Abstract
Background:
Hearing loss is associated with greater cognitive decline and incident dementia in older adults. Whether hearing intervention could reduce cognitive decline in cognitively-healthy older adults with hearing loss is unknown.
Method:
The ACHIEVE study is a multicentre, parallel group, unmasked randomised controlled trial (ClinicalTrials.gov: NCT03243422) of 70–84 year-old adults with untreated hearing loss and free from substantial cognitive impairment that took place at four community study sites across the United States. Participants were recruited from two study populations at each site: 1) older adults participating in a longstanding observational study of cardiovascular health (Atherosclerosis Risk in Communities [ARIC] study), and 2) healthy de novo community volunteers. Participants were randomised (1:1) to hearing intervention (HI; audiological counselling and provision of hearing aids) or a successful aging health education control intervention (SA; individual sessions with a health educator covering topics on chronic disease prevention) and followed semi-annually. The primary endpoint was 3-year change in a global cognition standardized factor score from a comprehensive neurocognitive battery. Analysis was by intention-to-treat.
Findings:
From November 9, 2017 to October 25, 2019, 3004 participants were screened for eligibility, and 977 participants (238 from ARIC, 739 de novo) underwent randomisation; 490 were assigned to HI and 487 to SA control. Participants from ARIC were older, had more risk factors for cognitive decline, and had lower baseline cognitive scores than the de novo cohort. In the primary analysis combining the ARIC and de novo cohorts, 3-year cognitive change (in standard deviation units) was not significantly different between HI and SA control (HI: −0·200 [95% confidence interval [CI]: −0·256, 0·144]; SA: −0·202 [95% CI: −0·258, −0·145]; Difference 0·002 [95% CI: −0·077, 0·081], p=0·96). However, a prespecified sensitivity analysis demonstrated a significant difference in the effect of HI on 3-year cognitive change between the ARIC and de novo cohorts (p for interaction=0·010). Other prespecified sensitivity analyses that varied analytical parameters used in the total cohort did not change the observed results. No significant adverse events attributed to the study were reported with either HI or SA control.
Interpretation:
Hearing intervention did not reduce 3-year cognitive decline in the primary analysis of the total cohort. However, a prespecified sensitivity analysis demonstrated that the effect differed between the two study populations that comprised the cohort. These findings suggest that hearing intervention may reduce cognitive change over 3 years in populations of older adults at increased risk for cognitive decline but not in populations at decreased risk for cognitive decline.
Funding:
This study was funded by grants from the National Institutes of Health.
Introduction
The global burden of dementia will increase rapidly over the next 30 years because of the aging of the world’s population. Over 150 million individuals are projected to be living with dementia by 2050 with the vast majority living in low and middle income countries.1 Efforts to address this global health challenge have increasingly focused on identifying potentially modifiable risk factors that could be addressed at scale to help reduce the risk of dementia and the cognitive decline that precedes dementia onset.
Over the past five years, consensus studies2–5 investigating these risk factors have consistently identified hearing loss, prevalent in 65% of adults over 60 years, 6 as being a key risk factor of interest. Reports from the Lancet Commission on Dementia have identified hearing loss as being the single largest potentially modifiable risk factor for dementia in both high and low-to-middle income countries. 2,5 Hypothesized mechanisms through which hearing loss and degraded peripheral sound encoding could affect cognitive decline and dementia risk include effects of hearing loss on cognitive load, brain structure, and/or reduced engagement in social and cognitively-stimulating activities. 7 Importantly, these pathways may be modifiable with existing interventions for hearing loss that remain underutilized (<10% of individuals in low-income countries and <20–30% in high-income countries with hearing loss use hearing aids8).
Prior studies on the role of hearing aids in dementia prevention have principally been based on observational data and have demonstrated encouraging results suggestive of a positive effect of hearing intervention on reducing risk for cognitive decline and dementia. 9,10 However, inferences from these observational studies are limited because measured (e.g., education, income) and unmeasured factors (e.g., health behaviours) may confound observed associations of hearing aid use with reduced cognitive decline. Therefore, we conducted a 3-year randomised controlled trial of hearing intervention (versus health education control) to determine its effects on cognitive decline among community-dwelling older adults.
Methods
Study design and participants
The Aging and Cognitive Health Evaluation in Elders (ACHIEVE) study is a 3-year multicentre, parallel group, unmasked randomised controlled trial that is based within the scientific and physical infrastructure of the Atherosclerosis Risk in Communities (ARIC) study, 11 an ongoing longitudinal study of adults who were aged 45–64 years when initially recruited in 1987–1989 (N=15,792) from a random sample of the surrounding communities at four community-based field sites in the United States (Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis suburbs, Minnesota; Washington County, Maryland). The main goal of the observational ARIC study is to understand risk factors for heart disease and stroke and the connections between cardiovascular and cognitive health. ARIC participants have been followed since 1989 at 6 in-person study visits during which neurocognitive testing was administered at 4 of the visits. The ACHIEVE study was carried out at the same four field sites, and both studies shared study personnel, protocols, and methods. The trial’s study design and methods have been previously published. 12
ACHIEVE participants were recruited from two populations at each site: 1) existing ARIC study participants; and 2) de novo from healthy volunteers in the communities of the four field sites. De novo participants were recruited through advertisements in local papers and radio, internet advertisements, and related means.12 Participants were prescreened by telephone and completed additional in-person screening. Main inclusion criteria were: 70 to 84 years old, adult-onset bilateral hearing loss with a better-ear 4-frequency (0.5 to 4 kHz) pure tone average (PTA) ≥30 and <70 dB, free of substantial cognitive impairment (Mini-Mental State Exam [MMSE] ≥23 for high school degree or less; ≥25 for some college or more), word recognition score in quiet ≥60% correct in the better-hearing ear, community-dwelling, and being a fluent English speaker. Main exclusion criteria were: self-reported disability in ≥2 activities of daily living, presenting visual acuity worse than 20/63 on the MNREAD acuity chart (Precision Vision, Woodstock, IL; corresponding to inability to comfortably read 14-point font), self-reported hearing aid use in the past year, permanent conductive hearing loss, medical contraindication to hearing aid use, or unwillingness to wear hearing aids on a regular basis. Audiologically-related inclusion and exclusion criteria were specified to identify individuals who would be expected to benefit from amplification with conventional hearing aids and related audiological services.
The ACHIEVE trial was approved by the institutional review boards of all participating study sites and academic centres. Participants provided written informed consent. An independent Data and Safety Monitoring Board (DSMB) met semi-annually to review study progress, adverse events, and changes to the study protocol and statistical analysis plan. A final version of the study protocol and analysis plan are available at clinicaltrials.gov.
Randomisation and masking
We randomised eligible participants using 1:1 permuted block randomisation, stratified by severity of hearing loss (PTA <40 dB or ≥ 40 dB), recruitment source (ARIC or de novo), and field site, to either hearing intervention (HI) or a successful aging (SA) health education control intervention from January 2018 to October 2019. Eligible participants who were spouses/partners were randomised as a unit, stratified by recruitment source and field site. The randomisation allocation schedule was developed by the coordinating centre at the University of North Carolina and completed within the Carolina Data Acquisition and Reporting Tool web-based data management system. Assignment to hearing intervention (which involves participants’ use of hearing aids) is by nature unmasked to participants and study staff collecting outcome data who may notice if a participant is wearing a hearing aid. To minimize potential bias, participants were masked to the study hypothesis and every participant was informed before randomisation that they would be offered both study interventions which could promote healthy aging during study follow-up. Participants were informed that either HI or SA intervention would be assigned randomly at baseline, and all participants would then receive the other intervention after 3-year follow-up. Other procedures to minimize bias included use of standardized protocols for training of data collectors and assessment of study outcomes; lack of access to cognitive testing results from prior study visits for data collectors and study coordinators to avoid unintentional and possibly unconscious bias by study staff during data collection; and masking of accumulating trial data from ACHIEVE investigators and study staff (except coordinating centre staff and one unblinded statistician).
Procedures
Participants randomised to HI completed four 1-hour sessions with a study audiologist held every 1 to 3 weeks post-randomisation. Participants received bilateral hearing aids fit to prescriptive targets using real-ear measures and other hearing assistive technologies to pair with the hearing aids (e.g., devices to stream cell phones and television, remote microphones to directly access other speakers in difficult listening environments). The intervention included systematic orientation and instruction in device use and hearing “toolkit” materials for self-management and communication strategies. Reinstruction in use of devices and hearing rehabilitative strategies was provided during booster visits held semi-annually. Complete details of the hearing intervention have been previously published.13
Participants assigned to SA control met individually with a certified health educator who administered the 10 Keys to Healthy Aging program,14 an evidence-based interactive health education program for older adults on topics relevant to chronic disease and disability prevention, which has been previously implemented as the control intervention in other trials. The format of the SA control intervention was designed to control for general levels of staff and participant time and attention and to parallel the intensity of the hearing intervention. Participants met with a health educator every 1 to 3 weeks for a total of four visits post-randomisation. Session content was tailored to each participant and included a standardized didactic education component as well as activities, goal-setting, optional extracurricular enrichment activities, and a 5- to 10-minute upper body extremity stretching program. Participants returned for booster sessions semi-annually.
After baseline assessment, randomisation, and provision of the assigned study intervention, participants were followed at semi-annual visits. From March 2020 to June 2021, all study sites were closed for in-person study visits because of the COVID-19 pandemic. During this period, visits continued with modified procedures for provision of phone-based intervention booster sessions and phone-based assessments of study outcomes.
Outcomes
The primary study endpoint is change (in standard deviation [SD] units) from baseline to year 3 in a global cognition standardized factor score derived from a comprehensive neurocognitive battery that was administered at baseline and annually for 3 years by psychometrists trained and supervised by a neuropsychologist. Tests included delayed word recall, digit symbol substitution, incidental learning, trail making parts A and B, logical memory, digit span backwards, Boston naming, word fluency, and animal naming (appendix p 9–11). Standardized factor scores were developed using a latent variable modelling approach that has been previously used and validated15. Compared with other summary measures, such as weighted averages (e.g., z-scores), factor scores better account for measurement error of individual tests and their relative difficulty and improves precision.16 In addition to the neurocognitive battery, the MMSE was administered at baseline and semiannually. During the period of COVID-related study site closures, a telephone-based adaptation of the neurocognitive battery and MMSE was developed and implemented for the annual neurocognitive assessments. This battery included Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) immediate and delayed word recall, digit span backward, oral trails A and B, word fluency, and animal naming. Final year 3 neurocognitive assessments were conducted in-person from June 2021 to November 2022. Procedures implemented to help ensure hearing loss would not affect cognitive testing accuracy were described previously.12 Of the 10 tests comprising the in-person neurocognitive battery, only 2 tests had exclusively auditory stimuli (digit span backward and logical memory). All other tests contained visual stimuli or both auditory and visual stimuli.
Secondary cognitive outcomes include 3-year change in cognitive domain-specific latent factor scores15 (executive function [trail making A and B, digit symbol substitution], language [Boston naming, word fluency, animal naming], and memory [delayed word recall, logical memory, incidental learning) and time until cognitive impairment defined as a composite outcome of: 1) adjudicated dementia determined from in-person or phone-based evaluations; 2) adjudicated mild cognitive impairment (MCI) determined from in-person evaluations; or 3) a 3-point drop compared to baseline in a 30-item MMSE administered in-person or the equivalent in a factor score derived from the 10-item MMSE orientation subscale and 11-item Blessed scale administered over the phone. Incident events of cognitive impairment defined by adjudicated MCI or a 3-point drop compared to baseline in the MMSE or phone equivalent required subsequent confirmation at the following assessment to ensure persistence of cognitive impairment. Diagnostic adjudication procedures for MCI and dementia diagnoses are provided in the appendix (p 16–20).
A measure of self-perceived communicative function (Hearing Handicap Inventory for the Elderly-Screening17 [HHI]) was also assessed at baseline and annually to evaluate for hearing intervention target engagement. This interviewer-administered 10-item scale assesses the influence of hearing loss on daily communicative function. An HHI score 0–8 indicates no communication impairment, 10–24 mild-to-moderate communication impairment, and 26–40 significant communication impairment.
Statistical analysis
Sample size and power were calculated on the following assumptions based on prior data from ARIC and other representative studies of older adults: 1) change in global cognition standardized factor score of −0·24 SD units over 3 years; 2) standard deviation of 3-year cognitive change of 0·27; 3) drop-in (individuals in SA control obtaining hearing aids outside of the study) and drop-out (individuals assigned to HI who discontinue hearing aid use entirely) net total of 15% over 3 years; 4) withdrawal or missing data from competing events of 27% over 3 years. Under these assumptions, a sample size of 850 participants provided 90% power with two-tailed α=0·05 to detect a 35% difference in the rate of 3-year cognitive change between HI and SA control. Prior to reaching this target sample size and based on the favourable rate of recruitment, the DSMB recommended a modest extension to the recruitment period to obtain a larger sample size to account for potential uncertainty. A 3-month extension of the recruitment period after the initial target sample size of 850 was reached allowed for a final sample size of 977.
Descriptive characteristics were compared by randomisation and recruitment source (ARIC or de novo). We estimated the effect of randomised treatment assignment on 3-year change in global cognition by fitting a three-level linear mixed effects model with an unstructured covariance matrix to data from the baseline and the year 3 in-person neurocognitive assessment. The model utilized restricted maximum likelihood with a Kenward-Roger correction to generate parameter estimates, 95% confidence intervals (CI), and p-values. A random intercept and time slope were specified at level two for participants and a random intercept was specified at level three for spouses/partners randomised as a unit. Neurocognitive data from in-person year 1 and 2 assessments were used when a participant died prior to year 3 but completed an assessment less than a year before death. Phone-based neurocognitive data were only used in sensitivity analyses. A prespecified imputation model generated values for missing covariates and global cognition factor scores at year 3. Time from baseline and an interaction between time and randomisation were included in the model along with prespecified, prognostic covariates of hearing loss severity (PTA <40 dB vs 40+ dB), recruitment source, field site, age, sex, education, and the presence of APOE ε4 allele(s) at baseline. An interaction with time was specified for each covariate except education. A three-way interaction between randomisation, recruitment source, and time was tested prior to conducting sensitivity analysis that stratified by recruitment source. The analysis was repeated for the secondary outcomes of executive function, language, and memory.
We used cumulative incidence curves that accounted for the competing risk of death to evaluate the secondary outcome of incident cognitive impairment. We employed a two-level, discrete-time, cause-specific proportional-hazards model with a complimentary log-log link to estimate the effect of treatment assignment on incident cognitive impairment. The model utilized maximum likelihood with a quadrature approximation and a bias-corrected sandwich estimator to generate hazard ratios, 95% CIs, and p-values. A random intercept was specified at level two for spouses/partners. The same prespecified baseline covariates were included in the model and missing covariates were imputed. A two-way interaction between randomization and recruitment source was tested before stratification by recruitment source.
Statistical significance for the primary outcome was defined as a two-tailed α<0·05. The four secondary outcomes were evaluated with a Hochberg modification to the Bonferroni adjustment in which estimates are considered statistically significant if the largest p-value is <0·05. If the largest p-value exceeds 0·05, then the second largest p-value is evaluated at <0·025 (0·05/2). If this p-value exceeds 0·025, then the third largest p-value is evaluated at <0·017 (0·05/3). Finally, if this p-value exceeds 0·017, the fourth p-value is evaluated at <0·0125 (0·05/4). The same approach was applied post-hoc to stratified analyses. The three-way interaction in mixed effects models and two-way interaction in proportional-hazards models were tested at a prespecified α<0·10.
Sensitivity analyses estimated the per-protocol and complier average causal effect (CACE) for each outcome, tested alternative methods of handling missing data, examined different definitions of the outcomes, and compared continuous and discretized time. All analyses were executed in SAS 9.4 (SAS Institute, Cary, NC) with the exception of multiple imputation (Stata 18.0, StataCorp, College Station, TX) and CACE (Mplus 8.8, Muthén & Muthén, Los Angeles, CA). The trial and analysis plan were registered at ClinicalTrials.gov (NCT03243422) before the unmasking of trial data.
Results
From November 9, 2017 to October 25, 2019, 3004 participants were screened for eligibility and 977 were randomised (238 participants from ARIC and 739 who were recruited de novo); 490 participants were assigned to HI and 487 to SA control (Figure 1). All participants were randomised and had received their assigned study intervention before COVID-19 pandemic-related closures of study sites for in-person visits from March 2020 to June 2021 during which semi-annual and annual visits were converted to phone-based visits. Sites re-opened for in-person visits in June 2021, and from June 1, 2021 to November 30, 2022, 862 participants (88·2%) returned for year 3 in-person visits, while 15 participants (1·5%) had phone-based year 3 visits. A total of 100 participants (10·2%; 50 who had been assigned to HI and 50 who had been assigned to SA control) did not complete a year 3 visit. Of these 100 participants, 24 were lost to follow-up by year 3, 26 had withdrawn from the study by year 3, 34 had died, and 16 did not complete neurocognitive assessment at year 3 (incomplete assessment).
Figure 1.
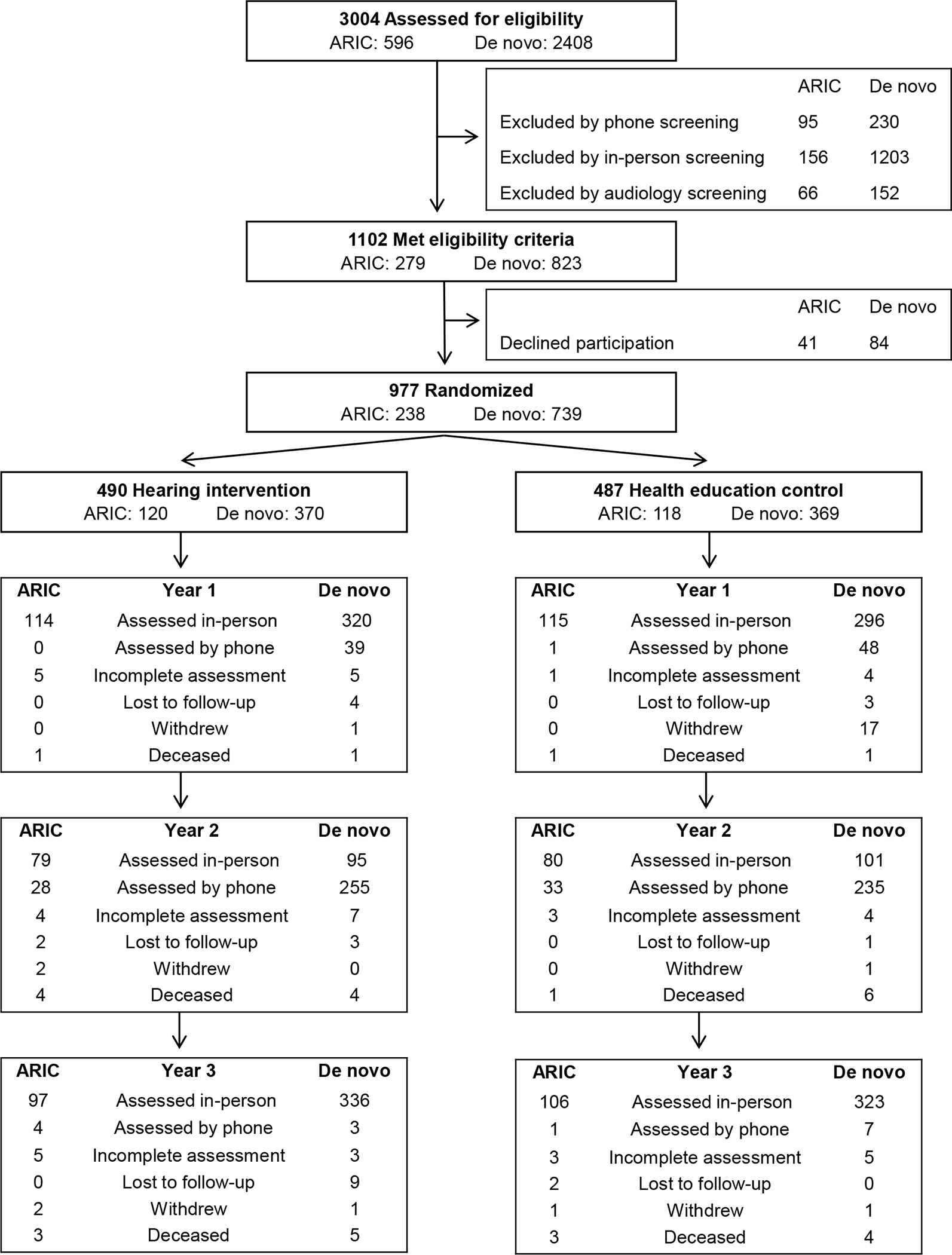
ACHIEVE Screening, Randomisation, and Follow-Up
Abbreviations: ACHIEVE, Aging and Cognitive Health Evaluation in Elders; ARIC, Atherosclerosis Risk in Communities.
Participants assigned to HI and SA control were similar at baseline (Table 1). The cohort had a mean age of 76·8 years (SD 4·0), was 53.5% female and 11.5% Black, had a mean 4-frequency pure tone average of 39·4 dB (SD 6·9), mean MMSE of 28·2 (SD 1·6), and mean self-perceived communication impairment (HHI) score of 15·3 (SD 9·8) indicative of mild-to-moderate communication impairment. There were substantial differences at baseline between participants from the ARIC versus de novo cohorts (appendix p 2). On average, participants from ARIC compared to de novo were more likely to be older, female, Black, have lower education and income, have higher rates of diabetes and hypertension, and to live alone. ARIC participants had slightly lower MMSE scores and significantly lower global cognition and cognitive domain factor scores at baseline compared to de novo participants. ARIC and de novo cohort participants had similar audiometric levels of hearing at baseline, but de novo participants had greater self-perceived communication impairment on the HHI.
Table 1.
Demographic and Clinical Characteristics at Baseline, Hearing Aid Use, and Cognitive Outcomes of ACHIEVE Participants Stratified by Randomised Treatment Assignment and Recruitment Source (N=977)
| Total (N=977) | ARIC (N=238) | De novo (N=739) | ||||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | |||
| N | All | (N=490) | (N=487) | (N=120) | (N=118) | (N=370) | (N=369) | |
|
|
|
|
|
|
||||
|
Baseline Age, mean (SD), y |
977 | 76.8 (4.0) | 76.5 (3.9) | 77.0 (4.0) | 79.2 (2.9) | 78.6 (2.9) | 75.7 (3.8) | 76.5 (4.2) |
| Female sex, No. (%) | 977 | 523 (53.5) | 264 (53.9) | 259 (53.2) | 74 (61.7) | 73 (61.9) | 190 (51.4) | 186 (50.4) |
| Black race, No. (%) | 977 | 112 (11.5) | 53 (10.8) | 59 (12.1) | 33 (27.5) | 35 (29.7) | 20 (5.4) | 24 (6.5) |
| White race, No. (%) Field site, No. (%) |
977 | 858 (87.8) | 434 (88.6) | 424 (87.1) | 86 (71.7) | 83 (70.3) | 348 (94.1) | 341 (92.4) |
| Forsyth County, North Carolina | 977 | 236 (24.2) | 117 (23.9) | 119 (24.4) | 31 (25.8) | 30 (25.4) | 86 (23.2) | 89 (24.1) |
| Jackson, Mississippi | 243 (24.9) | 120 (24.5) | 123 (25.3) | 30 (25.0) | 33 (28.0) | 90 (24.3) | 90 (24.4) | |
| Minneapolis, Minnesota | 236 (24.2) | 120 (24.5) | 116 (23.8) | 21 (17.5) | 22 (18.6) | 99 (26.8) | 94 (25.5) | |
| Washington County, Maryland Education, No. (%) |
262 (26.8) | 133 (27.1) | 129 (26.5) | 38 (31.7) | 33 (28.0) | 95 (25.7) | 96 (26.0) | |
| Less than high school | 976 | 37 (3.8) | 19 (3.9) | 18 (3.7) | 12 (10.1) | 10 (8.5) | 7 (1.9) | 8 (2.2) |
| High school, GED, or vocational school | 418 (42.8) | 206 (42.1) | 212 (43.5) | 48 (40.3) | 48 (40.7) | 158 (42.7) | 164 (44.4) | |
| College, graduate, or professional school | 521 (53.4) | 264 (54.0) | 257 (52.8) | 59 (49.6) | 60 (50.8) | 205 (55.4) | 197 (53.4) | |
| One or more APOE £4 alleles, No. (%) | 908 | 224 (24.7) | 110 (24.5) | 114 (24.8) | 26 (23.0) | 33 (28.2) | 84 (25.0) | 81 (23.7) |
| Diabetes, No. (%) | 977 | 195 (20.0) | 104 (21.2) | 91 (18.7) | 36 (30.0) | 32 (27.1) | 68 (18.4) | 59 (16.0) |
| Hypertension, No. (%) | 974 | 651 (66.8) | 333 (68.0) | 318 (65.7) | 87 (72.5) | 82 (71.3) | 246 (66.5) | 236 (64.0) |
| Living alone, No. (%) Income, No. (%) | 967 | 290 (30.0) | 153 (31.6) | 137 (28.4) | 44 (37.9) | 39 (33.9) | 109 (29.6) | 98 (26.6) |
| Under $25,000 | 950 | 147 (15.5) | 73 (15.3) | 74 (15.7) | 29 (25.4) | 31 (27.9) | 44 (12.1) | 43 (11.9) |
| $25,000–$49,999 | 283 (29.8) | 156 (32.6) | 127 (26.9) | 47 (41.2) | 30 (27.0) | 109 (29.9) | 97 (26.9) | |
| $50,000–$74,999 | 210 (22.1) | 91 (19.0) | 119 (25.2) | 22 (19.3) | 25 (22.5) | 69 (19.0) | 94 (26.0) | |
| $75,000–$100,000 | 140 (14.7) | 68 (14.2) | 72 (15.3) | 8 (7.0) | 13 (11.7) | 60 (16.5) | 59 (16.3) | |
| Over $100,000 | 170 (17.9) | 90 (18.8) | 80 (16.9) | 8 (7.0) | 12 (10.8) | 82 (22.5) | 68 (18.8) | |
| Pure tone average, mean (SD), dB Baseline & Follow-Up |
977 | 39.4 (6.9) | 39.5 (7.1) | 39.3 (6.7) | 39.5 (6.7) | 38.7 (6.7) | 39.6 (7.2) | 39.5 (6.8) |
| Hearing handicap inventory, mean (SD), Baseline | 970 | 15.3 (9.8) | 15.7 (10.2) | 14.9 (9.3) | 12.7 (10.3) | 11.4 (8.6) | 16.7 (9.9) | 16.0 (9.3) |
| Year One | 926 | 9.8 (9.0) | 5.7 (5.9) | 14.0 (9.8) | 5.1 (5.4) | 10.1 (7.3) | 5.9 (6.0) | 15.3 (10.1) |
| Year Two | 892 | 10.3 (9.2) | 6.6 (6.6) | 14.0 (9.9) | 5.1 (6.0) | 10.2 (8.3) | 7.1 (6.6) | 15.3 (10.1) |
| Year Three | 863 | 12.0 (9.6) | 7.8 (7.3) | 16.2 (9.9) | 7.6 (8.6) | 12.4 (9.2) | 7.8 (6.8) | 17.4 (9.8) |
| Mini-mental state exam, mean (SD), Baseline | 977 | 28.2 (1.6) | 28.2 (1.6) | 28.2 (1.6) | 28.1 (1.7) | 27.9 (1.8) | 28.3 (1.6) | 28.3 (1.5) |
| Year Three | 856 | 27.8 (2.3) | 27.9 (2.4) | 27.7 (2.2) | 26.9 (2.8) | 26.6 (2.7) | 28.2 (2.1) | 28.0 (1.8) |
| Global cognition, mean (SD), Baseline | 977 | 0.000 (0.926) | 0.012 (0.949) | −0.011 (0.902) | −0.411 (1.024) | −0.346 (1.062) | 0.149 (0.883) | 0096 (0.818) |
| Year Three | 859 | −0.161 (1.098) | −0.136 (1.139) | −0.186 (1.057) | −0.604 (1.274) | −0.643 (1.156) | −0.001 (1.060) | −0.037 (0.980) |
| Executive function, mean (SD), Baseline | 977 | −0.001 (0.888) | 0.020 (0.897) | −0.021 (0.879) | −0.327 (1.042) | −0.310 (0.958) | 0.132 (0.815) | 0.072 (0.833) |
| Year Three | 856 | −0.236 (1.060) | −0.224 (1.096) | −0.248 (1.025) | −0.608 (1.228) | −0.652 (1.122) | −0.112 (1.029) | −0.116 (0.956) |
| Language, mean (SD), Baseline | 977 | 0.000 (0.837) | −0.011 (0.851) | 0.012 (0.823) | −0.436 (0.883) | −0.352 (0.965) | 0.126 (0.794) | 0.128 (0.737) |
| Year Three | 859 | −0.115 (0.930) | −0.111 (0.949) | −0.119 (0.912) | −0.485 (1.026) | −0.563 (0.969) | −0.002 (0.898) | 0.025 (0.845) |
| Memory, mean (SD), Baseline | 977 | 0.000 (0.909) | 0.016 (0.938) | −0.016 (0.879) | −0.223 (0.918) | −0.159 (0.959) | 0.093 (0.933) | 0.030 (0.849) |
| Year Three Hours of hearing aid use per day, mean (SD) |
859 | 0.012 (1.070) | 0.067 (1.091) | −0.043 (1.046) | −0.220 (1.128) | −0.254 (1.116) | 0.151 (1.068) | 0.026 (1.015) |
| Year One | 470 | 8.1 (4.6) | 8.1 (4.6) | 7.2 (4.4) | 8.3 (4.7) | |||
| Year Two | 456 | 7.1 (5.0) | 7.1 (5.0) | 6.8 (4.8) | 7.2 (5.1) | |||
| Year Three | 431 | 7.2 (5.2) | 7.2 (5.2) | 5.8 (5.0) | 7.6 (5.2) | |||
| Intervention drop-in, No. (%) | 462 | 76 (16.5) | 76 (16.5) | 9 (7.8) | 67 (19.4) | |||
| Intervention drop-out, No. (%) | 488 | 10 (2.0) | 10 (2.0) | 5 (4.2) | 5 (1.4) | |||
|
|
|
|
|
|
||||
Abbreviations: ACHIEVE, Aging and Cognitive Health Evaluation in Elders; APOE, apolipoprotein E; ARIC, Atherosclerosis Risk in Communities; dB, decibels; GED, General educational development credential; SD, standard deviation; y, year. Sex (male/female) was based on self-report. Diabetes was defined as present if the participant reported using medication for diabetes or self-reported a physician diagnosis of diabetes. Sitting blood pressure was measured using a random zero sphygmomanometer. Hypertension was defined as present based on the use of antihypertensive medication, systolic blood pressure greater than or equal to 140 mm Hg, or diastolic blood pressure greater than or equal to 90 mm Hg. Income was based on participant self-report of all family income over the past 12 months. Factor scores of global cognition, executive function, language, and memory were developed using a validated latent variable modeling approach15 and standardized to the baseline with higher scores indicating better cognitive function. Hearing aid use is based on average self-reported hours of use per day.
The hearing intervention demonstrated evidence of target engagement based on self-reported hours of hearing aid use and reduction in self-perceived communication impairment after HI (Table 1). Participants receiving HI reported a mean of 7·2 hours (SD 5.2) of hearing aid use per day at year 3 and had HHI scores that declined from a mean of 15·7 (SD 10·2) at baseline to 7·8 (SD 7·3) at year 3 which is indicative of no communication impairment. In contrast, the HHI score among SA control participants increased from a mean of 14·9 (SD 9·3) at baseline to 16·2 (SD 9·9) at year 3. A similar pattern of HI target engagement was observed between the ARIC and de novo cohorts but with HI participants in the de novo cohort reporting more hours of daily hearing aid use. During follow-up, the rate of drop-out from the hearing intervention (i.e., discontinuance of hearing aid use) was 2%. Rates of drop-in (i.e., individuals assigned to SA control but choosing to obtain hearing aids on their own outside of the study) was 16·5% with a higher rate of drop-in observed among SA control participants in the de novo than the ARIC cohort (19·4% vs. 7·8%).
In the analysis of the primary outcome of 3-year global cognitive change combining both the ARIC and de novo cohorts, global cognitive change (in SD units) was not significantly different between HI and SA control (Figures 2 and 3; Difference 0·002 [95% CI: −0·077, 0·081], p=0·96). However, prespecified sensitivity analyses stratified by recruitment source demonstrated significant differences in the effect of HI on 3-year cognitive change between the ARIC and de novo cohorts (p for interaction=0·010, Figures 2 and 3). In the ARIC cohort, HI was associated with a 48% reduction in 3-year cognitive change compared to SA control (Difference 0·191 [95% CI: 0·022, 0·360], p=0·027). In the de novo cohort, 3-year cognitive change was not significantly different between HI and SA control (Difference −0·061 [95% CI: −0·151, 0·028], p=0·18). Other key distinctions between the ARIC and de novo cohorts include lower baseline cognitive scores among ARIC participants (Figure 3) and a 2.7-fold greater rate of 3-year cognitive change among SA control participants in the ARIC versus de novo cohort (−0·402 [95% CI: −0·536, −0·267] versus −0·151 [95% CI: −0·215, −0·087]). Sensitivity analyses that varied the analytical approach did not substantively change the observed results for the primary outcome (appendix p 3,5,6,8), although the protective effect of HI was greater in per-protocol and CACE analyses in the ARIC cohort (appendix p 3).
Figure 2.
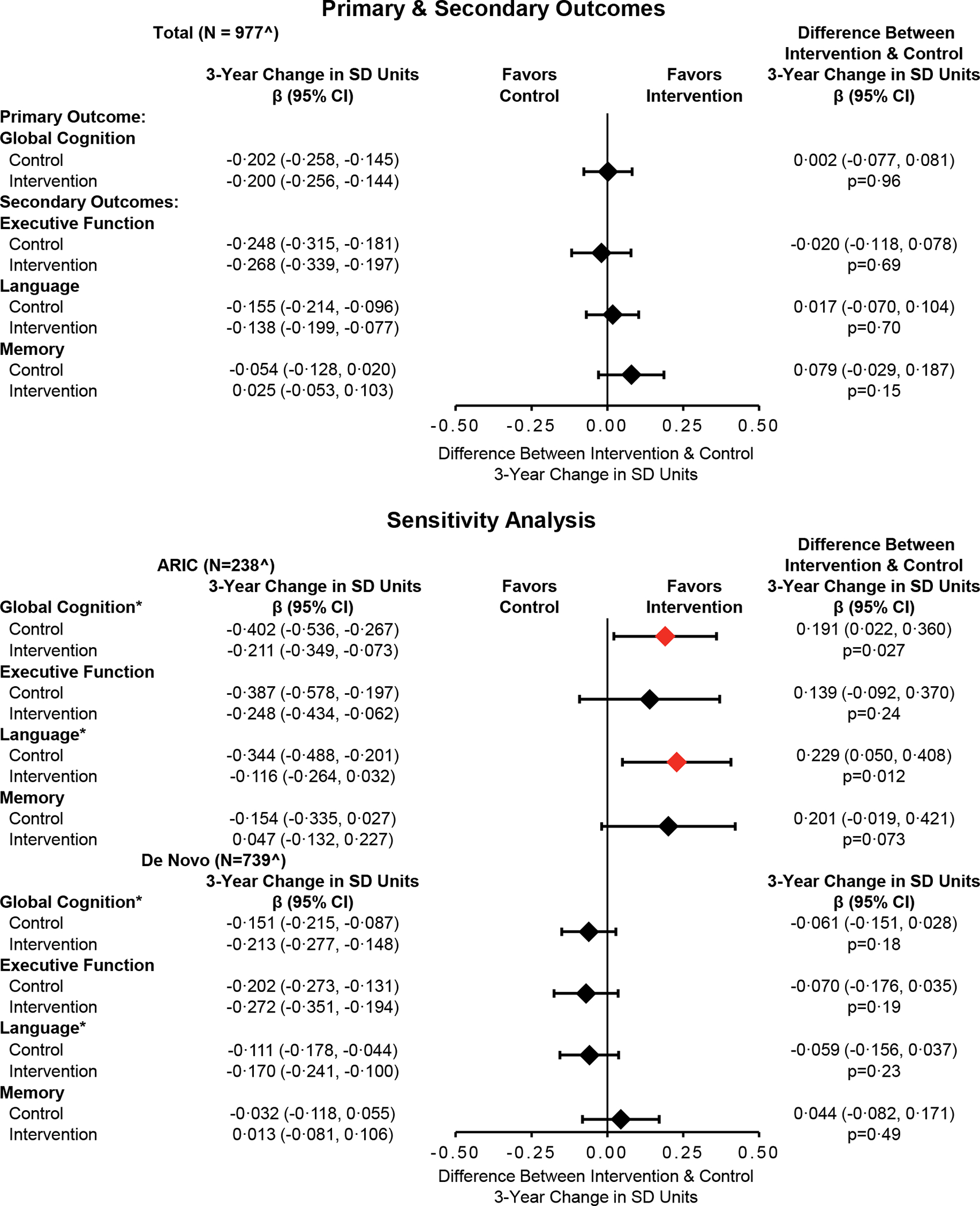
Covariate-Adjusted Analysis of Three-Year Cognitive Change by Randomised Treatment Assignment Among the Total Cohort and Stratified by Recruitment Source (N=977)
Symbols: *Statistically significant (p<·05) three-way interaction between randomisation, recruitment source, and time; ^ The analytic sample for the primary analysis comprised 977 in-person assessments from baseline, 862 in-person assessments from year 3 (203 ARIC, 659 De Novo), 9 in-person assessments (5 ARIC, 4 De Novo) from participants who died prior to year 3 but completed an assessment less than a year before death, and 106 missing year 3 assessments (30 ARIC, 76 De Novo) with values generated from a prespecified multiple imputation model. Abbreviations: ACHIEVE, Aging and Cognitive Health Evaluation in Elders; ARIC, Atherosclerosis Risk in Communities; CI, confidence intervals; SD, standard deviation. Parameter estimates, 95% confidence intervals, and p-values were calculated from a linear mixed effects models that adjusted for hearing loss (PTA <40 dB vs 40+ dB), recruitment source, field site, age, sex, education, and the presence of APOE e4 alleles at baseline. An interaction with time was specified for each covariate except education. A three-way interaction between randomisation, recruitment source, and time was tested for each model prior to stratification.
Figure 3.
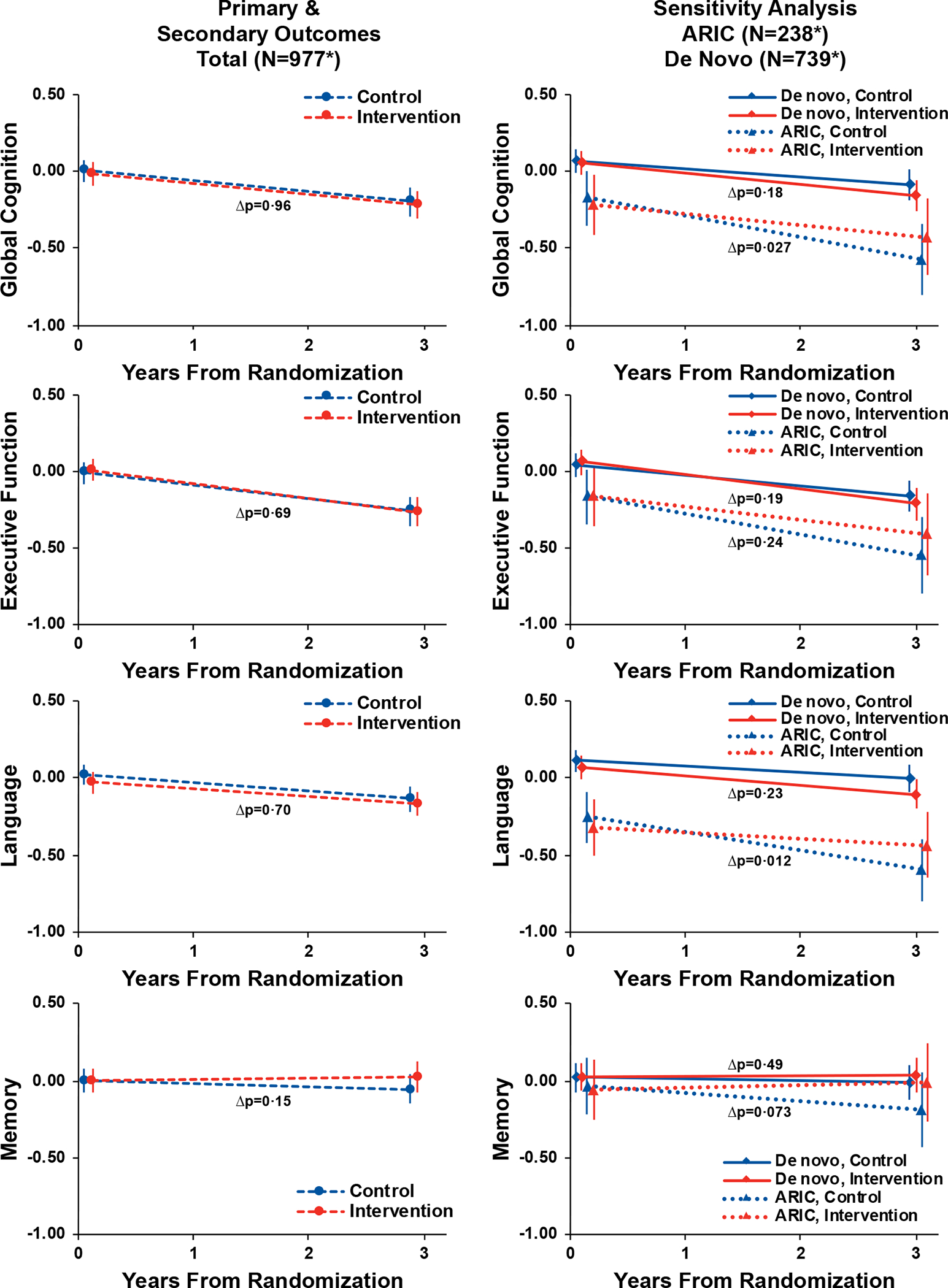
Trajectories and Pointwise Estimates of Cognitive Function by Randomised Treatment Assignment Among the Total Cohort and Stratified by Recruitment Source (N=977)
Symbols: *The analytic sample for the primary analysis comprised 977 in-person assessments from baseline, 862 in-person assessments from year 3 (203 ARIC, 659 De Novo), 9 in-person assessments (5 ARIC, 4 De Novo) from participants who died prior to year 3 but completed an assessment less than a year before death, and 106 missing year 3 assessments (30 ARIC, 76 De Novo) with values generated from a prespecified multiple imputation model. Abbreviations: ACHIEVE, Aging and Cognitive Health Evaluation in Elders; ARIC, Atherosclerosis Risk in Communities. Y-axis values are cognitive factor scores that were developed using a validated latent variable modeling approach15 and standardized to the baseline with higher scores indicating better cognitive function. Parameter estimates, 95% confidence intervals, and p-values were calculated from a linear mixed effects models that adjusted for hearing loss (PTA <40 dB vs 40+ dB), recruitment source, field site, age, sex, education, and the presence of APOE e4 alleles at baseline. An interaction with time was specified for each covariate except education. Visualization based on a hypothetical participant whose characteristics equalled the sample means. Δp refers to the p-value of the interaction between time and randomisation.
Analyses of the secondary outcomes of domain-specific cognitive factor scores in executive function, language, and memory domains did not demonstrate differences between HI and SA control in analyses of the combined ARIC and de novo cohorts (Figures 2 and 3). In a stratified analysis of the ARIC cohort, HI was significantly associated with reduced 3-year decline in the language domain (Difference 0·229 [95% CI: 0·050, 0·408], p=0·012) compared to SA control. No effect of HI on 3-year change in cognitive domains was observed in the de novo cohort. For the secondary outcome of incident cognitive impairment, the cumulative incidence of cognitive impairment was noted be greater in the ARIC versus de novo cohort by year 1. HI was not associated with a reduced hazard of cognitive impairment in analyses of the total cohort (Hazard ratio [HR] 0·90 [95% CI: 0·61, 1·33], p=0·59) or in analyses stratified by the ARIC (HR 0·94 [95% CI: 0·54, 1·64], p=0·83) or de novo cohort (HR 0·89 [95% CI: 0·48, 1·67], p=0·72).
Adverse events of otitis externa, cerumen impaction or ear foreign body requiring removal by a physician, and death from any cause were monitored by study investigators and the DSMB throughout the study. There were no adverse events that were unexpected and judged to be related to study participation.
Discussion
In this first-in-kind randomised trial investigating the long-term effects of hearing intervention on reducing cognitive decline, our results demonstrated differences in the effect of hearing intervention between the two study populations that comprised the trial cohort. The primary analysis of the total cohort which combined both study populations demonstrated no effect of hearing intervention on reducing cognitive decline. However, in prespecified stratified analyses, hearing intervention was associated with a 48% reduction in 3-year global cognitive decline in the ARIC cohort (n=238), but no effect of HI was observed in the de novo cohort (n=739). Compared to the de novo cohort of healthy volunteers, the ARIC cohort had more risk factors for cognitive decline and dementia, lower baseline cognitive scores, and faster rates of 3-year cognitive decline. Taken together, our results suggest that hearing intervention may differ in its effect on 3-year cognitive change across different populations. Hearing intervention in populations of older adults at increased risk for cognitive decline and dementia may have a significant effect on reducing cognitive change within 3 years. In contrast, hearing intervention may not have appreciable effects on reducing cognitive change within 3 years in populations at decreased risk for cognitive decline. A follow-up study of the ACHIEVE cohort is currently underway to study longer term effects of hearing intervention on cognition and other outcomes.
Results of the ACHIEVE trial are consistent with the findings of previous observational studies9,10,18,19 which have suggested that hearing loss treatment may have beneficial effects on reducing cognitive decline and dementia. A recent pooled meta-analysis of 126 903 participants in 8 observational studies with periods of follow-up ranging from 2 to 25 years found a lower hazard of cognitive decline in hearing aid users. 9 However, inferences from these larger observational studies are often limited by residual confounding and lack of information about the duration and characteristics of the hearing loss treatment. The ACHIEVE study now provides RCT-level evidence of the effect of a well-defined hearing intervention on cognitive decline. These findings are supportive of previous conclusions from the 2020 Lancet Commission on dementia2, the 2022 United States National Plan to Address Alzheimer’s Disease20, and recent research10,21 that has called for treating hearing loss in older adults to supplement existing national dementia risk reduction strategies. Results from the ACHIEVE study clarify that any benefits of hearing intervention in reducing cognitive change within 3 years will likely vary across populations depending on risk for cognitive decline.
Hypothesized mechanisms through which hearing loss could potentially increase risk for cognitive decline and dementia have been previously described7,22,23 and include cognitive load (information degradation hypothesis), structural effects on brain integrity (sensory deprivation hypothesis), and reduced social engagement and participation in cognitively-stimulating activities. These mechanisms are not mutually exclusive, and our findings in the ACHIEVE study suggest that hearing intervention could mitigate the effects of hearing loss on cognitive decline through one or more of these pathways. Future analyses of brain MRI and social engagement data that were collected in the ACHIEVE study will allow for further elucidation of the pathways through which hearing intervention may reduce cognitive decline.
A key finding from the ACHIEVE study is the notable difference between the effect of HI in the ARIC and de novo cohorts despite similar levels of baseline hearing and more pronounced evidence of target engagement with the HI in the de novo cohort (as evidenced by the larger drop in HHI scores and greater number of hours of hearing aid use). This finding may be attributable to the nearly 3-fold difference in rates of cognitive change observed in the control participants between the two cohorts. The annual rate of cognitive change observed in de novo control participants (−0·151 SD unit change over 3 years = −0·05 SD unit/year) is consistent with a slow rate of cognitive change (estimated at −0·04 SD unit/year in a previous study24), while the rate in ARIC control participants (−0·402 SD unit change over 3 years = −0·134 SD unit/year) is more consistent with a moderate rate of cognitive decline (estimated at −0·19 SD unit/year24). Based on the hypothesis that hearing intervention could potentially reduce cognitive decline, the slow rate of cognitive change observed in the de novo cohort may limit any effect of hearing intervention in potentially further reducing this decline within a relatively modest 3-year period of follow-up.
A possible explanation for the de novo cohort having a slower rate of cognitive change over 3 years compared to the ARIC cohort is that the de novo cohort was younger, had fewer risk factors for cognitive decline (e.g., higher education, less cardiovascular risk factors, less likely to be living alone), and higher baseline levels of cognition. These characteristics may be related to a ‘healthy volunteer’ effect of the de novo participants being newly recruited into this trial. A healthy volunteer effect has been described in previous cohort studies25,26 whereby participants who newly elect to participate in studies generally represent a healthier subset of the target population. In contrast, participants from ARIC were recruited more than 30 years ago over which time there would be expected to be declining differences27 between these participants and the potential target population of community-dwelling older adults who meet study inclusion criteria. Another possible explanation for the slower rate of cognitive decline in the de novo cohort relates to practice or learning effects with repeat neurocognitive testing in the de novo participants who were naïve to cognitive testing. Other large trials involving repeated assessments of cognition have demonstrated continued improvement in neurocognitive performance over 2 or more years, 28,29 and the magnitude of these practice effects may vary based on the type of neurocognitive test administered. 30 In contrast, participants in the ARIC cohort had already undergone numerous cognitive assessments prior to randomisation into ACHIEVE which would minimize benefits from continued practice effects.
This trial has limitations. Understanding the possible effects of hearing intervention on populations at decreased risk for cognitive decline will require longer-term follow-up of the de novo cohort beyond 3 years which is currently underway. Participants and study technicians also could not be feasibly masked to study intervention assignment which could possibly bias collected results. Two of the 10 tests that comprise the in-person neurocognitive battery also contained only auditory stimuli, and individuals receiving SA control with untreated hearing loss could potentially perform more poorly on these measures if the auditory stimuli were not correctly understood by the participant. However, in secondary analyses of the 3 cognitive domains, we note that the strongest effect of HI in ARIC participants was observed in the language domain which did not consist of any tests with exclusively auditory stimuli. Finally, we were not able to observe effects of HI on incident cognitive impairment, but these analyses may be underpowered given the relatively modest period of follow-up. Continued follow-up of the ACHIEVE cohort is presently underway to understand these longer term effects of HI on cognitive function.
Results from the ACHIEVE study add to the growing evidence base that suggests addressing modifiable risk factors for cognitive decline and dementia could be impactful in reducing the future global burden of dementia. Based on evidence from the ACHIEVE study, hearing loss may be a particularly important global public health target for dementia prevention efforts given that hearing loss is highly prevalent among older adults and is treatable with an established intervention (i.e., hearing aids and related support services). Such interventions are underutilized around the world, confer essentially no medical risk, and have now been demonstrated to reduce cognitive decline within 3 years when implemented in late-life for at-risk older adults.
Supplementary Material
Figure 4.
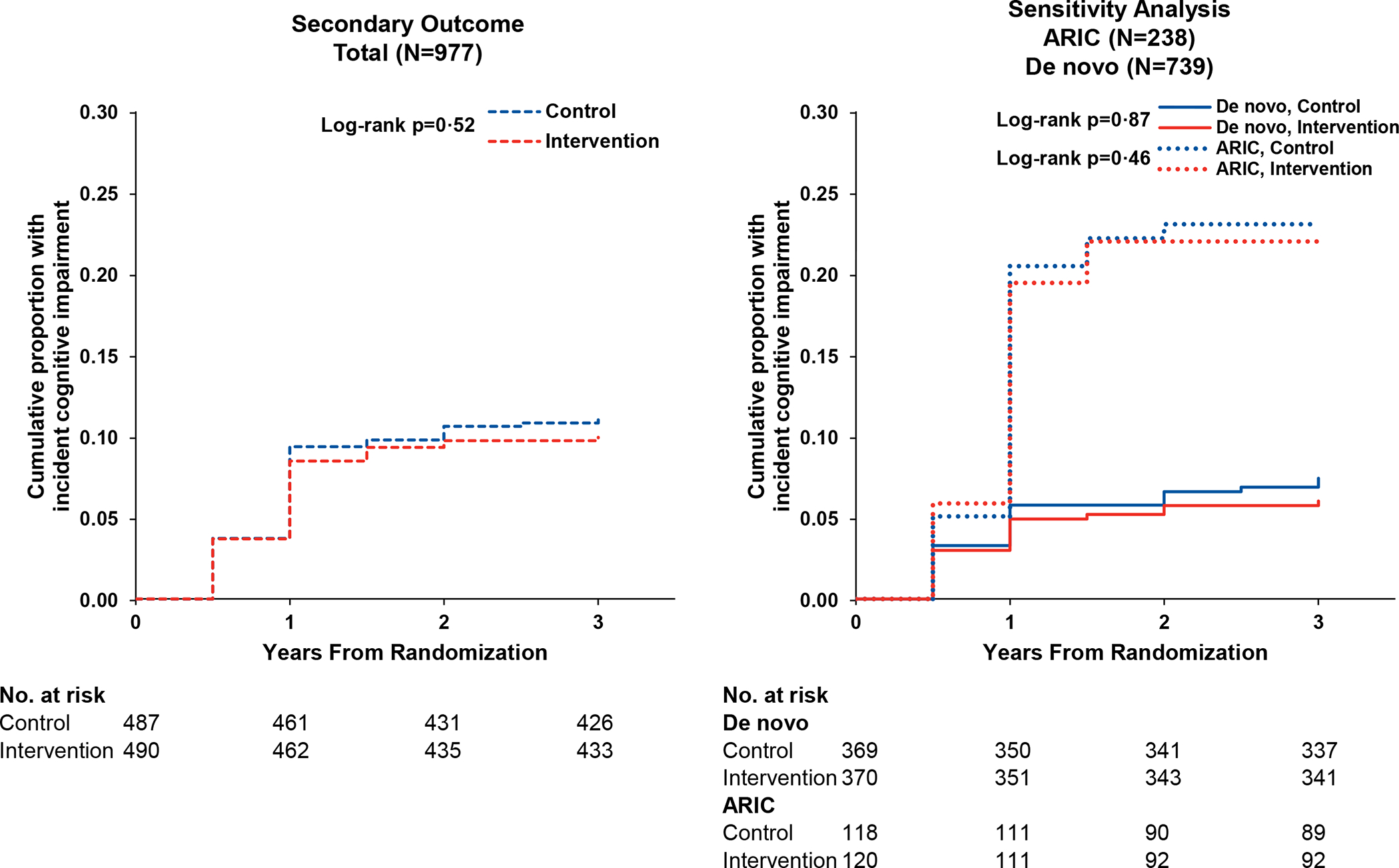
Cumulative Incidence of Cognitive Impairment by Randomised Treatment Assignment Among the Total Cohort and Stratified by Recruitment Source (N=977)
Abbreviations: ACHIEVE, Aging and Cognitive Health Evaluation in Elders; ARIC, Atherosclerosis Risk in Communities.
Cumulative incidence curves depict the proportion of participants with cognitive impairment after accounting for the competing risk of death.
Research in context.
Evidence before this study
We searched PubMed on May 22, 2023 using the search terms “(randomized trial) AND (hearing) AND (cognitive decline)” and further restricted retrieved studies to those that included a study population of adults without prevalent cognitive impairment or dementia, tested an intervention involving technologies or strategies for hearing loss treatment, had trial follow-up of >1 year, and had a primary outcome involving cognition. No published trials were identified that met these criteria. A recent meta-analysis published in February 2023 of 8 observational studies which had 126 903 participants and a follow-up duration ranging from 2 to 25 years, concluded that hearing loss intervention was associated with reduced hazard of long-term cognitive decline and that the “cognitive benefit of hearing restorative devices should be further investigated in randomized trials”.
Added value of this study
To the investigators’ knowledge, the ACHIEVE trial is the first randomised controlled trial to investigate whether hearing intervention can reduce long-term cognitive change in cognitively healthy older adults (primary prevention trial for cognitive decline and dementia). The primary analysis of the total cohort showed no reduction in 3-year cognitive decline with hearing intervention, but a prespecified sensitivity analysis revealed a difference in the effect of hearing intervention between the two distinct study populations that comprised the study cohort. Hearing intervention reduced 3-year cognitive change in the population of older adults at increased risk for cognitive decline but had no effect in those at decreased risk for cognitive decline.
Implications of all the available evidence
Taken together, our findings suggest that hearing loss may be a particularly important global public health target for dementia prevention efforts. Hearing loss is highly prevalent in older adults and is treatable with an established intervention (i.e., hearing aids and related support services) that is underutilized and confers essentially no medical risk. Results from this randomised trial suggest that hearing intervention can reduce cognitive change within 3 years when implemented in late-life for older adults at increased risk for cognitive decline.
Acknowledgments
Members of the ACHIEVE Collaborative Research Group are provided in the appendix (p 21).
The Aging and Cognitive Health Evaluation in Elders (ACHIEVE) study is supported by the National Institute on Aging (NIA) R01AG055426 and R01AG060502 with previous pilot study support from the NIA R34AG046548 and the Eleanor Schwartz Charitable Foundation, in collaboration with the Atherosclerosis Risk in Communities (ARIC) Study, supported by National Heart, Lung, and Blood Institute contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Neurocognitive data are collected by 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, NINDS, NIA and NIDCD), and with previous brain MRI examinations funded by R01HL70825 from the NHLBI. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funder of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit for publication.
The investigators thank the participants and staff of the ACHIEVE and ARIC studies for their important contributions and dedication to the study, Sonova/Phonak for in-kind donation of hearing technologies and training support of audiologists for the ACHIEVE study, members of the ACHIEVE DSMB (Doug Galasko [Chair, University of California San Diego], Julie Buring [Harvard University], Judy R. Dubno [Medical University of South Carolina], Tom Greene [University of Utah], and Larry Lustig [Columbia University]) for their guidance and insights during the course of the study, and Nae-Yuh Wang (Johns Hopkins University) for his review of the statistical analyses.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Declaration of Interests
FL reports research grants from the National Institutes of Health and Eleanor Schwartz Charitable Foundation, consulting fees from Frequency Therapeutics and Apple Inc, payment for expert testimony, participation on a scientific advisory board for Fondation Pour L’Audition and Sharper Sense, volunteer board member for Access HEARS, donation in-kind from Sonova/Phonak to Johns Hopkins University for hearing technologies used in the present study, and being the director of a public health research center funded in part by a philanthropic donation from Cochlear Ltd. to the Johns Hopkins Bloomberg School of Public Health. KH reports consulting fees from Fred Hutchinson Cancer Research Center, support for attending meetings (National Institute for Health Center for Scientific Review, Hebrew Senior Life), participation on the Wake Forest School of Medicine DSMB (unpaid), and leadership roles for peer-reviewed journals (Alzheimer’s & Dementia: Translational Research and Clinical Interventions and Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring [unpaid]). DK serves on a Data Safety Monitoring Board for the Dominantly Inherited Alzheimer Network Treatment Unit study. He served on a Data Safety monitoring Board for a tau therapeutic for Biogen (until 2021) but received no personal compensation. He is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals and the University of Southern California. He has served as a consultant for Roche, Samus Therapeutics, Magellan Health, Biovie and Alzeca Biosciences but receives no personal compensation. He attended an Eisai advisory board meeting for lecanemab on December 2, 2022, but received no compensation. VS reports industry-sponsored clinical research contract (to institution) to support research activity from Otonomy Inc., Frequency Therapeutics., Pipeline Therapeutics, Aerin Medical, Oticon Medical, Helen of Troy Ltd., consulting fees from Autifony Therapeutics, Boehringer Ingelheim, honoraria from Oticon Medical, Sonova Holding AG and Phonak USA, and hearing technology devices donated for educational or research proposes from Sonova Holding AG, and Phonak USA. MA reports consulting fees from GN Resound, NIDCD, and NIA, travel support from NIDCD and NIA, and receipt of equipment from Sonova AG. NR reports being editor of the American Journal of Audiology (paid) and Scientific Chair of the American Academy of Audiology, Advisory Board Member with stock Options for Neosensory, and being a member of the Scientific Advisory Board for Shoebox Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Frank R Lin, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Cochlear Center for Hearing and Public Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Otolaryngology-Head & Neck Surgery, Johns Hopkins School of Medicine, Baltimore, MD, USA; Center on Aging and Health, Johns Hopkins University, Baltimore, MD, USA.
James R Pike, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Marilyn S Albert, Department of Neurology, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Michelle Arnold, Sarasota, FL, USA.
Sheila Burgard, Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC, USA.
Theresa Chisolm, Department of Communication Sciences & Disorders, College of Behavioral & Community Sciences, University of South Florida, Tampa, FL, USA.
David Couper, Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC, USA.
Jennifer A Deal, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Cochlear Center for Hearing and Public Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Otolaryngology-Head & Neck Surgery, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Adele M Goman, School of Health and Social Care, Edinburgh Napier University, UK.
Nancy W Glynn, Department of Epidemiology, University of Pittsburgh School of Public Health, Pittsburgh, PA, USA.
Theresa Gmelin, Department of Epidemiology, University of Pittsburgh School of Public Health, Pittsburgh, PA, USA.
Lisa Gravens-Mueller, Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC, USA.
Kathleen M Hayden, Department of Social Sciences and Health Policy, Wake Forest University School of Medicine, Winston-Salem, NC, USA.
Alison R Huang, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Cochlear Center for Hearing and Public Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
David Knopman, Department of Neurology, Mayo Clinic, Rochester, MN, USA.
Christine M Mitchell, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Thomas Mosley, The MIND Center, University of Mississippi Medical Center, Jackson, MS, USA.
James S Pankow, Division of Epidemiology and Community Health, University of Minnesota School of Public Health, Minneapolis, MN, USA.
Nicholas S Reed, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Cochlear Center for Hearing and Public Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Otolaryngology-Head & Neck Surgery, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Victoria Sanchez, Department of Otolaryngology-Head & Neck Surgery, Morsani College of Medicine, University of South Florida, Tampa, FL, USA.
Jennifer A Schrack, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Center on Aging and Health, Johns Hopkins University, Baltimore, MD, USA.
B Gwen Windham, The MIND Center, University of Mississippi Medical Center, Jackson, MS, USA.
Josef Coresh, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Data Sharing
A deidentified dataset and data dictionary will be made available in 2024 on a publicly-available U.S. data repository pending approval by the funding sponsor (National Institute on Aging). Additional details on data access policies will be made available at www.achievestudy.org at that time. The study protocol and statistical analysis plan are available at www.clinicaltrials.gov. Access to ACHIEVE study manuals and forms are available by contacting the corresponding author.
References
- 1.Alzheimer’s Disease International. Numbers of people with dementia around the world. https://www.alzint.org/resource/numbers-of-people-with-dementia-worldwide. Accessed May 8, 2023.
- 2.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. August 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Risk reduction of cognitive decline and dementia : WHO guidelines. World Health Organization,; 2019:1 online resource (1 PDF file (xiv, 78 pages)). https://www.ncbi.nlm.nih.gov/books/NBK542796/ NLM Bookshelf Books [PubMed] [Google Scholar]
- 4.Lazar RM, Howard VJ, Kernan WN, et al. A Primary Care Agenda for Brain Health: A Scientific Statement From the American Heart Association. Stroke. Mar 2021:STR0000000000000367. doi: 10.1161/STR.0000000000000367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukadam N, Sommerlad A, Huntley J, Livingston G. Population attributable fractions for risk factors for dementia in low-income and middle-income countries: an analysis using cross-sectional survey data. Lancet Glob Health. May 2019;7(5):e596–e603. doi: 10.1016/S2214-109X(19)30074-9 [DOI] [PubMed] [Google Scholar]
- 6.GBD 2019 Hearing Loss Collaborators. Hearing loss prevalence and years lived with disability, 1990–2019: findings from the Global Burden of Disease Study 2019. Lancet. Mar 13 2021;397(10278):996–1009. doi: 10.1016/S0140-6736(21)00516-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin FR, Albert M. Hearing loss and dementia - who is listening? Aging Ment Health. 2014;18(6):671–3. doi: 10.1080/13607863.2014.915924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. World Report on Hearing 2021. Geneva. World Health Organization. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 9.Yeo BSY, Song HJJM, Toh EMS, et al. Association of Hearing Aids and Cochlear Implants With Cognitive Decline and Dementia: A Systematic Review and Meta-analysis. JAMA Neurol. Feb 01 2023;80(2):134–141. doi: 10.1001/jamaneurol.2022.4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang F, Mishra SR, Shrestha N, et al. Association between hearing aid use and all-cause and cause-specific dementia: an analysis of the UK Biobank cohort. Lancet Public Health. May 2023;8(5):e329–e338. doi: 10.1016/S2468-2667(23)00048-8 [DOI] [PubMed] [Google Scholar]
- 11.Wright JD, Folsom AR, Coresh J, et al. The ARIC (Atherosclerosis Risk In Communities) Study: JACC Focus Seminar 3/8. J Am Coll Cardiol. Jun 15 2021;77(23):2939–2959. doi: 10.1016/j.jacc.2021.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deal JA, Goman AM, Albert MS, et al. Hearing treatment for reducing cognitive decline: Design and methods of the Aging and Cognitive Health Evaluation in Elders randomized controlled trial. Alzheimers Dement (N Y). 2018;4:499–507. doi: 10.1016/j.trci.2018.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez VA, Arnold ML, Reed NS, et al. The Hearing Intervention for the Aging and Cognitive Health Evaluation in Elders Randomized Control Trial: Manualization and Feasibility Study. Ear Hear. Apr 2020;doi: 10.1097/AUD.0000000000000858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newman AB, Bayles CM, Milas CN, et al. The 10 keys to healthy aging: findings from an innovative prevention program in the community. J Aging Health. Aug 2010;22(5):547–66. doi: 10.1177/0898264310363772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross AL, Power MC, Albert MS, et al. Application of Latent Variable Methods to the Study of Cognitive Decline When Tests Change over Time. Research Support, N.I.H., Extramural. Epidemiology. Nov 2015;26(6):878–87. doi: 10.1097/EDE.0000000000000379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross AL, Sherva R, Mukherjee S, et al. Calibrating longitudinal cognition in Alzheimer’s disease across diverse test batteries and datasets. Neuroepidemiology. 2014;43(3–4):194–205. doi: 10.1159/000367970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinstein BE. Validity of a screening protocol for identifying elderly people with hearing problems. ASHA. May 1986;28(5):41–5. [PubMed] [Google Scholar]
- 18.Maharani A, Dawes P, Nazroo J, Tampubolon G, Pendleton N, group S-CW. Longitudinal Relationship Between Hearing Aid Use and Cognitive Function in Older Americans. J Am Geriatr Soc. July 2018;66(6):1130–1136. doi: 10.1111/jgs.15363 [DOI] [PubMed] [Google Scholar]
- 19.Ray J, Popli G, Fell G. Association of Cognition and Age-Related Hearing Impairment in the English Longitudinal Study of Ageing. JAMA Otolaryngol Head Neck Surg. Oct 01 2018;144(10):876–882. doi: 10.1001/jamaoto.2018.1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.United States Department of Health and Human Services. National Plan to Address Alzheimer’s Disease: 2021 Update. https://aspe.hhs.gov/reports/national-plan-2021-update. Accessed May 21, 2023 [Google Scholar]
- 21.Livingston G, Costafreda S. Preventing dementia through correcting hearing: huge progress but more to do. Lancet Public Health. May 2023;8(5):e319–e320. doi: 10.1016/S2468-2667(23)00058-0 [DOI] [PubMed] [Google Scholar]
- 22.Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res Rev. Sep 2015;23(Pt B):154–66. doi: 10.1016/j.arr.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 23.Rutherford BR, Brewster K, Golub JS, Kim AH, Roose SP. Sensation and Psychiatry: Linking Age-Related Hearing Loss to Late-Life Depression and Cognitive Decline. Am J Psychiatry. March 2018;175(3):215–224. doi: 10.1176/appi.ajp.2017.17040423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayden KM, Reed BR, Manly JJ, et al. Cognitive decline in the elderly: an analysis of population heterogeneity. Age Ageing. Nov 2011;40(6):684–9. doi: 10.1093/ageing/afr101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leening MJ, Heeringa J, Deckers JW, et al. Healthy volunteer effect and cardiovascular risk. Epidemiology. May 2014;25(3):470–1. doi: 10.1097/EDE.0000000000000091 [DOI] [PubMed] [Google Scholar]
- 26.Lindsted KD, Fraser GE, Steinkohl M, Beeson WL. Healthy volunteer effect in a cohort study: temporal resolution in the Adventist Health Study. J Clin Epidemiol. Jul 1996;49(7):783–90. doi: 10.1016/0895-4356(96)00009-1 [DOI] [PubMed] [Google Scholar]
- 27.Zheng Z, Rebholz CM, Matsushita K, et al. Survival advantage of cohort participation attenuates over time: results from three long-standing community-based studies. Ann Epidemiol. May 2020;45:40–46.e4. doi: 10.1016/j.annepidem.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. Jun 06 2015;385(9984):2255–63. doi: 10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- 29.Rebok GW, Langbaum JB, Jones RN, et al. Memory training in the ACTIVE study: how much is needed and who benefits? J Aging Health. Dec 2013;25(8 Suppl):21S–42S. doi: 10.1177/0898264312461937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Royall DR, Palmer R, Chiodo LK, Polk MJ. Normal rates of cognitive change in successful aging: the freedom house study. J Int Neuropsychol Soc. Nov 2005;11(7):899–909. doi: 10.1017/s135561770505109x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A deidentified dataset and data dictionary will be made available in 2024 on a publicly-available U.S. data repository pending approval by the funding sponsor (National Institute on Aging). Additional details on data access policies will be made available at www.achievestudy.org at that time. The study protocol and statistical analysis plan are available at www.clinicaltrials.gov. Access to ACHIEVE study manuals and forms are available by contacting the corresponding author.


