Abstract
Hyperglycemia and oxidative stress, enhanced in diabetes and aging, result in excessive accumulation of advanced glycation and glycoxidation end products (AGEs/AGOEs) in bone. AGEs/AGOES are considered to be “the missing link” in explaining increased skeletal fragility with diabetes, aging, and osteoporosis where increased fracture risk cannot be solely explained by bone mass and/or fall incidences. AGEs/AGOEs disrupt bone turnover and deteriorate bone quality through alterations of organic matrix (collagen and non-collagenous proteins), mineral, and water content. AGEs and AGOEs are also associated with bone fragility in other conditions such as Alzheimer’s disease, circadian rhythm disruption, and cancer. This review explains how AGEs and AGOEs accumulate in bone and impact bone quality and bone fracture, and how AGES/AGOEs are being targeted in preclinical and clinical investigations for inhibition or removal, and for prediction and management of diabetic, osteoporotic and insufficiency fractures.
Keywords: Non-enzymatic Glycation, Advanced glycation end-products, Glycoxidation, Bone quality, Fracture, Diabetes
Introduction
Increased propensity of fragility fractures is linked to conditions beyond osteoporosis, such as aging [1,2] and diabetes [3–5]. For example, both type 1 diabetes (T1D) and type 2 diabetes (T2D) increase fracture risk that cannot be solely explained by changes in bone mineral density (BMD) and fall incidences [3,6,7]. Insufficiency fractures, on the other hand, are caused by habitual daily loading in the elderly or in patients with bone fragility [8,9], and are also associated with osteoporosis, osteogenesis imperfecta, and diabetes [10,11]. To understand bone fragility in these conditions, changes in bone matrix quality need to be investigated.
For example, non-enzymatic glycation (NEG) (its classical pathway is also known as the Maillard reaction), a post-translational modification of proteins by sugars, is associated with diabetes and linked to an increase in bone fracture risk [12,13]. It is known that enhanced hyperglycemia in diabetes induces excessive accumulation of advanced glycation end products (AGEs) – the products of NEG – in bone [14,15], including but not limited to pentosidine (PEN) and carboxymethyl-lysine (CML). The formation CML and PEN is accelerated by increased oxidative stress due to overproduction of reactive oxygen species (ROS). Therefore, CML and PEN are also referred to as advanced glycoxidation end products (AGOEs) [16]. However, not all AGEs are AGOEs, and the proportion of AGEs and AGOEs may vary with the extent of hyperglycemia and oxidative stress. Various measures of NEG are reviewed in the next section.
Here, with a focus on diabetes and bone, we sought to review the role of AGEs/AGOEs in bone metabolism, bone matrix quality (such as alterations in organic matrix, mineral, and water content), and relate such changes to bone biomechanical properties. We also provide a clinical perspective on the use of circulating measurements of AGEs/AGOEs in diabetic population for predicting and managing bone fracture risk. This is a promising avenue to translate the work on AGEs/AGOEs in bone from bench to bedside. Furthermore, the review highlights the relationship between AGEs/AGOEs and bone fragility beyond diabetes. We discuss the role AGEs/AGOEs in skeletal fragility associated with aging, osteoporosis, and other conditions such as Alzheimer’s disease, circadian rhythm disruption, cancer, and osteogenesis imperfecta.
Formation and Identification of AGEs/AGOEs in Bone Matrix
In bone matrix, AGEs/AGOEs can be formed via different pathways. Maillard reaction, or the classical pathway starts with the process where an aldehyde form of a given sugar such as glucose reacts with ε-amino group of lysine or hydroxylysine to form a Schiff base. The rearrangement of the Schiff base generates diverse Amadori products, which goes through further rearrangements, cleavage and covalent binding to form irreversible and stable adducts or crosslinks as known as AGEs [17]. In addition to the Maillard reaction, oxidative stress plays an important role in the formation of diverse sugar degradation products such as glyoxal (precursor for CML, glyoxal-derived lysyl dimer (GOLD), etc.), 3-deoxyglucosone (precursor for CML, PEN, pyrraline, etc.), and methylglyoxal (precursor for carboxyethyl-lysine (CEL), methylglyoxal-derived hydroimidazolone (MG-H1), etc.) [18]. In these alternate glycoxidative pathways, these reactive carbonyl groups react with lysine or arginine to form AGEs/AGOEs [19–21].
AGEs/AGOEs are used as an umbrella term for a large group of complex chemical compounds (estimated at ~750 types). The identities and chemical structures of many AGEs/AGOEs are still not known. Collectively in skeletal tissue, a subgroup of nonspecific fluorescent AGEs/AGOEs (including PEN, vesperlysines, crosslines, etc.) can be assessed by their total fluorescence content, quantified at 360/460 nm excitation/emission and compared against the quinine standard and normalized to the collagen content [22]. Individually, PEN and CML are the two representative AGEs/AGOEs that can be directly measured in bone tissue.
PEN is a fluorescent crosslinking AGOE that forms between lysine and arginine residues [23]. It is the first specific AGOE that was linked to bone fragility [12], and has been widely used as a direct measurement of AGEs/AGOEs in bone. Higher level of PEN in bone matrix has been noted with increased age [24,25], hip fractures [26,27], T1D [14], and T2D [15,28]. In clinical studies, PEN measured in the circulatory system has been linked to increased fracture risk in osteoporosis [29,30], T2D [12,31] and chronic liver disease [32].
In contrast to PEN, CML is a non-fluorescent and non-crosslinking side-chain modification on the lysine residuals that has been recently identified, isolated and measured in bone matrix [21]. CML is 40–100 times more abundant than PEN in bone tissue [21]. As the most commonly used glycoxidative biomarker in long-lived proteins [33] that captures both the aforementioned classical and alternate pathways to achieve glycoxidation [34–36], CML can be considered to be a potential alternative glycoxidative marker and a dominant AGOEs in diabetic bone. Indeed CML accumulation is higher in T2D bone than in control [37], and greater levels of CML, measured in serum, are linked to T2D fracture risk [13].
Other AGEs/AGOEs, such as glucosepane [38], MG-H1 [20], and CEL [20], have been recently quantified in bone, but their contributions to bone fragility are not yet established. This is an area of active and future investigation. The techniques for identification and measurement of AGEs/AGOEs in bone including analytical biochemistry (chromatography [39], mass spectroscopy [20], ELISA [21]) and vibrational spectroscopy (Raman [38,40] and FTIR spectroscopy [41,42]) have been reviewed by Sroga et al. [16].
The physiological and pathophysiological range of AGEs/AGOEs is not fully established. AGEs/AGOEs accumulation is significantly higher in trabecular bone than in cortical bone due to its higher surface-to-volume ratio that allows more access for NEG to occur through bone surface [43]. Consequently, we recommend that, where possible, AGEs/AGOEs should be measured separately in cortical and trabecular bone. Gender differences of AGEs/AGOEs accumulation in bone have not been widely explored, with one aging study reporting no significant gender-specific influence [44]. Again, this is a promising avenue for future investigation as AGEs/AGOEs impact bone turnover and postmenopausal osteoporosis involves significant changes in bone turnover that can be impacted by accumulated AGEs/AGOEs (see next section for further details).
For mechanistic studies, the formation of AGEs/AGOEs in bone can be induced via in vitro glycation. In vitro models of NEG in bone allow to isolate and investigate contributions of AGEs/AGOEs on bone material with a short incubation period that enhances the AGEs/AGOEs to pathophysiological levels, shown in Figure 1. Ribose incubation supports the pathway to the formation of PEN, and has shown to be more effective in increasing the level of PEN and total fluorescent AGEs (fAGEs) compared to glucose incubation [23]. In contrast, glyoxal incubation supports enhanced formation of CML over other AGEs/AGOEs [45]. As the formation of various AGEs/AGOEs share the similar and spontaneous non-enzymatic pathways, dominant formation of a specific AGEs/AGOEs requires careful selection and optimization of incubation time, incubation conditions, type and amount of bone [23,45,46], such that AGEs/AGOEs form within the pathophysiological range associated with aging and diabetes (Figure 1).
Figure 1.
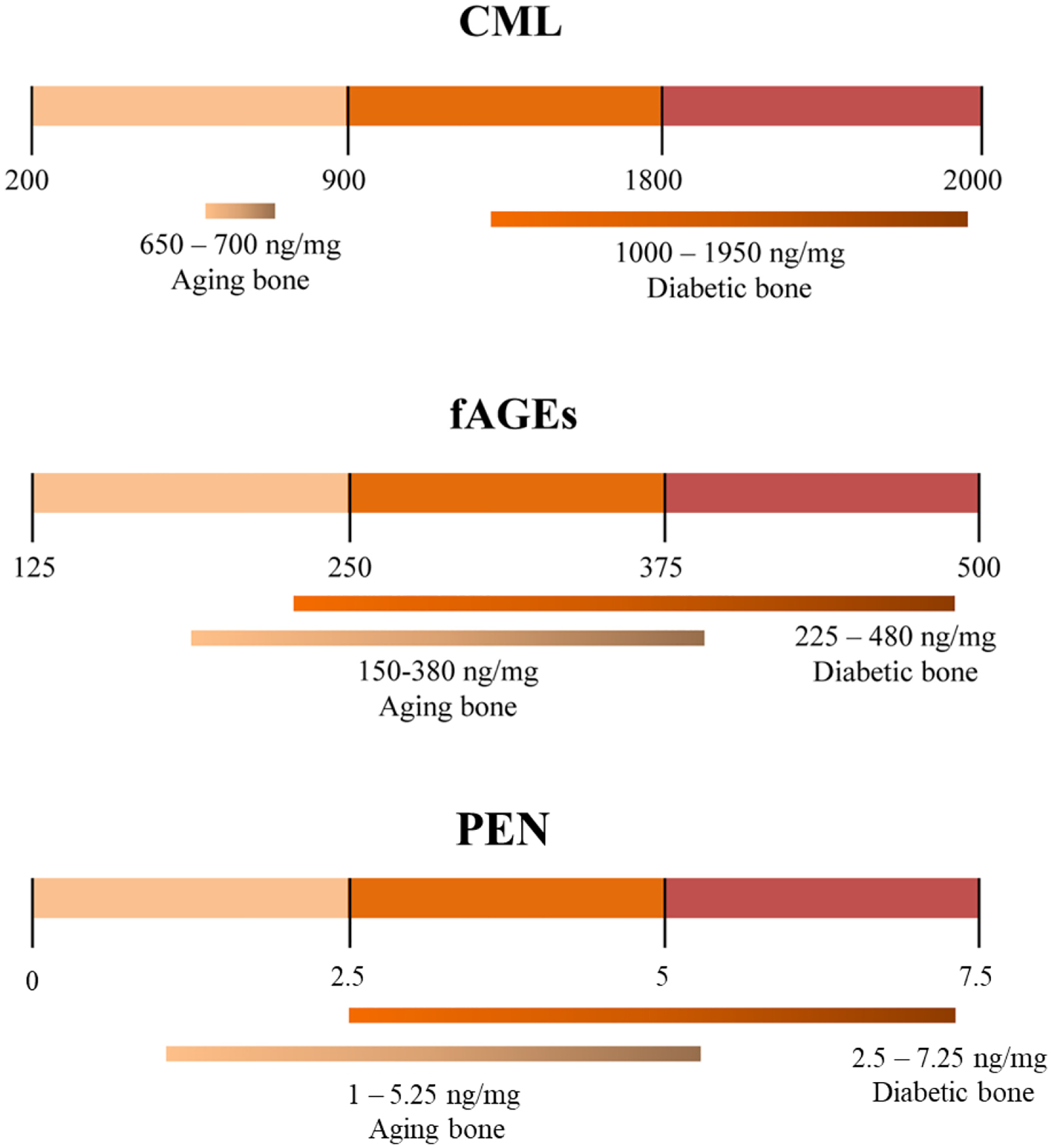
Pathophysiological and age-related levels of CML [21,37,47], fAGEs [48,49], and PEN [21,28,43]. Figure adapted from Sroga and Vashishth [45].
AGEs/AGOEs impact on Bone Metabolism and Bone Quality
Bone Metabolism
AGEs/AGOEs have been known to attenuate bone metabolism by suppressing bone formation and resorption. With the increase of the receptor of AGEs (RAGE) expression, in vitro studies showed the AGE-RAGE axis with its inflammation-enhancing activities is the major factor resulting in decreased bone formation by reducing osteoblastic differentiation and proliferation, downregulating matrix production and increasing osteoblast apoptosis [50–52].
In context of bone resorption, in vitro studies show that with augmented RAGE signaling, AGEs decrease the RANKL expression and osteoclastic differentiation [53,54]. Furthermore, in vitro cell study demonstrates that the dramatic decrease in RANK expression and the corresponding decrease in osteoclast-like cells resorption ability occurred with increased AGEs concentration at the early cell fusion stage; whereas in the mature stage, there was no effect of AGEs in RANK expression and a slight increase of resorption area was found in one of the AGE concentrations [55]. Since RANK signaling negatively impacts the osteoclast maturity and osteoblast-osteoclast crosstalk [56,57], increased AGEs would decrease bone resorption. In support of the above statement, in vitro bone resorption experiments report decreased osteoclastic bone resorption of devitalized bone tissue with higher AGEs content, even in the absence of osteocytes and cell signaling pathways [54,58].
However, when instead of bone slices, in vitro glycated bone particles were subjected to in vitro resorption in mouse bone cell cultures or in vivo resorption via subcutaneously in rats glycated bone was more readily resorbed [59]. Similarly, when human bone with naturally occurring levels of AGEs was subjected to in vitro resorption using mouse osteoclasts [60], a trend towards increase in bone resorption area and resorption pit density was observed in elderly but not in young donors. Differences between the studies could result from differences in use of bone slices vs particles and differences in levels on AGEs. Also, in vitro studies often fail to control variations in other bone matrix constituents including but not limited to non-collagenous proteins such as osteopontin that vary with tissue age [61] and control osteoclast attachment and detachment to bone matrix impacting the amount of bone resorption [62]. Thus, in our view spatially matched bone slices where AGEs have been selectively enhanced within the pathophysiological range (See Figure 1) serve as better substrate/model to investigate the impact of AGEs on bone resorption in vitro.
Given the mixed evidence from the in vitro studies, analysis of ex vivo human cortical bone containing natural levels of AGEs and resorption pits at time of donor death can be instructive. Such an analysis shows that increase in in vivo levels of AGEs is indeed associated with decrease of average in vivo resorption pit area as well as percent resorption area (indicating reduced bone resorption), independently of age [63]. This decrease in the resorption ability could be mechanistically explained by the low solubility and digestibility of AGEs/AGOEs by enzymes such as collagenase, as demonstrated for other tissues [64,65]. Similarly, other studies using in vivo animal models treated with bisphosphonates established negative correlation between PEN and activation frequency [66], as well as between total fluorescent AGEs and the number of labeled osteons [67] (both activation frequency and labeled osteon number are measures of bone turnover).
Decrease in bone formation with altered resorption could lead to altered remodeling and potentially explain low turnover as seen in T2D bone [68]. Such changes results in poor bone quality, accumulation of microcracks [69] and further increase in AGEs [66,67]. Investigating the impact of enhanced AGEs on bone quality in diabetes as well as in postmenopausal and disuse osteoporosis, where bone remodeling processes are significantly altered, is a promising area for future investigations.
Organic Matrix Quality
Among the post-translational modifications in bone collagen, enzymatic crosslinks, such as pyridinoline and deoxypyridinoline, stabilize bone collagen arrangement and improve bone mechanical properties [70]. Conversely, accumulation of AGEs/AGOEs impairs bone toughening mechanisms. The accumulation of crosslinking AGEs/AGOEs on collagen increases the stiffness of collagen matrix [22], reduces the post-yield deformation [71,72], decreases and alters morphology of microdamage [73,74], and leads to loss of energy dissipation [71,72,74] and post-yield toughness [25,72] in bone. At the level of collagen, in vitro glycation reduces interfibrillar energy dissipation. At the level of bone tissue, in vitro NEG of bone alters bone’s toughening mechanism by reducing density of microcracks, and increasing the length of crack segment and uncracked ligaments, making it easy to propagate a crack at a low energy threshold and cause catastrophic failure under impact at 3000–4000 μstrain [74]. These results highlight the role AGEs/AGOEs in potentially causing insufficiency fragility fractures during physiological activities [8,9,75]. Similar to in vitro results, a T2D rat model, where total fluorescent AGEs were 27% higher than control, showed a 40% reduction in ultimate collagen fibril strain indicating weakened ability for collagen to deform and dissipate energy at nanoscale [76].
In contrast to PEN and total fluorescent AGEs, that crosslink bone collagen, the role of CML, a dominant AGOE, in causing bone fragility has not been explored. It has been recently proposed that CML could alter the charge distribution of collagen [45], leading to a disruption of molecular organizations within the organic matrix, and thereby impair energy dissipation mechanisms (Figure 2). However, this mechanism needs to be tested experimentally in future studies.
Figure 2.
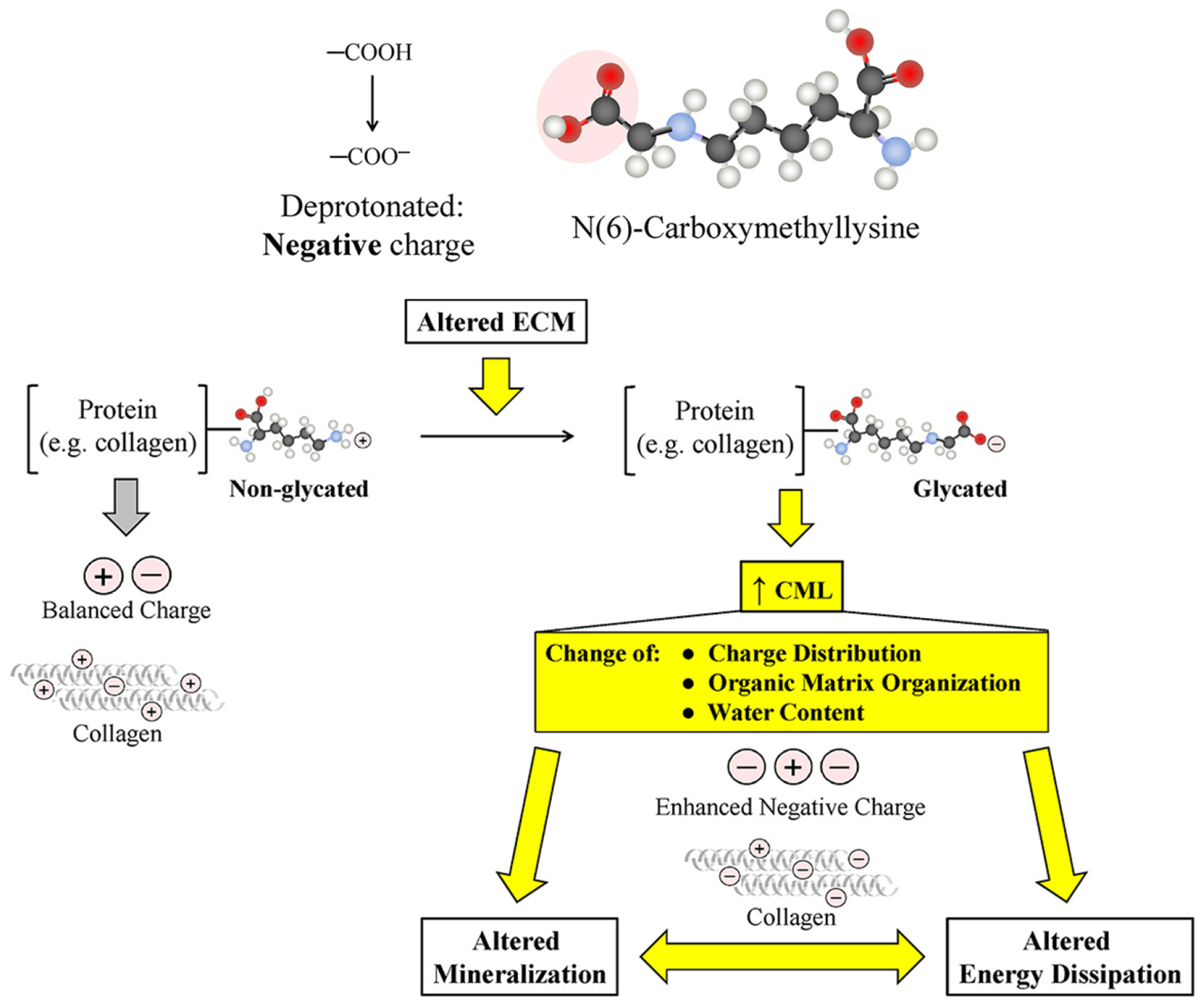
Proposed mechanisms on CML impacting organic matrix and mineral quality. Figure adapted from Sroga and Vashishth [45]. ECM = extracellular matrix.
Other than collagen, glycation also impacts non-collagenous proteins, specifically osteocalcin (OC) – a structural non-collagenous protein [77,78]. Using multiple mass spectroscopy fragmentation techniques our group showed that, during NEG, Amadori products are formed solely on osteocalcin’s N-terminus [79]. Thus, glycation is likely to interfere with OC’s interaction with collagen and osteopontin and consequently alter nanoscale energy dissipation mechanism in bone, including dilatational bands and diffuse damage that are generated at this scale [77]. Furthermore, a recent ultra-performance liquid chromatography (UPLC) study showed the evidence of in vivo modification of OC by PEN, by demonstrating the presence of PEN in the wild-type C57BL/6 mouse tibiae in the chromatogram, while PEN was not detected in mouse tibiae when OC was genetically knocked out. Moreover, in vitro glycation, caused a greater increase in total fluorescent AGEs in wild-type bone tissue than in OC deficient bone tissue, and consequently caused a greater decrease of fracture toughness by glycation in wild type bone tissue than in OC deficient mice bones. These results establish that AGEs/AGOEs could also modify the structural non-collagenous proteins and impair bone material properties. Moreover, it is suggested that loss of bone toughness during NEG is partially attributable to the negative impact of glycated OC on energy dissipation [80].
Mineral Quality
The mechanisms by which AGEs/AGOEs alter mineralization and bone mineral quality are less understood. And there have been inconsistencies in the observations of mineral quality changes that occur in concert with in vivo AGEs/AGOEs accumulation. For example, a bone biopsy study of T1D patients with fracture history found that the degree of bone mineralization (quantification of bone mineral substance in bone tissue alone that is independent of BMD [81]) was positively correlated to PEN content in trabecular bone, both of which were higher than control [14]. Similarly, for T2D, a non-obese animal model, which eliminates the confounding effect of obesity, demonstrated significantly higher crystallinity and crystal thickness in femora, where the crystallinity was positively correlated with CML and PEN [82]. A study on discarded femoral heads originating from patients who went through arthroplasty due to fragility hip fracture, found a near significant decrease in crystallinity (p = 0.07), however, with increased crystal width in T2D group compared to controls. Intriguingly, the mineral-to-matrix ratio was lower in T2D group compared to controls in this study despite an expected suppression of bone turnover in T2D (not measured). In this study, both control and T2D groups displayed low BMD at around the osteoporotic threshold. This unexpected outcome could be potentially explained by increased AGEs in bone, as the mineral-to-matrix ratio was negatively correlated to total fluorescent AGEs only in the T2D group [42]. However, another study using femora from cadavers showed significant increase of CML as well as higher periosteal mineral-to-matrix ratio in T2D than controls. This study also reported that in a T2D subgroup with high level of cortical porosity, bone tissue had low crystallinity where mineral appeared as non-apatite and poorly crystalized [37].
The above studies report alterations in tissue-level mineral quality as well as the presence and absence of the associations between AGEs/AGOEs and various measures of mineralization. However, in order to understand the impact of AGES/AGOEs on bone mineral quality, the mechanisms by which AGEs/AGOEs impact mineralization and affect mineral quantity need to be investigated and established. We proposed that enhanced CML may be involved in the mineralization process and may therefore play a role in determining bone mineral quality [45]. The negatively charged carboxyl group in CML tends to attract the positively charged calcium ions which may lead to enhanced mineralization albeit in an immature and imperfect non-apatite form. Therefore, acting as a ‘molecular link’, CML could bridge collagen and hydroxyapatite, and also alter energy dissipation mechanism through its involvement in the mineralization process [45] (Figure 2). Consistent with this hypothesis we find that in a preclinical mouse model of non-obese T2D, increased CML formation is indeed positively correlated with higher crystallinity (associated with elongated mineral crystal length [83]), supporting the role of this negatively charged side chain to attract calcium ions, promote growth of hydroxyapatite, and build a physical link between mineral and collagen [82]. Overall, direct literature evidence on the effect of AGEs/AGOEs on mineral growth and mineral quality is relatively scant.
Bound Water Content
In aging and diabetes, loss of bound water and NEG have been suggested as concurrent processes in collagenous tissues [84]. As a surrogate measure for bone quality and an important contributor to fracture resistance of bone tissue [85–87], the relationship between AGEs/AGOEs accumulation in bone and bound water content has been a subject of investigation. One in vitro glycation study showed ribose treatment significantly reduced the bound water content in bone which might coincide with the changes in secondary structure of collagen and perturb the hydrogen bounding ability [88]. Similarly, an in vivo model of aging showed that, compared to young group, cortical bone in the aged group consistently showed a significant increase in PEN with a decrease in bound water [89,90]. Subsequent work by the same group suggests that the mechanistic basis of bound water loss, in the above model, is likely another post-translational modification such as collagen deamidation, as BALB/c mice did not show age-related changes in non-crosslinking AGEs. However, studies with diabetic mouse models, such as streptozotocin induced T1D, do show loss of bound water [91] and abnormal collagen crosslinking [92]. In theory, CML accumulation in bone could enhance the hydrophilicity due to the negative charge from its carbonyl group [45], however, the net impact to attract or repel water will depend on all AGEs types. Future studies should evaluate the alteration in bound water content with selective enhancement of various AGEs/AGOEs including but not limited to CML.
Impact of AGEs/AGOEs on Mechanical Properties and Use of Agents to Inhibit or Cleave AGEs and Rescue Bone Fragility.
The direct impact of AGEs/AGOEs on mechanical properties of bone related to fracture is difficult to evaluate, because under the conditions of aging, osteoporosis, diabetes, and other conditions, several other bone quality parameters are altered. To this end, in vitro protocols to induce formation of AGEs/AGOEs have been developed to study their direct impact on bone. For example, one of the first studies, developed a dose-response curve wherein machined bone bovine bone specimens were exposed to ribosylation to dial in specific AGEs/AGOEs based on incubation time [22]. Subsequent applications of this approach focused on 7 days of incubation period that doubles the AGEs/AGOEs in human cortical and cancellous bone and mimicked the accumulation of AGEs/AGOEs within the pathophysiological shown in Figure 1 [71,72,74,93,94]. Because bone is known to accumulate cracks and not fracture [95], these studies have focused on measurement of post-yield properties rather than energy required to initiate a crack. Results show that enhanced formation of total fluorescent AGEs and/or PEN negatively impact biomechanical properties of bone including reduced propagation fracture toughness (the slope of R curve) [74], post-yield strain [93], damage fraction [71,93], etc. Increased levels of AGEs/AGOEs predict various measures of bone mechanical properties associated with human bone fragility [74,94]. Furthermore, mechanisms of reduced energy dissipation at multiple length scales, cited earlier in this review, including increased stiffness of collagen matrix, altered microdamage morphology [transition from self-limiting and efficient form of microcracking (diffuse damage) [75,96] to dominant and fracture prone linear crack and reduction in magnitude of microcracking [73], uncracked crack bridging [74] etc. were demonstrated due to formation and accumulation of AGEs/AGOEs within the pathophysiological range.
In contrast to the above, one recent study on human bone reported no change [97] in the mechanical properties, in fall-related or quasi-static loading rates, following 4 weeks of incubation in 0.1M ribose concentration resulting in supraphysiological levels of PEN [>20 mmol/mol of collagen or 2540% increase over PBS incubated group] in combination with a modest increase in total fluorescent AGEs below the diabetic threshold [200–250 total fluorescent AGEs/mol of collagen]. The inconsistencies in fracture toughness data could be attributed to the different protocols used for in vitro ribosylation as the level of total fluorescent AGEs in the group treated with ribose did not reach the diabetic threshold while the pentosidine levels, which represent a small fraction of AGEs/AGOEs in bone, exceeded the diabetic threshold > 3 times. Another in vitro glycation study similarly found no difference in J-integral fracture toughness between control and glycated samples under fall-like situation, while the reduction in plastic contribution of J-integral, damage fraction, post-yield strain, post-yield energy by in vitro glycation could be demonstrated under quasi-static loading rate [72].
On the contrary, a fracture toughness testing approach to capture the tendency of bone to resist the propagation of preexisting cracks, which accumulate with aging [95] and in diabetes [98], measures the changes in crack growth resistance. The results showed that distinctive effect of in vitro glycation, corresponding to doubling of fAGEs or three decades of in vivo aging, are manifested only in crack growth toughness and not at initiation [74]. Furthermore, under impact loading specimens in vitro glycated specimens, corresponding to three decades of aging, failed at 42% lower strain in proximity of 3000 μstrain that are seen during intense physiological activities [74]. As mentioned in this review, the in vitro incubation method using glyoxal to selectively form CML in bone has been established [45]. Future in vitro studies could, therefore, focus on including conditions to form CML that allow investigation of the impact of glycoxidative processes in bone.
To address the limitations associated with in vitro methods, an alternative approach has been to focus on the relationship between AGEs/AGOEs and bone fracture properties in in vivo diabetic animal models. For example, in a T1D mouse model, strong negative correlations were determined between propagation toughness with Raman-assessed CML (R = −0.99) and PEN (R = −0.97) [40]. Similarly, in the aforementioned non-obese T2D model [82], the increase of total fluorescent AGEs explained the loss in cracking toughness and work-to-fracture, and found inverse relationships between Raman-assessed CML and maximum toughness, and between Raman-assessed PEN and toughening effect (difference between initiation and propagation toughness) [82]. A diet-induced T2D mouse model also showed negative correlations between maximum toughness, and total fluorescent AGEs/Raman-assessed AGOEs.
More importantly, the study demonstrated the rescue of T2D bone skeletal fragility through cleavage of non-enzymatic crosslinks using in vitro treatment of phenacyl thiazolium chloride (PTC). The incubation of diabetic bone in PTC reduced total fluorescent AGEs content by 41% and increased the maximum toughness of PTC-treated samples by 35% compared to the untreated diabetic bones [99]. Interestingly, similar to in vitro ribosylation studies showing accumulation of AGEs/AGOEs and a consequent loss bone fracture properties, in vitro removal of total fluorescent AGEs and a consequent decrease in bone fragility demonstrate the causality between AGEs/AGOEs and bone fragility. Although not in context of diabetes, in vivo treatment of mice with chronic kidney diseases (CKD) with PTC has been shown to decrease bone AGEs [100]. In vivo PTC treatment of the diabetic BKS Cg-m+/+ Leprdb mice significantly reduced serum CML [101]. Taken together these results suggest that PTC may have several mechanisms of action. Future work is needed to determine if PTC could both inhibit and cleave AGEs.
In contrast to PTC, pyridoxamine acts as a post-Amadori inhibitor in the formation of AGEs and inhibits the formation of CML on protein during lipid peroxidation reactions [102]. Thus, PM has proven to be a potent inhibitor of AGE formation from glycated proteins [102,103]. A recent study [103] used an in vitro model to demonstrate that PM inhibited formation on AGEs and improved parameters associated with crack growth resistance in bone. These results suggest that PM can indeed prevent loss of bone’s mechanical properties. It is noteworthy that PM has been clinically tested in randomized, double-blind, placebo-controlled, multi-center Phase III studies [104]. Future work needs to be done to determine if PM can inhibit formation of AGEs in vivo.
Evaluation of the role of AGEs/AGOEs in ex vivo human bone studies remains challenging, as the bone mechanical properties are also likely to be affected by other heterogenous confounding factors. In one of the studies, T2D trabecular bone, obtained from individuals undergoing total hip replacement, demonstrated significant inverse correlations between total fluorescent AGEs and toughness, total fluorescent AGEs and post-yield energy, as well as FTIR-assessed nonenzymatic crosslinks ratio and post-yield strain [42]. Negative correlation between trabecular bone total fluorescent AGEs and post-yield displacement have also been reported [105]. Similarly in multiple regression models, PEN and total fluorescent AGEs have been shown to explain the variance in trabecular bone post-yield toughness (adjusted R2 = 0.74) [28]. However, the post-yield mechanical properties in these two studies were not different between T2D group and age-matched controls [28,105]. These studies are based on trabecular bone core assessment, where the mechanical testing is impacted by trabecular bone quantity and microarchitecture, and the post-yield mechanical behavior from compression test is subjected to more variation as the fracture point is not straightforward to determine compared to bending or tensile tests. Also, the duration of diabetes is an important variable that is not available for such ex vivo studies. To investigate the relationship of AGEs/AGOEs and fracture properties in ex vivo T2D bone tissue, future studies should place the emphasis on cortical bone tissue, diabetes duration and material-level mechanical assessments.
Clinical Implications of AGEs/AGOEs in Fracture Risk
Circulating AGEs/AGOEs levels reflect systemic measures of glycation and glycoxidation and these have been correlated to bone AGEs/AGOEs [105]. Investigations involving such measures not only contribute to the expanded mechanistic linkage between AGEs/AGOEs and bone fractures in large clinical cohorts but are of potential value to address the fracture risk underestimation in diabetes.
In the general population, not specifically related to diabetes, circulating PEN [29,30,106–108] and CML [109] have been separately shown to be associated with the risk of osteoporotic fractures. However, lack of significance in the association with fracture risk following adjustments was also reported in other studies for PEN [110] and CML [106], potentially due to the differences in population (ethnicities and lifestyles) and adjustment methods. In contrast, studies on diabetic fracture risk are more consistent. In Health Aging, Body and Composition (Health ABC) study, urinary PEN [12] and serum CML [13] have separately shown significant associations with the risk of incident clinical fractures specifically in the T2D cohort, while no such relationship was shown in the non-diabetic cohort. Urinary PEN is correlated with increased prevalence of vertebral fractures in T2D population [12] and this relationship persists following adjustment of BMD. Similar findings have been reported in a Japanese study where serum PEN is associated with vertebral fracture risk in diabetic women [31]. Moreover, a negative correlation between urinary PEN and trabecular bone score (measure of trabecular bone microarchitecture) was reported only in the T2D group [111]. Because urinary PEN was not different between T2D and non-diabetic group while serum CML was significantly higher in T2D than non-diabetic group in the Health ABC cohort, one can hypothesize that the pathway by which circulating PEN and CML impact fracture risk may be different, and CML could provide a more specific measure of bone fragility in the diabetic population. Nonetheless, both circulating AGOE markers have shown clear utility in evaluation of fracture risk particularly in T2D populations.
Alternatively, a simpler and more universal measurement, glycated hemoglobin (HbA1c), commonly and universally measured via simple blood test, could potentially serve as a surrogate measure for AGEs/AGOEs in bone and be used in diabetic fracture risk assessment. In particular, a recent study found a significant positive correlation between trabecular bone AGEs and serum HbA1c levels [42]. Additionally, in a postmenopausal T2D cohort, the long-term HbA1c level was found to inversely correlated with bone material strength index (BMSi, an in vivo assessment of bone material-level properties) [112]. A recent review [113] suggests that, compared to other biochemical markers such as bone turnover markers, HbA1c seems to be the only reliable predictor for diabetic fracture risk.
Consistent with the above results and the notions that NEG is a diffusion based process and long-term HbA1c measure may indeed capture both the impact of hyperglycemia and its duration, we evaluated various longitudinal HbA1c averages in large T2D population-based cohort study and found that longitudinal average of HbA1c, measured over two years, is indeed significantly associated with subsequent two-year fracture, where T2D individuals with poor glycemic control (longitudinal HbA1c ≥ 9%) had a 29% increase in adjusted fracture risk compared to T2D individuals with adequate glycemic control (longitudinal HbA1c between 6% and 9%) [114]. With adjustments of diabetic comorbidities (retinopathy, neuropathy, nephropathy, cardiovascular diseases, etc.) as well as osteoporosis, the increased fracture risk comparing poor glycemic control against adequate glycemic control in T2D was attenuated to 18% but remained significant. The remaining relationship adjusted for comorbidities more directly reflects the negative impact of glycation on bone fractures [114]. These findings open new avenues for using glycemic control over a moderate period of time, which potentially would capture the impact of AGEs/AGOEs in T2D bone, to address the underestimation of fracture risk in T2D by BMD.
AGEs and AGOEs Beyond Diabetes
Beyond diabetes, AGEs/AGOEs increase in bone with age [43], and the elevated AGEs/AGOEs levels are associated with weaker bone properties including toughness [115]. As mentioned earlier, increased concentration of AGEs/AGOEs in bone (PEN in particular) is associated with bone loss in non-diabetic individuals [44], which potentially links AGEs/AGOEs in bone to the prevalence of osteoporosis. Indeed, higher levels of circulating PEN and CML have been identified in patients with osteoporosis compared to healthy subjects [116]. Similar to human data, an animal study also found an increased pentosidine level in bone in the ovariectomized model compared to the sham group [117]. Moreover, excessive accumulation of total fluorescent AGEs and PEN in bone was found in a microgravity-induced osteoporosis animal model, where AGEs/AGOEs explained the deteriorated trabecular bone microarchitecture and disrupted bone metabolism [118]. Moreover, the inhibition of AGEs/AGOEs using irbesartan prevented bone loss due to microgravity and rescued bone fragility [119]. In addition, in two studies related to a cohort of Japanese postmenopausal women, higher levels of bone PEN were discovered in individuals with hip fracture than in non-fractured individuals for cortical bone with lower degree of mineralization [26], and for trabecular bone with higher degree of mineralization [27].
Recent evidence has revealed that AGEs/AGOEs explain bone fragility in other pathological conditions. For example, consistent with the notion of disruption of glucose metabolism in Alzheimer’s diseases [120], an animal study demonstrated significant associations of weaker bone fracture properties with increased AGEs/AGOEs accumulation in bone in a transgenic 5XFAD model of Alzheimer’s disease [121]. Similar results were also found under a combination of circadian rhythm and high-fat diet [122]. In metastatic bone diseases, the total fluorescent AGEs accumulation in human vertebral bone was found to be associated with lower vertebral strength and stiffness [123]. Additionally, in an osteogenesis imperfecta mouse model, where bones exhibit brittle fracture properties, a recent study found a 30% increase in the total fluorescent AGEs accumulation compared to healthy controls [124]. Taken together these studies highlight that AGEs/AGOEs may ubiquitously form and impact bone tissues in diseases and conditions involving alterations of hyperglycemia and/or oxidative stress.
Summary
In this review, we summarized the bone quality alterations at multiple scales due to the accumulation of AGEs/AGOEs. Although the deleterious effects of non-enzymatic crosslinks on collagen and non-collagenous proteins are known, the roles of non-crosslinking AGEs/AGOEs, such as CML on diabetic bone quality and related bone mechanical integrity are not yet fully established and require further investigations. Clinical measurements of systemic glycoxidation (circulating levels of PEN and CML) measures have been found to associate with diabetic fracture risk and these results provide new avenues to improve diabetic fracture risk prediction and to better manage and understand bone fragility as an important comorbidity of diabetes. The increased accumulation of AGEs/AGOEs in bone is being identified in diseases such as Alzheimer’s disease, cancer, osteogenesis imperfecta etc. and these results highlight the ubiquitous role that AGEs/AGOEs play in the heightened bone fragility under a variety of situations involving alterations in hyperglycemia and/or oxidative stress. Future studies should consider exploring the role of AGOEs such as CML on bone fragility, gender effect on accumulation of AGEs/AGOEs in bone and for use of circulating or surrogate markers of AGEs/AGOEs in bone (such as longitudinal glycemic control) to assess and predict fracture risk. The preventive therapeutics for inhibiting and/or removing existing AGEs/AGOEs from aging and diabetic bone tissue need to be developed to better manage bone fragility under myriad conditions including but not limited to diabetes, osteoporosis, Alzheimer’s disease, circadian rhythm disruption, osteogenesis imperfecta, cancer etc.
Highlights.
Hyperglycemia and oxidative stress are enhanced in diabetes and aging and result in accumulation of advanced glycation (AGEs) and glycoxidation (AGOEs) products in bone.
AGEs and AGOEs are associated with bone fragility in conditions such as diabetes, osteoporosis, Alzheimer’s disease, circadian rhythm disruption, and cancer.
AGEs/AGOEs can be useful for prediction and management of diabetic, osteoporotic and insufficiency fractures.
Targeting AGEs/AGOEs to inhibit their formation and/or accumulation could potentially rescue bone fragility.
Acknowledgements
NIH/NIA grants R01AG075654 and R21AG0630631.
NYSTAR Hi Tech Matching Grant Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Diab T, Sit S, Kim D, Rho J, Vashishth D, Age-dependent fatigue behaviour of human cortical bone, Eur. J. Morphol 42 (2005) 53–59. 10.1080/09243860500095539. [DOI] [PubMed] [Google Scholar]
- [2].Acevedo C, Stadelmann VA, Pioletti DP, Alliston T, Ritchie RO, Fatigue as the missing link between bone fragility and fracture, Nat. Biomed. Eng 2 (2018) 62–71. 10.1038/s41551-017-0183-9. [DOI] [PubMed] [Google Scholar]
- [3].Vestergaard P, Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis, Osteoporos. Int 18 (2007) 427–444. 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- [4].Moseley KF, Type 2 diabetes and bone fractures, Curr. Opin. Endocrinol. Diabetes Obes 19 (2012) 128–135. 10.1097/MED.0b013e328350a6e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Starup-Linde J, Frost M, Vestergaard P, Abrahamsen B, Epidemiology of fractures in diabetes, Calcif. Tissue Int 100 (2017) 109–121. 10.1007/s00223-016-0175-x. [DOI] [PubMed] [Google Scholar]
- [6].Compston J, Type 2 diabetes mellitus and bone, J. Intern. Med 283 (2018) 140–153. 10.1111/joim.12725. [DOI] [PubMed] [Google Scholar]
- [7].Starup-Linde J, Hygum K, Harsløf T, Langdahl B, Type 1 Diabetes and Bone Fragility: Links and Risks, Diabetes Metab. Syndr. Obes. Targets Ther 12 (2019) 2539–2547. 10.2147/DMSO.S191091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kaye RA, Insufficiency stress fractures of the foot and ankle in postmenopausal women, Foot Ankle Int. 19 (1998) 221–224. 10.1177/107110079801900406. [DOI] [PubMed] [Google Scholar]
- [9].Breer S, Krause M, Marshall RP, Oheim R, Amling M, Barvencik F, Stress fractures in elderly patients, Int. Orthop 36 (2012) 2581–2587. 10.1007/s00264-012-1708-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Krestan C, Hojreh A, Imaging of insufficiency fractures, Eur. J. Radiol 71 (2009) 398–405. 10.1016/j.ejrad.2008.04.059. [DOI] [PubMed] [Google Scholar]
- [11].Lenart BA, Neviaser AS, Lyman S, Chang CC, Edobor-Osula F, Steele B, van der Meulen MCH, Lorich DG, Lane JM, Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study, Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 20 (2009) 1353–1362. 10.1007/s00198-008-0805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schwartz AV, Garnero P, Hillier TA, Sellmeyer DE, Strotmeyer ES, Feingold KR, Resnick HE, Tylavsky FA, Black DM, Cummings SR, Harris TB, Bauer DC, Health, Aging, and Body Composition Study, Pentosidine and increased fracture risk in older adults with type 2 diabetes, J. Clin. Endocrinol. Metab 94 (2009) 2380–2386. 10.1210/jc.2008-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dhaliwal R, Ewing SK, Vashishth D, Semba RD, Schwartz AV, Greater Carboxy‐Methyl‐Lysine is Associated with Increased Fracture Risk in Type 2 Diabetes, J. Bone Miner. Res Manuscript accepted; In press (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Farlay D, Armas LAG, Gineyts E, Akhter MP, Recker RR, Boivin G, Nonenzymatic glycation and degree of mineralization are higher in bone from fractured patients with type 1 diabetes mellitus, J. Bone Miner. Res 31 (2016) 190–195. 10.1002/jbmr.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Oren TW, Botolin S, Williams A, Bucknell A, King KB, Arthroplasty in veterans: Analysis of cartilage, bone, serum, and synovial fluid reveals differences and similarities in osteoarthritis with and without comorbid diabetes, J. Rehabil. Res. Dev 48 (2011) 1195–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sroga GE, Stephen SJ, Wang B, Vashishth D, Techniques for advanced glycation end product measurements for diabetic bone disease: pitfalls and future directions, Curr. Opin. Endocrinol. Diabetes Obes 29 (2022) 333–342. 10.1097/MED.0000000000000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Avery NC, Bailey AJ, Enzymic and non-enzymic cross-linking mechanisms in relation to turnover of collagen: relevance to aging and exercise, Scand. J. Med. Sci. Sports 15 (2005) 231–240. 10.1111/j.1600-0838.2005.00464.x. [DOI] [PubMed] [Google Scholar]
- [18].Younus H, Anwar S, Prevention of non-enzymatic glycosylation (glycation): Implication in the treatment of diabetic complication, Int. J. Health Sci 10 (2016) 261–277. [PMC free article] [PubMed] [Google Scholar]
- [19].Nagai R, Shirakawa J, Fujiwara Y, Ohno R, Moroishi N, Sakata N, Nagai M, Detection of AGEs as markers for carbohydrate metabolism and protein denaturation, J. Clin. Biochem. Nutr 55 (2014) 1–6. 10.3164/jcbn.13-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Arakawa S, Suzuki R, Kurosaka D, Ikeda R, Hayashi H, Kayama T, Ohno R, Nagai R, Marumo K, Saito M, Mass spectrometric quantitation of AGEs and enzymatic crosslinks in human cancellous bone, Sci. Rep 10 (2020) 18774. 10.1038/s41598-020-75923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Thomas CJ, Cleland TP, Sroga GE, Vashishth D, Accumulation of carboxymethyl-lysine (CML) in human cortical bone, Bone. 110 (2018) 128–133. 10.1016/j.bone.2018.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP, Influence of nonenzymatic glycation on biomechanical properties of cortical bone, Bone. 28 (2001) 195–201. 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- [23].Sroga GE, Siddula A, Vashishth D, Glycation of human cortical and cancellous bone captures differences in the formation of Maillard reaction products between glucose and ribose, PloS One. 10 (2015) e0117240. 10.1371/journal.pone.0117240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nyman JS, Roy A, Tyler JH, Acuna RL, Gayle HJ, Wang X, Age-Related Factors Affecting the Post-Yield Energy Dissipation of Human Cortical Bone, J. Orthop. Res. Off. Publ. Orthop. Res. Soc 25 (2007) 646–655. 10.1002/jor.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang X, Shen X, Li X, Agrawal CM, Age-related changes in the collagen network and toughness of bone, Bone. 31 (2002) 1–7. 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- [26].Saito M, Fujii K, Soshi S, Tanaka T, Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of femoral neck fracture, Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 17 (2006) 986–995. 10.1007/s00198-006-0087-0. [DOI] [PubMed] [Google Scholar]
- [27].Saito M, Fujii K, Marumo K, Degree of mineralization-related collagen crosslinking in the femoral neck cancellous bone in cases of hip fracture and controls, Calcif. Tissue Int 79 (2006) 160–168. 10.1007/s00223-006-0035-1. [DOI] [PubMed] [Google Scholar]
- [28].Hunt HB, Torres AM, Palomino PM, Marty E, Saiyed R, Cohn M, Jo J, Warner S, Sroga GE, King KB, Lane JM, Vashishth D, Hernandez CJ, Donnelly E, Altered tissue composition, microarchitecture, and mechanical performance in cancellous bone from men with type 2 diabetes mellitus, J. Bone Miner. Res 34 (2019) 1191–1206. 10.1002/jbmr.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shiraki M, Kuroda T, Tanaka S, Saito M, Fukunaga M, Nakamura T, Nonenzymatic collagen cross-links induced by glycoxidation (pentosidine) predicts vertebral fractures, J. Bone Miner. Metab 26 (2008) 93–100. 10.1007/s00774-007-0784-6. [DOI] [PubMed] [Google Scholar]
- [30].Tanaka S, Saito M, Hagino H, Mori S, Nakamura T, Ohta H, Sone T, Takahashi K, Mitomo Y, Sugimoto T, Soen S, A.T. of O. (A-T.R. Group, Association of Urinary Pentosidine Levels With the Risk of Fractures in Patients With Severe Osteoporosis: The Japanese Osteoporosis Intervention Trial-05 (JOINT-05), JBMR Plus. 6 (2022) e10673. 10.1002/jbm4.10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T, Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes, J. Clin. Endocrinol. Metab 93 (2008) 1013–1019. 10.1210/jc.2007-1270. [DOI] [PubMed] [Google Scholar]
- [32].Saeki C, Saito M, Kanai T, Nakano M, Oikawa T, Torisu Y, Saruta M, Tsubota A, Plasma pentosidine levels are associated with prevalent fractures in patients with chronic liver disease, PLOS ONE. 16 (2021) e0249728. 10.1371/journal.pone.0249728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shaw JN, Baynes JW, Thorpe SR, N epsilon-(carboxymethyl)lysine (CML) as a biomarker of oxidative stress in long-lived tissue proteins, Methods Mol. Biol. Clifton NJ 186 (2002) 129–137. 10.1385/1-59259-173-6:129. [DOI] [PubMed] [Google Scholar]
- [34].Thorpe SR, Baynes JW, CML: a brief history, Int. Congr. Ser 1245 (2002) 91–99. 10.1016/S0531-5131(02)00881-6. [DOI] [Google Scholar]
- [35].Ahmed MU, Brinkmann Frye E, Degenhardt TP, Thorpe SR, Baynes JW, N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins., Biochem. J 324 (1997) 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Glomb MA, Monnier VM, Mechanism of Protein Modification by Glyoxal and Glycolaldehyde, Reactive Intermediates of the Maillard Reaction (*), J. Biol. Chem 270 (1995) 10017–10026. 10.1074/jbc.270.17.10017. [DOI] [PubMed] [Google Scholar]
- [37].Wölfel EM, Jähn-Rickert K, Schmidt FN, Wulff B, Mushumba H, Sroga GE, Püschel K, Milovanovic P, Amling M, Campbell GM, Vashishth D, Busse B, Individuals with type 2 diabetes mellitus show dimorphic and heterogeneous patterns of loss in femoral bone quality, Bone. 140 (2020) 115556. 10.1016/j.bone.2020.115556. [DOI] [PubMed] [Google Scholar]
- [38].Unal M, Uppuganti S, Leverant CJ, Creecy A, Granke M, Voziyan P, Nyman JS, Assessing glycation-mediated changes in human cortical bone with Raman spectroscopy, J. Biophotonics 11 (2018) e201700352. 10.1002/jbio.201700352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sroga GE, Vashishth D, UPLC methodology for identification and quantitation of naturally fluorescent crosslinks in proteins: a study of bone collagen, J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci 879 (2011) 379–385. 10.1016/j.jchromb.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rubin MR, Paschalis EP, Poundarik A, Sroga GE, McMahon DJ, Gamsjaeger S, Klaushofer K, Vashishth D, Advanced glycation endproducts and bone material properties in type 1 diabetic mice, PLOS ONE. 11 (2016) e0154700. 10.1371/journal.pone.0154700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schmidt FN, Zimmermann EA, Campbell GM, Sroga GE, Püschel K, Amling M, Tang SY, Vashishth D, Busse B, Assessment of collagen quality associated with non-enzymatic cross-links in human bone using Fourier-transform infrared imaging, Bone. 97 (2017) 243–251. 10.1016/j.bone.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sihota P, Yadav RN, Dhaliwal R, Bose JC, Dhiman V, Neradi D, Karn S, Sharma S, Aggarwal S, Goni VG, Mehandia V, Vashishth D, Bhadada SK, Kumar N, Investigation of mechanical, material, and compositional determinants of human trabecular bone quality in type 2 diabetes, J. Clin. Endocrinol. Metab 106 (2021) e2271–e2289. 10.1210/clinem/dgab027. [DOI] [PubMed] [Google Scholar]
- [43].Karim L, Tang SY, Sroga GE, Vashishth D, Differences in non-enzymatic glycation and collagen cross-links between human cortical and cancellous bone, Osteoporos. Int 24 (2013) 2441–2447. 10.1007/s00198-013-2319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Odetti P, Rossi S, Monacelli F, Poggi A, Cirnigliaro M, Federici M, Federici A, Advanced glycation end products and bone loss during aging, Ann. N. Y. Acad. Sci 1043 (2005) 710–717. 10.1196/annals.1333.082. [DOI] [PubMed] [Google Scholar]
- [45].Sroga GE, Vashishth D, Controlled formation of carboxymethyllysine in bone matrix through designed glycation reaction, JBMR Plus. 5 (2021) e10548. 10.1002/jbm4.10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Vaidya R, Rezaee T, Edwards T, Bender R, Vickneswaran A, Chalivendra V, Karim L, Accumulation of fluorescent advanced glycation end products and carboxymethyl-lysine in human cortical and trabecular bone, Bone Rep. 17 (2022) 101634. 10.1016/j.bonr.2022.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Woelfel EM, Jaehn K, Milovanovic P, Amling M, Busse B, Sroga G, Vashishth D, Wulff B, Mushumba H, Pueschel K, Individuals afflicted with type 2 diabetes mellitus show lower femoral endocortical Sclerostin expression along with higher fluorescent advanced glycation endproducts, in: Annu. Meet. Am. Soc. Bone Miner. Res, WILEY, 2019: pp. 284–285. [Google Scholar]
- [48].Sroga GE, Vashishth D, Phosphorylation of Extracellular Bone Matrix Proteins and Its Contribution to Bone Fragility, J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res 33 (2018) 2214–2229. 10.1002/jbmr.3552. [DOI] [PubMed] [Google Scholar]
- [49].Fan L, Wang B, Sroga G, Nickolas T, Vashishth D, Rubin M, Clinical measures of advanced glycation endproducts and bone tissue properties in type 2 diabetes, in: Annu. Meet. Am. Soc. Bone Miner. Res, WILEY, 2022: pp. 38–38. [Google Scholar]
- [50].Meng H-Z, Zhang W-L, Liu F, Yang M-W, Advanced glycation end products affect osteoblast proliferation and function by modulating autophagy via the receptor of advanced glycation end products/raf protein/mitogen-activated protein kinase/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase (rage/raf/mek/erk) pathway *, J. Biol. Chem 290 (2015) 28189–28199. 10.1074/jbc.M115.669499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yamamoto M, Sugimoto T, Advanced glycation end products, diabetes, and bone strength, Curr. Osteoporos. Rep 14 (2016) 320–326. 10.1007/s11914-016-0332-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Franke S, Rüster C, Pester J, Hofmann G, Oelzner P, Wolf G, Advanced glycation end products affect growth and function of osteoblasts, Clin. Exp. Rheumatol 29 (2011) 650–660. [PubMed] [Google Scholar]
- [53].Tanaka K, Yamaguchi T, Kanazawa I, Sugimoto T, Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells, Biochem. Biophys. Res. Commun 461 (2015) 193–199. 10.1016/j.bbrc.2015.02.091. [DOI] [PubMed] [Google Scholar]
- [54].Valcourt U, Merle B, Gineyts E, Viguet-Carrin S, Delmas PD, Garnero P, Non-enzymatic glycation of bone collagen modifies osteoclastic activity and differentiation, J. Biol. Chem 282 (2007) 5691–5703. 10.1074/jbc.M610536200. [DOI] [PubMed] [Google Scholar]
- [55].Li Z, Li C, Zhou Y, Chen W, Luo G, Zhang Z, Wang H, Zhang Y, Xu D, Sheng P, Advanced glycation end products biphasically modulate bone resorption in osteoclast-like cells, Am. J. Physiol.-Endocrinol. Metab 310 (2016) E355–E366. 10.1152/ajpendo.00309.2015. [DOI] [PubMed] [Google Scholar]
- [56].Zhang Y, Liang J, Liu P, Wang Q, Liu L, Zhao H, The RANK/RANKL/OPG system and tumor bone metastasis: Potential mechanisms and therapeutic strategies, Front. Endocrinol 13 (2022). https://www.frontiersin.org/articles/10.3389/fendo.2022.1063815 (accessed July 10, 2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Park JH, Lee NK, Lee SY, Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation, Mol. Cells 40 (2017) 706–713. 10.14348/molcells.2017.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Janeiro Colleen, The effect of bone quality on osteoclastic bone resorption, Rensselaer Polytechnic Institute, 2010. [Google Scholar]
- [59].Miyata T, Notoya K, Yoshida K, Horie K, Maeda K, Kurokawa K, Taketomi S, Advanced glycation end products enhance osteoclast-induced bone resorption in cultured mouse unfractionated bone cells and in rats implanted subcutaneously with devitalized bone particles., J. Am. Soc. Nephrol 8 (1997) 260. 10.1681/ASN.V82260. [DOI] [PubMed] [Google Scholar]
- [60].Dong XN, Qin A, Xu J, Wang X, In Situ Accumulation of Advanced Glycation Endproducts (AGEs) in Bone Matrix and Its Correlation with Osteoclastic Bone Resorption, Bone. 49 (2011) 174–183. 10.1016/j.bone.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sroga GE, Karim L, Colón W, Vashishth D, Biochemical characterization of major bone-matrix proteins using nanoscale-size bone samples and proteomics methodology, Mol. Cell. Proteomics MCP 10 (2011) M110.006718. 10.1074/mcp.M110.006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Franzén A, Hultenby K, Reinholt FP, Onnerfjord P, Heinegård D, Altered osteoclast development and function in osteopontin deficient mice, J. Orthop. Res. Off. Publ. Orthop. Res. Soc 26 (2008) 721–728. 10.1002/jor.20544. [DOI] [PubMed] [Google Scholar]
- [63].Ural A, Janeiro C, Karim L, Diab T, Vashishth D, Association between non-enzymatic glycation, resorption, and microdamage in human tibial cortices, Osteoporos. Int 26 (2015) 865–873. 10.1007/s00198-014-2938-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sakata N, Meng J, Jimi S, Takebayashi S, Nonenzymatic glycation and extractability of collagen in human atherosclerotic plaques, Atherosclerosis. 116 (1995) 63–75. 10.1016/0021-9150(95)05526-3. [DOI] [PubMed] [Google Scholar]
- [65].Hofmann B, Adam A-C, Jacobs K, Riemer M, Erbs C, Bushnaq H, Simm A, Silber R-E, Santos AN, Advanced glycation end product associated skin autofluorescence: A mirror of vascular function?, Exp. Gerontol 48 (2013) 38–44. 10.1016/j.exger.2012.04.011. [DOI] [PubMed] [Google Scholar]
- [66].Allen MR, Gineyts E, Leeming DJ, Burr DB, Delmas PD, Bisphosphonates alter trabecular bone collagen cross-linking and isomerization in beagle dog vertebra, Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 19 (2008) 329–337. 10.1007/s00198-007-0533-7. [DOI] [PubMed] [Google Scholar]
- [67].Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D, Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate, Osteoporos. Int 20 (2009) 887–894. 10.1007/s00198-008-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Rubin MR, Patsch JM, Assessment of bone turnover and bone quality in type 2 diabetic bone disease: current concepts and future directions, Bone Res. 4 (2016) 1–9. 10.1038/boneres.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Seref-Ferlengez Z, Kennedy OD, Schaffler MB, Bone microdamage, remodeling and bone fragility: how much damage is too much damage?, BoneKEy Rep. 4 (2015) 644. 10.1038/bonekey.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Garnero P, The contribution of collagen crosslinks to bone strength, BoneKEy Rep. 1 (2012) 182. 10.1038/bonekey.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Tang SY, Zeenath U, Vashishth D, Effects of non-enzymatic glycation on cancellous bone fragility, Bone. 40 (2007) 1144–1151. 10.1016/j.bone.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Jia S, Gong H, Cen H, Shi P, Zhang R, Li Z, Bi X, Influence of non-enzymatic glycation on the mechanical properties of cortical bone, J. Mech. Behav. Biomed. Mater 119 (2021) 104553. 10.1016/j.jmbbm.2021.104553. [DOI] [PubMed] [Google Scholar]
- [73].Tang SY, Vashishth D, Non-enzymatic glycation alters microdamage formation in human cancellous bone, Bone. 46 (2010) 148–154. 10.1016/j.bone.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Poundarik AA, Wu P-C, Evis Z, Sroga GE, Ural A, Rubin M, Vashishth D, A direct role of collagen glycation in bone fracture, J. Mech. Behav. Biomed. Mater 52 (2015) 120–130. 10.1016/j.jmbbm.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Diab T, Vashishth D, Effects of damage morphology on cortical bone fragility, Bone. 37 (2005) 96–102. 10.1016/j.bone.2005.03.014. [DOI] [PubMed] [Google Scholar]
- [76].Acevedo C, Sylvia M, Schaible E, Graham JL, Stanhope KL, Metz LN, Gludovatz B, Schwartz AV, Ritchie RO, Alliston TN, Havel PJ, Fields AJ, Contributions of material properties and structure to increased bone fragility for a given bone mass in the UCD-T2DM rat model of type 2 diabetes, J. Bone Miner. Res 33 (2018) 1066–1075. 10.1002/jbmr.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Poundarik AA, Diab T, Sroga GE, Ural A, Boskey AL, Gundberg CM, Vashishth D, Dilatational band formation in bone, Proc. Natl. Acad. Sci 109 (2012) 19178–19183. 10.1073/pnas.1201513109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Nikel O, Poundarik AA, Bailey S, Vashishth D, Structural role of osteocalcin and osteopontin in energy dissipation in bone, J. Biomech 80 (2018) 45–52. 10.1016/j.jbiomech.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Thomas CJ, Cleland TP, Zhang S, Gundberg CM, Vashishth D, Identification and characterization of glycation adducts on osteocalcin, Anal. Biochem 525 (2017) 46–53. 10.1016/j.ab.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Bailey S, Poundarik AA, Sroga GE, Vashishth D, Structural role of osteocalcin and its modification in bone fracture, Appl. Phys. Rev 10 (2023) 011410. 10.1063/5.0102897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Boivin G, Meunier PJ, The degree of mineralization of bone tissue measured by computerized quantitative contact microradiography, Calcif. Tissue Int 70 (2002) 503–511. 10.1007/s00223-001-2048-0. [DOI] [PubMed] [Google Scholar]
- [82].Tice MJL, Bailey S, Sroga GE, Gallagher EJ, Vashishth D, Non-obese MKR mouse model of type 2 diabetes reveals skeletal alterations in mineralization and material properties, JBMR Plus. 6 (2021) e10583. 10.1002/jbm4.10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Taylor EA, Mileti CJ, Ganesan S, Kim JH, Donnelly E, Measures of bone mineral carbonate content and mineral maturity/crystallinity for ft-ir and raman spectroscopic imaging differentially relate to physical–chemical properties of carbonate-substituted hydroxyapatite, Calcif. Tissue Int 109 (2021) 77–91. 10.1007/s00223-021-00825-4. [DOI] [PubMed] [Google Scholar]
- [84].Kerch G, Distribution of tightly and loosely bound water in biological macromolecules and age-related diseases, Int. J. Biol. Macromol 118 (2018) 1310–1318. 10.1016/j.ijbiomac.2018.06.187. [DOI] [PubMed] [Google Scholar]
- [85].Manhard MK, Uppuganti S, Granke M, Gochberg DF, Nyman JS, Does MD, MRI-derived bound and pore water concentrations as predictors of fracture resistance, Bone. 87 (2016) 1–10. 10.1016/j.bone.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Granke M, Does MD, Nyman JS, The Role of Water Compartments in the Material Properties of Cortical Bone, Calcif. Tissue Int 97 (2015) 292–307. 10.1007/s00223-015-9977-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Granke M, Makowski AJ, Uppuganti S, Does MD, Nyman JS, Identifying novel clinical surrogates to assess human bone fracture toughness, J. Bone Miner. Res 30 (2015) 1290–1300. 10.1002/jbmr.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Nyman JS, Uppuganti S, Unal M, Leverant CJ, Adabala S, Granke M, Voziyan P, Does MD, Manipulating the amount and structure of the organic matrix affects the water compartments of human cortical bone, JBMR Plus. 3 (2019) e10135. 10.1002/jbm4.10135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Uppuganti S, Granke M, Makowski AJ, Does MD, Nyman JS, Age-related changes in the fracture resistance of male Fischer F344 rat bone, Bone. 83 (2016) 220–232. 10.1016/j.bone.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Creecy A, Uppuganti S, Girard MR, Schlunk SG, Amah C, Granke M, Unal M, Does MD, Nyman JS, The age-related decrease in material properties of BALB/c mouse long bones involves alterations to the extracellular matrix, Bone. 130 (2020) 115126. 10.1016/j.bone.2019.115126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Minami M, Ikoma K, Horii M, Sukenari T, Onishi O, Fujiwara H, Ogi H, Itoh K, Kubo T, Usefulness of Sweep Imaging With Fourier Transform for Evaluation of Cortical Bone in Diabetic Rats, J. Magn. Reson. Imaging 48 (2018) 389–397. 10.1002/jmri.25955. [DOI] [PubMed] [Google Scholar]
- [92].Mieczkowska A, Mansur SA, Irwin N, Flatt PR, Chappard D, Mabilleau G, Alteration of the bone tissue material properties in type 1 diabetes mellitus: A Fourier transform infrared microspectroscopy study, Bone. 76 (2015) 31–39. 10.1016/j.bone.2015.03.010. [DOI] [PubMed] [Google Scholar]
- [93].Bradke BS, Vashishth D, N-phenacylthiazolium bromide reduces bone fragility induced by nonenzymatic glycation, PloS One. 9 (2014) e103199. 10.1371/journal.pone.0103199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Merlo K, Aaronson J, Vaidya R, Rezaee T, Chalivendra V, Karim L, In Vitro-Induced High Sugar Environments Deteriorate Human Cortical Bone Elastic Modulus and Fracture Toughness, J. Orthop. Res. Off. Publ. Orthop. Res. Soc 38 (2020) 972–983. 10.1002/jor.24543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Schaffler MB, Choi K, Milgrom C, Aging and matrix microdamage accumulation in human compact bone, Bone. 17 (1995) 521–525. 10.1016/8756-3282(95)00370-3. [DOI] [PubMed] [Google Scholar]
- [96].Diab T, Condon KW, Burr DB, Vashishth D, Age-related change in the damage morphology of human cortical bone and its role in bone fragility, Bone. 38 (2006) 427–431. 10.1016/j.bone.2005.09.002. [DOI] [PubMed] [Google Scholar]
- [97].Unal M, Uppuganti S, Dapaah DY, Ahmed R, Pennings JS, Willett TL, Voziyan P, Nyman JS, Effect of ribose incubation on physical, chemical, and mechanical properties of human cortical bone, J. Mech. Behav. Biomed. Mater 140 (2023) 105731. 10.1016/j.jmbbm.2023.105731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Mohsin S, Kaimala S, AlTamimi EKY, Tariq S, Adeghate E, In vivo Labeling of Bone Microdamage in an Animal Model of Type 1 Diabetes Mellitus, Sci. Rep 9 (2019) 16994. 10.1038/s41598-019-53487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].LLabre JE, Sroga GE, Tice MJL, Vashishth D, Induction and rescue of skeletal fragility in a high-fat diet mouse model of type 2 diabetes: An in vivo and in vitro approach, Bone. 156 (2022) 116302. 10.1016/j.bone.2021.116302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Chen NX, Srinivasan S, O’Neill K, Nickolas TL, Wallace JM, Allen MR, Metzger CE, Creecy A, Avin KG, Moe SM, Effect of Advanced Glycation End-Products (AGE) Lowering Drug ALT-711 on Biochemical, Vascular, and Bone Parameters in a Rat Model of CKD-MBD, J. Bone Miner. Res 35 (2020) 608–617. 10.1002/jbmr.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Peppa M, Brem H, Cai W, Zhang J-G, Basgen J, Li Z, Vlassara H, Uribarri J, Prevention and Reversal of Diabetic Nephropathy in db/db Mice Treated with Alagebrium (ALT-711), Am. J. Nephrol 26 (2006) 430–436. 10.1159/000095786. [DOI] [PubMed] [Google Scholar]
- [102].Metz TO, Alderson NL, Thorpe SR, Baynes JW, Pyridoxamine, an inhibitor of advanced glycation and lipoxidation reactions: a novel therapy for treatment of diabetic complications, Arch. Biochem. Biophys 419 (2003) 41–49. 10.1016/j.abb.2003.08.021. [DOI] [PubMed] [Google Scholar]
- [103].Abar O, Dharmar S, Tang SY, The effect of aminoguanidine (AG) and pyridoxamine (PM) on ageing human cortical bone, Bone Jt. Res 7 (2018) 105–110. 10.1302/2046-3758.71.BJR-2017-0135.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Rubin M, Advanced Glycation Endproducts and Bone Material Strength in Type 2 Diabetes Treated With Pyridoxamine, clinicaltrials.gov, 2022. https://clinicaltrials.gov/ct2/show/NCT03778580 (accessed June 4, 2023).
- [105].Karim L, Moulton J, Van Vliet M, Velie K, Robbins A, Malekipour F, Abdeen A, Ayres D, Bouxsein ML, Bone microarchitecture, biomechanical properties, and advanced glycation end-products in the proximal femur of adults with type 2 diabetes, Bone. 114 (2018) 32–39. 10.1016/j.bone.2018.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Nakano M, Nakamura Y, Suzuki T, Miyazaki A, Takahashi J, Saito M, Shiraki M, Pentosidine and carboxymethyl-lysine associate differently with prevalent osteoporotic vertebral fracture and various bone markers, Sci. Rep 10 (2020) 22090. 10.1038/s41598-020-78993-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Shiraki M, Kashiwabara S, Imai T, Tanaka S, Saito M, The association of urinary pentosidine levels with the prevalence of osteoporotic fractures in postmenopausal women, J. Bone Miner. Metab 37 (2019) 1067–1074. 10.1007/s00774-019-01017-9. [DOI] [PubMed] [Google Scholar]
- [108].Hagino H, Moriwaki K, Wada T, Osaki M, Nagashima H, Matsumoto H, Urinary pentosidine level is associated with the risk of fracture in community-dwelling older adults: a prospective observational study, Osteoporos. Int (2023). 10.1007/s00198-023-06816-5. [DOI] [PubMed] [Google Scholar]
- [109].Barzilay JI, Bůžková P, Zieman SJ, Kizer JR, Djoussé L, Ix JH, Tracy RP, Siscovick DS, Cauley JA, Mukamal KJ, Circulating levels of carboxy‐methyl‐ lysine (CML) are associated with hip fracture risk: the Cardiovascular Health Study, J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res 29 (2014) 1061–1066. 10.1002/jbmr.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Gineyts E, Munoz F, Bertholon C, Sornay-Rendu E, Chapurlat R, Urinary levels of pentosidine and the risk of fracture in postmenopausal women: the OFELY study, Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 21 (2010) 243–250. 10.1007/s00198-009-0939-5. [DOI] [PubMed] [Google Scholar]
- [111].Choi YJ, Ock SY, Jin Y, Lee JS, Kim SH, Chung Y-S, Urinary Pentosidine levels negatively associates with trabecular bone scores in patients with type 2 diabetes mellitus, Osteoporos. Int 29 (2018) 907–915. 10.1007/s00198-017-4359-7. [DOI] [PubMed] [Google Scholar]
- [112].Farr JN, Drake MT, Amin S, Melton LJ, McCready LK, Khosla S, In vivo assessment of bone quality in postmenopausal women with type 2 diabetes, J. Bone Miner. Res 29 (2014) 787–795. 10.1002/jbmr.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Meier C, Eastell R, Pierroz DD, Lane NE, Al-Daghri N, Suzuki A, Napoli N, Mithal A, Chakhtoura M, El-Hajj Fuleihan G, Ferrari S, Biochemical Markers of Bone Fragility in Patients with Diabetes. A Narrative Review by the IOF and the ECTS, J. Clin. Endocrinol. Metab (2023) dgad255. 10.1210/clinem/dgad255. [DOI] [PubMed] [Google Scholar]
- [114].Wang B, Wang Z, Poundarik AA, Zaki MJ, Bockman RS, Glicksberg BS, Nadkarni GN, Vashishth D, Unmasking fracture risk in type 2 diabetes: the association of longitudinal glycemic hemoglobin level and medications, J. Clin. Endocrinol. Metab 107 (2022) e1390–e1401. 10.1210/clinem/dgab882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Karim L, Vashishth D, Heterogeneous glycation of cancellous bone and its association with bone quality and fragility, PloS One. 7 (2012) e35047. 10.1371/journal.pone.0035047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Hein G, Wiegand R, Lehmann G, Stein G, Franke S, Advanced glycation end-products pentosidine and N epsilon-carboxymethyllysine are elevated in serum of patients with osteoporosis, Rheumatol. Oxf. Engl 42 (2003) 1242–1246. 10.1093/rheumatology/keg324. [DOI] [PubMed] [Google Scholar]
- [117].Saito M, Marumo K, Kida Y, Ushiku C, Kato S, Takao-Kawabata R, Kuroda T, Changes in the contents of enzymatic immature, mature, and non-enzymatic senescent cross-links of collagen after once-weekly treatment with human parathyroid hormone (1–34) for 18 months contribute to improvement of bone strength in ovariectomized monkeys, Osteoporos. Int 22 (2011) 2373–2383. 10.1007/s00198-010-1454-4. [DOI] [PubMed] [Google Scholar]
- [118].Liu C-J, Yang X, Mao Y, Zhang X-X, Wu X-T, Wang S-H, Fan Y-B, Sun L-W, The alteration of advanced glycation end products and its potential role on bone loss under microgravity, Acta Astronaut. 206 (2023) 114–122. 10.1016/j.actaastro.2023.02.019. [DOI] [Google Scholar]
- [119].Liu C-J, Yang X, Wang S-H, Wu X-T, Mao Y, Shi J-W, Fan Y-B, Sun L-W, Preventing Disused Bone Loss through Inhibition of Advanced Glycation End Products, Int. J. Mol. Sci 24 (2023) 4953. 10.3390/ijms24054953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].González A, Calfío C, Churruca M, Maccioni RB, Glucose metabolism and AD: evidence for a potential diabetes type 3, Alzheimers Res. Ther 14 (2022) 56. 10.1186/s13195-022-00996-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].LLabre JE, Gil C, Amatya N, Lagalwar S, Possidente B, Vashishth D, Degradation of Bone Quality in a Transgenic Mouse Model of Alzheimer′s Disease, J. Bone Miner. Res 37 (2022) 2548–2565. 10.1002/jbmr.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].LLabre JE, Trujillo R, Sroga GE, Figueiro MG, Vashishth D, Circadian rhythm disruption with high-fat diet impairs glycemic control and bone quality, FASEB J. 35 (2021) e21786. 10.1096/fj.202100610RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Bailey S, Stadelmann MA, Zysset PK, Vashishth D, Alkalay RN, Influence of Metastatic Bone Lesion Type and Tumor Origin on Human Vertebral Bone Architecture, Matrix Quality, and Mechanical Properties, J. Bone Miner. Res 37 (2022) 896–907. 10.1002/jbmr.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Carriero A, Zimmermann EA, Paluszny A, Tang SY, Bale H, Busse B, Alliston T, Kazakia G, Ritchie RO, Shefelbine SJ, How tough is Brittle Bone? Investigating Osteogenesis Imperfecta in Mouse Bone, J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res 29 (2014) 1392–1401. 10.1002/jbmr.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]


