Abstract
The worldwide population is aging exponentially, creating burdens to patients, their families and society. Increasing age is associated with higher risk of a wide range of chronic diseases, and aging of the vascular system is closely linked to the development of many age-related diseases. Endothelial glycocalyx is a layer of proteoglycan polymers on the surface of the inner lumen of blood vessels. It plays an important role in maintaining vascular homeostasis and protecting various organ functions. Endothelial glycocalyx loss happens through the aging process and repairing the endothelial glycocalyx may alleviate the symptoms of age-related diseases. Given the important role of the glycocalyx and its regenerative properties, it is posited that the endothelial glycocalyx may be a potential therapeutic target for aging and age-related diseases and repairing endothelial glycocalyx could play a role in the promotion of healthy aging and longevity. Here, we review the composition, function, shedding, and manifestation of the endothelial glycocalyx in aging and age-related diseases, as well as regeneration of endothelial glycocalyx.
Keywords: endothelial glycocalyx, aging, age-related diseases, healthy, older adults
The combination of greater life expectancy and reduced fertility rates has led to an ‘aging population’ where the number and proportion of older adults have increased rapidly. The World Population Prospects 2022 (https://population.un.org/wpp/) issued by the United Nations showed that currently, there are more than 1 billion adults aged 60 years and above globally. Moreover, by 2050 the population of adults over the age of 60 would grow to more than 2 billion in number, with 450 million people over the age of 80. According to the United Nations' World Population Ageing 2020 Highlights (www.un.org/development/desa/pd/content/ageing-1), it is expected that by 2050, 1 in 6 people in the world will be over the age of 65. While the increasing longevity of the human population is something worth celebrating, it is concerned that health does not extend with life expectancy. Older adults often suffer from frailty, deterioration of organ function, poor compensatory capacity, and degenerative diseases. Aging is a multifaceted phenomenon that is observable at molecular, cellular, and physiological levels. Identifying and understanding potential biological targets of healthy aging may allow us to develop preventive strategies to help delay the onset of age-related diseases, hence extending healthspan and improving quality of life.
In recent years, it has been widely recognized that the surface of a healthy endovascular lumen is covered with gelatinous villous material. This layer of proteoglycan polymer is called endothelial glycocalyx (EG). EG serves as a critical signaling platform to integrate chemical and haemodynamic signals [1]. EG plays an important role in regulating vascular permeability, and is involved in mechanical signal transduction, inflammation, oxidative stress, and the inhibition of coagulation and cell adhesion. The integrity of EG is crucial for the maintenance of normal physiological function of the organs. A thinner EG has been commonly associated with age-related chronic diseases [2-4]. This article reviews the composition, function, shedding, and manifestations of EG in aging and age-related diseases, and the potential role of EG regeneration in promoting healthy aging.
Composition and Function of EG
EG is mainly composed of glycosaminoglycans (GAGs), proteoglycans (PGs), glycoproteins (GPs) and plasma proteins. It is connected to endothelial cells through a cytoskeleton composed of PGs and GPs [5]. The thickness and structure of EG may vary across different species and organs [6]. Determining the thickness of EG is difficult as it can be easily disturbed during vessel handling and preparation protocols [7], leading to inaccurate measurements. However, in vivo immunolabeling and laser scanning confocal microscopy may be effective methods for measuring EG [8].
GAGs are the largest constituent of EG. The various groups of GAGs include heparan sulfate (HS), chondroitin sulfate (CS), keratan sulfate (KS), Dermatan sulfate (DS) and hyaluronic acid (HA) [7]. Among them, HS is the most abundant form accounting for 50-90% of the total GAGs [9]. Due to the highly sulphated nature of these components, EG has a net negative charge. On the other hand, PGs are composed of Syndecans and Glypicans, which are the membrane scaffold of GAGs [10]. Syndecans are transmembrane receptors with four members: syndecan-1, -2, -3 and -4, whereas Glypicans include six members: glypican-1, -2, -3, -4, -5 and -6.
Through covalent bonds, the core proteins of PGs can bind to a variety of GAG chains, primarily with HS and CS, and with DS and KS to a lesser extent [11]. These structures play an important role in signal transduction. HA is an uncharged non-sulfate that binds to the osteopontin receptor CD44 [12]. It is worth noting that aging and neurodegeneration alter sulfation in a fashion that is unpredictable. Therefore, there is a growing interest in finding out exactly how GAGs sulfation affects microvascular physiology in aging. EG can bind with plasmonic proteins and ions with a positive charge as it is negatively charged, and this affects the interaction between platelets and red blood cells [13]. These interactions play an important role in regulating the balance of fluid inside and outside blood vessels and is closely related to vascular permeability [5]. EG can repel negatively charged molecules and form an electrostatic barrier to plasma constituents, like a giant molecular sieve [14]. Studies have shown that the negative charge of EG can be neutralized by myeloperoxidase, and this neutralization affect vascular endothelial function and increase vascular permeability [15]. Macromolecules with molecular weight greater than 70KDa have difficulty penetrating the EG [14]. EG is also a mechanical sensor of blood shear force [16], playing a major role in mechanosensing and transducing in microvessel [17]. It transmits shear forces to endothelial cells via transmembrane domains and converts mechanical stimuli into intracellular signals that controls endothelial function. EG regulates a variety of important physiological functions such as the activation of endothelial nitric oxide synthase (eNOS) and mediates the release of nitric oxide (NO) [18, 19], where glypican-1 and HS are the main receptors that induce the regular release of NO [20]. EG can also combine with enzymes that scavenge oxygen radicals, such as extracellular superoxide dismutase (SOD), to maintain the bioavailability of NO and reduce oxidative stress [21].
GPs are the backbone proteins of EG, consisting of small branches of glucose side chains on the surface of endothelial cells, mostly ending with sialic acid (SA) [9]. GPs include members from the selectin family, integrin family, and immunoglobulin superfamily, each with a different role in cell adhesion. The selectin family is involved in the adhesion of leukocytes and endothelial cells [22], whereas the integrin family mediates the adhesion of endothelial cells to platelets. The immunoglobulin superfamily on the other hand is a ligand of integrins that mediate the return of leukocytes to the endodermis. Members of the superfamily mainly consist of intercellular adhesion molecules (ICAM), vascular cell adhesion molecule-1 (VCAM-1) and platelet endothelial cell adhesion molecule-1 (PECAM-1). If the integrity of EG is damaged, cell adhesion molecules will be exposed, and leukocytes and platelets will adhere to endothelial cells, and lead to platelet aggregation, oedema and loss of vascular reactivity [23].
Shedding of EG
Under normal conditions, EG is shed under the influence of biological factors and then regenerated by endothelial cell synthesis, maintaining its integrity through homeostasis. The enzyme that degrades EG includes matrix metalloproteinases (MMPs), A disintegrin and metalloproteinase (ADAM), heparanase (HPSE) and hyaluronidase (HAase) etc. MMPs are zinc-containing endopeptidases that cause the degradation of extracellular matrix and connective tissue proteins [11]. MMPs are the major players in degrading the skeletal components of EG [24]. Syndecan-1 and syndecan-2 are shed by MMP-7 [25], and IL-1β increases the shedding of syndecan-4 by MMP-9 in immortalized glomerular endothelial cells [26]. Oxidative stress can enhance the activity of histone deacetylase (HDAC), up-regulate the expression and activity of MMPs, and down-regulate tissue inhibitor of matrix metalloproteinases (TIMPs), thus eventually lead to the loss of EG [27]. Furthermore, it was also found that syndecan-1 and SOD3 shedding could be reversed through inhibition of MMPs and HDAC [27].
The HDAC family is divided into four different subgroups. Subgroups I, II and IV are Zn2+-dependent enzymes, and subgroup III, Sirtuins (SIRTs), are nicotinamide adenine dinucleotide-dependent enzymes that are not affected by classical HDAC inhibitors [28]. The Sirtuins consist of seven proteins (SIRT1-SIRT7), all of which are involved in key processes related to health and longevity such as chromatin regulation, DNA repair and cell metabolism [29-31], among them, SIRT1 pathway plays a critical role in aging [29-31]. NF-κB is one of the target proteins of SIRT1 deacetylation [32]. Normally, NF-κB forms a dimer structure of p50-p65 (NF-κB1/RelA). In an inactive state, NF-κB binds to the specific inhibitor IκB protein in the cytoplasm and interferes with its nuclear translocation [33]. SIRT1 binds to the p65 protein subunit of NF-κB and deacetylates Lys310 in p65, resulting in the loss of NF-κB transcriptional activity [30]. Increased SIRT1 activity inhibits NF-κB-dependent inflammatory responses [34]. Syndecan-4 is the target gene of NF-κB. In SIRT1endo-/- mice, when nuclear translocation of NF-κB is increased, transcription of syndecan-4 is also elevated [30]. However, it was found that the expression of syndecan-4 isolated from renal microvascular endothelial cells of SIRT1endo-/- mice decreased and its extracellular domain increased concurrently, which may be related to the disruption of the integrity of EG transmembrane scaffold [35]. ADAM17 is known to shed the extracellular domain of Syndecan-4 [36]. Lack of SIRT1 induces an increase in ADAM17 activity, resulting in greater shedding of syndecan-4 [35]. Moreover, ADAM15 cleaves CD44 to promote increased vascular permeability [37]. HPSE is the only known enzyme that degrades HS [38] HA can be degraded by HAase or non-enzyme. The non-enzymatic degradation of HA is mainly caused by reactive oxygen species (ROS) [39].
EG in Aging and Age-related Diseases
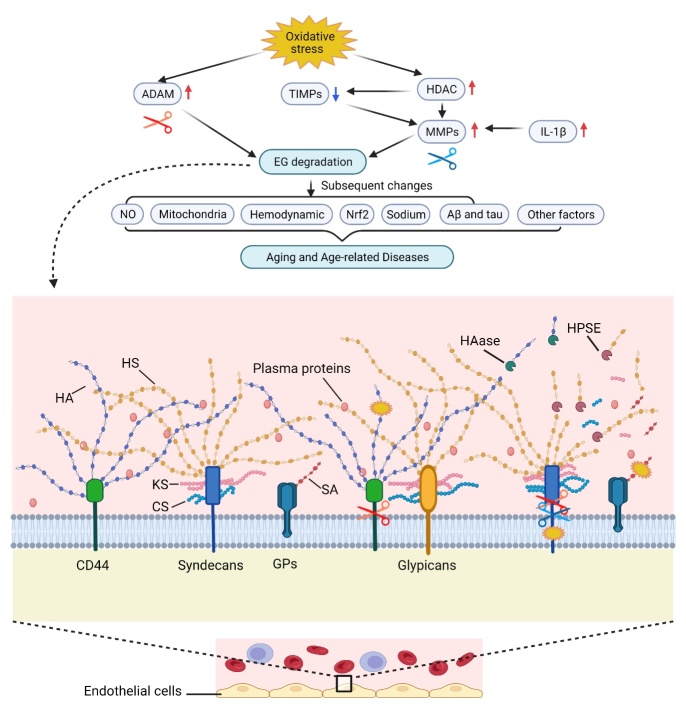
Aging is associated with increased risk of a wide range of diseases and such diseases are labeled as age-related diseases. By definition, aging is a complex process characterized by progressive loss of physiological integrity, and increased susceptibility to age-related diseases and death [40]. Aging is also driven by the consecutive impairment of the microcirculation [41]. Age-related diseases can be defined as the continuous deterioration of tissues and organs over time, ultimately resulting in tissue and organ failure [42]. Twelve hallmarks of aging are proposed recently according to the following three criteria: (a) the time-dependent alterations manifestation during aging; (b) the possibility to accelerate aging by experimental aggravation; and (c) the opportunity to decelerate, halt, or reverse aging by therapeutic interventions [43]. Based on these criteria and the characteristics of EG, EG is a potential new marker of aging and age-related diseases (Fig. 1).
Figure 1.
Endothelial Glycocalyx in Aging and Age-related Diseases. GPs, glycoproteins; HS, heparan sulfate; CS, chondroitin sulfate; KS, keratan sulfate; HA, hyaluronic acid; SA, sialic acid; HPSE, heparinase; HAase, hyaluronidase; EG, endothelial glycocalyx; MMPs, matrix metalloproteinases; ADAM, A disintegrin and metalloproteinase; HDAC, histone deacetylase; TIMPs, tissue inhibitor of matrix metalloproteinases; NO, nitric oxide; Nrf2, nuclear factor erythroid 2-related factor 2; Aβ, amyloid-beta. Created with BioRender.com.
Aging
Oxidative stress is widely recognized as an crucial factor associated with aging and age-related diseases [44]. EG components exposed to oxidative stress result in constant modifications in their structure, these changes in EG can be observed in aging. The expression of glypican-1, one of the main receptors that induce the regular release of NO [20], is inhibited in the aging process, in turn, vasoregulation can be damaged by reducing NO signaling [45]. Age-related deterioration of EG has been found in aged mice and humans. Studies showed that capillary density and EG thickness decreases in older adults, and the thickness of EG in sublingual microcirculation can decrease by 33% [46, 47]. In animal studies by observing the ultrastructure of rat tissue, it was found that EG thickness was decreased in aged rats [48]. It was also shown that the thickness of EG in the mesentery and skeletal muscle microvessels in aged mice is 51%-54% lower than that in young mice, which could be caused by age-related alterations in non-sulfated GAGs namely HA synthesis [47]. The same phenomenon was also found in aging mice, that degradation of HA increased in microvasculature of the cerebral cortex [49]. Similarly, SA is degraded from erythrocyte membranes at a higher rate in older adults compared to young adults, which may be associated with the increase in oxidative stress during aging [50]. In vitro study also has shown that aged cells have increased EG shedding and decreased EG layers, and under biomimetic shock conditions, EG shedding is further exacerbated in aged as compared to young endothelial cells [51]. Human brain is one of the two most abundant tissue sources of KS, and KS has neuroregulatory properties [52, 53]. The CS/KS PG aggrecan has anti-oxidant properties and play essential role in synaptic plasticity [52]. P-glycoprotein (P-gp) is expressed exclusively by endothelial cells in the brain as part of the blood-brain barrier (BBB). There is evidence that P-gp expression declines with age in the human BBB [54]. This is consistent with the decreasing trend of P-gp expression with age found in Fischer 344/Brown-Norway (F344/BN) aging model rats [55]. Overall, research on the direct role of EG in aging is still very limited.
Cardiovascular diseases
The main features of the pathogenesis of cardiovascular diseases are oxidative stress, endothelial activation, and endothelial dysfunction [56]. Age advancement is the independent and essential prognosticator of endothelial dysfunction and vascular dysfunction [57, 58]. Oxidative stress, which has been described an imbalance favoring the generation of ROS and/or reducing antioxidant defense systems [59], is a major cause of endothelial dysfunction [56]. Oxidative stress is normally related to EG degradation, proteolysis and increased permeability in the cardiovascular system [60]. Studies have found the phenomenon of decreased EG in age-related vascular diseases [2, 3]. EG in healthy individuals contains around 1.5 liters of plasma, which can replenish the blood volume if needed [61], making EG a very powerful fluid reservoir. When EG thickness declines during the aging process, the capacity of the cardiovascular reserve and the circulatory system regulation also decreases. Deteriorated EG can affect the efficiency of blood flow distribution, and this can lead to the derangement of microvascular perfusion [62, 63]. Once the microcirculation is impaired, cells would not be able to obtain adequate nutrients and oxygen, and the physiological functions of cells and organs will deteriorate [41], which may ultimately result in tissue and organ failure. Therefore, EG deterioration could be one of the triggers of age-related vascular diseases. There is also evidence that supports a possible hypothesis that age-related EG worsens and causes microvascular dysfunction, which in turn leads to macrovascular dysfunction and eventually cardiovascular disease (CVD) [4]. The deterioration of EG may precede traditional measurements of age-related impairment of vascular function such as large artery stiffness [4]. This could be the pathological mechanism of EG in the development of age-related CVD. Indeed, a 6-year follow-up study have revealed that the damage of EG predicted future cardiovascular events [64]. Similarly, other study supports that EG could be a key therapeutic target of age-related cardiovascular diseases, and the mechanisms may be related to the decreased activation of nuclear factor erythroid 2-related factor 2 (Nrf2) caused by SA reduction, leading to the reduction of antioxidants [65]. It is well known that Nrf2 is a positive regulator of antioxidant pathways [65]. Moreover, animal experiments have also shown that decreased SIRT-1 expression in blood vessels of aged mice is associated with age-related atherosclerosis of the great arteries [66], and age-related aortic stiffness was attenuated in mice with lifelong SIRT-1 overexpression [67]. In view of the above evidence, it is necessary to conduct more studies to further examine the role of EG in age-related vascular diseases.
Hypertension
Hypertension is a major risk factor for CVD, strokes, and has emerged as a pathogenic factor of Alzheimer’s disease (AD) [68, 69]. Excessive sodium intake has been shown to cause increase of blood pressure (BP) and been linked with hypertension and CVD [70, 71].The BP response to sodium is a predictor of severity of organ damage caused by hypertension [72] and sodium-dependent hypertension is related to EG degradation [73]. Importantly, GAGs are highly sulfated components with net negative charges, and have sodium-binding properties. Experiments in both mice and rats have identified that GAGs in the skin interstitium are responsible for sodium storage [74, 75], hence EG may have a significant role in sodium homeostasis. When EG integrity is lost, a sodium load would lead to water retention and increased BP [73]. NO is the major factor in maintaining vascular homeostasis and reduced bioavailability of NO marks the beginning of endothelial dysfunction [56]. In vitro studies have confirmed that on account of EG absence, plasma sodium concentration increases, which enhances endothelial stiffness and decreases the release of NO, eventually leading to elevated BP [76]. Study also showed in rat model of pulmonary arterial hypertension, heparin supplementation improved the integrity of EG and slowed down the development of hypertension [77]. This is also illustrated by the inverse correlation between EG volume and BP in patients with type II diabetes [78]. Besides, impaired EG and reduced thickness are associated with increased arterial stiffness in hypertensive patients [79, 80]. It was also found that hypertensive individuals have decreased erythrocyte membranes SA compared to normotensive individuals [50], and this supports that hemodynamic properties of the blood may be affected by decrease in SA content [81].
Strokes
Strokes can be caused by imbalance in the regulation of oxidative stress, over production of ROS and mitochondrial dysfunction [44]. There are two types of strokes, the ischemic stroke and the hemorrhagic stroke. Ischemic stroke is caused by occlusion of cerebral arteries and accounts for approximately 87% of all strokes [82]. After stroke, astrocytes and microglia are rapidly activated, producing huge amounts of ROS, and causing BBB damage [83]. Impairment of the BBB integrity occurs early in the pathogenies of strokes. EG function as an essential component of the BBB [84], that in concert with the endothelial cell layer, abluminal basement membrane, and astrocyte endplates, plays an important role in the maintenance of BBB integrity [85]. Intact EG is critical for the modulation of BBB permeability [86]. Like studies in aging brains, technically it is challenging to study EG in the brains of strokes. In vivo measurement of EG in brain is almost impossible, hence sublingual microvasculature measurement of EG dimensions is used as an alternative method. Lacunar stroke patients with white matter lesions have an increased perfused boundary region (PBR) compared with healthy controls, which indicate EG impairment [87]. However, this phenomenon does not exist in lacunar stroke patients without white matter lesions, which could be due to the improvement of endothelial function by statins and antihypertensive [87, 88]. The shed EG components may serve as potential markers of EG impairment [89, 90]. The degree of EG damage can be assessed by measuring the levels of circulating EG components in plasma, such as syndecan-1, HS, CS [91]. And it has been shown that plasma level of syndecan-1 increased in acute ischemic stroke and syndecan-1 level predicted the prognosis in stroke patients [92]. The plasma level of SA is also an indicator of the degree of EG breakdown. The combination of elevated SA plasma levels and reduced SA erythrocyte concentrations is one of the markers of ischemic stroke [93]. Mitochondrial dysregulation is common in neurological diseases such as strokes [94, 95] and AD [96]. HA is hydrophilic [97], it binds to the osteopontin receptor CD44. HA-aggrecan interactions in brain protect mitochondria from oxidative damage [52]. Intriguingly, the synthesis of HA increases in the injured areas of the stroke brain [98, 99], and the reason may be related to CD44 affecting BBB function by regulating BBB permeability in response to fluid shear stress [100]. With current evidence from both animal and human studies, it appears that EG has great potential as a therapeutic target for BBB recovery following strokes.
Neurodegenerative diseases
BBB breakdown in aging can lead to brain accumulation of serum proteins and several vasculotoxic and/or neurotoxic macromolecules, which contribute to the development of neuronal degenerative changes [44]. AD is the most common neurodegenerative diseases worldwide, characterized by the accumulation of amyloid-beta (Aβ) peptide and hyperphosphorylated tau [101, 102]. Oxidative stress is involved in the development of AD by facilitating Aβ deposition and the hyperphosphorylation of tau [103]. A human study found that EG was reduced in AD brains, leading to non-productive neutrophil adhesion to the vasculature and subsequent vascular oxidative stress [104]. HA level was elevated within damaged brain tissues in neurodegenerative diseases [105] and the increase of HA in AD brain was associated with Aβ and hyper-phosphorylated tau [106]. Interestingly, one study found that HA in cerebrospinal fluid (CSF) increased in vascular dementia patients as compared with controls while there was no difference between AD patients and controls [107]. There is a positive correlation between levels of HA in CSF and the CSF/ serum albumin ratio, an indicator of BBB integrity, in patients with vascular dementia and AD [107]. Furthermore, GAGs and PGs play pivotal roles in neuronal cell development and function [108, 109]. HSPGs are formed by the binding of HS to PGs [110]. HSPGS expression is altered in aging [45]. HSPGs may promote Aβ and tau fibrilization in AD brain [111] and it was observed the levels of some HSPGs were associated with lesions in AD [112]. This may be related to the fact that HSPGs play a role in synaptic stabilization [113, 114]. HS is relevant to almost every step of Aβ pathogenesis in AD [115]. It is possible that blocking the binding of Aβ to HS or desulfation of syndecan-2 can reduce the formation of amyloid plaques, and hence represent a potential therapeutic strategy for AD [116]. Given very limited and indefinitive findings on the role of EG in AD pathology, more research, especially human studies should be conducted in the future to shed light on the uncertainties.
Regeneration of EG
EG plays an important role in maintaining physiological stability, therefore, regeneration of EG may be one potential solution to healthy aging. EG can be repaired by exogenous supplementation of EG components. Dietary supplementation with EG precursors has been shown to restore EG in patients with type II diabetes [78]. Previous study has also shown that dietary supplementation of EG precursor GP for 10 weeks in aged mice restored EG barrier function [117]. Endothelial vesicles contain abundant deposits of EG [118, 119], and it is hypothesized that the externalization of these substances can bring the components of EG back to the endothelial surface [120]. Cutler et al. have shown that metabolites of blueberries could restore GAGs, suggesting that blueberries could regenerate EG [121]. Therefore, older adults can increase blueberry intake in their diet to enhance their health and the effects could partially be attributed to regenerated EG.
Heparin and heparinoids may have a role in EG regeneration. Heparin is a sulfated glycine aminoglycan that can directly bind to endothelium to form EG [122], probably because it increases HS production [123]. Sulodexide (SDX) is a glycosaminoglycan mixture consisting of 80% HS and 20% DS [12] that provides precursors of HS and a material source for the restoration of EG [120]. Study has shown that SDX can reconstruct EG in a rat model with carotid artery balloon injury [124]. As a supplement of GAGs, SDX could enhance EG and activate the nuclear factor Nrf2 signal and provide protection against ischemia-reperfusion injury [125].
In addition, in vitro studies have shown that metformin could down-regulate the expression of ICAM and E-selectin and repair EG [126]. Plasma resuscitation was shown to enhance lung syndecan-1mRNA expression in a rat model of hemorrhagic shock, which is an early sign of EG recovery [127]. On the other hand, sphingosine-1-phosphate (S1P), a sphingolipid in the plasma mainly derived from red blood cells, can protect EG and maintain normal vascular permeability [128]. Clinical studies have also shown that albumin can bind to EG to stabilize its structure, reducing the degradation of EG by the associated enzymes [10]. Albumin acts on EG primarily through modulating S1P [129].
Physical training has also been shown to have a protective and pro-repair effect on EG, and this may contribute to its anti-aging effects. Short-term respiratory muscle training improves respiratory and functional capacity as well as reducing plasma syndecan-1 concentrations in hemodialysis patients [130]. High-intensity interval training increases EG thickness [131] while moderate-intensity endurance training protects EG by a mechanism that may be related to reduced oxidative stress and enhanced antioxidant defense [132].
In addition to nutritional, pharmacological, albumin, S1P-based and physical training interventions, there is also evidence that liposomal nanocarriers of preassembled EG were able to restore endothelial mechanotransduction [133]. It is possible that combining different approaches may achieve synergy in delaying aging and age-related diseases and future research should test different combinations.
Conclusion
To date, there have been countless explorative efforts in finding solutions that can extend the human lifespan. With the economic developments and technological advances in medicine, people are not only concerned about extending lifespan but also paying more attention to improving the quality of their late-life. The concept of healthy aging has emerged in response to this changing era. The World Health Organization (WHO) first introduced the concept of healthy aging in 1990 and defined it in 2015 as a process of developing and maintaining bodily functions to enable older adults achieve well-being [134]. Diseases that come with aging affect every family. Although the question of whether the maximum human lifespan can be achieved remains open, the prevention, delay and, in some cases, reversal of the pathology of aging may be achieved through the study of physiopathology of aging, thus enabling older adults to live not only longer but also healthier lives [135]. One of the important pathophysiological processes of aging is the aging of blood vessels and the accumulation of vascular dysfunction [136]. EG is the main functional barrier to the vascular endothelium and plays an important role in maintaining selective vascular permeability [137]. Decreased microvascular density and loss of EG have been found during the aging process [51]. It has been shown in previous experiments that EG deficiency is closely linked to age-related diseases, thus protecting and repairing the EG is essential for healthy aging. Given the important role of EG in vascular homeostasis and the protection of various organs, as well as its regenerative properties [138], it is potentially an important therapeutic target for aging and age-related diseases. As previously mentioned, several drugs, foods and other measures have been shown to protect and restore EG. Although there is still a lack of definitive evidence, findings from early studies could guide future research on EG treatment. If more effective interventions can be developed to replenish EG in a timely manner, it may help to prevent age-related diseases and preserve physiological functions during human aging. Although many studies have greatly expanded our understanding of the role of EG in promoting healthy aging, much work remains to be done before we can clinically apply EG treatments to improve the health of older adults.
Acknowledgements
LS was supported by the province-school Joint Training Program of Shandong Province.
Footnotes
Competing interests
The authors have no conflicts of interest to report.
References
- [1].Zeng Y (2017). Endothelial glycocalyx as a critical signalling platform integrating the extracellular haemodynamic forces and chemical signalling. J Cell Mol Med, 21:1457-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Weinbaum S, Cancel LM, Fu BM, Tarbell JM (2021). The Glycocalyx and Its Role in Vascular Physiology and Vascular Related Diseases. Cardiovasc Eng Technol, 12:37-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liew H, Roberts MA, Pope A, McMahon LP (2021). Endothelial glycocalyx damage in kidney disease correlates with uraemic toxins and endothelial dysfunction. BMC Nephrol, 22:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Machin DR, Phuong TT, Donato AJ (2019). The role of the endothelial glycocalyx in advanced age and cardiovascular disease. Curr Opin Pharmacol, 45:66-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jedlicka J, Becker B F, and Chappell D (2020). Endothelial Glycocalyx. Care Clin., 36:217-232. [DOI] [PubMed] [Google Scholar]
- [6].Dogné S, Flamion B (2020). Endothelial Glycocalyx Impairment in Disease: Focus on Hyaluronan Shedding. Am J Pathol, 190:768-780. [DOI] [PubMed] [Google Scholar]
- [7].Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG (2007). The endothelial glycocalyx: composition, functions, and visualization. Eur. [J]. Physiol., 454:345-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yen WY, Cai B, Zeng M, Tarbell JM, Fu BM (2012). Quantification of the endothelial surface glycocalyx on rat and mouse blood vessels. Microvasc Res, 83:337-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhao F, Zhong L, Luo Y (2021). Endothelial glycocalyx as an important factor in composition of blood-brain barrier. CNS Neurosci Ther, 27:26-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Aldecoa C, Llau JV, Nuvials X, Artigas A (2020). Role of albumin in the preservation of endothelial glycocalyx integrity and the microcirculation: a review. Ann Intensive Care, 10:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jin J, Fang F, Gao W, Chen H, Wen J, Wen X, et al. (2021). The Structure and Function of the Glycocalyx and Its Connection With Blood-Brain Barrier. Frontiers in Cellular Neuroscience, 15:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Masola V, Zaza G, Arduini A, Onisto M, Gambaro G (2021). Endothelial Glycocalyx as a Regulator of Fibrotic Processes. Int J Mol Sci, 22:2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chelazzi C, Villa G, Mancinelli P, De Gaudio AR, Adembri C (2015). Glycocalyx and sepsis-induced alterations in vascular permeability. Crit Care, 19:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Alphonsus CS, Rodseth RN (2014). The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia, 69:777-784. [DOI] [PubMed] [Google Scholar]
- [15].Kolářová H, Víteček J, Černá A, Černík M, Přibyl J, Skládal P, et al. (2021). Myeloperoxidase mediated alteration of endothelial function is dependent on its cationic charge. Free Radic Biol Med, 162:14-26. [DOI] [PubMed] [Google Scholar]
- [16].Cosgun ZC, Fels B, Kusche-Vihrog K (2020). Nanomechanics of the Endothelial Glycocalyx: From Structure to Function. Am J Pathol, 190:732-741. [DOI] [PubMed] [Google Scholar]
- [17].Yen W, Cai B, Yang J, Zhang L, Zeng M, Tarbell JM, et al. (2015). Endothelial surface glycocalyx can regulate flow-induced nitric oxide production in microvessels in vivo. PLoS One, 10:e0117133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lyu N, Du Z, Qiu H, Gao P, Yao Q, Xiong K, et al. (2020). Mimicking the Nitric Oxide-Releasing and Glycocalyx Functions of Endothelium on Vascular Stent Surfaces. Adv Sci (Weinh), 7:2002330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lupu F, Kinasewitz G, Dormer K (2020). The role of endothelial shear stress on haemodynamics, inflammation, coagulation and glycocalyx during sepsis. J Cell Mol Med, 24:12258-12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bartosch AMW, Mathews R, Tarbell JM (2017). Endothelial Glycocalyx-Mediated Nitric Oxide Production in Response to Selective AFM Pulling. Biophys J, 113:101-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Haeren RH, van de Ven SE, van Zandvoort MA, Vink H, van Overbeeke JJ, Hoogland G, et al. (2016). Assessment and Imaging of the Cerebrovascular Glycocalyx. Curr Neurovasc Res, 13:249-260. [DOI] [PubMed] [Google Scholar]
- [22].Sperandio M (2006). Selectins and glycosyltransferases in leukocyte rolling in vivo. Febs j, 273:4377-4389. [DOI] [PubMed] [Google Scholar]
- [23].Iba T, Levy JH (2019). Derangement of the endothelial glycocalyx in sepsis. J Thromb Haemost, 17:283-294. [DOI] [PubMed] [Google Scholar]
- [24].Song J, Wu C, Korpos E, Zhang X, Agrawal SM, Wang Y, et al. (2015). Focal MMP-2 and MMP-9 activity at the blood-brain barrier promotes chemokine-induced leukocyte migration. Cell Rep, 10:1040-1054. [DOI] [PubMed] [Google Scholar]
- [25].Kwon MJ, Hong E, Choi Y, Kang DH, Oh ES (2014). Interleukin-1α promotes extracellular shedding of syndecan-2 via induction of matrix metalloproteinase-7 expression. Biochem Biophys Res Commun, 446:487-492. [DOI] [PubMed] [Google Scholar]
- [26].Reine TM, Lanzalaco F, Kristiansen O, Enget AR, Satchell S, Jenssen TG, et al. (2019). Matrix metalloproteinase-9 mediated shedding of syndecan-4 in glomerular endothelial cells. Microcirculation: e12534. [DOI] [PubMed] [Google Scholar]
- [27].Ali MM, Mahmoud AM, Le Master E, Levitan I, Phillips SA (2019). Role of matrix metalloproteinases and histone deacetylase in oxidative stress-induced degradation of the endothelial glycocalyx. Am J Physiol Heart Circ Physiol, 316:H647-h663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Clocchiatti A, Florean C, Brancolini C (2011). Class IIa HDACs: from important roles in differentiation to possible implications in tumourigenesis. J Cell Mol Med, 15:1833-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Young Hong J, Cao J, Lin H (2019). Fluorogenic Assays for the Defatty-Acylase Activity of Sirtuins. Methods Mol Biol, 2009:129-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lipphardt M, Song JW, Goligorsky MS (2020). Sirtuin 1 and endothelial glycocalyx. Pflugers Arch, 472:991-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yu M, Zhang H, Wang B, Zhang Y, Zheng X, Shao B, et al. (2021). Key Signaling Pathways in Aging and Potential Interventions for Healthy Aging. Cells, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xie J, Zhang X, Zhang L (2013). Negative regulation of inflammation by SIRT1. Pharmacol Res, 67:60-67. [DOI] [PubMed] [Google Scholar]
- [33].Bonizzi G, Karin M (2004). The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol, 25:280-288. [DOI] [PubMed] [Google Scholar]
- [34].Pan W, Yu H, Huang S, Zhu P (2016). Resveratrol Protects against TNF-α-Induced Injury in Human Umbilical Endothelial Cells through Promoting Sirtuin-1-Induced Repression of NF-κB and p38 MAPK. PLoS One, 11:e0147034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lipphardt M, Song JW, Ratliff BB, Dihazi H, Müller GA, Goligorsky MS (2018). Endothelial dysfunction is a superinducer of syndecan-4: fibrogenic role of its ectodomain. Am J Physiol Heart Circ Physiol, 314:H484-h496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Piperigkou Z, Mohr B, Karamanos N, Götte M (2016). Shed proteoglycans in tumor stroma. Cell Tissue Res, 365:643-655. [DOI] [PubMed] [Google Scholar]
- [37].Yang X, Meegan JE, Jannaway M, Coleman DC, Yuan SY (2018). A disintegrin and metalloproteinase 15-mediated glycocalyx shedding contributes to vascular leakage during inflammation. Cardiovasc Res, 114:1752-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vlodavsky I, Friedmann Y, Elkin M, Aingorn H, Atzmon R, Ishai-Michaeli R, et al. (1999). Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat Med, 5:793-802. [DOI] [PubMed] [Google Scholar]
- [39].Marinho A, Nunes C, Reis S (2021). Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules, 11:1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013). The hallmarks of aging. Cell, 153:1194-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jin K (2019). A Microcirculatory Theory of Aging. Aging Dis, 10:676-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhou RP, Chen Y, Wei X, Yu B, Xiong ZG, Lu C, et al. (2020). Novel insights into ferroptosis: Implications for age-related diseases. Theranostics, 10:11976-11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2023). Hallmarks of aging: An expanding universe. Cell, 186:243-278. [DOI] [PubMed] [Google Scholar]
- [44].Xu X, Wang B, Ren C, Hu J, Greenberg DA, Chen T, et al. (2017). Recent Progress in Vascular Aging: Mechanisms and Its Role in Age-related Diseases. Aging Dis, 8:486-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pretorius D, Richter RP, Anand T, Cardenas JC, Richter JR (2022). Alterations in heparan sulfate proteoglycan synthesis and sulfation and the impact on vascular endothelial function. Matrix Biol Plus, 16:100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Groen BB, Hamer HM, Snijders T, van Kranenburg J, Frijns D, Vink H, et al. (2014). Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J Appl Physiol (1985), 116:998-1005. [DOI] [PubMed] [Google Scholar]
- [47].Machin DR, Bloom SI, Campbell RA, Phuong TTT, Gates PE, Lesniewski LA, et al. (2018). Advanced age results in a diminished endothelial glycocalyx. Am J Physiol Heart Circ Physiol, 315:H531-h539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zolla V, Nizamutdinova IT, Scharf B, Clement CC, Maejima D, Akl T, et al. (2015). Aging-related anatomical and biochemical changes in lymphatic collectors impair lymph transport, fluid homeostasis, and pathogen clearance. Aging Cell, 14:582-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Reed MJ, Vernon RB, Damodarasamy M, Chan CK, Wight TN, Bentov I, et al. (2017). Microvasculature of the Mouse Cerebral Cortex Exhibits Increased Accumulation and Synthesis of Hyaluronan With Aging. J Gerontol A Biol Sci Med Sci, 72:740-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mehdi MM, Singh P, Rizvi SI (2012). Erythrocyte sialic acid content during aging in humans: correlation with markers of oxidative stress. Dis Markers, 32:179-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Carge MJ, Liberati DM, Diebel LN (2021). A biomimetic shock model on the effect of endothelial aging on vascular barrier properties. J Trauma Acute Care Surg, 91:849-855. [DOI] [PubMed] [Google Scholar]
- [52].Melrose J (2019). Keratan sulfate (KS)-proteoglycans and neuronal regulation in health and disease: the importance of KS-glycodynamics and interactive capability with neuroregulatory ligands. J Neurochem, 149:170-194. [DOI] [PubMed] [Google Scholar]
- [53].Melrose J (2019). Functional Consequences of Keratan Sulfate Sulfation in Electrosensory Tissues and in Neuronal Regulation. Adv Biosyst, 3:e1800327. [DOI] [PubMed] [Google Scholar]
- [54].Chiu C, Miller MC, Monahan R, Osgood DP, Stopa EG, Silverberg GD (2015). P-glycoprotein expression and amyloid accumulation in human aging and Alzheimer's disease: preliminary observations. Neurobiol Aging, 36:2475-2482. [DOI] [PubMed] [Google Scholar]
- [55].Silverberg GD, Messier AA, Miller MC, Machan JT, Majmudar SS, Stopa EG, et al. (2010). Amyloid efflux transporter expression at the blood-brain barrier declines in normal aging. J Neuropathol Exp Neurol, 69:1034-1043. [DOI] [PubMed] [Google Scholar]
- [56].Incalza MA, D'Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F (2018). Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol, 100:1-19. [DOI] [PubMed] [Google Scholar]
- [57].Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF Jr, et al. (2004). Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension, 44:134-139. [DOI] [PubMed] [Google Scholar]
- [58].Xu X, Wang B, Ren C, Hu J, Greenberg DA, Chen T, et al. (2017). Age-related Impairment of Vascular Structure and Functions. Aging Dis, 8:590-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL (2017). Oxidative Stress in Atherosclerosis. Curr Atheroscler Rep, 19:42. [DOI] [PubMed] [Google Scholar]
- [60].Foote CA, Soares RN, Ramirez-Perez FI, Ghiarone T, Aroor A, Manrique-Acevedo C, et al.2022. Endothelial Glycocalyx. In Comprehensive Physiology. 3781-3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Woodcock TE, Woodcock TM (2012). Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth, 108:384-394. [DOI] [PubMed] [Google Scholar]
- [62].Pries AR, Secomb TW (2005). Microvascular blood viscosity in vivo and the endothelial surface layer. Am J Physiol Heart Circ Physiol, 289:H2657-2664. [DOI] [PubMed] [Google Scholar]
- [63].McClatchey PM, Schafer M, Hunter KS, Reusch JE (2016). The endothelial glycocalyx promotes homogenous blood flow distribution within the microvasculature. Am J Physiol Heart Circ Physiol, 311:H168-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ikonomidis I, Thymis J, Simitsis P, Koliou GA, Katsanos S, Triantafyllou C, et al. (2022). Impaired Endothelial Glycocalyx Predicts Adverse Outcome in Subjects Without Overt Cardiovascular Disease: a 6-Year Follow-up Study. J Cardiovasc Transl Res, 15:890-902. [DOI] [PubMed] [Google Scholar]
- [65].Psefteli PM, Kitscha P, Vizcay G, Fleck R, Chapple SJ, Mann GE, et al. (2021). Glycocalyx sialic acids regulate Nrf2-mediated signaling by fluid shear stress in human endothelial cells. Redox Biol, 38:101816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, et al. (2013). Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell, 12:772-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Machin DR, Auduong Y, Gogulamudi VR, Liu Y, Islam MT, Lesniewski LA, et al. (2020). Lifelong SIRT-1 overexpression attenuates large artery stiffening with advancing age. Aging (Albany NY), 12:11314-11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Scheltens P, Blennow K, Breteler MMB, de Strooper B, Frisoni GB, Salloway S, et al. (2016). Alzheimer's disease. The Lancet, 388:505-517. [DOI] [PubMed] [Google Scholar]
- [69].Fuchs FD, Whelton PK (2020). High Blood Pressure and Cardiovascular Disease. Hypertension, 75:285-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Strazzullo P, D'Elia L, Kandala NB, Cappuccio FP (2009). Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. Bmj, 339:b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Grillo A, Salvi L, Coruzzi P, Salvi P, Parati G (2019). Sodium Intake and Hypertension. Nutrients, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bihorac A, Tezcan H, Ozener C, Oktay A, Akoglu E (2000). Association between salt sensitivity and target organ damage in essential hypertension. Am J Hypertens, 13:864-872. [DOI] [PubMed] [Google Scholar]
- [73].Olde Engberink RH, Rorije NM, Homan van der Heide JJ, van den Born BJ, Vogt L (2015). Role of the vascular wall in sodium homeostasis and salt sensitivity. J Am Soc Nephrol, 26:777-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Titze J, Shakibaei M, Schafflhuber M, Schulze-Tanzil G, Porst M, Schwind KH, et al. (2004). Glycosaminoglycan polymerization may enable osmotically inactive Na+ storage in the skin. Am J Physiol Heart Circ Physiol, 287:H203-208. [DOI] [PubMed] [Google Scholar]
- [75].Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, et al. (2010). Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med, 362:590-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Oberleithner H, Riethmüller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M (2007). Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci U S A, 104:16281-16286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Guo J, Yang ZC, Liu Y (2019). Attenuating Pulmonary Hypertension by Protecting the Integrity of Glycocalyx in Rats Model of Pulmonary Artery Hypertension. Inflammation, 42:1951-1956. [DOI] [PubMed] [Google Scholar]
- [78].Broekhuizen LN, Lemkes BA, Mooij HL, Meuwese MC, Verberne H, Holleman F, et al. (2010). Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia, 53:2646-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Triantafyllidi H, Benas D, Vlachos S, Vlastos D, Pavlidis G, Schoinas A, et al. (2018). HDL cholesterol levels and endothelial glycocalyx integrity in treated hypertensive patients. J Clin Hypertens (Greenwich), 20:1615-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Ikonomidis I, Voumvourakis A, Makavos G, Triantafyllidi H, Pavlidis G, Katogiannis K, et al. (2018). Association of impaired endothelial glycocalyx with arterial stiffness, coronary microcirculatory dysfunction, and abnormal myocardial deformation in untreated hypertensives. J Clin Hypertens (Greenwich), 20:672-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hadengue AL, Del-Pino M, Simon A, Levenson J (1998). Erythrocyte disaggregation shear stress, sialic acid, and cell aging in humans. Hypertension, 32:324-330. [DOI] [PubMed] [Google Scholar]
- [82].Barthels D, Das H (2020). Current advances in ischemic stroke research and therapies. Biochim Biophys Acta Mol Basis Dis, 1866:165260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zhu G, Wang X, Chen L, Lenahan C, Fu Z, Fang Y, et al. (2022). Crosstalk Between the Oxidative Stress and Glia Cells After Stroke: From Mechanism to Therapies. Front Immunol, 13:852416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ando Y, Okada H, Takemura G, Suzuki K, Takada C, Tomita H, et al. (2018). Brain-Specific Ultrastructure of Capillary Endothelial Glycocalyx and Its Possible Contribution for Blood Brain Barrier. Sci Rep, 8:17523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kutuzov N, Flyvbjerg H, Lauritzen M (2018). Contributions of the glycocalyx, endothelium, and extravascular compartment to the blood-brain barrier. Proc Natl Acad Sci U S A, 115:E9429-e9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Nian K, Harding IC, Herman IM, Ebong EE (2020). Blood-Brain Barrier Damage in Ischemic Stroke and Its Regulation by Endothelial Mechanotransduction. Front Physiol, 11:605398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Martens RJ, Vink H, van Oostenbrugge RJ, Staals J (2013). Sublingual microvascular glycocalyx dimensions in lacunar stroke patients. Cerebrovasc Dis, 35:451-454. [DOI] [PubMed] [Google Scholar]
- [88].Tomasoni L, Sitia S, Borghi C, Cicero AF, Ceconi C, Cecaro F, et al. (2010). Effects of treatment strategy on endothelial function. Autoimmun Rev, 9:840-844. [DOI] [PubMed] [Google Scholar]
- [89].Fels J, Jeggle P, Liashkovich I, Peters W, Oberleithner H (2014). Nanomechanics of vascular endothelium. Cell and Tissue Research, 355:727-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Fels J, Kusche-Vihrog K (2019). Endothelial Nanomechanics in the Context of Endothelial (Dys)function and Inflammation. Antioxid Redox Signal, 30:945-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Cusack R, Leone M, Rodriguez AH, Martin-Loeches I (2022). Endothelial Damage and the Microcirculation in Critical Illness. Biomedicines, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Zhao F, Wang R, Huang Y, Li L, Zhong L, Hu Y, et al. (2022). Elevated plasma syndecan-1 as glycocalyx injury marker predicts unfavorable outcomes after rt-PA intravenous thrombolysis in acute ischemic stroke. Front Pharmacol, 13:949290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Nanetti L, Vignini A, Raffaelli F, Taffi R, Silvestrini M, Provinciali L, et al. (2008). Sialic acid and sialidase activity in acute stroke. Dis Markers, 25:167-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Liu F, Lu J, Manaenko A, Tang J, Hu Q (2018). Mitochondria in Ischemic Stroke: New Insight and Implications. Aging Dis, 9:924-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Yang JL, Mukda S, Chen SD (2018). Diverse roles of mitochondria in ischemic stroke. Redox Biol, 16:263-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Alexiou A, Soursou G, Chatzichronis S, Gasparatos E, Kamal MA, Yarla NS, et al. (2019). Role of GTPases in the Regulation of Mitochondrial Dynamics in Alzheimer's Disease and CNS-Related Disorders. Mol Neurobiol, 56:4530-4538. [DOI] [PubMed] [Google Scholar]
- [97].Nelson A, Berkestedt I, Bodelsson M (2014). Circulating glycosaminoglycan species in septic shock. Acta Anaesthesiol Scand, 58:36-43. [DOI] [PubMed] [Google Scholar]
- [98].Al'Qteishat A, Gaffney J, Krupinski J, Rubio F, West D, Kumar S, et al. (2006). Changes in hyaluronan production and metabolism following ischaemic stroke in man. Brain, 129:2158-2176. [DOI] [PubMed] [Google Scholar]
- [99].Wang H, Zhan Y, Xu L, Feuerstein GZ, Wang X (2001). Use of suppression subtractive hybridization for differential gene expression in stroke: discovery of CD44 gene expression and localization in permanent focal stroke in rats. Stroke, 32:1020-1027. [DOI] [PubMed] [Google Scholar]
- [100].DeOre BJ, Partyka PP, Fan F, Galie PA (2020). CD44 regulates blood-brain barrier integrity in response to fluid shear stress. bioRxiv. [Google Scholar]
- [101].Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL (2015). Alzheimer's disease. Nature Reviews Disease Primers, 1:15056. [DOI] [PubMed] [Google Scholar]
- [102].Chételat G, Villemagne VL, Bourgeat P, Pike KE, Jones G, Ames D, et al. (2010). Relationship between atrophy and beta-amyloid deposition in Alzheimer disease. Ann Neurol, 67:317-324. [DOI] [PubMed] [Google Scholar]
- [103].Chen Z, Zhong C (2014). Oxidative stress in Alzheimer's disease. Neurosci Bull, 30:271-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Smyth LCD, Murray HC, Hill M, van Leeuwen E, Highet B, Magon NJ, et al. (2022). Neutrophil-vascular interactions drive myeloperoxidase accumulation in the brain in Alzheimer's disease. Acta Neuropathol Commun, 10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Sherman LS, Matsumoto S, Su W, Srivastava T, Back SA (2015). Hyaluronan Synthesis, Catabolism, and Signaling in Neurodegenerative Diseases. Int J Cell Biol, 2015:368584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Reed MJ, Damodarasamy M, Pathan JL, Chan CK, Spiekerman C, Wight TN, et al. (2019). Increased Hyaluronan and TSG-6 in Association with Neuropathologic Changes of Alzheimer's Disease. J Alzheimers Dis, 67:91-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Nägga K, Hansson O, van Westen D, Minthon L, Wennström M (2014). Increased levels of hyaluronic acid in cerebrospinal fluid in patients with vascular dementia. J Alzheimers Dis, 42:1435-1441. [DOI] [PubMed] [Google Scholar]
- [108].Hayes AJ, Melrose J (2018). Glycans and glycosaminoglycans in neurobiology: key regulators of neuronal cell function and fate. Biochem J, 475:2511-2545. [DOI] [PubMed] [Google Scholar]
- [109].Hayes AJ, Melrose J (2023). HS, an Ancient Molecular Recognition and Information Storage Glycosaminoglycan, Equips HS-Proteoglycans with Diverse Matrix and Cell-Interactive Properties Operative in Tissue Development and Tissue Function in Health and Disease. Int J Mol Sci, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Coulson-Thomas VJ (2016). The role of heparan sulphate in development: the ectodermal story. Int J Exp Pathol, 97:213-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].van Horssen J, Wesseling P, van den Heuvel LP, de Waal RM, Verbeek MM (2003). Heparan sulphate proteoglycans in Alzheimer's disease and amyloid-related disorders. Lancet Neurol, 2:482-492. [DOI] [PubMed] [Google Scholar]
- [112].Lorente-Gea L, García B, Martín C, Ordiales H, García-Suárez O, Piña-Batista KM, et al. (2020). Heparan Sulfate Proteoglycans Undergo Differential Expression Alterations in Alzheimer Disease Brains. J Neuropathol Exp Neurol, 79:474-483. [DOI] [PubMed] [Google Scholar]
- [113].Basu A, Patel NG, Nicholson ED, Weiss RJ (2022). Spatiotemporal diversity and regulation of glycosaminoglycans in cell homeostasis and human disease. Am J Physiol Cell Physiol, 322:C849-c864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Xu D, Esko JD (2014). Demystifying heparan sulfate-protein interactions. Annu Rev Biochem, 83:129-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Zhang GL, Zhang X, Wang XM, Li JP (2014). Towards understanding the roles of heparan sulfate proteoglycans in Alzheimer's disease. Biomed Res Int, 2014:516028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Leonova EI, Galzitskaya OV (2015). Role of Syndecans in Lipid Metabolism and Human Diseases. Adv Exp Med Biol, 855:241-258. [DOI] [PubMed] [Google Scholar]
- [117].Machin DR, Nguyen D, Bramwell RC, Lesniewski LA, Donato AJ (2019). Dietary Glycocalyx Precursor Supplementation Ameliorates Age-Related Vascular Dysfunction. The FASEB Journal, 33:828.821-828.821. [Google Scholar]
- [118].Becker BF, Chappell D, Bruegger D, Annecke T, Jacob M (2010). Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res, 87:300-310. [DOI] [PubMed] [Google Scholar]
- [119].Becker BF, Chappell D, Jacob M (2010). Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Basic Res Cardiol, 105:687-701. [DOI] [PubMed] [Google Scholar]
- [120].Becker BF, Jacob M, Leipert S, Salmon AH, Chappell D (2015). Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br J Clin Pharmacol, 80:389-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Cutler BR, Gholami S, Chua JS, Kuberan B, Anandh Babu PV (2018). Blueberry metabolites restore cell surface glycosaminoglycans and attenuate endothelial inflammation in diabetic human aortic endothelial cells. Int J Cardiol, 261:155-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Carroll BJ, Piazza G, Goldhaber SZ (2019). Sulodexide in venous disease. Journal of Thrombosis and Haemostasis, 17:31-38. [DOI] [PubMed] [Google Scholar]
- [123].Nader HB, Toma L, Pinhal MA, Buonassisi V, Colburn P, Dietrich CP (1991). Effect of heparin and dextran sulfate on the synthesis and structure of heparan sulfate from cultured endothelial cells. Semin Thromb Hemost, 17 Suppl 1:47-56. [PubMed] [Google Scholar]
- [124].Li T, Liu X, Zhao Z, Ni L, Liu C (2017). Sulodexide recovers endothelial function through reconstructing glycocalyx in the balloon-injury rat carotid artery model. Oncotarget, 8:91350-91361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Gabryel B, Bontor K, Jarząbek K, Plato M, Pudełko A, Machnik G, et al. (2020). Sulodexide up-regulates glutathione S-transferase P1 by enhancing Nrf2 expression and translocation in human umbilical vein endothelial cells injured by oxygen glucose deprivation. Arch Med Sci, 16:957-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Targosz-Korecka M, Malek-Zietek KE, Kloska D, Rajfur Z, Stepien E, Grochot-Przeczek A, et al. (2020). Metformin attenuates adhesion between cancer and endothelial cells in chronic hyperglycemia by recovery of the endothelial glycocalyx barrier. Biochim Biophys Acta Gen Subj, 1864:129533. [DOI] [PubMed] [Google Scholar]
- [127].Kozar RA, Peng Z, Zhang R, Holcomb JB, Pati S, Park P, et al. (2011). Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg, 112:1289-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Zhang L, Zeng M, Fan J, Tarbell JM, Curry FR, Fu BM (2016). Sphingosine-1-phosphate Maintains Normal Vascular Permeability by Preserving Endothelial Surface Glycocalyx in Intact Microvessels. Microcirculation, 23:301-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Adamson RH, Clark JF, Radeva M, Kheirolomoom A, Ferrara KW, Curry FE (2014). Albumin modulates S1P delivery from red blood cells in perfused microvessels: mechanism of the protein effect. Am J Physiol Heart Circ Physiol, 306:H1011-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Campos NG, Marizeiro DF, Florêncio ACL, Silva Í C, Meneses GC, Bezerra GF, et al. (2018). Effects of respiratory muscle training on endothelium and oxidative stress biomarkers in hemodialysis patients: A randomized clinical trial. Respir Med, 134:103-109. [DOI] [PubMed] [Google Scholar]
- [131].Schmitz B, Niehues H, Lenders M, Thorwesten L, Klose A, Krüger M, et al. (2019). Effects of high-intensity interval training on microvascular glycocalyx and associated microRNAs. Am J Physiol Heart Circ Physiol, 316:H1538-h1551. [DOI] [PubMed] [Google Scholar]
- [132].Majerczak J, Grandys M, Duda K, Zakrzewska A, Balcerczyk A, Kolodziejski L, et al. (2017). Moderate-intensity endurance training improves endothelial glycocalyx layer integrity in healthy young men. Exp Physiol, 102:70-85. [DOI] [PubMed] [Google Scholar]
- [133].Zhang X, Sun D, Song JW, Zullo J, Lipphardt M, Coneh-Gould L, et al. (2018). Endothelial cell dysfunction and glycocalyx - A vicious circle. Matrix Biol, 71-72:421-431. [DOI] [PubMed] [Google Scholar]
- [134].Rudnicka E, Napierała P, Podfigurna A, Męczekalski B, Smolarczyk R, Grymowicz M (2020). The World Health Organization (WHO) approach to healthy ageing. Maturitas, 139:6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E (2019). From discoveries in ageing research to therapeutics for healthy ageing. Nature, 571:183-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Donato AJ, Machin DR, Lesniewski LA (2018). Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circulation Research, 123:825-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Moore KH, Murphy HA, George EM (2021). The glycocalyx: a central regulator of vascular function. Am J Physiol Regul Integr Comp Physiol, 320:R508-r518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Potter DR, Jiang J, Damiano ER (2009). The recovery time course of the endothelial cell glycocalyx in vivo and its implications in vitro. Circ Res, 104:1318-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]