Abstract
Background:
Sodium glucose cotransporter 2 (SGLT2) inhibitors reduce risk of hospitalization for heart failure (HF) in patients with HF and preserved ejection fraction (HFpEF), but the hemodynamic mechanisms underlying these benefits remain unclear. This study sought to determine if treatment with dapagliflozin affects pulmonary capillary wedge pressure (PCWP) at rest and during exercise in patients with HFpEF.
Methods:
This was a single-center, double-blinded, randomized, placebo-controlled trial testing the effects of dapagliflozin 10 mg once daily in patients with HFpEF. Patients with class II-III HF, EF≥50%, and elevated PCWP during exercise were recruited. Cardiac hemodynamics were measured at rest and during exercise using high-fidelity micromanometers at baseline and following 24 weeks of treatment. The primary endpoint was change from baseline in rest and peak exercise PCWP incorporating both measurements, compared using a mixed model likelihood ratio test (LRT). Key secondary endpoints included body weight and directly-measured blood and plasma volumes. Expired gas analysis was performed evaluate oxygen transport in tandem with arterial lactate sampling.
Results:
Among 38 patients completing baseline assessments (median age 68, 66% women, 71% obese), 37 completed the trial. Treatment with dapagliflozin resulted in reduction in the primary endpoint of change in PCWP at rest and during exercise at 24 weeks relative to treatment with placebo (LRT for overall changes in PCWP p<0.001), with lower PCWP at rest (estimated treatment difference, ETD −3.5 mmHg, 95% CI: −6.6 to −0.4; p=0.029) and maximal exercise (ETD −5.7 mmHg, 95% CI: −10.8 to −0.7; p=0.027). Body weight was reduced with dapagliflozin (ETD −3.5 kg; 95% CI: −5.9 to −1.1; p=0.006), as was plasma volume (ETD −285 ml; 95% CI: −510 to −60; p=0.014), but there was no significant effect on red blood cell volume. There were no differences in oxygen consumption at 20W or peak exercise, but dapagliflozin decreased arterial lactate at 20W (−0.70±0.77 vs +0.37±1.29 mM, p=0.006).
Conclusions and Relevance:
In patients with HFpEF, treatment with dapagliflozin reduces resting and exercise pulmonary capillary wedge pressures, along with favorable effects plasma volume and body weight. These findings provide new insight into the hemodynamic mechanisms of benefit with SGLT2 inhibitors in HFpEF.
Keywords: heart failure, HFpEF, mechanism, diastolic function, exercise
Introduction
Sodium glucose cotransporter-2 inhibitors (SGLT2i) have been shown to reduce the risk of heart failure (HF) hospitalization or cardiovascular death and improve quality of life in patients with HF and preserved ejection fraction (HFpEF).1, 2 However, the mechanisms underlying these salutary effects remain unclear.3–5 Natriuretic effects may reduce plasma and blood volumes, erythropoiesis increases, and myocardial function may improve, but accumulating evidence suggests that SGLT2i also work in part by enhancing nutrient deprivation signaling and reducing nutrient surplus signaling pathways in patients with HFpEF.5, 6 Chronic nutrient surplus relative to energy expenditure causes obesity, a major risk factor for HFpEF.7
Assessment of cardiac filling pressures at rest and during exercise serves as the gold standard for diagnosis of HFpEF8 and provides the most direct means to evaluate the ability of the heart to function adequately as a pump that fills with and ejects blood at normal physiologic pressures.9, 10 Patients with HFpEF characteristically display elevation in cardiac diastolic filling pressures, assessed by the pulmonary capillary wedge pressure (PCWP), which leads to secondary elevations in pulmonary artery pressure.10–12 Greater increases in PCWP with activity are associated with impairments in exercise capacity11, 13 as well as increases in morbidity and mortality,14–16 but no study has evaluated the effects of SGLT2i on exercise hemodynamics in patients with HFpEF.
This randomized controlled trial tested the hypothesis that treatment with the SGLT2i dapagliflozin would reduce PCWP at rest and during exercise in patients with HFpEF, and further evaluated the effects of dapagliflozin on directly measured blood and plasma volumes, other hemodynamic measures, and oxygen transport.
Methods
Study overview
The data that support the findings of this study are available from the corresponding author upon reasonable request. This was an investigator initiated, prospective, randomized clinical trial of dapagliflozin 10 mg once daily compared with placebo in patients with HFpEF. All participants provided written informed consent. The Mayo Clinic institutional review board reviewed and approved the study. Investigators, patients, care providers and outcome assessors were blinded to treatment assignment. The study was investigator initiated and the authors had complete access to the data. Funding was provided by AstraZeneca, with a contract that provided no limitations on data to be considered for publication. The study protocol and statistical analysis plan are provided in Supplement 1 and Supplement 2, respectively.
Study Patients
Ambulatory patients with a diagnosis of HFpEF were eligible if they were 18 years of age or older, with symptoms of exertional dyspnea (NYHA class II-III), and left ventricular EF≥50%. Patients were required to display elevated PCWP during exercise (≥25 mmHg) at the baseline invasive exercise test following consent. Patients not meeting this criterion were considered screen failures. Other exclusion criteria included type 1 diabetes or type 2 diabetes with poor control (HbA1c≥10%), primary cardiomyopathy or pericardial disease, significant left-sided valvular heart disease, dyspnea primarily due to lung disease or ischemic heart disease, and severe anemia, liver, or kidney disease (estimated GFR<30). Full eligibility criteria are provided in Supplement 3.
Study Design
Following consent, participants underwent phlebotomy, echocardiography, measurement of blood and plasma volume, and then right heart catheterization at rest and with exercise to volitional exhaustion. Patients meeting the hemodynamic eligibility criteria were then randomized to treatment with dapagliflozin 10 mg once daily or matching placebo. Video or telephone visits (based upon patient preference) were conducted at 2 days, 1 week, 2 weeks, and then every 4 weeks for the 24 week study duration to assess for adverse events and encourage adherence with study medication. At 24 weeks, participants again underwent phlebotomy, echocardiography, blood/plasma volume measures, and rest/exercise right heart catheterization. A final follow up telephone/video visit was carried out 1 week following the 24 week visit.
Intervention and Randomization
Participants were treated with dapagliflozin 10 mg by mouth once daily or matching placebo for 24 weeks. The Mayo Clinic Research Pharmacy was provided with a block randomization sequence that was used to randomize enrolled patients 1:1 to dapagliflozin or placebo. Placebo and dapagliflozin tablets (provided in bulk by AstraZeneca) had an identical appearance to ensure that medical staff and patients remained blinded.
Outcome Measures
The primary endpoint was the change in pulmonary capillary wedge pressure (PCWP) at rest and during maximal exercise from the baseline study to the 24 week visit. The rationale for including both rest and exercise PCWP in the primary endpoint definition is that dapagliflozin may work on either or both components due to pleiotropic effects on plasma volume, myocardial reserve, or vascular loading conditions with stress. Participants had a wide range of exercise capacity, which limited direct comparisons between groups at each of the intermediate workloads due to the censoring of subsequent values once exhausted. To address this, the primary outcome values incorporate PCWP data from the rest phase prior to exercise and at peak exercise level defined as the highest workload reached. Key secondary endpoints included changes in right atrial and pulmonary artery pressures incorporating both rest and maximal exercise values, along with changes in plasma volume, total blood volume, red cell volume, and body weight. Measurements of oxygen transport including oxygen consumption (VO2), arterial-venous O2 content difference (Ca-vO2), and arterial lactate were examined as exploratory endpoints to provide further perspective.
Experimental Protocol
Hemodynamics including PCWP were measured using a high fidelity, solid state 2 Fr micromanometer advanced through a 7 Fr balloon tipped catheter as previously described, which provides higher frequency response than standard fluid-filled catheters (Figure S1).17, 18 Pressures were measured at end expiration and represent the mean of 3 replicate measurements during rest and exercise. Oxygen consumption (VO2) was measured using expired gas analysis at rest and during exercise (MedGraphics, St. Paul, MN). Arterial and mixed venous (pulmonary artery) blood samples were obtained at rest and during each stage of exercise to measure O2 content, Ca-vO2, and lactate. Cardiac output was determined using the direct Fick method as the quotient of VO2 and Ca-vO2. Transthoracic echocardiography was performed at the time of catheterization by a cardiologist to measure ejection fraction. Total blood volume, plasma volume, and red blood cell volume were directly measured using the radiolabeled iodinated albumin (131I, 5–25 μCu) indicator dilution technique (BVA-100 Blood Volume Analyzer, Daxor Corp, NY) as previously described.19
Statistical analysis
Based on preliminary data from our previous studies,17, 18 the standard deviation of change in PCWP was estimated to be ~5 mmHg, with some proportionality to the mean expected. Little improvement was expected in PCWP in the control group, and the assumed average exercise PCWP in patients with HFpEF was 30 mmHg, expecting a 20% (~6 mmHg) reduction in exercise PCWP in patients treated with dapagliflozin compared with patients with placebo. Based on two sample t test, 36 patients (18 receiving dapagliflozin and 18 placebo) would provide 93% power to detect a difference of 6mmHg or greater in PCWP during exercise workloads between the dapagliflozin and placebo groups, with a common standard deviation of 5 mmHg, at 0.05 significance level. Up to 51 patients were planned to be enrolled if necessary to allow for at least 36 to complete the trial.
The primary outcome measure was PCWP. This outcome was measured at rest and at maximal exercise intensity during both the baseline and end of treatment study visits. The primary measure of treatment effect, denoted as the estimated treatment difference (ETD), was defined as the between group differences of the changes from baseline to the end of study in PCWP at both rest and maximal exercise. The primary analysis was conducted on the intention to treat analysis set using all available data. The statistical model used was a linear mixed model consisting of eight repeated measures for PCWP (2 treatment groups × 2 study visits × 2 e×ercise levels). To account for the a priori assumption that the variance of PCWP would be larger at maximal exercise, an unstructured variance-covariance variance structure was used to allow for separate standard errors at each combination of study visit and exercise intensity. To address the design consideration that the primary outcome measure (PCWP) was measured at two exercise intensities, an overall test for treatment effect was constructed using a likelihood ratio test (LRT) under maximum likelihood estimation. This testing approach compared two statistical models: one model fully specified (i.e., 8 estimated means as noted above) with treatment and its associated interactions (treatment × study period; treatment × e×ercise level; and the treatment × study period × e×ercise level) and a second model with only 4 estimated means (2 study visits × 2 e×ercise levels) that removed all of the treatment related variables from the model. Thus, the LRT provided a means to simultaneously test a global null hypothesis of no treatment effect vs. the alternative that there was a treatment effect observed for at least one of the exercise levels or study periods. The ETDs measured at maximal exercise intensity and rest were constructed by comparing the appropriate model-based means between the end of study and baseline using the fully specified model with treatment and interactions after rejecting the global null hypothesis with a LRT.
This same modeling framework was used for the remaining secondary endpoints that were measured both at rest and peak exercise intensity. For endpoints measured only once at the final visit, a simplified repeated measures mixed model was fit using four estimated means (2 treatment groups × 2 study visits). Similar to the primary analysis, model-based contrasts were used to provide estimates of the differences in changes in outcomes over the study period between groups (e.g., effectively two-sample t-tests on the change values while allowing for missing data). All p-values are two-sided. P<0.05 was taken as statistically signficiant, and no corrections for multiple testing have been added to any reported p-values or confidence intervals. Analyses were performed using R 4.2.2 using the glmmTMB (version 1.1.7) package for the mixed model estimation. See Supplement 2 (Statistical Analysis Plan) for additional details.
Results
Study participants
Between February 2021 and May 2022, a total of 43 patients were consented, and 38 patients received treatment with study drug, with one patient (randomized to placebo) withdrawing from all follow up measurements (Figure 1). Patients were predominantly women (25/38, 66%) older aged (median age 68 years), with severe symptoms of heart failure (NYHA functional class 3 in 68% (26/38) of patients) and most were obese (27/38, 71%)(Table 1). Pulmonary capillary wedge pressure was normal (<15 mmHg) at rest in 37% or patients (14/38). Comorbidities were common including hypertension, atrial fibrillation and coronary disease, in keeping with typical HFpEF characteristics observed in the community.
Figure 1:
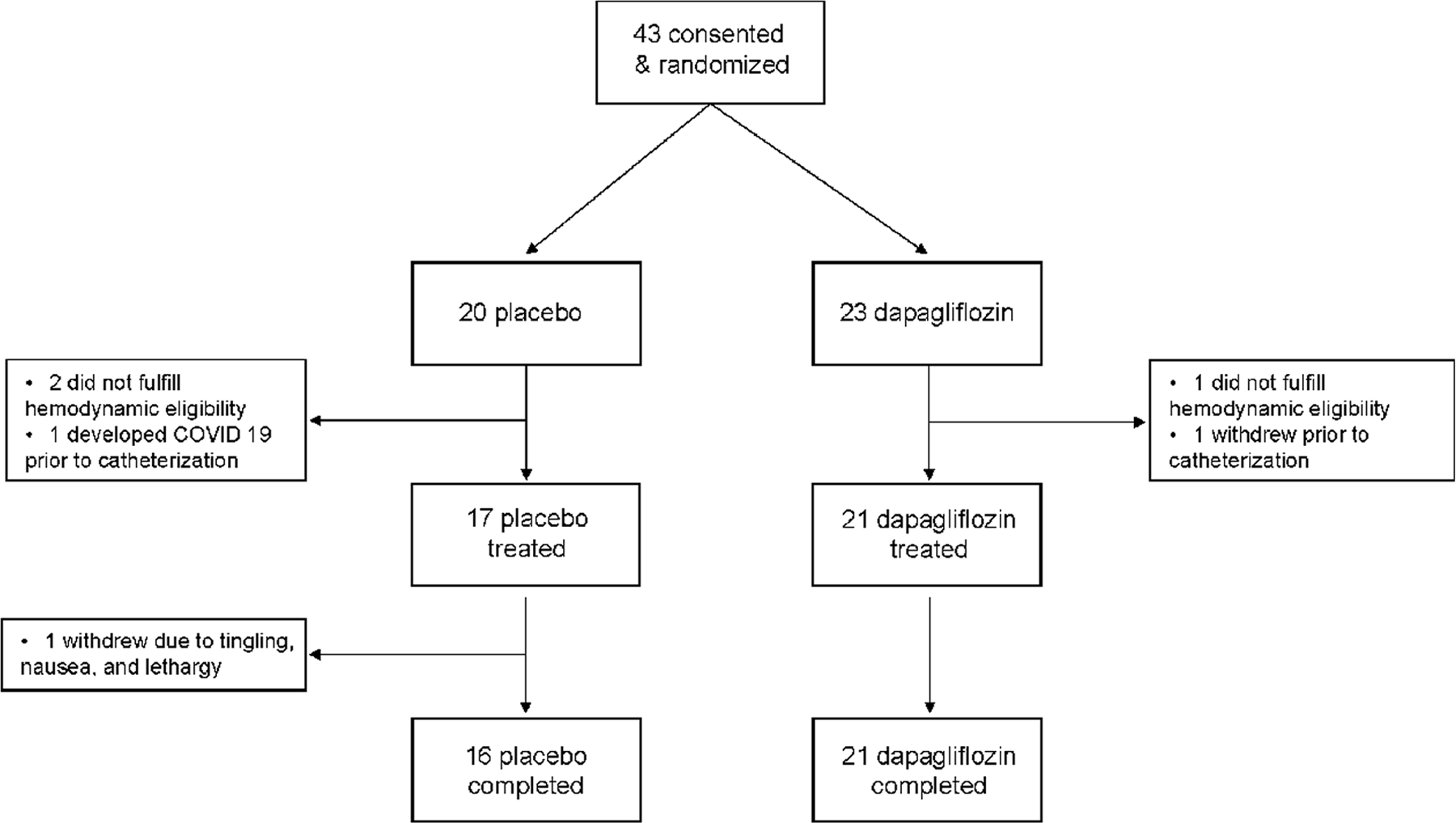
Flow of participants through the trial.
Table 1:
Baseline Characteristics
| Placebo (n=17) |
Dapagliflozin (n=21) |
|
|---|---|---|
| Age, years | 67 (9) | 67 (9) |
| Women, n (%) | 11 (65%) | 14 (67%) |
| Men, n (%) | 6 (35%) | 7 (33%) |
| Body weight, kg | 98.1 (18.2) | 100.8 (26.5) |
| Body mass index, kg/m2 | 34.5 (5.7) | 35.0 (7.2) |
| NYHA functional class, n (%) | ||
| Class II | 5 (29%) | 7 (33%) |
| Class III | 12 (71%) | 14 (67%) |
| Left ventricular ejection fraction, % | 63 (6) | 61 (6) |
| Resting PCWP <15 mmHg, n (%) | 6 (35%) | 8 (38%) |
| Comorbidities | ||
| Obesity (BMI≥30), n (%) | 12 (71%) | 15 (71%) |
| Hypertension, n (%) | 10 (59%) | 14 (67%) |
| Diabetes, n (%) | 1 (6%) | 6 (29%) |
| Atrial Fibrillation, n (%) | 6 (35%) | 8 (38%) |
| Coronary artery disease, n (%) | 5 (29%) | 4 (19%) |
| Medications | ||
| Diuretic, n (%) | 12 (71%) | 12 (57%) |
| ACEi/ARB/ARNI, n (%) | 4 (24%) | 6 (29%) |
| Beta-blocker, n (%) | 9 (53%) | 6 (29%) |
| Mineralocorticoid antagonist, n (%) | 6 (35%) | 7 (33%) |
| Laboratories | ||
| Hemoglobin, g/dl | 13.6 (1.4) | 13.0 (1.5) |
| Hematocrit, % | 40.7 (3.5) | 39.1 (4.0) |
| eGFR, ml/min/m1.73 | 73 (16) | 71 (17) |
| eGFR<60 ml/min/m1.73, n (%) | 3 (18%) | 5 (24%) |
| NTproBNP, pg/ml | 118 (76, 226) | 235 (102, 394) |
| Blood and Plasma Volumes a | ||
| Total blood volume deviation from normal, % | +2 (−2, 9) | +4 (−2, 11) |
| Red blood cell volume deviation from normal, % | −6 (−14, 6) | −8 (−20, 1) |
| Plasma volume deviation from normal, % | +5 (2, 14) | +12 (5, 22) |
Values represent mean (standard deviation) or median (25th, 75th)
Abbreviations: ACEi – angiotensin converting enzyme inhibitor; ARB – angiotensin receptor blocker; ARNI – angiotensin receptor blocker/neprilysin antagonist; BMI – body mass index; eGFR – estimated glomerular filtration rate; NTproBNP – N Terminal pro Brain Natriuretic Peptide; NYHA – New York Heart Association (higher class indicates more severe heart failure severity)
Blood and plasma volumes expressed as deviation from expected normal values based upon weight and sex, negative sign indicates a net deficit compared to normal and positive sign indicates a net excess compared to normal values.
On average, participants had mildly elevated resting right atrial pressure, pulmonary artery pressure, and PCWP prior to treatment, with marked elevation in cardiac filling pressures and pulmonary artery pressures during exercise (Table 2). Total blood volume and plasma volumes were mildly increased at baseline assessment, indicating low-grade congestion, and red blood cell volume was mildly reduced at baseline (Tables 1 and 2). During the study period there were diuretic escalations in 3/17 patients in the placebo group and 5/21 in the dapagliflozin group (p=0.71), and diuretics were reduced in 1/17 and 1/21 patients in the placebo and dapagliflozin groups, respectively (p=1.0).
Table 2:
Primary and Secondary Endpoints
| Dapagliflozin | Placebo | Estimated Treatment Differencea | ||||||
|---|---|---|---|---|---|---|---|---|
| Endpoint Classification | Measurement | Study Period | N | Mean (SD) | N | Mean (SD) | (95% CI) | P-value |
| Exercise | PCWP, mmHgb | Baseline | 21 | 33.5 (7.5) | 17 | 32.0 (5.8) | -- | -- |
| End of Study | 21 | 27.0 (6.8) | 16 | 30.8 (8.1) | -- | -- | ||
| Change from baseline | 21 | −6.6 (7.2) | 16 | −0.4 (8.4) | −5.7 (−10.8 to −0.7) | 0.027 | ||
| RA pressure, mmHgc | Baseline | 21 | 19.9 (7.0) | 17 | 18.2 (6.3) | -- | -- | |
| End of Study | 21 | 16.5 (6.8) | 16 | 18.7 (7.1) | -- | -- | ||
| Change from baseline | 21 | −3.4 (4.0) | 16 | 0.8 (5.6) | −4.1 (−7.2 to −1.0) | 0.011 | ||
| Mean PA pressure, mmHgd | Baseline | 21 | 49.9 (10.2) | 17 | 49.4 (10.6) | -- | -- | |
| End of Study | 21 | 44.7 (10.6) | 16 | 49.4 (10.6) | -- | -- | ||
| Change from baseline | 21 | −5.2 (6.6) | 16 | 0.7 (8.6) | −5.7 (−10.7 to −0.8) | 0.024 | ||
| Rest | PCWP, mmHgb | Baseline | 21 | 16.0 (3.9) | 17 | 15.8 (4.6) | -- | -- |
| End of Study | 21 | 13.5 (3.7) | 16 | 16.8 (5.5) | -- | -- | ||
| Change from baseline | 21 | −2.5 (3.7) | 16 | 1.1 (5.9) | −3.5 (−6.6 to −0.4) | 0.029 | ||
| RA pressure, mmHgc | Baseline | 21 | 10.4 (2.4) | 17 | 9.6 (3.2) | -- | -- | |
| End of Study | 21 | 8.8 (2.4) | 16 | 9.8 (3.6) | -- | -- | ||
| Change from baseline | 21 | −1.6 (2.6) | 16 | 0.3 (4.0) | −1.8 (−4.0 to −0.3) | 0.088 | ||
| Mean PA pressure, mmHgd | Baseline | 21 | 26.5 (6.9) | 17 | 26.2 (7.6) | -- | -- | |
| End | 21 | 24.7 (7.1) | 16 | 27.1 (6.1) | -- | -- | ||
| Change from baseline | 21 | −1.8 (4.0) | 16 | 1.1 (7.4) | −2.8 (−6.6 to 0.9) | 0.136 | ||
| Body weight, kg | Baseline | 21 | 100.8 (26.5) | 17 | 98.1 (18.2) | -- | -- | |
| End of Study | 21 | 97.1 (24.6) | 16 | 97.7 (18.8) | -- | -- | ||
| Change from baseline | 21 | −3.7 (4.2) | 16 | −0.2 (2.6) | −3.5 (−5.9 to −1.1) | 0.006 | ||
| Total blood volume, ml | Baseline | 21 | 5639 (1405) | 17 | 5533 (1088) | -- | -- | |
| Change from baseline | 21 | −118 (542) | 16 | 142 (399) | −259 (−586 to 68) | 0.12 | ||
| Red cell volume, ml | Baseline | 21 | 1859 (514) | 17 | 1889 (1709) | -- | -- | |
| End of Study | 21 | 1911 (468) | 16 | 1907 (422) | -- | -- | ||
| Change from baseline | 21 | 52 (285) | 16 | 26 (129) | 26 (−130 to 182) | 0.73 | ||
| Plasma volume, ml | Baseline | 21 | 3779 (946) | 17 | 3643 (755) | -- | -- | |
| End of Study | 21 | 3609 (889) | 16 | 3759 (712) | -- | -- | ||
| Change from baseline | 21 | −170 (343) | 16 | 115 (322) | −285 (−510 to −60) | 0.014 |
The treatment effect is estimated using a repeated measures mixed model that included repeated measures for baseline and end of study. For outcomes measured at rest and peak exercise intensity, a single model with 8 estimated means was constructed (i.e., 2 treatment groups (Dapagliflozin, placebo) × 2 measurement times (baseline, end of study) × 2 e×ercise levels (peak, rest)). For the remaining secondary outcomes, the model estimated only four means (2 treatment groups × 2 measurement times). A model-based contrast was used to estimate the treatment effect which was defined as the difference in the changes from baseline to end of study between treatment groups.
The primary endpoint was the change in PCWP over the course of the 24 week study at both rest and peak exercise intensity. The likelihood ratio test examining the statistical significance of the randomized drug was statistically significant (p<0.001) indicating the profiles of changes in PCWP were different between groups.
The secondary endpoint of change in right atrial pressure at rest and peak exercise by likelihood ratio was statistically significant (p<.001).
The secondary endpoint of change in mean pulmonary artery at rest and peak exercise by likelihood was statistically significant (p<.001).
Values represent mean (standard deviation) unless otherwise specified. Abbreviations: PCWP – pulmonary capillary wedge pressure; RA – right atrial; PA – pulmonary arterial
Primary Endpoint
Treatment with dapagliflozin was found to result in statistically significant reductions in PCWP incorporating values at both rest and maximal exercise intensity (p<0.001). Following 24 weeks of treatment, as compared with placebo, there was a significant reduction in PCWP with dapagliflozin incorporating values measured at rest (ETD −3.5 mmHg, 95% CI: −6.6 to −0.4, p=0.029) and at peak exercise (ETD −5.7 mmHg, 95% CI: −10.8 to −0.7, p=0.027) (Table 2, Figure 2).
Figure 2:
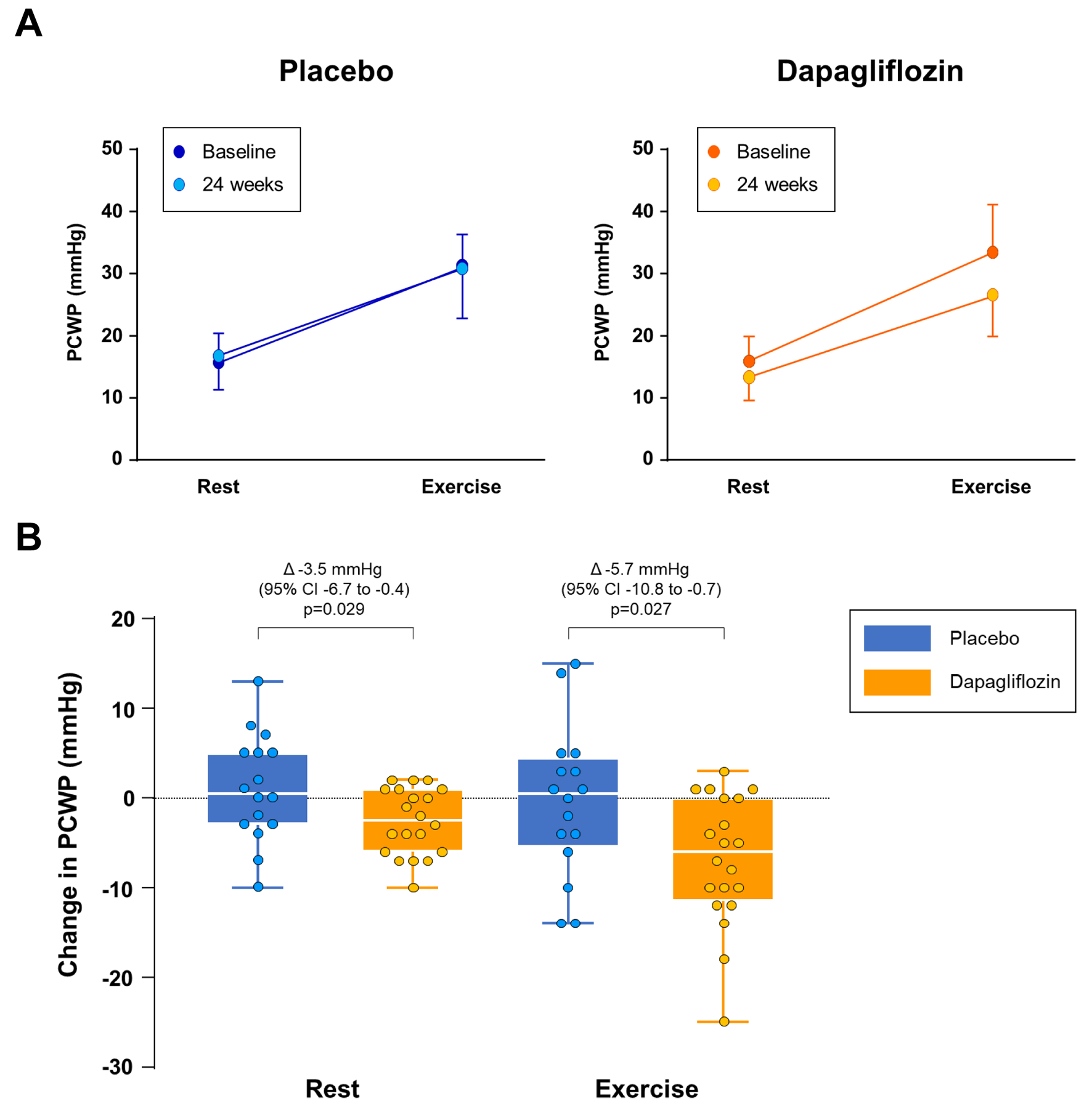
[A] Pulmonary capillary wedge pressure (PCWP) at rest and during exercise prior to treatment and following 24 weeks of treatment with placebo (left panels, blue) or dapagliflozin (right panels, orange). [B] Changes in PCWP at rest and exercise compared to the baseline cardiac catheterization in patients treated with placebo (blue) or dapagliflozin (orange). Error bars in panel [A] reflect standard deviation and [B] Tukey box plots represent median and interquartile range with whiskers representing minimum and maximum values, with individual patient data superimposed.
Secondary Endpoints
Treatment with dapagliflozin was found to result in statistically significant reductions in right atrial and mean pulmonary artery pressure incorporating values at both rest and maximal exercise intensity (LRT p<0.001, Table 2). As compared with placebo, treatment with dapagliflozin resulted in a significant reduction in body weight (ETD −3.5 kg, 95% CI: −5.9 to −1.1; p=.006, Table 2, Figure 3). Although treatment with dapagliflozin significantly reduced plasma volume as compared with placebo (ETD −285 ml, 95% CI: −510 to −60; p=.015, Table 2), there was not a statistically significant effect on total blood volume or red blood cell volume.
Figure 3:
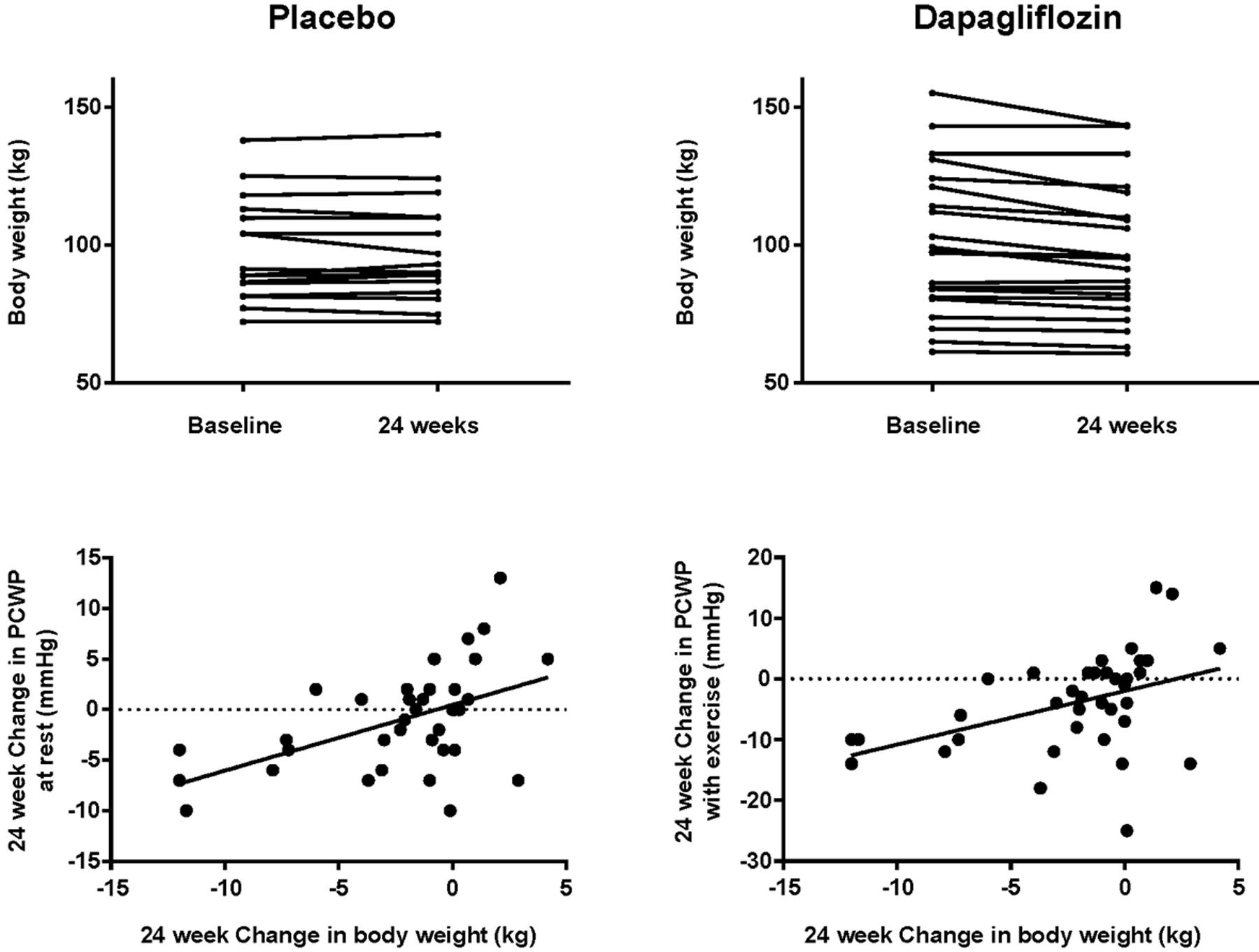
Changes in body weight from baseline to 24 weeks in patients treated with placebo and dapagliflozin (top panels). Bottom panels show correlations between changes in body weight and absolute reductions in pulmonary capillary wedge pressure (PCWP) at rest (r=.51, p=.001) and during exercise (r=.43, p=.009).
Exploratory and Mechanistic analyses
Treatment with dapagliflozin increased hematocrit and decreased NTproBNP levels compared with placebo but had no statistically significant effect on estimated glomerular filtration rate (Table 3) or systolic blood pressure (Table S1). Dapagliflozin had no effect on exercise capacity measured by peak VO2, peak workload, cardiac output, or arterial-venous oxygen content difference (Table 4). Dapagliflozin reduced arterial lactate during the matched 20W workload intensity (−0.70±0.77 vs +0.37±1.29 mM, p=.006) even as there were no differences in 20W VO2 (−18±231 vs −47±193 ml/min, p=.69; Figure S2). Arterial lactate was numerically lower at peak exercise following treatment with dapagliflozin but this was not significantly different than placebo (Table 4). Pulmonary capillary wedge pressure at a common workload of 20W was also reduced by dapagliflozin compared with placebo (−6.7±6.9 vs −0.5±7.3 mmHg, p=0.012) consistent with the beneficial effects observed at peak exercise.
Table 3:
Effects of Dapagliflozin on Laboratory Values
| n | Placebo | n | Dapagliflozin | p | |
|---|---|---|---|---|---|
| Baseline hematocrit (%) | 17 | 40.7 (3.5) | 21 | 39.1 (4.0) | 0.20 |
| Final hematocrit (%) | 16 | 41.2 (3.7) | 21 | 41.3 (4.7) | 0.95 |
| Change in hematocrit (%) | 16 | +0.3 (2.9) | 21 | +2.2 (2.5) | 0.046 |
| Baseline NTproBNP (pg/ml) | 15 | 118 (76, 226) | 21 | 235 (102, 394) | 0.18 |
| Final NTproBNP (pg/ml) | 16 | 153 (76, 282) | 21 | 163 (90, 444) | 0.94 |
| Change in NTproBNP (pg/ml) | 14 | +22 (1, 67) | 21 | −7 (−75, 7) | 0.037 |
| Baseline eGFR, mL/min/1.73 m2 | 17 | 73 (16) | 21 | 71 (17) | 0.73 |
| Final eGFR, mL/min/1.73 m2 | 16 | 68 (18) | 21 | 69 (18) | 0.83 |
| Change in eGFR, mL/min/1.73 m2 | 16 | −5 (10) | 21 | −2 (9) | 0.35 |
Values represent mean (standard deviation) or median (25th, 75th), and the corresponding p-values are from a two-sample t-test or Wilcoxon rank sum test, respectively.
eGFR – estimated glomerular filtration rate; NTproBNP – N Terminal pro Brain Natriuretic Peptide
Table 4:
Exercise Performance, Oxygen Transport and Metabolic Data
| N | Placebo | N | Dapagliflozin | p | |
|---|---|---|---|---|---|
| Baseline Assessment | |||||
| Resting cardiac output (l/min) | 16 | 5.6 (1.1) | 19 | 5.3 (1.9) | 0.65 |
| Peak cardiac output (l/min) | 17 | 10.3 (1.8) | 19 | 10.2 (3.1) | 0.92 |
| Peak O2 consumption (ml/kg/min) | 17 | 12.2 (3.1) | 20 | 11.8 (3.4) | 0.68 |
| Peak arterial venous O2 difference (ml/dl) | 17 | 11.5 (2.2) | 21 | 11.7 (3.0) | 0.76 |
| Peak workload (W) | 17 | 69 (24) | 21 | 82 (37) | 0.22 |
| Peak arterial lactate (mmol/l) | 16 | 5.3 (2.8) | 21 | 5.8 (3.0) | 0.60 |
| Final (24 week) Assessment | |||||
| Resting cardiac output (l/min) | 15 | 5.1 (1.3) | 20 | 4.8 (1.3) | 0.48 |
| Peak cardiac output (l/min) | 15 | 9.8 (2.4) | 21 | 9.7 (3.1) | 0.89 |
| Peak O2 consumption (ml/kg/min) | 16 | 11.3 (2.9) | 21 | 11.6 (4.0) | 0.79 |
| Peak arterial venous O2 difference (ml/dl) | 16 | 10.7 (1.7) | 21 | 11.3 (2.0) | 0.38 |
| Peak workload (W) | 16 | 65 (27) | 21 | 78 (42) | 0.28 |
| Peak arterial lactate (mmol/l) | 16 | 4.4 (2.5) | 20 | 4.6 (2.5) | 0.77 |
| Changes at Final visit compared with Baseline | |||||
| Resting cardiac output (l/min) | 15 | −0.3 (1.0) | 18 | −0.6 (1.2) | 0.52 |
| Peak cardiac output (l/min) | 15 | −0.4 (1.7) | 19 | −0.2 (1.7) | 0.75 |
| Peak O2 consumption (ml/kg/min) | 16 | −0.8 (2.4) | 20 | −0.1 (2.1) | 0.37 |
| Peak arterial venous O2 difference (ml/dl) | 16 | −0.6 (1.4) | 21 | −0.5 (1.9) | 0.77 |
| Peak workload (W) | 16 | −4 (21) | 21 | −4 (16) | 0.99 |
| Peak arterial lactate (mmol/l) | 15 | −0.6 (2.4) | 20 | −1.2 (2.1) | 0.42 |
Values reflect mean (standard deviation) and the corresponding p-values are from a two-sample t-test.
In correlation analyses, the change in PCWP at rest and with exercise was correlated with the change in body weight (r=0.51, p=.001 and r=0.43, p=0.009; Figure 3). Change in plasma volume was also correlated with change in body weight (r=0.51, p=0.001), but changes in rest and exercise PCWP were poorly correlated with the change in plasma volume (r=0.29, p=0.08 and 0.34, p=0.04).
Safety
One patient who was randomized to placebo withdrew from the study because of symptoms of medication intolerance (Table S2). A total of 4 serious adverse events in those treated with dapagliflozin and none in the group treated with placebo. These were all non-heart failure hospitalizations and none were judged to be related to treatment with study drug.
Discussion
In this study, treatment with the SGLT2 inhibitor dapagliflozin was shown to favorably reduce PCWP at rest and during exercise, improving the fundamental hemodynamic abnormality underlying this disorder. Treatment with dapagliflozin also decreased exertional right atrial pressures and pulmonary artery pressures, and led to reductions in body weight and plasma volume. Changes in body weight were correlated with changes in PCWP and to lesser extent, changes in plasma volume. Dapagliflozin had no effect on rest or exercise cardiac output, Ca-vO2, or peak VO2, but arterial lactate during 20W exercise was reduced compared with placebo. These findings provide new insight into the mechanisms underlying the favorable clinical effects of dapagliflozin in patients with HFpEF.
For years there was no clearly effective treatment for HFpEF20 until the recent publications of two large trials showing that the SGLT2i’s empagliflozin and dapagliflozin reduce the risk of hospitalization for HF or cardiovascular death by 18–21%.1, 2 Treatment with dapagliflozin has also been shown to improve submaximal exercise capacity as measured by 6 minute walk distance and patient reported health status in patients with HFpEF,21 and improvements in health status appear to be greater among patients with higher body mass.22 Potential hemodynamic mechanisms associated with these improvements have remained unclear.
Exercise intolerance in HFpEF is caused by complex interplay between the heart and extracardiac organs,23, 24 but an inability of the heart to pump blood during exercise without a pathologic rise in PCWP is common to all patients. Elevation in PCWP increases hydrostatic pressure in the lung capillaries to cause pulmonary congestion during exercise, while simultaneously increasing pulmonary artery pressures and the afterload seen by the right ventricle.10 Greater increases in PCWP with exercise in patients with HFpEF are associated with more severe symptoms of dyspnea, pulmonary reserve limitations, impairments in aerobic capacity, and increased risk for HF hospitalization and death, making this an important therapeutic target.11–16
Three prior studies have evaluated hemodynamic effects of SGLT2i in other patient populations. In patients with diabetes, treatment with empagliflozin for 13 weeks had no effect on the primary endpoint of PCWP at 25 W exercise, but there was a reduction in PCWP at rest.25 In patients with HF and reduced ejection fraction (HFrEF), treatment with empagliflozin did not reduce the ratio of PCWP to cardiac index at peak exercise (primary endpoint), nor did it affect PCWP at rest or exercise as compared with placebo.26 In another trial that enrolled patients with HF regardless of EF who also had preexisting implantable pulmonary artery pressure sensors,27 treatment with dapagliflozin was shown to result in a modest reduction in pulmonary artery diastolic pressure at rest (−1.7 mmHg). However, the sensor utilized in the prior study is only able to measure pressures in the pulmonary artery (rather than PCWP), and importantly, measurements could not be obtained during exercise. This is significant because approximately one-third of patients with HFpEF develop elevation in PCWP only during the stress of exertion, where symptoms are typically noted.16 The present data show improved ability of the heart to function as a pump that can perfuse the tissues at low filling pressures at rest and during the stress of exercise, with no reduction in cardiac output. The reduction in exercise PCWP (−5.7 mmHg) observed is clinically meaningful, as prior studies have shown that the risk of HF hospitalization or death increases by ~60% for increases in exercise PCWP of this magnitude.16
The magnitude of hemodynamic efficacy observed in patients with HFpEF exceeds the results reported in earlier studies that predominantly included patients with HFrEF. This is consistent with evidence that HFpEF is pathophysiologically more strongly tied to excess body fat than HFrEF.7, 28 The present findings relating body weight reduction to greater reduction in PCWP may provide mechanistic insight into recent findings from the DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) trial, which showed greater improvements in patient-reported health status and greater absolute weight reduction with dapagliflozin among patients with higher body mass.22
Weight reduction is sometimes used as a surrogate measure of decongestion in HF,29 but while the present data show correlations between weight loss and reduction in plasma volume, it is notable that the reduction in body weight (mean −3.5 kg) far exceeds the weight loss expected based upon plasma loss (−285 ml), though certainly reduction in interstitial fluid may also have contributed. Only one prior study has directly evaluated changes in plasma volume with SGLT2i in HF using the I-131 albumin indicator dilution technique, which also showed reductions over 2 weeks,30 consistent with the present study evaluating changes over 24 weeks of treatment. Plasma volume reduction with SGLT2 inhibitors has been hypothesized to occur as a compensatory adaptation to accommodate an increase in red cell mass.5 Here, using direct measures, it is shown that that plasma volume was reduced with dapagliflozin, with no increase red cell mass, arguing against this possibility. The lack of increase in red cell mass in the present study was unexpected given the known erythropoeitc effects of SGLT2i, and may relate to undiagnosed iron deficiency among participants. Notably, the reduction in PCWP observed here was only weakly correlated with the change in plasma volume, indicating that volume contraction alone is not a sufficient explanation for the hemodynamic improvements observed with dapagliflozin.
Dapagliflozin had no effect on measures of O2 transport including cardiac output, Ca-vO2, or VO2 at rest or exercise as compared to placebo. This may reflect a lack of effect on these determinants of exercise tolerance, but may also relate to type II error, as this study was not powered on these endpoints. Indeed, the point estimates for the mean change in peak VO2 was numerically greater for dapagliflozin (−0.1 vs −0.8 ml/kg/min, p=0.37). It may also be that exercise tolerance is improved to greater extent during lower workload, which is consistent with the previously demonstrated improvement in 6 minute hall walk distance with dapagliflozin.21 Reduction in cardiac filling pressures may be more relevant to these submaximal workloads as compared to the point of volitional exhaustion. The significant reduction in arterial lactate during 20W exercise with dapagliflozin is also interesting in this regard, even as VO2 and cardiac output were unchanged, perhaps suggesting other favorable metabolic effects.
Limitations
The study was single center, owing to the highly specialized nature of invasive exercise testing. While this may reduce generalizability, this is not a significant limitation because clinical efficacy for SGLT2 inhibitors has already been demonstrated in large-scale multicenter trials. The goal of this study was to evaluate hemodynamic effects, both at rest, and during exercise, where abnormalities become most apparent in HFpEF, and the greater precision and accuracy of hemodynamic assessments using high fidelity micromanometer catheters reduces variability and enhances scientific rigor. By designing the protocol to evaluate hemodynamic effects at rest and during exercise, the operationalization of the primary endpoint increased in complexity. The analysis approach used a simultaneous test (likelihood ratio test) to test for any differences in the changes of PCWP over the study duration. This overall test was supplemented with separate tests at rest and at peak exercise intensity, which could increase the overall study type I error rate due to multiple testing. Using a multiple testing strategy that accounts for the correlation present in the changes in PCWP at rest and at peak exericse,31 the correlation adjusted level of significance would be 0.037 (0.05/g* where g*=(g+1)–(1+(g-1) ICC), for g=2, ICC=0.6583066, which was the intraclass correlation (ICC) for the change in PCWP at rest and peak exercise). The estimated treatment effects at rest and peak exercise met statistical significance at this level of significance.
Additional limitations included that the cohort studied is relatively small, but sample size was selected to provide adequate power to test the study hypothesis while balancing cost, risk, and participant burden, which was significant, including two invasive procedures. This design decision, while necessary from a human subjects perspective, meant that some key mechanistic hypotheses and potential outcome measures such as peak oxygen consumption would not be adequately powered.
Conclusion
Among patients with HFpEF, treatment with dapagliflozin reduces both resting and exercise pulmonary capillary wedge pressures, along with favorable effects on pulmonary artery and right heart filling pressures, plasma volume, and body weight. These findings provide new insight into the hemodynamic mechanisms of benefit with SGLT2 inhibitors in HFpEF.
Supplementary Material
Clinical Perspectives.
What is New?
In this randomized clinical trial, treatment with the sodium glucose cotransporter-2 (SGLT2) inhibitor dapagliflozin reduced pulmonary capillary wedge pressure at rest and during exercise compared with placebo in patients with heart failure and preserved ejection fraction (HFpEF).
Dapagliflozin also reduced exertional pulmonary artery and right atrial pressure, as well as body weight and plasma volume compared with placebo.
What are the Clinical Implications?
These data provide new insight into the mechanisms of benefit for SGLT2 inhibitors in HFpEF, showing favorable hemodynamic effects at rest and during exercise.
Acknowledgements
This investigator-initated trial was conducted with support from AstraZeneca Pharmaceuticals LP. AstraZeneca was not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication. Dr. Borlaug is also supported in part by research grants from the NIH (R01 HL128526, R01 HL162828, and U01 HL160226) and the United States Department of Defense (W81XWH2210245).
Disclosures
Dr. Borlaug receives research support from the National Institutes of Health (NIH) and the United States Department of Defense, as well as research grant funding from AstraZeneca, Axon, GlaxoSmithKline, Medtronic, Mesoblast, Novo Nordisk, and Tenax Therapeutics. Dr. Borlaug has served as a consultant for Actelion, Amgen, Aria, Axon Therapies, BD, Boehringer Ingelheim, Cytokinetics, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, NGM, NXT, and VADovations, and is named inventor (US Patent no. 10,307,179) for the tools and approach for a minimally invasive pericardial modification procedure to treat heart failure.
Non-standard Abbreviations and Acronyms
- Ca-vO2
arterial-venous oxygen content difference
- ETD
estimated treatment difference
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- LRT
likelihood ratio test
- PCWP
pulmonary capillary wedge pressure
- SGLT2i
sodium glucose cotransporter-2 inhibitor
Footnotes
Trial Registration: clinicaltrials.gov Identifier NCT04730947
References
- 1.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Pina IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M and Investigators EM-PT. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021. [Google Scholar]
- 2.Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, Jhund PS, Belohlavek J, Chiang CE, Borleffs CJW, Comin-Colet J, Dobreanu D, Drozdz J, Fang JC, Alcocer-Gamba MA, Al Habeeb W, Han Y, Cabrera Honorio JW, Janssens SP, Katova T, Kitakaze M, Merkely B, O’Meara E, Saraiva JFK, Tereshchenko SN, Thierer J, Vaduganathan M, Vardeny O, Verma S, Pham VN, Wilderang U, Zaozerska N, Bachus E, Lindholm D, Petersson M, Langkilde AM, Committees DT and Investigators. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med. 2022. [Google Scholar]
- 3.Cowie MR and Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. 2020;17:761–772. [DOI] [PubMed] [Google Scholar]
- 4.Lopaschuk GD and Verma S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl Sci. 2020;5:632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Packer M Critical Reanalysis of the Mechanisms Underlying the Cardiorenal Benefits of SGLT2 Inhibitors and Reaffirmation of the Nutrient Deprivation Signaling/Autophagy Hypothesis. Circulation. 2022;146:1383–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zannad F, Ferreira JP, Butler J, Filippatos G, Januzzi JL, Sumin M, Zwick M, Saadati M, Pocock SJ, Sattar N, Anker SD and Packer M. Effect of empagliflozin on circulating proteomics in heart failure: mechanistic insights into the EMPEROR programme. Eur Heart J. 2022;43:4991–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borlaug BA, Jensen MD, Kitzman DW, Lam CSP, Obokata M and Rider OJ. Obesity and heart failure with preserved ejection fraction: new insights and pathophysiological targets. Cardiovasc Res. 2023;118:3434–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske-Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P and Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40:3297–3317. [DOI] [PubMed] [Google Scholar]
- 9.Hsu S, Fang JC and Borlaug BA. Hemodynamics for the Heart Failure Clinician: A State-of-the-Art Review. J Card Fail. 2022;28:133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verbrugge FH, Guazzi M, Testani JM and Borlaug BA. Altered Hemodynamics and End-Organ Damage in Heart Failure: Impact on the Lung and Kidney. Circulation. 2020;142:998–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obokata M, Olson TP, Reddy YNV, Melenovsky V, Kane GC and Borlaug BA. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J. 2018;39:2810–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy YNV, Obokata M, Wiley B, Koepp KE, Jorgenson CC, Egbe A, Melenovsky V, Carter RE and Borlaug BA. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J. 2019;40 3721–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddy YNV, Olson TP, Obokata M, Melenovsky V and Borlaug BA. Hemodynamic Correlates and Diagnostic Role of Cardiopulmonary Exercise Testing in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2018;6:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorfs S, Zeh W, Hochholzer W, Jander N, Kienzle RP, Pieske B and Neumann FJ. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3103–12. [DOI] [PubMed] [Google Scholar]
- 15.Eisman AS, Shah RV, Dhakal BP, Pappagianopoulos PP, Wooster L, Bailey C, Cunningham TF, Hardin KM, Baggish AL, Ho JE, Malhotra R and Lewis GD. Pulmonary Capillary Wedge Pressure Patterns During Exercise Predict Exercise Capacity and Incident Heart Failure. Circ Heart Fail. 2018;11:e004750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omote K, Verbrugge FH, Sorimachi H, Omar M, Popovic D, Obokata M, Reddy YNV and Borlaug BA. Central haemodynamic abnormalities and outcome in patients with unexplained dyspnoea. Eur J Heart Fail. 2023;25:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borlaug BA, Melenovsky V and Koepp KE. Inhaled Sodium Nitrite Improves Rest and Exercise Hemodynamics in Heart Failure With Preserved Ejection Fraction. Circ Res. 2016;119:880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borlaug BA, Koepp KE and Melenovsky V. Sodium Nitrite Improves Exercise Hemodynamics and Ventricular Performance in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2015;66:1672–82. [DOI] [PubMed] [Google Scholar]
- 19.Miller WL, Sorimachi H, Grill DE, Fischer K and Borlaug BA. Contributions of Cardiac Dysfunction and Volume Status to Central Hemodynamics in Chronic Heart Failure. Eur J Heart Fail. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfeffer MA, Shah AM and Borlaug BA. Heart Failure With Preserved Ejection Fraction In Perspective. Circ Res. 2019;124:1598–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, Khariton Y, Malik AO, Khumri T, Umpierrez G, Lamba S, Sharma K, Khan SS, Chandra L, Gordon RA, Ryan JJ, Chaudhry SP, Joseph SM, Chow CH, Kanwar MK, Pursley M, Siraj ES, Lewis GD, Clemson BS, Fong M and Kosiborod MN. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adamson C, Kondo T, Jhund PS, de Boer RA, Cabrera Honorio JW, Claggett B, Desai AS, Alcocer Gamba MA, Al Habeeb W, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Langkilde AM, Lindholm D, Bachus E, Litwin SE, Martinez F, Petersson M, Shah SJ, Vaduganathan M, Nguyen Vinh P, Wilderang U, Solomon SD and McMurray JJV. Dapagliflozin for heart failure according to body mass index: the DELIVER trial. Eur Heart J. 2022;43:4406–4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507–15. [DOI] [PubMed] [Google Scholar]
- 24.Pandey A, Shah SJ, Butler J, Kellogg DL Jr., Lewis GD, Forman DE, Mentz RJ, Borlaug BA, Simon MA, Chirinos JA, Fielding RA, Volpi E, Molina AJA, Haykowsky MJ, Sam F, Goodpaster BH, Bertoni AG, Justice JN, White JP, Ding J, Hummel SL, LeBrasseur NK, Taffet GE, Pipinos II and Kitzman D Exercise Intolerance in Older Adults With Heart Failure With Preserved Ejection Fraction: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;78:1166–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolsk E, Jurgens M, Schou M, Ersboll M, Hasbak P, Kjaer A, Zerahn B, Brandt NH, Gaede PH, Rossing P, Faber J, Inzucchi SE, Kistorp CM and Gustafsson F. Randomized Controlled Trial of the Hemodynamic Effects of Empagliflozin in Patients With Type 2 Diabetes at High Cardiovascular Risk: The SIMPLE Trial. Diabetes. 2022;71:812–820. [DOI] [PubMed] [Google Scholar]
- 26.Omar M, Jensen J, Frederiksen PH, Kistorp C, Videbaek L, Poulsen MK, Moller S, Ali M, Gustafsson F, Kober L, Borlaug BA, Schou M and Moller JE. Effect of Empagliflozin on Hemodynamics in Patients With Heart Failure and Reduced Ejection Fraction. J Am Coll Cardiol. 2020;76:2740–2751. [DOI] [PubMed] [Google Scholar]
- 27.Nassif ME, Qintar M, Windsor SL, Jermyn R, Shavelle DM, Tang F, Lamba S, Bhatt K, Brush J, Civitello A, Gordon R, Jonsson O, Lampert B, Pelzel J and Kosiborod MN. Empagliflozin Effects on Pulmonary Artery Pressure in Patients With Heart Failure: Results From the EMBRACE-HF Trial. Circulation. 2021;143:1673–1686. [DOI] [PubMed] [Google Scholar]
- 28.Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, Allen NB, de Lemos JA, Carnethon M, Greenland P and Berry JD. Relationship Between Physical Activity, Body Mass Index, and Risk of Heart Failure. J Am Coll Cardiol. 2017;69:1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biegus J, Voors AA, Collins SP, Kosiborod MN, Teerlink JR, Angermann CE, Tromp J, Ferreira JP, Nassif ME, Psotka MA, Brueckmann M, Salsali A, Blatchford JP and Ponikowski P. Impact of empagliflozin on decongestion in acute heart failure: the EMPULSE trial. Eur Heart J. 2023;44:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffin M, Rao VS, Ivey-Miranda J, Fleming J, Mahoney D, Maulion C, Suda N, Siwakoti K, Ahmad T, Jacoby D, Riello R, Bellumkonda L, Cox Z, Collins S, Jeon S, Turner JM, Wilson FP, Butler J, Inzucchi SE and Testani JM. Empagliflozin in Heart Failure: Diuretic and Cardio-Renal Effects. Circulation. 2020;142:1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi Q, Pavey ES and Carter RE. Bonferroni-based correction factor for multiple, correlated endpoints. Pharm Stat. 2012;11:300–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


