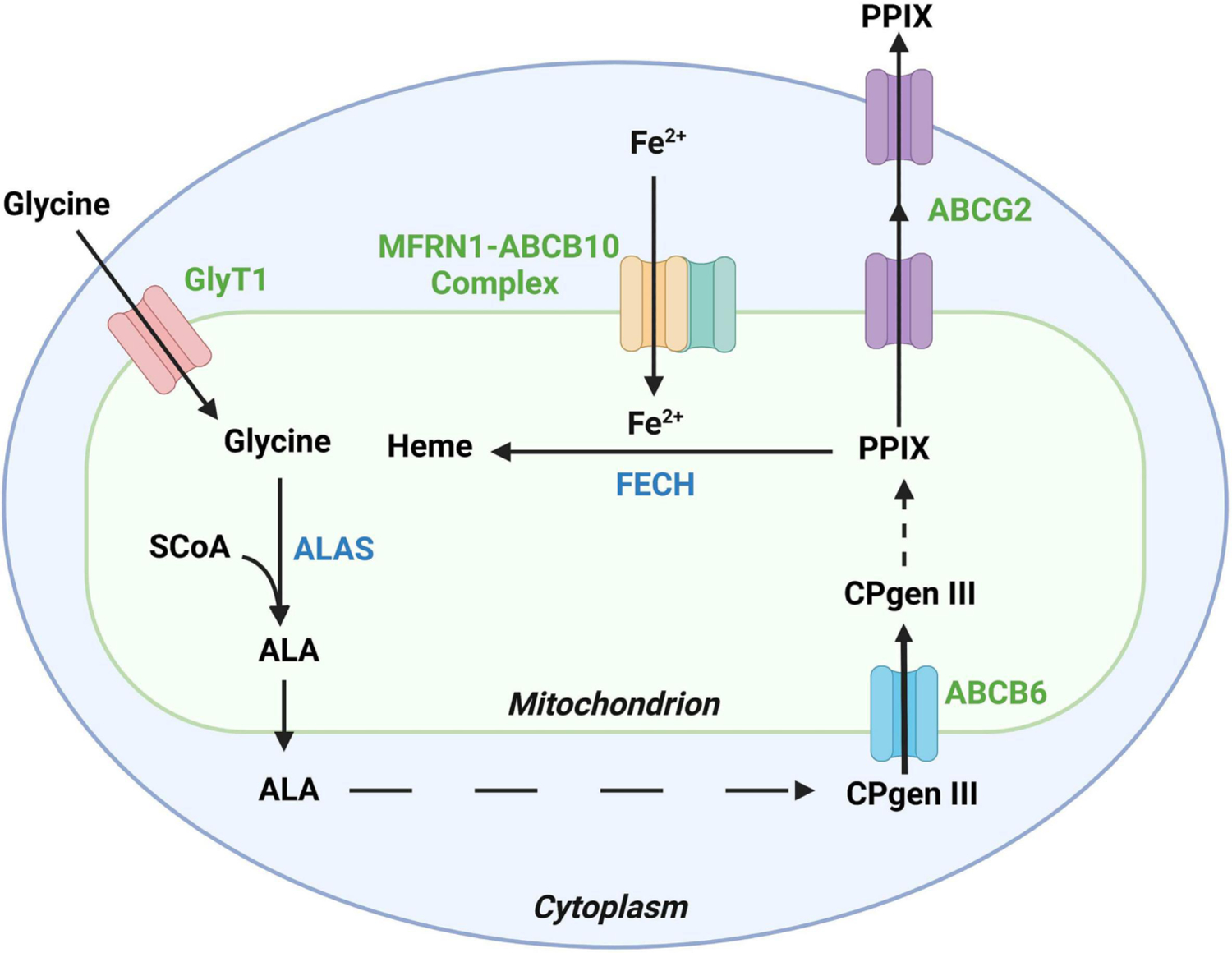
Major enzymes and transporters that are associated with protoporphyrin IX (PPIX) production and disposition. PPIX is an intermediate in the heme biosynthesis pathway, in which aminolevulinic acid synthase (ALAS) is the rate-limiting enzyme and ferrochelatase (FECH) is the enzyme that converts PPIX to heme. The ATP-binding cassette subfamily G member 2 (ABCG2) transports PPIX out of mitochondrion and the cell for excretion. Glycine transporter 1 (GlyT1), ABCB6, and the mitoferrin 1(MFRN1)-ABCB10 complex are also involved in the heme biosynthesis pathway, which transport glycine, coproporphyrinogen III (CPgen III), and iron, respectively. SCoA, Succinyl-coenzyme A. Created by Biorender.
In addition to enzymes, several transporters are involved in PPIX production and disposition (Fig. 1). The glycine transporter 1 (GlyT1) is located on the mitochondrial membrane, which feeds glycine into mitochondria to start heme biosynthesis (King and Gunn, 1989; Winter et al., 2016). ATP-binding cassette transporter subfamily B member 6 (ABCB6) is located on the outer mitochondrial membrane, which transports coproporphyrinogen III from cytoplasm back into mitochondria for the last three steps of heme biosynthesis (Krishnamurthy et al., 2006; Ulrich et al., 2012). Mitoferrin-1 (MFRN1) is a mitochondrial transporter responsible for transporting ferrous iron into mitochondria for the last step of heme biosynthesis (Chen et al., 2009). The catalytic activity of MFRN1 requires physical interaction with another mitochondrial transporter ABCB10 to form a MFRN1-ABCB10 complex (Chen et al., 2009). ATP-binding cassette transporter subfamily G member 2 (ABCG2) is expressed in both mitochondrial cristae and cell membrane (Austin Doyle et al., 1998; Gottesman et al., 2002; Solazzo et al., 2009). ABCG2 pumps PPIX out of bone marrow cells and red blood cells into plasma (Jonker et al., 2002b), and then PPIX is taken up by hepatocytes and excreted into the bile ducts. ABCG2 also contributes to the excretion of PPIX from hepatocytes to the biliary system and feces, the major pathway for the elimination of PPIX from the body (Ibrahim and Watson, 1968; Thapar and Bonkovsky, 2008; Wang et al., 2019).
3. Conditions that lead to accumulation of PPIX
3.1. FECH mutations and EPP
FECH is responsible for the insertion of iron into PPIX to form heme so that when FECH activity drops to less than 30 percent of the normal level, accumulation of PPIX significantly increases (Balwani, 2019). EPP is caused by loss-of-function mutations of FECH (Labbé et al., 1999). Up to 2003, more than 190 different mutations have been identified for the FECH gene (Stenson et al., 2003). In recent years, several novel FECH mutations have been discovered (Munemoto et al., 2022; Saito et al., 2020; Weiss et al., 2019). Among all the possible mutation types that have been observed in human FECH, including exon skipping, missense, nonsense, and deletion, exon 10 skipping caused by a transversion of A to T in intron 10 is a relatively common type of FECH mutation in EPP patients (Chen et al., 2002; Wang et al., 1993).
3.2. ALAS2 mutations and XLP
XLP is caused by a gain-of-function mutation of ALAS2, which greatly increases the rate of ALA formation in the bone marrow (Whatley et al., 2008a). The mutation increases the enzymatic activity of ALAS2 approximately 1.6- to 3.1-fold compared to the normal ALAS2 activity (Bishop et al., 2013). Specifically, this gain-of-function mutation is caused by deletion-induced frameshift mutation in exon 11 of ALAS2, leading to the change of 19–20 C-terminal amino acids and overall alternation of the secondary structure of ALAS2 (Whatley et al., 2008b). Exon 11 is a key regulator region for ALAS2 so that once mutated, this regulation is no longer functional and ALA becomes overproduced (Fratz et al., 2015). After ALAS2, FECH is the next limiting enzyme in the pathway, so even a mild increase in ALAS2 activity is enough to cause significant PPIX accumulation (Shah et al., 2012).
3.3. Chemicals that causes PPIX accumulation through ALAS induction
Genetic and chemical factors that cause PPIX overproduction in marrow erythroid precursor cells and hepatocytes are quite distinct. The liver endoplasmic reticulum is rich in cytochrome P450 enzymes (CYPs) that turn over rapidly and account for most of the large amounts of heme synthesized in the liver. Most hepatic CYPs are inducible by exogenous and endogenous chemicals, and these inducers can upregulate hepatic ALAS1 expression, resulting in increased synthesis of intermediates in the heme synthesis pathway, including the upstream intermediates that can cause acute attacks of porphyrias and the downstream intermediate PPIX that may potentate the symptoms of EPP (Fraser et al., 2003; Podvinec et al., 2004). Preclinical studies showed that many porphyrinogenic drugs, such as barbiturates, glutethimide, and 2-propyl-2-isopropylacetamide, significantly induced both hepatic CYPs and ALAS (Hamilton et al., 1988). Co-treatment of rifampicin and isoniazid upregulates ALAS1 in mouse liver, leading to hepatic accumulation of PPIX (Li et al., 2013). In addition, ALA loading, for example, during the photodynamic therapy, can bypass the rate limiting enzyme ALAS1, leading to accumulation of PPIX in many tissues including tumors (Kennedy et al., 1990).
3.4. Chemicals that cause PPIX accumulation through FECH suppression or iron deficiency
FECH inhibitors such as salicylic acid and alkylated-PPIX can cause PPIX accumulation (Cole and Marks, 1984; Gupta et al., 2013). Certain porphyrinogenic molecules such as griseofulvin potently inhibit FECH and result in hepatic PPIX accumulation through formation of N-methyl protoporphyrin IX (Holley et al., 1991; Liu et al., 2015). Another group of porphyrinogenic molecules, 5-diethoxycarbonyl-1,4-dihydro-2,4,6-trimethylpyridine and its 4-alkyl substituted analogs, show various potencies in both inducing formation of N-alkyl PPIX and directly inhibiting FECH activity, leading to hepatic PPIX accumulation (Cole et al., 1981; McCluskey et al., 1992; Ortiz de Montellano et al., 1981). Isoniazid, a common drug used as a part of tuberculosis treatment, is reported to cause PPIX accumulation by downregulating FECH protein in mouse liver (Sachar et al., 2016). The significance of these preclinical findings on chemical-induced PPIX accumulation are not clear for EPP/XLP patients. However, these chemicals may potentiate the symptoms of EPP/XLP because of their effects on PPIX accumulation.
Metal ions such as lead, gallium, copper, cadmium, mercury, and aluminum ions can also inhibit FECH with variable potencies (Schauder et al., 2010). In addition, iron chelators such as deferoxamine, ethylenediaminetetraacetic acid, and thiosemicarbazone derivatives, can cause PPIX accumulation by limiting iron availability for FECH activity (Amo et al., 2009; Berg et al., 1996; Gawecki et al., 2019; Troadec et al., 2011b; Yamamoto et al., 2014). Furthermore, deficiency and/or inhibition of iron transporters MFRN1 and ABCB10 restrain the supply of iron and thus reduce FECH activity, leading to hepatic PPIX accumulation (Troadec et al., 2011a).
3.5. Other conditions that lead to PPIX accumulation
PPIX is mainly excreted from the body by biliary excretion (Ibrahim and Watson, 1968; Thapar and Bonkovsky, 2008). Cholestasis is a type of liver disease that results from intra- or extrahepatic biliary obstruction, which impairs hepatic and biliary excretion of PPIX and can lead to progressive increases in circulating levels of PPIX and accelerated progression of liver damage (Yang et al., 2018). Any conditions causing cholestasis such as taking estrogen-based oral contraceptives may therefore increase the risk of PPIX accumulation (Chen et al., 2013). In addition, genetic deficiency of ABCG2 and ABCB6 would decrease PPIX excretion which in turn causes PPIX accumulation (Fukuda et al., 2016; Jonker et al., 2002a; Sakiyama et al., 2021; Tamura et al., 2006). Furthermore, fasting increases the activity of proliferator-activated receptor-γ coactivator 1α, which upregulates ALAS expression, thereby causing PPIX overproduction and accumulation (Handschin et al., 2005).
4. Symptoms of PPIX-induced phototoxicity and individual differences
4.1. Symptoms of PPIX-induced phototoxicity
In EPP and XLP patients with the elevation of circulating levels of PPIX, which is usually life long, sunlight exposure can cause prodromal itching and burning followed by a severely painful non-blistering type of photosensitivity accompanied by erythema, edema, and systemic symptoms. Time to onset of first symptoms can vary from a few minutes to several hours of outdoor activities (Maitra et al., 2019a; Thapar and Bonkovsky, 2008). Prolonged sun exposure may cause vesicles and scarring, and repeated exposure may cause leathery skin thickening, especially on the knuckles and face (Thapar and Bonkovsky, 2008). A “priming effect” of phototoxicity in EPP is well-known, whereby sun exposure on one day enhances sensitivity to sunlight on the following days (Poh-Fitzpatrick, 1989). In a survey regarding the impact of EPP on quality of life, 85% of patients reported the priming effect (Holme et al., 2006). Compared to non-sunlight-primed skin, sunlight-primed skin has significantly more limited tolerance to further sunlight exposure (Heerfordt et al., 2020). The cause of this priming effect is believed to be a light-mediated release of PPIX from erythrocytes to the skin, leading to the increase of photosensitivity for a prolonged period as the skin concentration of PPIX remains high (Heerfordt and Wulf, 2016).
Aside from the primary effects of PPIX-induced phototoxicity, various secondary effects are not negligible. In a cross-sectional study, 46% of EPP patients were found to be deficient in vitamin D because they avoided sunlight exposure (Spelt et al., 2010). This may contribute to low bone mineral density, osteoporosis, and osteopenia in EPP (Allo et al., 2013; Biewenga et al., 2017). In addition, patients with EPP and XLP usually alter their activities and lifestyles to avoid sunlight, which can limit employment opportunities and/or cause depression (Naik et al., 2019a). Children and young adults find it difficult to adapt to their disease and explain it to others (Naik et al., 2019b). Overall, PPIX-induced phototoxicity in EPP and XLP causes a significant decrease in quality of life. Furthermore, roughly 10 to 20 percent of EPP patients develop hepatic dysfunctions and in about 2 to 5 percents of patients, the continued progression of liver damage can lead to death due to cirrhosis or liver failure (Anstey and Hift, 2007; Hagiwara et al., 2022). Moreover, a significant portion of EPP patients developed mild anemia (Wahlin et al., 2011).
4.2. Individual differences in PPIX-induced phototoxicity
Light tolerability varies among individual patients with EPP or XLP. Most patients develop phototoxic symptoms within 30 minutes of sun exposure while some patients can tolerate exposure for several hours (Di Pierro et al., 2022). The detailed mechanisms of individual differences in the development of PPIX-induced phototoxicity remain unclear, but multiple factors have been identified, including genetic modifiers, liver diseases, and chemical modifiers from diets and drugs.
4.2.1. Genetic factors that modulate PPIX-induced phototoxicity
Dysfunctional variants of ABCB6 were found to be strongly correlated with more severe porphyria phenotypes (Fukuda et al., 2016). As for ABCG2, the efflux transporter of PPIX, over 80 polymorphic variants have been reported and 3 of these (Q126stop, S441N, and F489L) are of clinical importance due to their ability to disrupt ABCG2 function (Tamura et al., 2006). These loss-of-function mutants of ABCG2 showed phototoxicity phenotypes when exposed to pheophorbide, an exogenous porphyrin, and reduced hematoporphyrin transportation (Tamura et al., 2006). In another study conducted with a small group of healthy volunteers, 2 dysfunctional variants of ABCG2 (Q126stop and Q141K) were found to be correlated with higher PPIX accumulation in erythrocytes and photosensitivity (Sakiyama et al., 2021). In contrast, genetic dysfunction of ABCG2 protected against phototoxicity in an EPP mouse model by trapping PPIX inside erythrocytes and reducing its exposure to the skin (Wang et al., 2019).
4.2.2. Chemical factors that potentiate PPIX-induced phototoxicity
CYP and ALAS inducers, FECH inhibitors, and iron chelators from diets and medications may enhance phototoxicity by increasing PPIX accumulation (Blakely et al., 2019; Cole and Marks, 1984; Cole et al., 1981; Gupta et al., 2013; Holley et al., 1991; Li et al., 2013; Liu et al., 2015; McCluskey et al., 1992; Ortiz de Montellano et al., 1981; Richarz et al., 2017). In addition, certain medications, such as amiodarone, chlorpromazine, doxycycline, hydrochlorothiazide, and nalidixic acid, have photosensitizing effects and hence, they may exacerbate phototoxicity in porphyria patients (Blakely et al., 2019; Richarz et al., 2017). Furthermore, impaired antioxidant defense may contribute to variability in the severity of PPIX-induced phototoxicity (Böhm et al., 2001).
4.2.3. Liver diseases that potentiate PPIX-induced phototoxicity
Damage to the liver, especially by cholestasis, can reduce its capacity to eliminate PPIX from the body (Sachar et al., 2016; Wagner et al., 2003). Therefore, severe cholestasis in EPP and XLP patients potentiate PPIX-induced phototoxicity (Lyoumi et al., 2011). In addition, albumin is synthesized in the liver and it is an endogenous carrier of PPIX (Cohen and Margalit, 1990; Seery and Muller Eberhard, 1973; Sułkowski et al., 2016). Liver diseases, such as cirrhosis, can decrease albumin production and increase free PPIX levels in the blood, resulting in more severe phototoxicity (Wen et al., 2022). Furthermore, chronic liver diseases decrease antioxidant defense that can potentiate PPIX-induced phototoxicity due to diminished quenching of ROS generated by photodynamic reactions (Li et al., 2021).
5. Mechanisms of PPIX-induced phototoxicity
Phototoxicity is well documented in EPP and XLP, although its molecular mechanisms are not fully understood (Kosenow and Treibs, 1953; Sachar et al., 2016). Here we outlined the current understanding of PPIX-induced phototoxicity in four steps: distribution of PPIX to the skin, light-mediated PPIX excitation, cellular damages, and aberrant repair processes.
5.1. Distribution of PPIX to the skin
The bone marrow is the major site of PPIX synthesis and erythrocytes are reported as the bulk carrier of excess PPIX in the protoporphyrias (Brun and Sandberg, 1991; Puy et al., 2010). ABCG2 is expressed in bone marrow erythroid cells and circulating erythrocytes, contributing to the efflux of PPIX from these cells into plasma (Jonker et al., 2002b). PPIX is increased 10-fold in erythrocytes of ABCG2-deficient mice, indicating that ABCG2 is critical in PPIX efflux (Jonker et al., 2002a). Genetic dysfunction of ABCG2 in normal human subjects was recently reported to be correlated with higher levels of PPIX in erythrocytes, which further supports an important role for ABCG2 in PPIX efflux from these cells (Sakiyama et al., 2021).
The release of PPIX from erythrocytes increases with light exposure (Brun and Sandberg, 1991; Sandberg et al., 1983). It was proposed that loss of affinity of PPIX to intracellular carrier globin proteins due to light exposure causes the release of PPIX through membrane-bound efflux transporters (Brun and Sandberg, 1991). In fact, in EPP patients, this phenomenon of light-induced PPIX-release is believed to occur whenever erythrocytes pass through sunlight-exposed dermal capillaries, which increases the risk of phototoxicity for local endothelial cells (Brun and Sandberg, 1991). Possibly due to the shorter daylight time during winter, higher levels of PPIX trapped in erythrocytes of EPP patients has been observed in the winter (Brun and Sandberg, 1991).
Albumin is the most abundant plasma protein, which binds to PPIX and is considered as a major endogenous carrier for extracellular PPIX (Cohen and Margalit, 1990; Seery and Muller Eberhard, 1973; Sułkowski et al., 2016). In the presence of light, albumin facilitates the transfer of PPIX from erythrocytes to endothelial cells (Brun and Sandberg, 1991). In addition, PPIX also binds to hemopexin and immunoglobulin G in plasma (Brancaleon and Moseley, 2002; Seery and Muller Eberhard, 1973). Furthermore, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) may bind to PPIX in plasma (Beltramini et al., 1987). Though lipoprotein concentration is less than 5% of albumin, their contributions as PPIX carrier may be important because one LDL molecule can bind to fifty molecules of PPIX and these lipoproteins have the potential to transport PPIX to various tissues that express lipoprotein receptors (Brun and Sandberg, 1991).
Dermal endothelial cells, as the primary target of PPIX-induced phototoxicity (Brun and Sandberg, 1991), might be exposed to PPIX through multiple ways (Fig. 2). Dermal endothelial cells could uptake PPIX from plasma, because these cells are surrounded by plasma that is enriched with PPIX (Brun and Sandberg, 1991). In addition, since erythrocytes (diameter 7 μm) need to be squeezed while passing through dermal capillaries (diameter 4 – 5 μm), PPIX may be directly transferred to endothelial cells due to such close proximity to erythrocytes and its large concentration gradient (~ 1 mM) (Brun and Sandberg, 1991; Brun et al., 1990). Apart from the external sources of PPIX from plasma and erythrocytes, skin cells may produce their own PPIX because subcutaneous injection of ALA into mice showed fluorescence in the skin suggesting in situ synthesis (Sima et al., 1981). In addition, topical application of ALA-based photodynamic therapy causes vascular damage in the skin, indicating the synthesis and accumulation of PPIX in endothelial cells (De Bruijn et al., 2007; de Bruijn et al., 2008). Further studies are needed to determine which pathway, including transporter-dependent and independent pathways, is more important for the distribution of PPIX to dermal endothelial cells.
Fig. 2.

Pathways that PPIX is exposed to the endothelial cells of dermal capillaries in EPP. Three mechanisms have been proposed: (1) PPIX is directly transferred from plasma into endothelial cells down the concentration gradient; (2) PPIX in red blood cell (RBC) is transferred into endothelial cells during the squeezing movement of RBC through the contact of cell membranes; and (3) de novo synthesis of PPIX in endothelial cells. N, nucleus. Created by Biorender.
5.2. Light-mediated PPIX excitation
PPIX needs to be excited by light before inducing phototoxicity in the skin (Maitra et al., 2019a; Peterka et al., 1965). When chronic skin lesions of EPP patients were analyzed by electron microscopy, the main abnormality was found to be associated with small blood vessels in the upper part of the dermis (Ryan and Madill, 1968). Such skin abnormalities were absent in the cloth-covered areas of the skin, suggesting that exposure to sunlight is critical for PPIX-associated skin damage (Ryan and Madill, 1968). The maximal absorption wavelength area of PPIX is 400–600 nm, which can easily penetrate the skin and trigger photodynamic reaction in dermal endothelial cells (Mahmoud et al., 2008; Schnait et al., 1975).
PPIX-mediated endothelial damage was found to be specific to long-wave UV (UV-A) light but not short-wave UV light (UV-B) (Schnait et al., 1975). Long-wave UV light comes naturally from sunlight or glass-filtered artificial lamp and matches within the action spectrum of EPP at around 400 nm (Magnus et al., 1961; Schnait et al., 1975). The short-wave UV light (< 310 nm) which spares the dermal areas from injury actually damages the epidermal areas. Since epidermis was reported to contain very little PPIX, the epidermal lesion observed was suggested to be a typical sunburn instead of PPIX-induced phototoxicity (Gog and Schothorst, 1973; Schnait et al., 1975). The acute flare symptoms observed in EPP patients was reproduced in griseofulvin-induced protoporphyria mouse models, which confirmed that exposure to UV-A irradiation and PPIX are two pre-requisites for endothelial damage in dermal vessels (Konrad et al., 1975).
5.3. Cellular damages in the skin caused by PPIX
The acute skin lesion of EPP patients showed epidermal vacuolization and intercellular edema together with endothelial vacuolization and cytolysis in the superficial blood vessels (Lecha, 2003). Over time, accumulation of hyaline material around dermal blood vessels due to the change of vasopermeability causes capillary basement membranes thickening (Di Pierro et al., 2022; Schnait et al., 1975). The mechanisms of skin damages in EPP is associated with light-triggered production of reactive oxygen species (ROS) from PPIX (Ishizuka et al., 2011). Photodynamic reactions of PPIX lead to the formation of ROS including singlet oxygen, hydroxyl radicals, and super oxide anion radicals (Takeshita et al., 2004). The photodynamic reactions of PPIX were proposed to occur by either type-I or type-II photosensitized reactions (Brun and Sandberg, 1991). ROS is well-known for inducing various types of cell damages by reacting with cellular proteins, lipids, and nucleic acids (Girotti, 2001; Kurz et al., 2021). Overall, dermal endothelial cells come to be the primary target of PPIX-induced phototoxicity because: (1) dermal penetrance of long-wave UV light which aligns with the absorbance maxima of PPIX; (2) endothelial cells possesses the highest partial pressure of oxygen which is essential for the photodynamic process of PPIX, and (3) overt sensitivity of endothelial cells to oxidative stress (Brun and Sandberg, 1991).
Apart from the non-specific damage by light-induced PPIX excitation and ROS formation, organelle-specific protein aggregation was also observed in nucleus, endoplasmic reticulum (ER), and lysosome (Maitra et al., 2019a; Maitra et al., 2021). Such organelle-selective protein aggregation occurs through a “porphyrination-deporphyrination” cycle (Maitra et al., 2021). In porphyrination step, PPIX binds to target proteins and distorts tertiary structures (Belcher et al., 2009; Fernandez et al., 2008). If light exposed, photosensitization of this protein-PPIX complex generates singlet oxygen, which oxidizes methionine to form methionine sulfone or sulfoxide, leading to protein aggregation (Maitra et al., 2019b). As PPIX-induced protein aggregations was also noticed in internal organs, which are not exposed to light, endogenous ROS formed due to various reasons such as inflammation may also contribute to PPIX-induced protein aggregation (Maitra et al., 2021).
PPIX-induced phototoxicity is more severe when coupled with perivascular mast cell degranulation (Konrad et al., 1975). Previously, the H1-receptor antagonist terfenadine was shown to reduce the acute flare reaction in EPP patients when irradiated with blue light (Farr et al., 1990). However, phototoxicity of hematoporphyrin derivative in mast-cell deficient mice was similar to controls, suggesting the role of mast cells may not be critical for PPIX-induced phototoxicity (Lim et al., 1986).
5.4. Aberrant repair processes in PPIX-induced phototoxicity
When EPP patients are exposed to sunlight repeatedly, they usually develop thick skin in their cloth-uncovered areas (Murphy, 2003). Electron microscopy of the thick skin areas of EPP patients revealed that the basement membrane adjacent to dermal endothelial cells was largely replicated, layered, and fragmented (Ryan and Madill, 1968). This phenotype was suggested to be the result of consecutive repairs after endothelial injury (Ryan and Madill, 1968). Great amount of mast cells and extravascular erythrocytes were found in this area, indicating the increase of capillary permeability which causes prolonged edema (Ryan and Madill, 1968). This was further supported by the presence of plasma-derived products in the composition of amorphous hyaline deposits within the dermis as previously shown by histochemistry from chronically sun-exposed skin of EPP patients (Ryan, 1966; Ryan and Madill, 1968; Schnait et al., 1975; Timonen et al., 2000).
ER stress was observed in response to PPIX-induced protein aggregations (Elenbaas et al., 2016; Maitra et al., 2019a). In an acute protoporphyria model of zebrafish larvae, PPIX accumulation caused hepatic protein aggregation with multi-organelle alterations including ER (Elenbaas et al., 2016). As a part of an adaptive response, ER stress is well-known to upregulate ER chaperones proteins to repair defective proteins (Ron and Walter, 2007; Rutkowski and Kaufman, 2004). However, persistent ER stress leads to cell death (Lee et al., 2018). In addition, PPIX accumulation inactivates the chaperones resulting in a novel form of ER damage, which distorts the shape of ER compartment as identified under electron microscope (Elenbaas et al., 2016; Maitra et al., 2019a). Such protein aggregation-induced ER stress and ER damage are also likely to occur in PPIX-associated skin phototoxicities. Indeed, inhibition of ER stress was found to protect against cell death in a blue light-induced skin injury model, suggesting a relation between skin phototoxicity and ER stress-induced cell death (Zhu et al., 2022).
6. Therapeutic approaches for PPIX-induced phototoxicity
Since PPIX, light, and ROS are key players in the phototoxicity experienced by patients with EPP and XLP, current therapeutic approaches aim to shield the skin from light penetration, prevent PPIX excitation, and scavenge free radicals (Minder et al., 2009; Tintle et al., 2014). In recent years, novel strategies were tested in vitro and in animal models with the aim of reducing PPIX accumulation by restoring FECH activity or modulating the transporters related to PPIX biosynthesis or disposition. Here, we summarize these approaches and discuss their mechanism of action, efficacy, and limitations for the management of PPIX-induced phototoxicity.
6.1. Current approaches
6.1.1. β-carotene
β-carotene was reported to offer photoprotection to patients with EPP in 1970 (Mathews-Roth et al., 1970). After oral administration, this carotenoid compound accumulates in the epidermis and reduces light penetration and scavenges free radicals (Mathews-Roth, 1998; Tintle et al., 2014). Typical oral β-carotene dosing is 100–300 mg/day in adults, with a target serum carotene level of 6–8 mg/L (Tintle et al., 2014). Improved light tolerance and reduced light sensitivity was found in 86% patients within 1–3 months of starting treatment (Minder et al., 2009; Tintle et al., 2014). However, another randomized controlled study found no significant efficacy of β-carotene in EPP patients (Corbett et al., 1977). Regarding the side effects of β-carotene, it makes the skin yellowish and may increase the risk of pulmonary malignancy in smokers (Mathews-Roth, 1998; Minder et al., 2009; Tintle et al., 2014).
6.1.2. Phototherapy
Narrowband UV-B phototherapy can improve light tolerances in EPP patients (Roelandts, 1995; Tintle et al., 2014), although it may potentiate erythema, pruritus, skin photoaging, and risk of skin cancer (Collins and Ferguson, 1995). Improved light tolerance by UV-B phototherapy is believed to result from increased skin pigmentation and epidermal thickening (Tintle et al., 2014; Warren and George, 1998). However, the sample size was small in these uncontrolled studies using narrowband UV-B therapy and phototoxic symptoms were only improved in some but not all the patients (Collins and Ferguson, 1995; Sivaramakrishnan et al., 2014). Larger-scale and controlled studies are needed in the future to evaluate the safety and efficacy of UV-B phototherapy in EPP.
6.1.3. Afamelanotide
Afamelanotide is an α-melanocyte-stimulating hormone analog that induces the synthesis of melanin and eumelanin, leading to epidermal hyperpigmentation (Tintle et al., 2014), and is FDA-approved for treatment of EPP (Minder et al., 2009; Wu and Cotliar, 2021). Afamelanotide also has antioxidant and anti-inflammatory activity (Tintle et al., 2014). A three-year observational study showed that afamelanotide increased phototoxic burn tolerance time and quality of life in EPP patients (Barman-Aksozen et al., 2020). The most common adverse effects observed in clinical studies were nausea (19%), headache (20%), and implant site reactions, including discoloration, pain, hematoma or erythema (21%) (Langendonk et al., 2015). A potential drawback is that implantation by a health care professional is required, which may be inconvenient for some patients (Langendonk et al., 2015).
6.1.4. Bone marrow transplantation
Since the bone marrow is the major site of PPIX production, bone marrow transplantation is often discussed as treatment for protoporphyrias (Casanova-Gonzalez et al., 2010). It was reported in 2021 that 9 patients underwent successful bone marrow transplantation and were cured of EPP and no longer had phototoxic symptoms (Wang et al., 2021). One case who was cured of protoporphyria was a child with XLP (Butler et al., 2015). Cost and the risk of bone marrow transplantation are the major drawbacks of this therapeutic strategy (Wang et al., 2021). In addition, it is plausible that some patients could not be cured by bone marrow transplantation, especially for liver toxicity, because PPIX is also produced in the liver.
6.1.5. Other available approaches
Antioxidants, such cysteine, N-acetylcysteine, and vitamin C, have been reported to benefit EPP patients by quenching ROS formed in PPIX-induced phototoxicity (Boffa et al., 1996; Mathews-Roth et al., 1994; Minder et al., 2009). However, their effectiveness is limited because they do not reduce PPIX accumulation in tissues or prevent light-induced PPIX excitation. Nevertheless, antioxidants are sometimes used as adjuvants together with the above-mentioned approaches for EPP therapy. In addition, opaque clothing is commonly used by EPP patients, which can block visible light (Heerfordt et al., 2020). Special sunscreen with high sun protection factor is also frequently used for EPP patients with phototoxic symptoms (Diffey and Farr, 1991; Thapar and Bonkovsky, 2008). It has been shown that microfine titanium dioxide sunscreen significantly increased light tolerance in EPP patients (Diffey and Farr, 1991). In recent couple decades, a number of improved sunscreens have been developed with similar or greater ability to protect EPP patients from sunlight (Moseley et al., 2001; Petersen et al., 2014). Furthermore, blood therapy to exchange red blood cells can effectively lower circulating PPIX concentration, but it may cause infection and iron overload in a long term (Leaf and Dickey, 2023). Additionally, red blood cell exchange and plasmapheresis are ineffective in preventing liver damage or reversing the progression towards liver failure in EPP patients (Eichbaum et al., 2005; Pagano et al., 2012).
6.2. Approaches under development
6.2.1. Dersimelagon
Dersimelagon (MT-7117), is currently undergoing a phase 3 clinical trial for treatment of EPP and XLP (Mitsubishi Tanabe Pharma Development America, 2021). It is a potent melanocortin 1 receptor agonist that induces the synthesis of eumelanin, leading to skin hyperpigmentation (Suzuki et al., 2022). Various studies on dersimelagon showed good tolerance and favorable pharmacokinetic profiles among healthy participants with diverse ethnicities and age groups (Ogasawara et al., 2023; Ogawa et al., 2023; Tsuda et al., 2023). A phase 2 clinical trial demonstrated that the time to initial symptoms after sunlight exposure was significantly increased by dersimelagon (Balwani et al., 2020). Another controlled phase 2 trial confirmed the efficacy of dersimelagon at increasing safe sunlight exposure time for both XLP and EPP patients (Balwani et al., 2023). Common adverse effects of dersimelagon include skin hyperpigmentation, nausea, headache, freckles, and ephelides (Balwani et al., 2020; Balwani et al., 2023).
6.2.2. Bitopertin
Currently, a phase 2 clinical trial of bitopertin for EPP treatment is in progress (Disc Medicine, 2022). Bitopertin selectively and potently inhibits GlyT1, the transporter supplies intracellular glycine for heme biosynthesis (Winter et al., 2016). In vitro studies showed that inhibition of GlyT1 decreased the total amount of PPIX synthesized and reduced the extent of PPIX-induced cytotoxicity and oxidative stress (Halloy et al., 2021). Bitopertin was also effective in reducing cholestasis and liver fibrosis in an EPP mouse model (Wu et al., 2022).
6.2.3. Gene therapy
Most EPP patients harbor a hypomorphic allele, which produces an unstable mRNA with a premature stop codon, resulting in reduced FECH activity (Gouya et al., 1999). Transfecting EPP patient-derived cells by an antisense oligonucleotide targeting this defective allele of FECH gene restored the activity of FECH, resulting in decreased PPIX levels (Oustric et al., 2014). With an aim to specifically target erythrocyte cells, a nanocomplex delivery system was recently developed combining this antisense oligonucleotide with bifunctional transferrin receptor 1 ligand-peptides, which showed the reduction of PPIX levels in the treated primary culture cells derived from the erythroid cells of an EPP patient (Erwin and Balwani, 2021; Mirmiran et al., 2019). Thus, this approach holds promise for future in vivo trials.
6.2.4. ABCG2 inhibitors
PPIX-induced phototoxicity was fully prevented in an EPP mouse model deficient in ABCG2 (Wang et al., 2019), leading to the hypothesis that ABCG2 inhibitors will suppress EPP and XLP associated phototoxicity. Deficiency of ABCG2 decreased PPIX distribution to the skin by trapping PPIX in erythrocytes, resulting in a lower level of PPIX in the plasma and less phototoxicity (Wang et al., 2019). In addition, deficiency of ABCG2 also trapped PPIX in hepatocytes and attenuated PPIX-induced cholestatic liver injury (Wang et al., 2019). Further studies are needed to evaluate the efficacy and safety of ABCG2 inhibitors for EPP and XLP therapy.
7. Summary and perspective
The current review summarized PPIX-induced phototoxicity as related to PPIX production and disposition, PPIX accumulation, symptoms, underlying mechanisms, and therapeutic approaches in EPP and XLP. PPIX accumulation stems from mutations of heme biosynthetic enzymes (ALAS2 and FECH) and is potentiated by additional factors such as ALAS inducers and FECH inhibitors. Accumulated PPIX in erythrocytes and plasma damages the skin in the presence of light by forming ROS. Endothelial cells in the skin are suggested to be the main target for PPIX phototoxicity. Individual differences in PPIX phototoxicity are significant, and may be caused by genetic differences, liver damage, chlorophyll derivatives, various drugs, and impaired antioxidant defense.
EPP and XLP patients who suffer from PPIX-induced phototoxicity have significantly diminished overall quality of life. Because EPP and XLP patients learn to avoid sunlight by limiting outdoor activities and wearing protective clothes, secondary effects develop to further threaten their health such as vitamin D deficiency, osteoporosis, social stress, and depression. Current approaches used to manage PPIX-induced phototoxicity include light-shielding, drugs that increase skin pigmentation, antioxidants, sunscreens, phototherapy, blood therapy, and bone marrow transplantation. Some novel potential approaches, such as GlyT1 inhibitors and ABCG2 inhibitors, have been proposed and are under further investigation.
Although understanding of the pathophysiology of PPIX-induced phototoxicity in EPP and XLP has advanced, the detailed mechanisms for such toxicity remain unclear, especially at the molecular level. Multiple therapeutic approaches have been used in EPP and XLP patients to manage PPIX-induced phototoxicity, but side effects and limitations of these available approaches have also been noted. Future research will better elucidate the molecular mechanisms of PPIX-induced phototoxicity as well as mechanism-based approaches to alleviate or eliminate phototoxic symptoms in EPP and XLP.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK126875), and in part by the National Institute of Allergy and Infectious Diseases (R01AI131983). Figures were created using BioRender.
Abbreviations:
- ABCB6/10
ATP-binding cassette subfamily B member 6/10
- ABCG2
ATP-binding cassette subfamily G member 2
- ALA
δ-aminolevulinic acid
- ALAS
ALA synthase
- EPP
erythropoietic protoporphyria
- FECH
ferrochelatase
- MFRN1
mitoferrin 1
- PPIX
protoporphyrin IX
- ROS
reactive oxygen species
- XLP
X-linked protoporphyria
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
Xiaochao Ma and Junjie Zhu are inventors on a patent (WO2020236901) and hold equity in Portal Therapeutics, Inc. The authors declare no other competing interests.
References
- Allo G, del Carmen Garrido-Astray M, Mendez M, De Salamanca RE, Martinez G and Hawkins F (2013) Bone mineral density and vitamin D levels in erythropoietic protoporphyria. Endocrine 44:803–807. [DOI] [PubMed] [Google Scholar]
- Amo T, Kawanishi N, Uchida M, Fujita H, Oyanagi E, Utsumi T, Ogino T, Inoue K, Shuin T, Utsumi K and Sasaki J (2009) Mechanism of cell death by 5-aminolevulinic acid-based photodynamic action and its enhancement by ferrochelatase inhibitors in human histiocytic lymphoma cell line U937. Cell Biochem Funct 27:503–515. [DOI] [PubMed] [Google Scholar]
- Anstey AV and Hift RJ (2007) Liver disease in erythropoietic protoporphyria: insights and implications for management. Postgrad Med J 83:739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin Doyle L, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK and Ross DD (1998) A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci 95:15665–15670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balwani M (2019) Erythropoietic Protoporphyria and X-Linked Protoporphyria: pathophysiology, genetics, clinical manifestations, and management. Mol Genet Metab 128:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balwani M, Bonkovsky HL, Belongie KJ, Anderson KE, Takahashi F, Irizarry A, Amster M, Bissell DM, Wang B, Hazan L, Parker CJ, Cordasco E, Levy C and Desnick RJ (2020) Erythropoietic Protoporphyria: Phase 2 Clinical Trial Results Evaluating the Safety and Effectiveness of Dersimelagon (MT-7117), an Oral MC1R Agonist. Blood 136:51–51. [Google Scholar]
- Balwani M, Bonkovsky HL, Levy C, Anderson KE, Bissell DM, Parker C, Takahashi F, Desnick RJ, Belongie K and Endeavor I (2023) Dersimelagon in Erythropoietic Protoporphyrias. N Engl J Med 388:1376–1385. [DOI] [PubMed] [Google Scholar]
- Barman-Aksozen J, Nydegger M, Schneider-Yin X and Minder AE (2020) Increased phototoxic burn tolerance time and quality of life in patients with erythropoietic protoporphyria treated with afamelanotide - a three years observational study. Orphanet J Rare Dis 15:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher J, Sansone S, Fernandez NF, Haskins WE and Brancaleon L (2009) Photoinduced unfolding of beta-lactoglobulin mediated by a water-soluble porphyrin. J Phys Chem B 113:6020–6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramini M, Firey PA, Ricchelli F, Rodgers MAJ and Jori G (1987) Steady-state and time-resolved spectroscopic studies on the hematoporphyrin-lipoprotein complex. Biochem 26:6852–6858. [DOI] [PubMed] [Google Scholar]
- Berg K, Anholt H, Bech O and Moan J (1996) The influence of iron chelators on the accumulation of protoporphyrin IX in 5-aminolaevulinic acid-treated cells. Br J Cancer 74:688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biewenga M, Matawlie RHS, Friesema ECH, Koole-Lesuis H, Langeveld M, Wilson JHP and Langendonk JG (2017) Osteoporosis in patients with erythropoietic protoporphyria. Br J Dermatol 177:1693–1698. [DOI] [PubMed] [Google Scholar]
- Bishop DF, Tchaikovskii V, Nazarenko I and Desnick RJ (2013) Molecular expression and characterization of erythroid-specific 5-aminolevulinate synthase gain-of-function mutations causing X-linked protoporphyria. Mol Med 19:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely KM, Drucker AM and Rosen CF (2019) Drug-induced photosensitivity—an update: culprit drugs, prevention and management. Drug Saf 42:827–847. [DOI] [PubMed] [Google Scholar]
- Boffa M, Ead R, Reed P and Weinkove C (1996) A double-blind, placebo-controlled, crossover trial of oral vitamin C in erythropoietic protoporphyria. Photodermatol Photoimmunol Photomed 12:27–30. [DOI] [PubMed] [Google Scholar]
- Bogorad L (1958a) The enzymatic synthesis of porphyrins from porphobilinogen. I. Uroporphyrin I. J Biol Chem 233:501–509. [PubMed] [Google Scholar]
- Bogorad L (1958b) The enzymatic synthesis of porphyrins from porphobilinogen. II. Uroporphyrin III. J Biol Chem 233:510–515. [PubMed] [Google Scholar]
- Böhm F, Edge R, Foley S, Lange L and Truscott TG (2001) Antioxidant inhibition of porphyrin-induced cellular phototoxicity. J Photochem Photobiol B 65:177–183. [DOI] [PubMed] [Google Scholar]
- Brancaleon L and Moseley H (2002) Effects of photoproducts on the binding properties of protoporphyrin IX to proteins. Biophys Chem 96:77–87. [DOI] [PubMed] [Google Scholar]
- Brun A and Sandberg S (1991) Mechanisms of photosensitivity in porphyric patients with special emphasis on erythropoietic protoporphyria. J Photochem Photobiol B 10:285–302. [DOI] [PubMed] [Google Scholar]
- Brun A, Western A, Malik Z and Sandberg S (1990) Erythropoietic protoporphyria: photodynamic transfer of protoporphyrin from intact erythrocytes to other cells. Photochem Photobiol 51:573–577. [DOI] [PubMed] [Google Scholar]
- Burch JS, Marcero JR, Maschek JA, Cox JE, Jackson LK, Medlock AE, Phillips JD and Dailey HA (2018) Glutamine via a-ketoglutarate dehydrogenase provides succinyl-CoA for heme synthesis during erythropoiesis. Blood 132:987–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler DF, Ginn KF, Daniel JF, Bloomer JR, Kats A, Shreve N and Myers GD (2015) Bone marrow transplant for X-linked protoporphyria with severe hepatic fibrosis. Pediatr Transplant 19:E106–110. [DOI] [PubMed] [Google Scholar]
- Cable E, Miller T and Isom H (2000) Regulation of heme metabolism in rat hepatocytes and hepatocyte cell lines: delta-aminolevulinic acid synthase and heme oxygenase are regulated by different heme-dependent mechanisms. Arch Biochem Biophys 384:280–295. [DOI] [PubMed] [Google Scholar]
- Casanova-Gonzalez MJ, Trapero-Marugan M, Jones EA and Moreno-Otero R (2010) Liver disease and erythropoietic protoporphyria: a concise review. World J Gastroenterol 16:4526–4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FP, Risheg H, Liu Y and Bloomer J (2002) Ferrochelatase gene mutations in erythropoietic protoporphyria: focus on liver disease. Cell Mol Biol (Noisy-le-grand) 48:83–89. [PubMed] [Google Scholar]
- Chen J, Zhao K and Liu G (2013) Estrogen-induced cholestasis: pathogenesis and therapeutic implications. Hepatogastroenterology 60:1289–1296. [DOI] [PubMed] [Google Scholar]
- Chen W, Paradkar PN, Li L, Pierce EL, Langer NB, Takahashi-Makise N, Hyde BB, Shirihai OS, Ward DM, Kaplan J and Paw BH (2009) Abcb10 physically interacts with mitoferrin-1 (Slc25a37) to enhance its stability and function in the erythroid mitochondria. Proc Natl Acad Sci U S A 106:16263–16268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S and Margalit R (1990) Binding of porphyrin to human serum albumin. Structure-activity relationships. Biochem J 270:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S and Marks G (1984) Ferrochelatase and N-alkylated porphyrins. Mol Cell Biochem 64:127–137. [DOI] [PubMed] [Google Scholar]
- Cole SP, Massey TE, Marks GS and Racz WJ (1981) Effects of porphyrin-inducing drugs on ferrochelatase activity in isolated mouse hepatocytes. Can J Physiol Pharmacol 59:1155–1158. [DOI] [PubMed] [Google Scholar]
- Collins P and Ferguson J (1995) Narrow-band UVB (TL-01) phototherapy: an effective preventative treatment for the photodermatoses. Br J Dermatol 132:956–963. [DOI] [PubMed] [Google Scholar]
- Corbett M, Herxheimer A, Magnus I, Ramsay C and Kobza-Black A (1977) The long term treatment with beta-carotene in erythropoietic protoporphyria: a controlled trial. Br J Dermatol 97:655–662. [DOI] [PubMed] [Google Scholar]
- De Bruijn HS, Kruijt B, Van Der Ploeg - Van Den Heuvel A, Sterenborg HJCM and Robinson DJ (2007) Increase in protoporphyrin IX after 5-aminolevulinic acid based photodynamic therapy is due to local re-synthesis. Photochem Photobiol Sci 6:857–864. [DOI] [PubMed] [Google Scholar]
- de Bruijn HS, Meijers C, van der Ploeg - van den Heuvel A, Sterenborg HJCM and Robinson DJ (2008) Microscopic localisation of protoporphyrin IX in normal mouse skin after topical application of 5-aminolevulinic acid or methyl 5-aminolevulinate. J Photochem Photobiol B 92:91–97. [DOI] [PubMed] [Google Scholar]
- Di Pierro E, Granata F, De Canio M, Rossi M, Ricci A, Marcacci M, De Luca G, Sarno L, Barbieri L, Ventura P and G G (2022) Recognized and emerging features of erythropoietic and X-linked protoporphyria. Diagnostics (Basel) 12:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey AK, Naik H, Keel SB, Levy C, Beaven SW, Elmariah SB, Erwin AL, Goddu RJ, Hedstrom K, Leaf RK, Kazamel M, Mazepa M, Philpotts LL, Quigley J, Raef H, Rudnick SR, Saberi B, Thapar M, Ungar J, Wang B, Balwani M and Porphyrias Consortium of the Rare Diseases Clinical Research N (2022) Evidence-based consensus guidelines for the diagnosis and management of erythropoietic protoporphyria and X-linked protoporphyria. J Am Acad Dermatol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffey BL and Farr PM (1991) Sunscreen protection against UVB, UVA and blue light: an in vivo and in vitro comparison. Br J Dermatol 124:258–263. [DOI] [PubMed] [Google Scholar]
- Disc Medicine I (2022) Study of Bitopertin to Evaluate the Safety, Tolerability, Efficacy, and PPIX Concentrations in Participants With EPP, https://ClinicalTrials.gov/show/NCT05308472.
- Eichbaum QG, Dzik WH, Chung RT and Szczepiorkowski ZM (2005) Red blood cell exchange transfusion in two patients with advanced erythropoietic protoporphyria. Transfusion 45:208–213. [DOI] [PubMed] [Google Scholar]
- Elder G, Harper P, Badminton M, Sandberg S and Deybach J (2013) The incidence of inherited porphyrias in Europe. J Inherit Metab Dis 36:849–857. [DOI] [PubMed] [Google Scholar]
- Elenbaas JS, Maitra D, Liu Y, Lentz SI, Nelson B, Hoenerhoff MJ, Shavit JA and Omary MB (2016) A precursor-inducible zebrafish model of acute protoporphyria with hepatic protein aggregation and multiorganelle stress. FASEB J 30:1798–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin AL and Balwani M (2021) Porphyrias in the age of targeted therapies. Diagnostics (Basel) 11:1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr PM, Diffey BL and Matthews JNS (1990) Inhibition of photosensitivity in erythropoietic protoporphyria with terfenadine. Br J Dermatol 122:809–815. [DOI] [PubMed] [Google Scholar]
- Fernandez NF, Sansone S, Mazzini A and Brancaleon L (2008) Irradiation of the porphyrin causes unfolding of the protein in the protoporphyrin IX/beta-lactoglobulin noncovalent complex. J Phys Chem B 112:7592–7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D, Zumsteg A and Meyer U (2003) Nuclear receptors constitutive androstane receptor and pregnane X receptor activate a drug-responsive enhancer of the murine 5-aminolevulinic acid synthase gene. J Biol Chem 278:39392–39401. [DOI] [PubMed] [Google Scholar]
- Fratz EJ, Clayton J, Hunter GA, Ducamp S, Breydo L, Uversky VN, Deybach JC, Gouya L, Puy H and Ferreira GC (2015) Human Erythroid 5-Aminolevulinate Synthase Mutations Associated with X-Linked Protoporphyria Disrupt the Conformational Equilibrium and Enhance Product Release. Biochemistry 54:5617–5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y, Cheong PL, Lynch J, Brighton C, Frase S, Kargas V, Rampersaud E, Wang Y, Sankaran VG, Yu B, Ney PA, Weiss MJ, Vogel P, Bond PJ, Ford RC, Trent RJ and Schuetz JD (2016) The severity of hereditary porphyria is modulated by the porphyrin exporter and Lan antigen ABCB6. Nat Commun 7:12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawecki R, Malarz K, Rejmund M, Polanski J and Mrozek-Wilczkiewicz A (2019) Impact of thiosemicarbazones on the accumulation of PpIX and the expression of the associated genes. J Photochem Photobiol B 199:111585. [DOI] [PubMed] [Google Scholar]
- Ged C, Megarbane H, Chouery E, Lalanne M, Megarbane A and de Verneuil H (2004) Congenital erythropoietic porphyria: report of a novel mutation with absence of clinical manifestations in a homozygous mutant sibling. J Invest Dermatol 123:589–591. [DOI] [PubMed] [Google Scholar]
- Gibson KD, Laver WG and Neuberger A (1958) Initial stages in the biosynthesis of porphyrins. 2. The formation of delta-aminolaevulic acid from glycine and succinyl-coenzyme A by particles from chicken erythrocytes. Biochem J 70:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson KD, Neuberger A and Scott JJ (1955) The purification and properties of delta-aminolaevulic acid dehydrase. Biochem J 61:618–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotti AW (2001) Photosensitized oxidation of membrane lipids: Reaction pathways, cytotoxic effects, and cytoprotective mechanisms. J Photochem Photobiol B 63:103–113. [DOI] [PubMed] [Google Scholar]
- Gog Hv and Schothorst AA (1973) Determination of very small amounts of protoporphyrin in epidermis, plasma, and blister fluids. J Invest Dermatol 61:42–45. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T and Bates SE (2002) Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat Rev Cancer 2:48–58. [DOI] [PubMed] [Google Scholar]
- Gouya L, Puy H, Lamoril J, Da Silva V, Grandchamp B, Nordmann Y and Deybach JC (1999) Inheritance in erythropoietic protoporphyria: a common wild-type ferrochelatase allelic variant with low expression accounts for clinical manifestation. Blood 93:2105–2110. [PubMed] [Google Scholar]
- Gupta V, Liu S, Ando H, Ishii R, Tateno S, Kaneko Y, Yugami M, Sakamoto S, Yamaguchi Y and Nureki O (2013) Salicylic acid induces mitochondrial injury by inhibiting ferrochelatase heme biosynthesis activity. Mol Pharmacol 84:824–833. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Nishida N, Ida H, Ueshima K, Minami Y, Takita M, Aoki T, Morita M, Chishina H, Komeda Y, Yoshida A, Park AM, Sato M, Kawada A, Nakano H, Nakagawa H and Kudo M (2022) Role of phlebotomy in the treatment of liver damage related to erythropoietic porphyria. Sci Rep 12:6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloy F, Iyer PS, Ghidini A, Lysenko V, Barman-Aksozen J, Grubenmann CP, Jucker J, Wildner-Verhey van Wijk N, Ruepp MD, Minder EI, Minder AE, Schneider-Yin X, Theocharides APA, Schumperli D and Hall J (2021) Repurposing of glycine transport inhibitors for the treatment of erythropoietic protoporphyria. Cell Chem Biol 28:1221–1234 e1226. [DOI] [PubMed] [Google Scholar]
- Hamilton JW, Bement WJ, Sinclair PR, Sinclair JF and Wetterhahn KE (1988) Expression of 5-aminolaevulinate synthase and cytochrome P-450 mRNAs in chicken embryo hepatocytes in vivo and in culture. Effect of porphyrinogenic drugs and haem. Biochem J 255:267–275. [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Lin J, Rhee J, Peyer A, Chin S, Wu P, Meyer U and Spiegelman B (2005) Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1alpha. Cell 122:505–515. [DOI] [PubMed] [Google Scholar]
- Heerfordt IM, Heydenreich J, Philipsen PA, Lerche CM and Wulf HC (2020) Light-provoked skin symptoms on the hands of erythropoietic protoporphyria patients related to personal dosimeter measurements, skin symptoms, light protection and priming. J Photochem Photobiol B 213:112054. [DOI] [PubMed] [Google Scholar]
- Heerfordt IM, Lerche CM, Philipsen PA and Wulf HC (2023) Experimental and approved treatments for skin photosensitivity in individuals with erythropoietic protoporphyria or X-linked protoporphyria: A systematic review. Biomed Pharmacother 158:114132. [DOI] [PubMed] [Google Scholar]
- Heerfordt IM and Wulf HC (2016) Protoporphyrin IX in the skin measured noninvasively predicts photosensitivity in patients with erythropoietic protoporphyria. Br J Dermatol 175:1284–1289. [DOI] [PubMed] [Google Scholar]
- Holley AE, Frater Y, Gibbs AH, De Matteis F, Lamb JH, Farmer PB and Naylor S (1991) Isolation of two N-monosubstituted protoporphyrins, bearing either the whole drug or a methyl group on the pyrrole nitrogen atom, from liver of mice given griseofulvin. Biochem J 274 (Pt 3):843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holme SA, Anstey AV, Finlay AY, Elder GH and Badminton MN (2006) Erythropoietic protoporphyria in the U.K.: clinical features and effect on quality of life. Br J Dermatol 155:574–581. [DOI] [PubMed] [Google Scholar]
- Ibrahim GW and Watson CJ (1968) Enterohepatic circulation and conversion of protoporphyrin to bile pigment in man. Proc Soc Exp Biol Med 127:890–895. [DOI] [PubMed] [Google Scholar]
- Ishizuka M, Abe F, Sano Y, Takahashi K, Inoue K, Nakajima M, Kohda T, Komatsu N, Ogura S and Tanaka T (2011) Novel development of 5-aminolevurinic acid (ALA) in cancer diagnoses and therapy. Int Immunopharmacol 11:358–365. [DOI] [PubMed] [Google Scholar]
- Jonker J, Buitelaar M, Wagenaar E, Van Der Valk M, Scheffer G, Scheper R, Plosch T, Kuipers F, Elferink R and Rosing H (2002a) The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci USA 99:15649–15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RP, Rosing H, Beijnen JH and Schinkel AH (2002b) The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci U S A 99:15649–15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J, Pottier R and Pross D (1990) Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience. J Photochem Photobiol B 6:143–148. [DOI] [PubMed] [Google Scholar]
- King PA and Gunn RB (1989) Na- and Cl-dependent glycine transport in human red blood cells and ghosts. A study of the binding of substrates to the outward-facing carrier. J Gen Physiol 93:321–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluri S, Sadlon T, May B and Bonkovsky H (2005) Haem repression of the housekeeping 5-aminolaevulinic acid synthase gene in the hepatoma cell line LMH. Biochem J 392:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, Honigsmann H, Gschnait F and Wolff K (1975) Mouse model for protoporphyria. II. Cellular and subcellular events in the photosensitivity flare of the skin. J Invest Dermatol 65:300–310. [DOI] [PubMed] [Google Scholar]
- Kosenow W and Treibs A (1953) Light hypersensitivity and porphyrinemia. Z Kinderheilkd 73:82–92. [PubMed] [Google Scholar]
- Krishnamurthy PC, Du G, Fukuda Y, Sun D, Sampath J, Mercer KE, Wang J, Sosa-Pineda B, Murti KG and Schuetz JD (2006) Identification of a mammalian mitochondrial porphyrin transporter. Nature 443:586–589. [DOI] [PubMed] [Google Scholar]
- Kurz B, Ivanova I, Bäumler W and Berneburg M (2021) Turn the light on photosensitivity. J Photochem Photobiol 8:100071. [Google Scholar]
- Labbé R, Vreman H and Stevenson D (1999) Zinc protoporphyrin: a metabolite with a mission. Clin Chem 45:2060–2072. [PubMed] [Google Scholar]
- Langendonk JG, Balwani M, Anderson KE, Bonkovsky HL, Anstey AV, Bissell DM, Bloomer J, Edwards C, Neumann NJ, Parker C, Phillips JD, Lim HW, Hamzavi I, Deybach JC, Kauppinen R, Rhodes LE, Frank J, Murphy GM, Karstens FPJ, Sijbrands EJG, de Rooij FWM, Lebwohl M, Naik H, Goding CR, Wilson JHP and Desnick RJ (2015) Afamelanotide for Erythropoietic Protoporphyria. N Engl J Med 373:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaf RK and Dickey AK (2023) How I treat erythropoietic protoporphyria and X-linked protoporphyria. Blood. [DOI] [PubMed] [Google Scholar]
- Lecha M (2003) Erythropoietic protoporphyria. Photodermatol Photoimmunol Photomed 19:142–146. [DOI] [PubMed] [Google Scholar]
- Lee YS, Lee DH, Choudry HA, Bartlett DL and Lee YJ (2018) Ferroptosis-induced endoplasmic reticulum stress: Cross-talk between ferroptosis and apoptosis. Mol Cancer Res 16:1073–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Lu J, Cheng J, Wang L, Matsubara T, Csanaky IL, Klaassen CD, Gonzalez FJ and Ma X (2013) Human PXR modulates hepatotoxicity associated with rifampicin and isoniazid co-therapy. Nat Med 19:418–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Hussain Z, Zhu J, Lei S, Lu J and Ma X (2021) Role of CYP2A6 in methimazole bioactivation and hepatotoxicity. Chem Res Toxicol 34:2534–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HW (1989) Mechanisms of phototoxicity in porphyria cutanea tarda and erythropoietic protoporphyria. Immunol Ser 46:671–685. [PubMed] [Google Scholar]
- Lim HW, Hagan M and Gigli I (1986) Phototoxicity Induced By Hematoporphyrin Derivative in C5-Deficient, Mast Cell-Deficient and Leukopenic Mice. Photochem Photobiol 44:175–180. [DOI] [PubMed] [Google Scholar]
- Liu K, Yan J, Sachar M, Zhang X, Guan M, Xie W and Ma X (2015) A metabolomic perspective of griseofulvin-induced liver injury in mice. Biochem Pharmacol 98:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoumi S, Abitbol M, Rainteau D, Karim Z, Bernex F, Oustric V, Millot S, Letteron P, Heming N, Guillmot L, Montagutelli X, Berdeaux G, Gouya L, Poupon R, Deybach JC, Beaumont C and Puy H (2011) Protoporphyrin retention in hepatocytes and Kupffer cells prevents sclerosing cholangitis in erythropoietic protoporphyria mouse model. Gastroenterology 141:1509–1519, 1519 e1501–1503. [DOI] [PubMed] [Google Scholar]
- Magnus IA, Jarrett A, Prankerd TA and Rimington C (1961) Erythropoietic protoporphyria. A new porphyria syndrome with solar urticaria due to protoporphyrinaemia. Lancet 2:448–451. [DOI] [PubMed] [Google Scholar]
- Mahmoud BH, Hexsel CL, Hamzavi IH and Lim HW (2008) Effects of visible light on the skin. Photochem Photobiol 84:450–462. [DOI] [PubMed] [Google Scholar]
- Maitra D, Bragazzi Cunha J, Elenbaas JS, Bonkovsky HL, Shavit JA and Omary MB (2019a) Porphyrin-Induced Protein Oxidation and Aggregation as a Mechanism of Porphyria-Associated Cell Injury. Cell Mol Gastroenterol Hepatol 8:535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra D, Carter EL, Richardson R, Rittie L, Basrur V, Zhang H, Nesvizhskii AI, Osawa Y, Wolf MW, Ragsdale SW, Lehnert N, Herrmann H and Omary MB (2019b) Oxygen and Conformation Dependent Protein Oxidation and Aggregation by Porphyrins in Hepatocytes and Light-Exposed Cells. Cell Mol Gastroenterol Hepatol 8:659–682 e651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra D, Pinsky BM, Soherawardy A, Zheng H, Banerjee R and Omary MB (2021) Protein-aggregating ability of different protoporphyrin-IX nanostructures is dependent on their oxidation and protein-binding capacity. J Biol Chem 297:100778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews-Roth M, Pathak M, Fitzpatrick T, Harber L and Kass E (1970) Beta-carotene as a photoprotective agent in erythropoietic protoporphyria. N Engl J Med 282:1231–1234. [DOI] [PubMed] [Google Scholar]
- Mathews-Roth M, Rosner B, Benfell K and Roberts J (1994) A double-blind study of cysteine photoprotection in erythropoietic protoporphyria. Photodermatol Photoimmunol Photomed 10:244–248. [PubMed] [Google Scholar]
- Mathews-Roth MM (1998) Treatment of the Cutaneous Porphyrias. Clin Dermatol 16:295–298. [DOI] [PubMed] [Google Scholar]
- McCluskey SA, Riddick DS, Mackie JE, Kimmett SM, Whitney RA and Marks GS (1992) Inactivation of cytochrome P450 and inhibition of ferrochelatase by analogues of 3,5-diethoxycarbonyl-1,4-dihydro-2,4,6-trimethylpyridine with 4-nonyl and 4-dodecyl substituents. Can J Physiol Pharmacol 70:1069–1074. [DOI] [PubMed] [Google Scholar]
- Melefors O, Goossen B, Johansson H, Stripecke R, Gray N and Hentze M (1993) Translational control of 5-aminolevulinate synthase mRNA by iron-responsive elements in erythroid cells. J Biol Chem 268:5974–5978. [PubMed] [Google Scholar]
- Minder EI, Schneider-Yin X, Steurer J and Bachmann LM (2009) A systematic review of treatment options for dermal photosensitivity in erythropoietic protoporphyria. Cell Mol Biol 55:84–97. [PubMed] [Google Scholar]
- Mirmiran A, Schmitt C, Lefebvre T, Manceau H, Daher R, Oustric V, Poli A, Lacapère JJ, Moulouel B, Puy H, Karim Z, Peoc’h K, Lenglet H, Simonin S, Deybach JC, Nicolas G and Gouya L (2019) Erythroid-progenitor-targeted gene therapy using bifunctional TFR1 ligand-peptides in human erythropoietic protoporphyria. Am J Hum Genet 104:341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsubishi Tanabe Pharma Development America I (2021) Extension Study to Evaluate Safety and Tolerability of Oral Dersimelagon (MT-7117) in Subjects With Erythropoietic Protoporphyria (EPP) or X-Linked Protoporphyria (XLP), https://ClinicalTrials.gov/show/NCT05005975.
- Moseley H, Cameron H, MacLeod T, Clark C, Dawe R and Ferguson J (2001) New sunscreens confer improved protection for photosensitive patients in the blue light region. Br J Dermatol 145:789–794. [DOI] [PubMed] [Google Scholar]
- Munemoto S, Tsuchihara K, Fujishima C, Hioki C, Sasaki H, Yoshida H, Akasaka E, Nakano H and Kudo H (2022) Novel mutation of the ferrochelatase gene in a Japanese boy with erythropoietic protoporphyria. J Dermatol 49:e179–e180. [DOI] [PubMed] [Google Scholar]
- Murphy GM (2003) Diagnosis and management of the erythropoietic porphyrias. Dermatol Ther 16:57–64. [DOI] [PubMed] [Google Scholar]
- Naik H, Overbey JR, Desnick RJ, Anderson KE, Bissell DM, Bloomer J, Bonkovsky HL, Phillips JD, Wang B, Singal A and Balwani M (2019a) Evaluating quality of life tools in North American patients with erythropoietic protoporphyria and X-linked protoporphyria. JIMD Rep 50:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik H, Shenbagam S, Go AM and Balwani M (2019b) Psychosocial issues in erythropoietic protoporphyria - the perspective of parents, children, and young adults: A qualitative study. Mol Genet Metab 128:314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara A, Ogawa K, Ide R, Ikenaga Y, Fukunaga C, Nakayama S and Tsuda M (2023) Results from a first-in-human study of dersimelagon, an investigational oral selective MC1R agonist. Eur J Clin Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Ide R, Belongie K, Tsuda M, Kawanishi H, Teng R and Ogasawara A (2023) The Oral Bioavailability and Effect of Various Gastric Conditions on the Pharmacokinetics of Dersimelagon in Healthy Adult Volunteers. Clin Pharmacol Drug Dev 12:493–501. [DOI] [PubMed] [Google Scholar]
- Ortiz de Montellano PR, Beilan HS and Kunze KL (1981) N-Alkylprotoporphyrin IX formation in 3,5-dicarbethoxy-1,4-dihydrocollidine-treated rats. Transfer of the alkyl group from the substrate to the porphyrin. J Biol Chem 256:6708–6713. [PubMed] [Google Scholar]
- Oustric V, Manceau H, Ducamp S, Soaid R, Karim Z, Schmitt C, Mirmiran A, Peoc’H K, Grandchamp B, Beaumont C, Lyoumi S, Moreau-Gaudry F, Guyonnet-Dupérat V, De Verneuil H, Marie J, Puy H, Deybach JC and Gouya L (2014) Antisense oligonucleotide-based therapy in human erythropoietic protoporphyria. Am J Hum Genet 94:611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano MB, Hobbs W, Linenberger M and Delaney M (2012) Plasma and red cell exchange transfusions for erythropoietic protoporphyria: a case report and review of the literature. J Clin Apher 27:336–341. [DOI] [PubMed] [Google Scholar]
- Peterka ES, Fusaro RM and Goltz RW (1965) Erythropoietic protoporphyria. Arch Dermat 92:357–361. [DOI] [PubMed] [Google Scholar]
- Petersen B, Wiegell SR and Wulf HC (2014) Light protection of the skin after photodynamic therapy reduces inflammation: an unblinded randomized controlled study. Br J Dermatol 171:175–178. [DOI] [PubMed] [Google Scholar]
- Phillips JD (2019) Heme biosynthesis and the porphyrias. Mol Genet Metab 128:164–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli S, Lamola AA, Poh-Fitzpatrick MF, Seaman C and Harber LC (1975) Erythropoietic protoporphyria and lead intoxication: the molecular basis for difference in cutaneous photosensitivity. I. Different rates of disappearance of protoporphyrin from the erythrocytes, both in vivo and in vitro. J Clin Invest 56:1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podvinec M, Handschin C, Looser R and Meyer U (2004) Identification of the xenosensors regulating human 5-aminolevulinate synthase. Proc Natl Acad Sci USA 101:9127–9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh-Fitzpatrick M (1989) The “priming phenomenon” in the acute phototoxicity of erythropoietic protoporphyria. J Am Acad Dermatol 21:311. [DOI] [PubMed] [Google Scholar]
- Puy H, Gouya L and Deybach JC (2010) Porphyrias. Lancet 375:924–937. [DOI] [PubMed] [Google Scholar]
- Ramanujam V and Anderson K (2015) Porphyria Diagnostics-Part 1: A Brief Overview of the Porphyrias. Curr Protoc Hum Genet 86:17.20.11–17.20.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richarz NA, Aguilera J, Castillo G, Fuente MJ, Ferrándiz C and Carrascosa JM (2017) Phototoxic reaction to a combined oral contraceptive (levonorgestrel/ethinylestradiol). Photochem Photobiol Sci 16:1381–1383. [DOI] [PubMed] [Google Scholar]
- Riethmueller G and Tuppy H (1964) [Heme Synthetase (Ferrochelatase) in Saccharomyces Cerevisiae after Aerobic and Anaerobic Growth]. Biochem Z 340:413–420. [PubMed] [Google Scholar]
- Roelandts R (1995) Photo(chemo)therapy and general management of erythropoietic protoporphyria. Dermatology 190:330–331. [DOI] [PubMed] [Google Scholar]
- Ron D and Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529. [DOI] [PubMed] [Google Scholar]
- Rutkowski DT and Kaufman RJ (2004) A trip to the ER: coping with stress. Trends Cell Biol 14:20–28. [DOI] [PubMed] [Google Scholar]
- Ryan EA (1966) Histochemistry of the skin in erythropoietic protoporphyria. Br J Dermatol 78:501–518. [DOI] [PubMed] [Google Scholar]
- Ryan EA and Madill GT (1968) Electron microscopy of the skin in erythropoietic protoporphyria. Br J Derm 80:561–570. [DOI] [PubMed] [Google Scholar]
- Sachar M, Li F, Liu K, Wang P, Lu J and Ma X (2016) Chronic Treatment with Isoniazid Causes Protoporphyrin IX Accumulation in Mouse Liver. Chem Res Toxicol 29:1293–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Okiyama N, Inoue S, Kubota N, Nakamura Y, Ishitsuka Y, Watanabe R, Nakano H and Fujisawa Y (2020) Novel mutation of the ferrochelatase gene in a Japanese family with erythropoietic protoporphyria. J Dermatol 47:e114–e116. [DOI] [PubMed] [Google Scholar]
- Sakiyama M, Matsuo H, Toyoda Y, Yonekura Y, Ishikawa T, Nakayama A, Higashino T, Kawamura Y, Fujimoto N, Shinomiya N and Satoh T (2021) Porphyrin accumulation in humans with common dysfunctional variants of ABCG2, a porphyrin transporter: potential association with acquired photosensitivity. Human Cell 34:1082–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg S, Talstad I, Hovding G and Bjelland N (1983) Light-induced release of protoporphyrin, but not of zinc protoporphyrin, from erythrocytes in a patient with greatly elevated erythrocyte protoporphyrin. Blood 62:846–851. [PubMed] [Google Scholar]
- Schauder A, Avital A and Malik Z (2010) Regulation and gene expression of heme synthesis under heavy metal exposure–review. J Environ Pathol Toxicol Oncol 29:137–158. [DOI] [PubMed] [Google Scholar]
- Schnait FG, Wolff K and Konrad K (1975) Erythropoietic protoporphyria-submicroscopic events during the acute photosensitivity flare. Br J Derm 92:545–557. [DOI] [PubMed] [Google Scholar]
- Seery VL and Muller Eberhard U (1973) Binding of porphyrins to rabbit hemopexin and albumin. J Biol Chem 248:3796–3800. [PubMed] [Google Scholar]
- Shah DI, Takahashi-Makise N, Cooney JD, Li L, Schultz IJ, Pierce EL, Narla A, Seguin A, Hattangadi SM, Medlock AE, Langer NB, Dailey TA, Hurst SN, Faccenda D, Wiwczar JM, Heggers SK, Vogin G, Chen W, Chen C, Campagna DR, Brugnara C, Zhou Y, Ebert BL, Danial NN, Fleming MD, Ward DM, Campanella M, Dailey HA, Kaplan J and Paw BH (2012) Mitochondrial Atpif1 regulates haem synthesis in developing erythroblasts. Nature 491:608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sima AAf, Kennedy JC, Blakeslee D and Robertson DM (1981) Experimental Porphyric Neuropathy: A Preliminary Report. Can J Neurol Sci 8:105–113. [DOI] [PubMed] [Google Scholar]
- Sivaramakrishnan M, Woods J and Dawe R (2014) Narrowband ultraviolet B phototherapy in erythropoietic protoporphyria: case series. Br J Dermatol 170:987–988. [DOI] [PubMed] [Google Scholar]
- Solazzo M, Fantappiè O, D’Amico M, Sassoli C, Tani A, Cipriani G, Bogani C, Formigli L and Mazzanti R (2009) Mitochondrial expression and functional activity of breast cancer resistance protein in different multiple drug-resistant cell lines. Cancer Res 69:7235–7242. [DOI] [PubMed] [Google Scholar]
- Spelt JM, de Rooij FW, Wilson JH and Zandbergen AA (2010) Vitamin D deficiency in patients with erythropoietic protoporphyria. J Inherit Metab Dis 33 Suppl 3:S1–4. [DOI] [PubMed] [Google Scholar]
- Stenson PD, Ball EV, Mort M, Phillips AD, Shiel JA, Thomas NS, Abeysinghe S, Krawczak M and Cooper DN (2003) Human Gene Mutation Database (HGMD): 2003 update. Hum Mutat 21:577–581. [DOI] [PubMed] [Google Scholar]
- Strand LJ, Felsher BF, Redeker AG and Marver HS (1970) Heme biosynthesis in intermittent acute prophyria: decreased hepatic conversion of porphobilinogen to porphyrins and increased delta aminolevulinic acid synthetase activity. Proc Natl Acad Sci U S A 67:1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sułkowski L, Pawełczak B, Chudzik M and Maciazek-Jurczyk M (2016) Characteristics of the protoporphyrin IX binding sites on human serum albumin using molecular docking. Molecules 21:1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Kawano Y, Matsumoto A, Kondo M, Funayama K, Tanemura S, Miyashiro M, Nishi A, Yamada K, Tsuda M, Sato A, Morokuma K and Yamamoto Y (2022) Melanogenic effect of dersimelagon (MT-7117), a novel oral melanocortin 1 receptor agonist. Skin Health Dis 2:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita K, Takajo T, Hirata H, Ono M and Utsumi H (2004) In vivo oxygen radical generation in the skin of the protoporphyria model mouse with visible light exposure: An L-band ESR study. J Invest Dermatol 122:1463–1470. [DOI] [PubMed] [Google Scholar]
- Tamura A, Watanabe M, Saito H, Nakagawa H, Kamachi T, Okura I and Ishikawa T (2006) Functional validation of the genetic polymorphisms of human ATP-binding cassette (ABC) transporter ABCG2: identification of alleles that are defective in porphyrin transport. Mol Pharmacol 70:287–296. [DOI] [PubMed] [Google Scholar]
- Thapar M and Bonkovsky HL (2008) The diagnosis and management of erythropoietic protoporphyria. Gastroenterology and Hepatology 4:561–566. [PMC free article] [PubMed] [Google Scholar]
- Timonen K, Kariniemi AL, Niemi KM, Teppo AM, Tenhunen R and Kauppinen R (2000) Vascular changes in erythropoietic protoporphyria: Histopathologic and immunohistochemical study. J Am Acad Dermatol 43:489–497. [DOI] [PubMed] [Google Scholar]
- Tintle S, Alikhan A, Horner ME, Hand JL and Davis DMR (2014) Cutaneous porphyrias part II: treatment strategies. Int J Dermatol 53:3–24. [DOI] [PubMed] [Google Scholar]
- To-Figueras J, Badenas C, Mascaro JM, Madrigal I, Merino A, Bastida P, Lecha M and Herrero C (2007) Study of the genotype-phenotype relationship in four cases of congenital erythropoietic porphyria. Blood Cells Mol Dis 38:242–246. [DOI] [PubMed] [Google Scholar]
- Troadec MB, Warner D, Wallace J, Thomas K, Spangrude GJ, Phillips J, Khalimonchuk O, Paw BH, Ward DM and Kaplan J (2011a) Targeted deletion of the mouse Mitoferrin1 gene: from anemia to protoporphyria. Blood 117:5494–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troadec MB, Warner D, Wallace J, Thomas K, Spangrude GJ, Phillips J, Khalimonchuk O, Paw BH, Ward DMV and Kaplan J (2011b) Targeted deletion of the mouse Mitoferrin1 gene: From anemia to protoporphyria. Blood 117:5494–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschudy DP, Valsamis M and Magnussen CR (1975) Acute intermittent porphyria: clinical and selected research aspects. Ann Intern Med 83:851–864. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Ogawa K, Endou T, Goto T, Ogasawara Y and Ogasawara A (2023) Absorption, metabolism, and excretion of [(14) C]dersimelagon, an investigational oral selective melanocortin 1 receptor agonist, in preclinical species and healthy volunteers. Pharmacol Res Perspect 11:e01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich DL, Lynch J, Wang Y, Fukuda Y, Nachagari D, Du G, Sun D, Fan Y, Tsurkan L, Potter PM, Rehg JE and Schuetz JD (2012) ATP-dependent mitochondrial porphyrin importer ABCB6 protects against phenylhydrazine toxicity. J Biol Chem 287:12679–12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Fickert P, Zollner G, Fuchsbichler A, Silbert D, Tsybrovskyy O, Zatloukal K, Guo GL, Schuetz JD, Gonzalez FJ, Marschall HU, Denk H and Trauner M (2003) Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology 125:825–838. [DOI] [PubMed] [Google Scholar]
- Wahlin S, Floderus Y, Stal P and Harper P (2011) Erythropoietic protoporphyria in Sweden: demographic, clinical, biochemical and genetic characteristics. J Intern Med 269:278–288. [DOI] [PubMed] [Google Scholar]
- Wang P, Sachar M, Lu J, Shehu AI, Zhu J, Chen J, Liu K, Anderson KE, Xie W, Gonzalez FJ, Klaassen CD and Ma X (2019) The essential role of the transporter ABCG2 in the pathophysiology of erythropoietic protoporphyria. Sci Adv 5:eaaw6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Poh-Fitzpatrick M, Carriero D, Ostasiewicz L, Chen T, Taketani S and Piomelli S (1993) A novel mutation in erythropoietic protoporphyria: an aberrant ferrochelatase mRNA caused by exon skipping during RNA splicing. Biochim Biophys Acta 1181:198–200. [DOI] [PubMed] [Google Scholar]
- Wang YM, Gloude NJ, Davies SM, Lucky AW and Nelson AS (2021) Hematopoietic stem cell transplant for erythropoietic porphyrias in pediatric patients. Pediatr Blood Cancer 68:e29231. [DOI] [PubMed] [Google Scholar]
- Warren L and George S (1998) Erythropoietic protoporphyria treated with narrow-band (TL-01) UVB phototherapy. Australas J Dermatol 39:179–182. [DOI] [PubMed] [Google Scholar]
- Weiss Y, Balwani M, Chen B, Yasuda M, Nazarenko I and Desnick RJ (2019) Congenital erythropoietic porphyria and erythropoietic protoporphyria: Identification of 7 uroporphyrinogen III synthase and 20 ferrochelatase novel mutations. Mol Genet Metab 128:358–362. [DOI] [PubMed] [Google Scholar]
- Wen J, Chen X, Wei S, Ma X and Zhao Y (2022) Research progress and treatment status of liver cirrhosis with hypoproteinemia. Evid Based Complement Alternat Med 2022:2245491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley S, Ducamp S, Gouya L, Grandchamp B, Beaumont C, Badminton M, Elder G, Holme S, Anstey A and Parker M (2008a) C-terminal deletions in the ALAS2 gene lead to gain of function and cause X-linked dominant protoporphyria without anemia or iron overload. Am J Hum Genet 83:408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatley SD, Ducamp S, Gouya L, Grandchamp B, Beaumont C, Badminton MN, Elder GH, Holme SA, Anstey AV, Parker M, Corrigall AV, Meissner PN, Hift RJ, Marsden JT, Ma Y, Mieli-Vergani G, Deybach JC and Puy H (2008b) C-terminal deletions in the ALAS2 gene lead to gain of function and cause X-linked dominant protoporphyria without anemia or iron overload. Am J Hum Genet 83:408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter M, Funk J, Korner A, Alberati D, Christen F, Schmitt G, Altmann B, Pospischil A and Singer T (2016) Effects of GlyT1 inhibition on erythropoiesis and iron homeostasis in rats. Exp Hematol 44:964–974 e964. [DOI] [PubMed] [Google Scholar]
- Wu J and Cotliar R (2021) Afamelanotide: An Orphan Drug with Potential for Broad Dermatologic Applications. J Drugs Dermatol 20:290–294. [DOI] [PubMed] [Google Scholar]
- Wu M, Ducamp S, Xiang Y, Putra J, Fleming MD, MacDonald B and Schmidt PJ (2022) Bitopertin, a Selective Glycine Transporter 1 Inhibitor, Reduced Protoporphyrin IX (PPIX) Level and Improved Liver Fibrosis in a Mouse Model of Erythropoietic Protoporphyria (EPP). Blood 140:8192–8193. [Google Scholar]
- Yamamoto M, Arimura H, Fukushige T, Minami K, Nishizawa Y, Tanimoto A, Kanekura T, Nakagawa M, Akiyama S-i and Furukawa T (2014) Abcb10 Role in Heme Biosynthesis In Vivo : Abcb10 Knockout in Mice Causes Anemia with Protoporphyrin IX and Iron Accumulation Mol Cell Biol 34:1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Zhao Q, Hu DD, Xiao XR, Huang JF and Li F (2018) Metabolomic analysis of cholestatic liver damage in mice. Food Chem Toxicol 120:253–260. [DOI] [PubMed] [Google Scholar]
- Zhu S, Li X, Wu F, Cao X, Gou K, Wang C and Lin C (2022) Blue light induces skin apoptosis and degeneration through activation of the endoplasmic reticulum stress-autophagy apoptosis axis: Protective role of hydrogen sulfide. J Photochem Photobiol B 229:112426. [DOI] [PubMed] [Google Scholar]


