Abstract
Purpose of Review:
To review the pathophysiology and treatment of ocular itch and pain, encompassing nociceptive and neuropathic categories.
Recent Findings:
Ocular itch and pain are sensations that arise from activation of ocular surface polymodal nerves. Nociceptive itch, commonly comorbid with ocular pain complaints, is mainly driven by a histamine-mediated type 1 hypersensitivity reaction. Beyond topical therapy, novel drug delivery systems are being explored to improve ocular residence time of non-steroidal anti-inflammatory drugs (NSAIDs) and antihistamines. Nociceptive ocular pain can be driven by a variety of factors. Treatment focuses on addressing the causative sources of pain. Neuropathic ocular itch and pain are driven by nerve damage and dysfunction and as such, topical and oral neuromodulation have been explored as treatments. Oral neuromodulators include alpha 2 delta ligands, tricyclic antidepressants (TCAs), and low dose naltrexone. Novel therapies are being evaluated for both modalities such as difelikefalin (κ-opioid receptor agonist) for neuropathic itch and libvatrep (TRPV1 antagonist) for neuropathic pain.
Summary:
Both ocular itch and pain can be driven by nociceptive and/or neuropathic mechanisms. Identifying contributors to abnormal ocular sensations is vital for precise medical care. Novel therapeutics for these conditions aim to improve patient outcomes and quality of life.
Keywords: Nociceptive Pain, Nociceptive Itch, Neuropathic Pain, Neuropathic Itch, Nerve Dysregulation, Conjunctive Allergic Conjunctivitis, Neuromodulators, TRPV1 Antagonist
Introduction:
The sensations of itch and pain, two distinct types of sensory symptoms, can have a significant impact on quality of life. These sensations can be experienced in the presence of ocular surface diseases. Ocular itch, a common symptom reported in individuals with allergic conjunctivitis (AC) and Demodex infestation, can also be experienced outside the purview of ocular surface disease and in this setting, is thought to have neuropathic contributors (2, 3, 4). Ocular pain can be described using various terms, commonly “dryness”, “grittiness”, “aching”, “tenderness”, and “burning”, to name a few (5). It can be seen in the setting of various ocular surface diseases including aqueous tear deficiency, Meibomian gland dysfunction, and anatomic abnormalities (pterygium, Salzmann nodule), but also independently of notable ocular surface disease. Neuropathic contributors to pain are thought to be involved in the latter scenario. Despite quality differences, shared mechanisms between ocular itch and pain include inflammation, immune system activation, and nerve abnormalities. In this paper, we explore the immunologic and neural basis of ocular itch and pain and discuss current and potential treatments for these symptoms. By gaining a better understanding of the immune and nervous systems’ role in ocular itch and pain, we hope to identify new strategies that can improve diagnosis, treatment, and management of these challenging conditions.
Nociceptive Itch and Nociceptive Pain
Nociceptive Itch of the Ocular Surface
Ocular itch, a common symptom of various eye-related conditions, causes a subjective feeling of discomfort or irritation through environmental, mechanical, psychological, and immunological factors (6). Our focus is on the impact of immunological mediators in the context of allergic conjunctivitis, an inflammation of the conjunctiva that occurs when exposed to an external allergen. The two most common subsets are seasonal allergic conjunctivitis and perennial allergic conjunctivitis. Seasonal allergic conjunctivitis usually happens in spring, summer, or fall due to allergens such as grass and tree pollen. Perennial allergic conjunctivitis, on the other hand, occurs throughout the year and is often triggered by indoor allergens like pet hair, mold, and dust mites (7, 8).
Molecular Components of Nociceptive Itch
The symptoms of seasonal and perennial allergic conjunctivitis are driven by a type 1 hypersensitivity reaction. In seasonal allergic conjunctivitis, outdoor allergens are triggers for this reaction, while in perennial allergic conjunctivitis, indoor allergens are responsible. Initially, in both cases, the allergens interact with IgE molecules on mast cells' surface, causing degranulation of mast cells and subsequent release of proinflammatory molecules like histamine. These molecules are the main culprits behind the itching and tearing of the eye (1, 9).
Furthermore, in the case of perennial allergic conjunctivitis, due to continued exposure to the allergen on the ocular surface, the primed mast cells' IgE-FcerRL complexes are constantly cross-linked. This ongoing process leads to persistent mast cell degranulation and the continued release of histamine (1, 9, 11).
Mast cells also produce and secrete various pro-inflammatory mediators, such as cytokines (IL-4 / TNF-a), chemokines, and arachidonic acid metabolites, through de novo synthesis (12). Cytokines such as IL-4 have been implicated in the involvement of mast-cell mediated allergic reactions (13). Prostaglandins are also known to cause local inflammation through their vasodilatory effect (14). In a study on vernal keratoconjunctivitis (VKC), tear samples were collected from five VKC patients and five healthy controls to measure the levels of Prostaglandin E2 (PGE2). Results showed a statistically significant elevation (p < 0.04) in the average tear PGE2 level in VKC patients (190 pg/mL) compared to control subjects (6.0 pg/mL). After a 4-week treatment of ketotifen fumarate and dexamethasone, the PGE2 levels were reduced in the VKC patients to a mean of 62.6 pg/mL (p < 0.04), along with a decrease in symptoms (15). These findings suggest that molecules involved in allergic processes contribute to ocular itch in individuals with allergic ocular surface disease.
Treatment Approaches of Nociceptive Itch
Despite affecting up to 40% of the US population, allergic conjunctivitis is underdiagnosed and undertreated. Improving an individual’s quality of life, reducing recurrences, and preventing potential complications highlight the importance of early detection and management of allergic conjunctivitis (16, 17). Though primarily diagnosed clinically, suspicions can be complemented with laboratory tests such as skin allergy testing or IgE level detection in tears (18).
Non-pharmacological treatments for allergic conjunctivitis aim to reduce allergen interaction on the ocular surface by methods such as wearing large protective eyeglasses or using a high efficiency air (HEPA) filter. Also frequently washing clothes and bedding can reduce encounters with triggers like dust mites and pet dander (1, 16). Cold compresses aid by vasoconstricting vessels in the conjunctiva to reduce hyperemia and edema. Lastly, artificial tears help flush the ocular surface of allergens. However, non-pharmacological methods have shown varying degrees of efficiency causing most patients to rely on pharmacological options (16, 19).
Pharmacological therapies for allergic conjunctivitis include antihistamines, mast cell stabilizers, NSAIDs, and corticosteroids to name a few. Topical antihistamines, such as olopatadine, alcaftadine, and epinastine, competitively inhibit histamine receptors. While topical agents provide acute relief, their short therapeutic timeframe requires frequent dosing to achieve sustained relief leading to decreased compliance (1, 20).
Mast cell stabilizers like lodoxamide, tromethamine, and pemirolast potassium inhibit mast cell degranulation, reducing symptoms by blocking the release of histamine and other factors. However, these treatments are limited to prophylactic use and cannot provide retroactive relief of acute symptoms (3, 21). Combination solutions of mast cell stabilizers and antihistamines, such as ketotifen, bepotastine, and olopatadine overcome this limitation. Bepotastine, a newer combination medication, provides relief within 15 minutes and up to 8 hours after administration (1). NSAIDs block prostaglandin release for symptomatic relief but should be used short-term due to potential adverse effects such as corneal ulceration and keratitis (1, 22). Corticosteroids, such as prednisolone and dexamethasone, primarily alleviate symptoms by exerting anti-inflammatory effects. These effects involve the inhibition of histamine production, the upregulation of anti-inflammatory mediators, and the suppression of histidine decarboxylase activity, which is necessary for histamine synthesis in mast cells (1, 23). However, due to their immunosuppressive effect and safety concerns, including increased infection risk, cataract development, and raised intraocular pressure, chronic use should be limited (24, 25).
Eye drops are effective for managing ocular diseases but anatomical barriers, including the tear film's lipid and aqueous mucin layers, hinder drug diffusion. Additionally, eye drops trigger a rapid tear secretion rate of 1.2-2 μl/minute that increases by 100-fold with reflexive blinking, in turn decreasing drug bioavailability (26, 27, 29). Additionally, older individuals may face compliance issues due to decreased manual dexterity when applying eye drops (32).
Researchers aim to overcome these challenges by enhancing drug bioavailability and therapeutic efficacy for ocular diseases. Contact lenses have been an exciting novel drug delivery system to achieve a consistent and prolonged release of medication, with increased residence time and bioavailability (28). A study in rabbits compared the effectiveness of bromfenac loaded lenses to bromfenac eye drops for drug delivery to the eyes. Results showed 26 times higher bioavailability and 155 times higher residence time with the lenses, suggesting a promising solution to challenges of administering drugs solely through eye drops. (33, 34).
Johnson and Johnson launched Acuvue Theravision in 2022, a daily disposable contact lenses loaded with the NSAID ketotifen. A randomized control study was conducted with three groups of established lenses wearers with a history of ocular allergies. Group 1 (41 patients) received the test lenses in one eye and the control lenses in the other, group 2 (39 patients) received test lenses bilaterally, and group 3 (40 patients) received control lenses bilaterally. The subjects underwent the conjunctival allergen challenge (CAC) test, and their ocular itch scores were recorded at different time intervals. The lenses loaded with ketotifen (average itch score of 0.71, p < 0.001) were found to reduce ocular itch compared to control lenses (average itch score of 1.83, p < 0.001), while providing appropriate vision correction (28, 35).
To conclude, most of the treatment of nociceptive itch focus on targeting molecules and cells shown to mediate itch, namely histamine, mast cells, and prostaglandins. Current therapies provide these therapies via topical medications, but studies are underway to deliver products in novel ways that increase contract time and provide a more stable and long-term release.
Nociceptive Pain of the Ocular Surface
Etiologies of nociceptive pain at the ocular surface arise from sources such as acute trauma (by a foreign body, ultraviolet keratitis, or chemical injury) or chronic insults (aqueous tear deficiency, anatomic disturbances such as entropion, ectropion) (36). Nociceptive ocular pain can also be co-morbid with systemic conditions, such as Sjögrens and graft-versus-host disease (GVHD), diseases that often present with aqueous tear deficiency, ocular surface inflammation, and epithelial disruption (36). Treatment of nociceptive ocular pain focuses on addressing the acute injury (e.g., removal of a foreign body) and/or the chronic insults noted on exam (e.g., addressing ocular surface inflammation, fixing anatomic abnormalities).
Neural Ocular Surface Anatomy and Its Relationship to the Development of Neuropathic Itch and Pain
The cornea, a densely innervated structure, is supplied with sensory innervation mainly by ophthalmic branch of the trigeminal nerve (37, 38). These branches comprise of unmyelinated C-fibers and some sparsely myelinated Aδ fibers (37, 39). The nerves contain receptors which include about 70% polymodal nociceptors such as polymodal C-nociceptors (PmC) which are responsible for sensing thermal, chemical, and endogenous inflammatory mediators; 15% are mechanonociceptors such as Aδ-mechanoreceptors which sense mechanical stimuli; and another 15% are thermoreceptors such as C-fiber cold thermoreceptors for sensing a decrease in temperature below 33°C (40, 41). Myelinated Aδ fibers propagate signals at about 20 meters/second while un-myelinated C fiber propagate at around 2 meters/second (42, 43). Transient receptor potential (TRP) channels, particularly transient receptor potential vanilloid 1 (TRPV1) and transient receptor potential ankyrin 1 (TRPA1), are cation channels on the terminal endings of polymodal nociceptors, that play a crucial role in allergic and pain responses and symptoms (45, 46). The ocular surface nerves continuously sense their environment and their activation leads to the sensation of itch and pain (36). Ocular surface nerves undergo changes in their structure and/or function after acute or chronic injury (e.g., acute surgical trauma, chronic aqueous tear deficiency, chronic ocular surface inflammation). Neuroplasticity of the primary sensory neurons of the peripheral nervous system, termed peripheral sensitization, and spinal cord/brain, termed central sensitization, contribute to the development and maintenance of neuropathic itch and pain, with decreased thresholds and enhanced responses to stimuli noted (5, 47).
Neuropathic Itch and Neuropathic Pain
Neuropathic Itch of the Ocular Surface
Unlike nociceptive itch, neuropathic itch is typically a non-histamine mediated process that occurs from dysregulation and damage of sensory neurons which transmit signals between the eye and the brain (37, 47). Such dysregulation occurs from the impact of endogenous inflammatory mediators, nerve compression, or degeneration of fibers through disease processes such as chronic allergic conjunctivitis, trigeminal neuralgia, or tumors (48, 49).
Molecular Components of Neuropathic Itch:
Many factors contribute to a phenotype of chronic neuropathic ocular surface itch. In this review, we focus on mechanisms caused by chronic exposure to immune factors, such as with chronic allergic conjunctivitis. As noted above, persistent exposure to immunological mediators leads to modulation of sensory nerves. Mast cell degeneration releases pro-inflammatory factors that can change the function of TRPV1 ion channels, ultimately lowering their activation thresholds. While histamine typically mediates TRPV1 activation, studies conducted in mice suggest that the cytokine IL-31 can also activate these channels. In fact, when researchers injected IL-31 intrathecally into the cerebrospinal fluid at the lumbar level of mice deficient in TRPV1 and into control mice, the TRPV1-deficient mice scratched less, suggesting a histamine-independent activation of TRPV1 (50). Neuropeptides, substance P and nerve growth factor (NGF), contribute to neuropathic itch by interacting with mast cell receptors to cause additional degranulation creating a cycle of repetitive mast cell degranulation, release of pro-inflammatory mediators, nerve damage, and sensitization, ultimately leading to chronic neuropathic itch (47, 51, 52).
Treatment Approaches and Future Directions for Neuropathic itch:
Antihistamines have limited effectiveness for neuropathic itch caused by nerve dysregulation or damage (49). It is not yet known which therapeutic approaches are best for managing neuropathic ocular surface itch. However, therapeutics approaches outside the purview of the ocular surface may prove to have benefit. For example, gabapentin, used to manage pruritus in chronic kidney disease, idiopathic itch, and trigeminal trophic syndrome, blocks the α2-δ-subunit calcium channels in nociceptive neurons, preventing depolarization (53, 54, 55). However, large studies are lacking. Antidepressants like mirtazapine, paroxetine, and doxepin have also been shown to have antipruritic effects caused by diseases such as polycythemia vera, atopic dermatitis, and somatoform pruritus (56, 57, 58).
Some newer molecules are being explored in clinical studies. A randomized, masked, placebo-controlled study (NCT03422653) was conducted in 378 hemodialysis patients with pruritus to assess the efficacy of κ-opioid receptor (KOR) agonists (difelikefalin), in the management of chronic itch. Using The Worst Itching Intensity-Numerical Rating Scale (WI-NRS, scale 0 to 10), a baseline itch level was established. Intravenous difelikefalin 0.5 μg/kg or placebo, delivered into the venous port of the dialysis circuit, after hemodialysis sessions, three times a week for 12 weeks, showed that 49% of individuals in the test group experienced a decrease of ≥3 points in itch compared to 28% in the placebo group (p < 0.001) (59). Another compound being explored for chronic itch is oral dronabinol, a synthetic form of tetrahydrocannabinol (THC). In one case, a patient with a chronic itch history resulting in multiple wounds experienced a significant reduction in itch score from 10 to 0-1 on a visual analog scale with the use of 2.5 mg of oral dronabinol (twice daily) after other methods failed (60). However, it is not known which, if any, of these molecules will be effective for the management of chronic neuropathic ocular surface itch.
Neuropathic Pain of the Ocular Surface
Neuropathic pain is a pain syndrome caused by a variety of diseases or lesions that affect the somatosensory nervous system (61). As applied to the ocular surface, this involves changes in the structure and function of peripheral (corneal and conjunctival) and central nerves in the trigeminal pain pathway. Based on this definition, it becomes apparent that neuropathic itch is closely related to neuropathic ocular surface pain. Neuropathic ocular pain can be perceived by patients in various ways, often described as burning, but also as “sharp, stabbing”, “stinging”, or “itching”(62). As noted above, neuropathic sensations can also include itch. Hypersensitivity to pain is often seen in neuropathic pain, with moving air and light as common triggers for ocular pain (63). Neuropathic ocular pain (NOP) is often co-morbid with chronic non-ocular disorders, such as migraine and fibromyalgia (64, 65, 66).
Molecular Components of Neuropathic Pain:
A peripheral nerve injury is often the inciting cause of neuropathic pain. One of any number of insults can lead to peripheral nerve changes (e.g., sensitization) in susceptible patients, including ocular surgery, infectious keratitis, chemical injury, or tear instability, to name a few (67). Damaged epithelial cells release inflammatory mediators (IL-2, IL-4, IL-5, IL-6, IL-8, IL10, and tumor necrosis factor (TNF)) along with damaged peripheral neurons (substance P, calcitonin gene-related peptide (CGRP), and glutamate). These mediators activate TRPV1 and TRPA1 receptors on neighboring neurons which can induce the increased release of substance P, CGRP (47, 68, 69). Over time, a pro-inflammatory state can become self-propagating involving the continuous release bradykinin, prostaglandins, serotonin, and histamine which not only bind on neighboring nerve cells, but also surrounding tissue (47, 68, 70). The pro-inflammatory state induces the release of neurotrophins, like nerve growth factor (NGF), which upregulate the production of substance P and glutamate, and increase the expression of TRPV1 on the neuronal surface (47).
Central sensitization can follow these events by elevated substance P levels binding to G coupled neurokinin 1 receptors (NK1R) while elevated glutamate and other excitatory amino acid levels binding to N-methyl-d-aspartate receptors (NMDAR), and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR). The activation of NK1R, NMDAR, and AMPAR, leads to intracellular calcium release that increases the phosphorylation, production and insertion of excitatory ion channels on sensory neurons membranes (68, 70). This change in peripheral nerve structure and function can over time lead to changes within central nerves, leading to central sensitization.
Treatment Approaches and Future Directions for Neuropathic pain
Even individuals with neuropathic pain have a nociceptive source of pain which must first be addressed. If pain is not adequately managed, then neuromodulatory strategies are considered to reduce peripheral and central nerve sensitivity. Targeting inflammation is a first line therapeutic strategy as inflammation and neuropathic changes are closely related (70). Topical neuromodulators are used for individuals with a peripheral component to pain (determined by the anesthetic challenge in which pain significantly improves after placement of topical anesthetic). One option is topical nerve growth factor, used for the treatment of neurotrophic keratitis (lack of corneal sensation), is now being explored for the treatment of peripheral neuropathic pain (71). TRPV1 activation plays a central role in mediating neuropathic pain. Various animal studies show that reducing expression or activation of TRPV1 via antagonism or downregulation creates analgesic effects (72). A recent clinical trial with the TRPV1 antagonist SAF312 (libvatrep) explored the impact of this agent in addressing chronic post-surgical ocular pain. Prior studies have demonstrated that TRPV1 antagonists can reduce acute ocular surface pain, as well. In 40 patients with post–photorefractive keratectomy (PRK) pain, average pain was measured from 0-12 hours after surgery with the visual analog scale (VAS). At both 6 hours post operatively (p=0.005) and over 0-12 hours (p=0.017) the mean VAS score was reduced by 25% and 22% respectively. Patients also reported taking less oral rescue medication between 0- and 72-hours post operation (73).
Oral neuromodulators can be considered in individuals with a suspected central component to pain, that is, persistent pain after topical anesthesia placement and/or cutaneous allodynia (pain to light touch around the eye). Pregabalin and gabapentin have been used in this regard with some success. Within a case series involving eight individuals, gabapentin and pregabalin were administered in varying doses. Gabapentin was started at 300mg oral (daily) and titrated up to 600-900mg (thrice a day), while pregabalin was started at 75mg oral (daily) and titrated up to 150mg (twice a day). Two individuals experienced complete relief from pain, three had marked relief, one experienced mild relief, and two showed no improvement (74). In the study, patients with marked relief also took oral serotonin norepinephrine reuptake inhibitors (SNRIs), suggesting a multifaceted approach could benefit those with chronic ocular surface pain. (74, 75). Tricyclic antidepressants (TCA) have also been used with success. In one study evaluating the effect of oral nortriptyline at a dose of 10mg titrated up to 100mg on neuropathic corneal pain in 30 individuals, mean ocular pain decreased significantly from 5.7 ± 2.1 to 3.6 ± 2.1 (p < 0.0001). Forty percent of patients had equal to or more than 50% improvement in pain (76). Low dose naltrexone (LDN) is often added as an adjuvant therapy to the above neuromodulators. In a case studying LDN as a monotherapy or as an adjunct in therapy, LDN resulted in 49% decrease on mean ocular pain score in 59 patients treated with 4.5mg of oral LDN (77). With a comprehensive approach combining LDN with traditional topical management patients reported a decrease in pain score with a 5.79 ± 0.30 at the first visit to 3.70 ± 0.74 at the last visit on the ocular pain assessment survey (OPAS) questionnaire (77). There was also better reported quality of life improving from 5.84 ± 2.57 at the first visit to 3.77 ± 2.91 at the last visit (77). Other strategies currently being used to treat neuropathic ocular pain include transcutaneous stimulation and botulinum toxin (78, 79).
Other compounds are being investigated in clinical trials for the treatment of neuropathic ocular pain. One such compound is PL265, an inhibitor of enkephalinase, that has been shown to reduce corneal inflammation and decrease neuronal injury marker (ATF3) in the afferent sensory neurons in the trigeminal ganglion. Administration of 10 μL at 10 mM topical PL265 on healthy corneas did not have any effect on corneal mechanical or chemical sensitivity. However, when tested on murine models of corneal pain, repeated application of PL265 resulted in a significant reduction of corneal mechanical and chemical hypersensitivity in mouse models of corneal pain (80).
Conclusion:
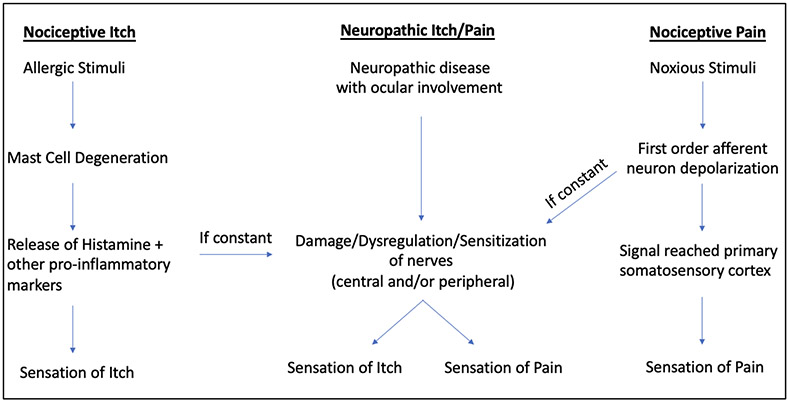
Both ocular itch and pain arise from nociceptive and neuropathic etiologies (Figure 1). Nociceptive ocular itch manifests as an allergic response predominantly mediated by mast cells while nociceptive ocular pain occurs via activation of diverse sensory neurons from factors including trauma, aqueous tear deficiency, and abnormal ocular surface anatomy. Prolonged nerve stimulation in both settings can cause peripheral and central abnormalities, resulting in neuropathic pain and/or neuropathic itch. A push to better understand mechanisms that contribute to the acute to chronic pain transition, molecules involved in the maintenance of chronic pain, and diagnostic strategies to identify neuropathic pain will hopefully lead to improved treatment strategies that deliver personalized targeted therapies. As exploration continues in addressing ocular itch and pain, further characterization of these abnormal sensations can help guide diagnostics and treatment strategies.
Figure 1.
Understanding the Triggers and Progression of Sensory Experiences: A Flowchart of Nociceptive Itch, Neuropathic Itch/Pain, and Nociceptive Pain
Figure 1 is a flowchart that provides a simplified overview of the complex sensory experiences associated with three categories: Nociceptive Itch, Neuropathic Itch/Pain, and Nociceptive Pain.
Nociceptive itch is usually triggered by an external allergen, such as pollen or dust mites, which leads to a Type 1 hypersensitivity reaction. This causes mast cells to release pro-inflammatory mediators, including histamine, resulting in an inflammatory response that ultimately leads to the sensation of itch.
Nociceptive pain is caused by noxious stimuli, such as acute trauma, aqueous tear deficiency, or anatomical disturbances, which lead to the depolarization of first-order afferent neurons. This ultimately sends a signal through the trigeminal nerve (V1) to the primary somatosensory cortex, resulting in the feeling of pain. Nociceptive itch and pain most often resolve when the offending insult is removed.
Neuropathic itch and pain occur due to damage and/or dysregulation of the peripheral and/or central sensory nerves/pathways. Continuous release of pro-inflammatory mediators and/or persistent depolarization of the primary afferent neurons can result in neuropathic itch, pain, or both which can persist despite resolution of the initial insult.
Key Points:
Pain and itch within the ocular surface can arise from nociceptive or neuropathic origins.
Itch, when nociceptive in origin, is most often driven by an IgE mediated type 1 hypersensitivity reaction.
Nociceptive itch is managed by controlling inflammation and release of pro-inflammatory mediators with antihistamines, mast cell stabilizers, non-steroid anti-inflammatory drugs, and corticosteroids.
When neuropathic in origin, itch is driven by nerve dysregulation and damage through non-histamine dependent pathways. As such, antihistamines have limited therapeutic benefit but gabapentin and antidepressants have shown some efficacy. Further research is needed to develop management strategies for neuropathic itch.
Nociceptive pain on the ocular surface can stem from acute or chronic insults that disrupt the tear film and epithelial layer. Current treatment relies on removing the caustic agent or treating the underlying disease.
Neuropathic ocular pain occurs due to a lesion or disease of the somatosensory nervous system in peripheral and/or central nerves of the trigeminal nerve pathway.
Topical and oral neuromodulators have been used to treat neuropathic ocular pain but more research is needed on therapeutic strategies for the condition.
Financial Support:
Supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences R&D (CSRD) I01 CX002015 (Dr. Galor), Biomedical Laboratory R&D (BLRD) Service I01 BX004893 (Dr. Galor), Rehabilitation R&D (RRD) I21 RX003883 (Dr. Galor), Department of Defense Gulf War Illness Research Program (GWIRP) W81XWH-20-1-0579 (Dr. Galor) and Vision Research Program (VRP) W81XWH-20-1-0820 (Dr. Galor), National Eye Institute U01 EY034686 (Dr. Galor), R01EY026174 (Dr. Galor), R61EY032468 (Dr. Galor), NIH Center Core Grant P30EY014801 (institutional) and Research to Prevent Blindness Unrestricted Grant GR004596-1 (institutional).
Footnotes
Conflict of Interest:
No conflicting relationship exists for any author.
References:
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Labib BA, Chigbu DI. Therapeutic Targets in Allergic Conjunctivitis. Pharmaceuticals (Basel). 2022;15(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng AM, Hwang J, Dermer H, Galor A. Prevalence of Ocular Demodicosis in an Older Population and Its Association With Symptoms and Signs of Dry Eye. Cornea. 2021;40(8):995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.La Rosa M, Lionetti E, Reibaldi M, Russo A, Longo A, Leonardi S, et al. Allergic conjunctivitis: a comprehensive review of the literature. Italian journal of pediatrics. 2013;39:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galor A, Small L, Feuer W, Levitt RC, Sarantopoulos KD, Yosipovitch G. The Relationship Between Ocular Itch, Ocular Pain, and Dry Eye Symptoms (An American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc. 2017;115:T5. [PMC free article] [PubMed] [Google Scholar]
- 5.Kalangara JP, Galor A, Levitt RC, Covington DB, McManus KT, Sarantopoulos CD, et al. Characteristics of Ocular Pain Complaints in Patients With Idiopathic Dry Eye Symptoms. Eye Contact Lens. 2017;43(3):192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leonardi A, Bogacka E, Fauquert JL, Kowalski ML, Groblewska A, Jedrzejczak-Czechowicz M, et al. Ocular allergy: recognizing and diagnosing hypersensitivity disorders of the ocular surface. Allergy. 2012;67(11):1327–37. [DOI] [PubMed] [Google Scholar]
- 7.Bilkhu PS, Wolffsohn JS, Naroo SA. A review of non-pharmacological and pharmacological management of seasonal and perennial allergic conjunctivitis. Cont Lens Anterior Eye. 2012;35(1):9–16. [DOI] [PubMed] [Google Scholar]
- 8.Iordache A, Boruga M, Musat O, Jipa DA, Tataru CP, Musat GC. Relationship between allergic rhinitis and allergic conjunctivitis (allergic rhinoconjunctivitis) - review. Rom J Ophthalmol. 2022;66(1):8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ackerman S, Smith LM, Gomes PJ. Ocular itch associated with allergic conjunctivitis: latest evidence and clinical management. Ther Adv Chronic Dis. 2016;7(1):52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chigbu D, Minhas BK. Immunopathology of allergic conjunctivitis. Eur Med J. 2018;3:76–83. [Google Scholar]
- 11.Elieh Ali Komi D, Rambasek T, Bielory L. Clinical implications of mast cell involvement in allergic conjunctivitis. Allergy. 2018;73(3):528–39. [DOI] [PubMed] [Google Scholar]
- 12.McLeod JJ, Baker B, Ryan JJ. Mast cell production and response to IL-4 and IL-13. Cytokine. 2015;75(1):57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strait RT, Morris SC, Smiley K, Urban JF, Finkelman FD. IL-4 exacerbates anaphylaxis. The Journal of Immunology. 2003;170(7):3835–42. [DOI] [PubMed] [Google Scholar]
- 14.Murrant CL, Dodd JD, Foster AJ, Inch KA, Muckle FR, Ruiz DA, et al. Prostaglandins induce vasodilatation of the microvasculature during muscle contraction and induce vasodilatation independent of adenosine. The Journal of Physiology. 2014;592(6):1267–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamanishi R, Okada N, Shimizu E, Fujishima H. Elevated levels of prostaglandin E(2) in the tears of patients with severe allergic conjunctivitis and primary cultured conjunctival cells are suppressed by ketotifen and dexamethasone. BMJ Open Ophthalmol. 2021;6(1):e000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupuis P, Prokopich CL, Hynes A, Kim H. A contemporary look at allergic conjunctivitis. Allergy, Asthma & Clinical Immunology. 2020;16(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villegas BV, Benitez-Del-Castillo JM. Current Knowledge in Allergic Conjunctivitis. Turk J Ophthalmol. 2021;51(1):45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mimura T, Usui T, Mori M, Funatsu H, Noma H, Yamamoto H, et al. Relation Between Total Tear IgE and Specific Serum IgE in Seasonal Allergic Conjunctivitis. Cornea. 2011;30(7):790–5. [DOI] [PubMed] [Google Scholar]
- 19.Jia-Ying L, Zhao C, Jia-Jun G, Zi-Jun G, Xiao L, Bao-Qing S. Efficacy of air purifier therapy in allergic rhinitis. Asian Pacific journal of allergy and immunology. 2018;36(4):217–21. [DOI] [PubMed] [Google Scholar]
- 20.Kimchi N, Bielory L. The allergic eye: recommendations about pharmacotherapy and recent therapeutic agents. Current Opinion in Allergy and Clinical Immunology. 2020;20(4):414–20. [DOI] [PubMed] [Google Scholar]
- 21.Finn D, Walsh J. Twenty-first century mast cell stabilizers. British journal of pharmacology. 2013;170(1):23–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rigas B, Huang W, Honkanen R. NSAID-induced corneal melt: Clinical importance, pathogenesis, and risk mitigation. Survey of Ophthalmology. 2020;65(1):1–11. [DOI] [PubMed] [Google Scholar]
- 23.Comstock TL, DeCory HH. Advances in corticosteroid therapy for ocular inflammation: loteprednol etabonate. International Journal of Inflammation. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prinz J, Maffulli N, Fuest M, Walter P, Bell A, Migliorini F. Efficacy of Topical Administration of Corticosteroids for the Management of Dry Eye Disease: Systematic Review and Meta-Analysis. Life. 2022;12(11):1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bielory BP, O’Brien TP, Bielory L. Management of seasonal allergic conjunctivitis: guide to therapy. Acta ophthalmologica. 2012;90(5):399–407. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Xue Y, Hu G, Lin T, Gou J, Yin T, et al. A comprehensive review on contact lens for ophthalmic drug delivery. J Control Release. 2018;281:97–118. [DOI] [PubMed] [Google Scholar]
- 27.Wang C, Pang Y. Nano-based eye drop: Topical and noninvasive therapy for ocular diseases. Adv Drug Deliv Rev. 2023;194:114721. [DOI] [PubMed] [Google Scholar]
- 28.Abdi B, Mofidfar M, Hassanpour F, Kirbas Cilingir E, Kalajahi SK, Milani PH, et al. Therapeutic contact lenses for the treatment of corneal and ocular surface diseases: advances in extended and targeted drug delivery. Int J Pharm. 2023:122740. [DOI] [PubMed] [Google Scholar]
- 29.Willcox MDP, Argueso P, Georgiev GA, Holopainen JM, Laurie GW, Millar TJ, et al. TFOS DEWS II Tear Film Report. Ocul Surf. 2017;15(3):366–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carvalho IM, Marques CS, Oliveira RS, Coelho PB, Costa PC, Ferreira DC. Sustained drug release by contact lenses for glaucoma treatment-a review. J Control Release. 2015;202:76–82. [DOI] [PubMed] [Google Scholar]
- 31.Lee K, Lee G, Lee S, Park CY. Advances in ophthalmic drug delivery technology for postoperative management after cataract surgery. Expert Opin Drug Deliv. 2022;19(8):945–64. [DOI] [PubMed] [Google Scholar]
- 32.Tsai T, Robin AL, Smith JP 3rd. An evaluation of how glaucoma patients use topical medications: a pilot study. Trans Am Ophthalmol Soc. 2007;105:29–33; discussion −5. [PMC free article] [PubMed] [Google Scholar]
- 33.DiPasquale SA, Wuchte LD, Mosley RJ, Demarest RM, Voyles ML, Byrne ME. One Week Sustained In Vivo Therapeutic Release and Safety of Novel Extended-Wear Silicone Hydrogel Contact Lenses. Adv Healthc Mater. 2022;11(7). [DOI] [PubMed] [Google Scholar]
- 34.Yang YJ, Lockwood A. Topical ocular drug delivery systems: Innovations for an unmet need. Exp Eye Res. 2022;218. [DOI] [PubMed] [Google Scholar]
- 35.Pall B, Gomes P, Yi F, Torkildsen G. Management of ocular allergy itch with an antihistamine-releasing contact lens. Cornea. 2019;38(6):713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehra D, Cohen NK, Galor A. Ocular Surface Pain: A Narrative Review. Ophthalmol Ther. 2020;9(3):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andersen HH, Yosipovitch G, Galor A. Neuropathic symptoms of the ocular surface: dryness, pain, and itch. Current Opinion in Allergy and Clinical Immunology. 2017;17:373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galor A, Levitt RC, Felix ER, Martin ER, Sarantopoulos CD. Neuropathic ocular pain: an important yet underevaluated feature of dry eye. Eye. 2015;29:301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Surv Ophthalmol. 2014;59(3):263–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebrahimiadib N, Yousefshahi F, Abdi P, Ghahari M, Modjtahedi BS. Ocular Neuropathic Pain: An Overview Focusing on Ocular Surface Pains. Clin Ophthalmol. 2020;14:2843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rózsa AJ, Beuerman RW. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain. 1982;14(2):105–20. [DOI] [PubMed] [Google Scholar]
- 42.Cruccu G, Iannetti GD, Agostino R, Romaniello A, Truini A, Manfredi M. Conduction velocity of the human spinothalamic tract as assessed by laser evoked potentials. NeuroReport. 2000;11(13):3029–32. [DOI] [PubMed] [Google Scholar]
- 43.Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. The Journal of Physiology. 1985;359(1):31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goyal S, Hamrah P. Understanding Neuropathic Corneal Pain--Gaps and Current Therapeutic Approaches. Semin Ophthalmol. 2016;31(1-2):59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sukhotinsky I, Ben-Dor E, Raber P, Devor M. Key role of the dorsal root ganglion in neuropathic tactile hypersensibility. European Journal of Pain. 2004;8. [DOI] [PubMed] [Google Scholar]
- 46.Lieu T, Myers AC, Meeker SN, Undem BJ. TRPV1 induction in airway vagal low-threshold mechanosensory neurons by allergen challenge and neurotrophic factors. American journal of physiology Lung cellular and molecular physiology. 2012;302 9:L941–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuruvilla M, Kalangara J, Lee FEE. Neuropathic Pain and Itch Mechanisms Underlying Allergic Conjunctivitis. J Investig Allergol Clin Immunol. 2019;29(5):349–56. [DOI] [PubMed] [Google Scholar]
- 48.Stumpf A, Ständer S. Neuropathic itch: diagnosis and management. Dermatologic Therapy. 2013;26. [DOI] [PubMed] [Google Scholar]
- 49.Pereira MP, Wiegmann H, Agelopoulos K, Ständer S. Neuropathic Itch: Routes to Clinical Diagnosis. Frontiers in Medicine. 2021;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal AS, et al. , editors. A Sensory Neuron-expressed Interleukin-31 Receptor Mediates T helper Cell-dependent Itch : Involvement of TRPV 1 and TRPA 1 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rogerio AP, Andrade EL, Calixto JB. C-fibers, but not the transient potential receptor vanilloid 1 (TRPV1), play a role in experimental allergic airway inflammation. Eur J Pharmacol. 2011;662(1-3):55–62. [DOI] [PubMed] [Google Scholar]
- 52.Raap U, Braunstahl GJ. The role of neurotrophins in the pathophysiology of allergic rhinitis. Curr Opin Allergy Clin Immunol. 2010;10(1):8–13. [DOI] [PubMed] [Google Scholar]
- 53.Gunal AI, Ozalp G, Yoldas TK, Gunal SY, Kirciman E, Celiker H. Gabapentin therapy for pruritus in haemodialysis patients: a randomized, placebo-controlled, double-blind trial. Nephrology Dialysis Transplantation. 2004;19(12):3137–9. [DOI] [PubMed] [Google Scholar]
- 54.Yesudian PD, Wilson NJE. Efficacy of Gabapentin in the Management of Pruritus of Unknown Origin. Archives of Dermatology. 2005;141(12):1507–9. [DOI] [PubMed] [Google Scholar]
- 55.Nakamizo S, Miyachi Y, Kabashima K. Treatment of neuropathic itch possibly due to trigeminal trophic syndrome with 0.1% topical tacrolimus and gabapentin. Acta dermato-venereologica. 2010;90 6:654–5. [DOI] [PubMed] [Google Scholar]
- 56.Davis MP, Frandsen JL, Walsh D, Andresen S, Taylor S. Mirtazapine for pruritus. J Pain Symptom Manage. 2003;25(3):288–91. [DOI] [PubMed] [Google Scholar]
- 57.Shohrati M, Tajik A, Harandi AA, Davoodi SM, Akmasi M. Comparison of hydroxyzine and doxepin in treatment of pruritus due to sulfur mustard. Skinmed. 2007;6(2):70–2. [DOI] [PubMed] [Google Scholar]
- 58.Tefferi A, Fonseca R. Selective serotonin reuptake inhibitors are effective in the treatment of polycythemia vera-associated pruritus. Blood. 2002;99(7):2627. [DOI] [PubMed] [Google Scholar]
- 59.Fishbane S, Jamal A, Munera C, Wen W, Menzaghi F. A Phase 3 Trial of Difelikefalin in Hemodialysis Patients with Pruritus. New England Journal of Medicine. 2019;382(3):222–32. [DOI] [PubMed] [Google Scholar]
- 60.Morin CB, Raef HS, Elmariah SB. Neuropathic itch treated with oral cannabinoids: A case series. JAAD Case Reports. 2021;17:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain. 2019;160(1):19–27. [DOI] [PubMed] [Google Scholar]
- 62.Feng Y, Simpson TL. Nociceptive sensation and sensitivity evoked from human cornea and conjunctiva stimulated by CO2. Invest Ophthalmol Vis Sci. 2003;44(2):529–32. [DOI] [PubMed] [Google Scholar]
- 63.Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol. 2016;100(1):128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Farhangi M, Feuer W, Galor A, Bouhassira D, Levitt RC, Sarantopoulos CD, et al. Modification of the Neuropathic Pain Symptom Inventory for use in eye pain (NPSI-Eye). Pain. 2019;160(7):1541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crane AM, Levitt RC, Felix ER, Sarantopoulos KD, McClellan AL, Galor A. Patients with more severe symptoms of neuropathic ocular pain report more frequent and severe chronic overlapping pain conditions and psychiatric disease. Br J Ophthalmol. 2017;101(2):227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schuster AK, Wettstein M, Gerhardt A, Eich W, Bieber C, Tesarz J. Eye Pain and Dry Eye in Patients with Fibromyalgia. Pain Med. 2018;19(12):2528–35. [DOI] [PubMed] [Google Scholar]
- 67.Dermer H, Lent-Schochet D, Theotoka D, Paba C, Cheema AA, Kim RS, et al. A Review of Management Strategies for Nociceptive and Neuropathic Ocular Surface Pain. Drugs. 2020;80(6):547–71. [DOI] [PubMed] [Google Scholar]
- 68. Galor A, Hamrah P, Haque S, Attal N, Labetoulle M. Understanding chronic ocular surface pain: An unmet need for targeted drug therapy. Ocul Surf. 2022;26:148–56. * This study details the obstacles understanding and treating ocular surface pain. Not only are there unmet therapy needs, but also there are a multitude of institutional and social factors that must be addressed which complicate the identification of chronic ocular surface pain in patients
- 69.Hirata H, Oshinsky ML. Ocular dryness excites two classes of corneal afferent neurons implicated in basal tearing in rats: involvement of transient receptor potential channels. J Neurophysiol. 2012;107(4):1199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Galor A, Moein HR, Lee C, Rodriguez A, Felix ER, Sarantopoulos KD, et al. Neuropathic pain and dry eye. Ocul Surf. 2018;16(1):31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eftimiadi G, Soligo M, Manni L, Di Giuda D, Calcagni ML, Chiaretti A. Topical delivery of nerve growth factor for treatment of ocular and brain disorders. Neural Regen Res. 2021;16(9):1740–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gunthorpe MJ, Chizh BA. Clinical development of TRPV1 antagonists: targeting a pivotal point in the pain pathway. Drug Discov Today. 2009;14(1-2):56–67. [DOI] [PubMed] [Google Scholar]
- 73. Thompson V, Moshirfar M, Clinch T, Scoper S, Linn SH, McIntosh A, et al. Topical Ocular TRPV1 Antagonist SAF312 (Libvatrep) for Postoperative Pain After Photorefractive Keratectomy. Transl Vis Sci Technol. 2023;12(3):7. ** The TRPV1 receptor plays a central role in ocular surface pain. In recent clinical trials, SAF312 (Libvatrep) a TRPV1 antagonist is being shown to significantly reduce ocular surface pain. This article demonstrates the powerful development of next generation treatments that target ocular surface pain.
- 74.Patel S, Mittal R, Felix ER, Sarantopoulos KD, Levitt RC, Galor A. Differential Effects of Treatment Strategies in Individuals With Chronic Ocular Surface Pain With a Neuropathic Component. Front Pharmacol. 2021;12:788524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Small LR, Galor A, Felix ER, Horn DB, Levitt RC, Sarantopoulos CD. Oral Gabapentinoids and Nerve Blocks for the Treatment of Chronic Ocular Pain. Eye Contact Lens. 2020;46(3):174–81. [DOI] [PubMed] [Google Scholar]
- 76.Ozmen MC, Dieckmann G, Cox SM, Rashad R, Paracha R, Sanayei N, et al. Efficacy and tolerability of nortriptyline in the management of neuropathic corneal pain. Ocul Surf. 2020;18(4):814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dieckmann G, Ozmen MC, Cox SM, Engert RC, Hamrah P. Low-dose naltrexone is effective and well-tolerated for modulating symptoms in patients with neuropathic corneal pain. Ocul Surf. 2021;20:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sivanesan E, Levitt RC, Sarantopoulos CD, Patin D, Galor A. Noninvasive Electrical Stimulation for the Treatment of Chronic Ocular Pain and Photophobia. Neuromodulation. 2018;21(8):727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Venkateswaran N, Hwang J, Rong AJ, Levitt AE, Diel RJ, Levitt RC, et al. Periorbital botulinum toxin A improves photophobia and sensations of dryness in patients without migraine: Case series of four patients. Am J Ophthalmol Case Rep. 2020;19:100809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reaux-Le Goazigo A, Poras H, Ben-Dhaou C, Ouimet T, Baudouin C, Wurm M, et al. Dual enkephalinase inhibitor PL265: a novel topical treatment to alleviate corneal pain and inflammation. Pain. 2019;160(2):307–21. [DOI] [PubMed] [Google Scholar]