Abstract
Background:
Higher neighborhood socioeconomic status has been favorably associated with stroke outcomes. This may be due to these areas having more beneficial resources such as recreational centers. We aimed to determine if neighborhood density of recreation centers is favorably associated with stroke outcomes.
Methods:
We conducted analyses of data from the Brain Attack Surveillance in Corpus Christi (BASIC) project, a cohort of stroke-survivors ≥45 years of age residing in Nueces County, Texas (2009–2020). We included non-Hispanic Whites and Mexican Americans with incident stroke, who were not institutionalized pre-stroke and completed baseline and follow-up assessments (N=1,392). We calculated the density of fitness and recreational sports centers within their residential census tract during year of their stroke. Outcomes included function (self-ratings on activities of daily living (ADL) and instrumental ADL), cognition (modified mini-mental state exam), depression (Patient Health Questionnaire 8), and quality of life (abbreviated Stroke-Specific Quality of Life scale (SS-QoL)). We fit confounder-adjusted gamma distributed mixed generalized linear models with a log link for each outcome and considered an interaction with stroke severity.
Results:
On average, participants were 65 years old, 53% male, and 63% Mexican American. Median recreational centers was 1.60 per square mile (IQR: 0.41 to 3.06). Among moderate-severe stroke survivors, greater density of recreation centers (75th versus 25th percentile) was associated with more favorable function and possibly quality of life (ADL/Instrumental ADL: - 4.8% change; 95% CI: −0.11, −9.27%; SS-QoL: 3.7% change, 95% CI: −0.7%, 8.2%). Minimal non-significant differences were observed among the overall stroke population and those with mild stroke.
Conclusions:
Availability of recreation centers may be beneficial for post-stroke function and quality of life among those with moderate-severe stroke. If further research confirms recreation centers to be beneficial, this could inform rehabilitation following stroke.
Introduction
Approximately 7.6 million non-institutionalized Americans (2.7% of adults) report surviving a stroke; by 2030, this number is expected to rise to 11 million (National Health and Nutrition Examination Survey 2011–2018).1,2 Stroke survivors have higher rates of functional and cognitive impairment, depression, and decreased quality of life than stroke-free adults.1 Given the rising prevalence and high burden of stroke, it’s crucial to increase our understanding of what factors may improve outcomes following stroke.
Community factors likely influence post-stroke outcomes.3–5 Many stroke survivors reside in the community (non-institutionalized settings).6,7 Often community-dwelling survivors report they do not have sufficient opportunities for social or physical activities beneficial for recovery.3 Studies have demonstrated that lower neighborhood socioeconomic status (SES) is associated with worse post-stroke outcomes, particularly among those with moderate-severe stroke severity.5,8,9 This association may result from fewer beneficial establishments, such as recreation centers. Recreation centers may benefit recovery by providing opportunities for physical activity, socialization, and cognitive practice.10,11
Few studies have considered the influence of recreation centers on stroke outcomes.11–15 Two review papers of primarily qualitative studies reported shorter distance to destinations and places to exercise and walk were beneficial for recovery.14,15 An exploratory study reported that the number of and distance to recreational facilities were among top self-reported barriers for exercise among stroke survivors.11 Three studies investigated the effect of recreation centers on post-stroke physical activity among stroke survivors capable of walking.11–13 Since the study populations were restricted to those capable of walking their findings may not reflect the broader population of stroke survivors, particularly those with more severe stroke.12,13
To overcome these limitations and build on existing research, we studied the associations between availability of recreation centers and stroke outcomes in a population-based stroke cohort. We hypothesized greater availability of recreation centers would be associated with more favorable post-stroke outcomes. We further hypothesized these associations would be stronger among those who had a moderate-severe stroke, as observed with neighborhood SES.
Methods
Study Population
We included non-Hispanic White and Mexican American first-time stroke (ischemic or intracerebral hemorrhage) survivors enrolled in the Brain Attack Surveillance in Corpus Christi (BASIC) project from 2009–2020, a population-based surveillance cohort in Nueces County, Texas. Potential stroke cases ≥45 years of age who reside in Nueces County are identified through active surveillance of daily admission logs, medical and intensive care units, and passive surveillance of discharges.16 All identified stroke cases were validated by a stroke fellowship-trained physician.16 The institutional review board at the University of Michigan and the two local hospital systems approved the BASIC project and written informed consent was obtained from all participants.
Prevalent stroke cases were excluded based on medical record documentation of prior stroke. Non-Hispanic Black, Asian, American Indian, and unknown race/ethnicity survivors were excluded due to small sample size (figure 1). Most of Nueces County, Texas residents are Hispanic (65.2%) or non-Hispanic White (28.0%).17 We further excluded those who resided in an institutionalized setting prior to stroke, those who did not complete or had a proxy (e.g., relative) interview at baseline or follow-up, and those whose residence was not matched to a census tract (figure 1). For analysis specific to outcomes of cognition, quality of life, and depression we further excluded participants enrolled in year(s) when these measures were not collected (2020, 2009, and 2009–2010, respectively).
Figure 1.
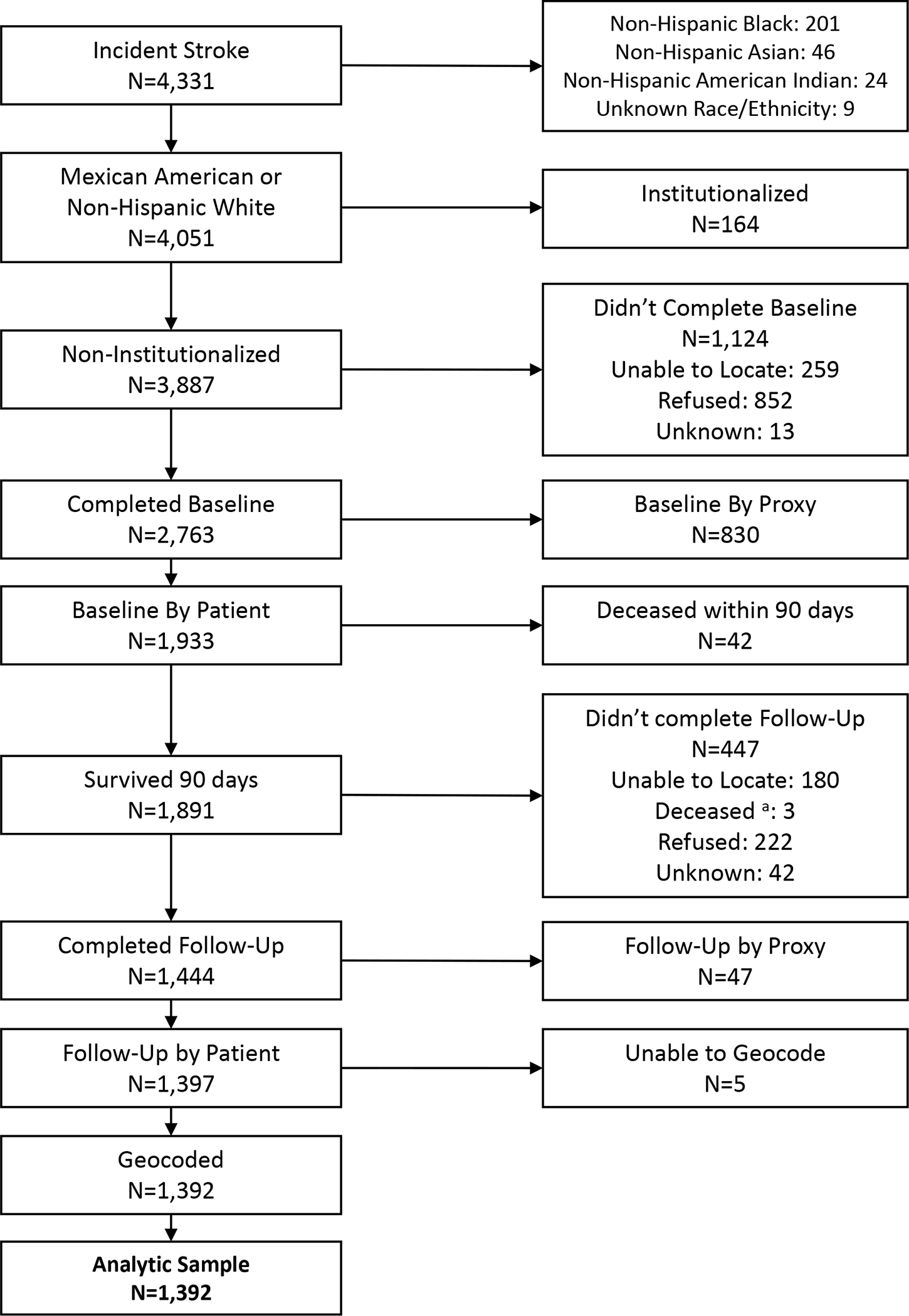
Eligibility flow chart.
a. Survived 90 days after stroke; however, died prior to completion of follow-up interview
Exposure
We assessed availability of recreation centers by density per square land mile within census tracts using data publicly available from the National Neighborhood Data Archive (NaNDA; URL: https://nanda.isr.umich.edu/), described elsewhere.18 The stroke survivors’ physical address was abstracted from their medical records and geocoded to identify the census tract 2010 FIPS code. We matched recreation center density by census tract and year of stroke for those that occurred from 2009–2017. Those that occurred from 2018–2020 (351 eligible participants) were matched with 2017, the most recent year available. Recreation centers were defined by the North American Industry Classification System code “7139” indicating all fitness and recreational sports centers (e.g., health clubs, exercise facilities, and recreational sports centers).18
Outcome
Post-stroke outcomes were assessed approximately 3-months following stroke.5,16 Function was assessed by average score (range 1–4, lower is better) of self-reported ratings for 7 activities of daily living (ADL) items and 15 instrumental ADL items (IADL).5,16 Cognitive performance was assessed using the Modified Mini-mental State Exam (3MSE), a measure of global cognitive function (range 0–100, higher is better).16 Depressive symptoms were assessed by self-report on the Patient Health Questionnaire Eight (PHQ-8; range 0–24, lower is better).5,19 Average score from the abbreviated Stroke-Specific Quality of Life scale (SS-QoL) was used to assess physical and psychosocial dimensions of quality of life (range 1–5, higher is better).5,20
Covariates
We reviewed previous related publications and created a directed acyclic graph to identify confounders to adjust for in the multivariable models.21,22 Selected confounders were assessed at baseline (shortly following the stroke).
Individual level variables were assessed by interview or medical record abstraction. Demographic variables consisted of age in years (categorized by quartiles), sex (male or female), and self-reported race/ethnicity (non-Hispanic White or Mexican American). Measures of SES included: self-reported educational attainment (less than high school, high school, post-high school) and insurance status (insured or not). Pre-stroke disability was assessed with the modified Rankin scale (range 0–4+, lower is better).23 Pre-stroke cognition was assessed by the informant questionnaire on cognitive decline in the elderly (IQCODE; range 1–5, lower is better).24 Pre-stroke depression was present if self-reported ever told by provider had depression or ever taken medication for depression. Medical records were used to determine comorbidity score and smoking status (ever smoker or not). The comorbidity score was a count of the following conditions present: amyotrophic lateral sclerosis (ALS), atrial fibrillation, cancer, chronic obstructive pulmonary disease, coronary heart disease or myocardial infarction, dementia or Alzheimer’s disease, diabetes, end-stage renal disease, epilepsy, excessive alcohol use, heart failure, hyperlipidemia, and Parkinson’s disease.5 Stroke type was classified as ischemic or intracerebral hemorrhage using standard clinical definitions.16 Initial stroke severity was scored using the NIH stroke scale (NIHSS) abstracted from medical records or determined by validated algorithm.25 This score was categorized as mild (≤5) or moderate-severe (>5) to facilitate evaluation of effect modification, as done previously.5,26,27
Interpersonal variables were assessed during the interview. Self-reported marital status was categorized as single/never married, married/living with someone, widowed, divorced/separated. A 7-item questionnaire was used to assess social support, as described previously (range 0–14, higher is better).5
Neighborhood disadvantage and affluence scores were ascertained from NaNDA.28 The disadvantage score reflects proportions of households in poverty, unemployed, female-headed, and receiving public assistance income.28,29 The affluence score reflects proportions of households with college education, incomes of at least $75,000, and employed in managerial /professional occupations.28,29 These scores were computed with data available from the US Census Bureau’s American Community Survey and provides estimates for 5-year periods (2008–2012 and 2013–2017).28 This score was linked by census tract and 5-year period closest to the year of stroke.
Data Analyses
We conducted descriptive analysis for the overall population and stratified on recreation center density quartile. Means/standard deviations, medians/interquartile ranges (IQR), and counts/percentages are provided for normally or not normally distributed continuous variables and categorical variables, respectively. Differences by recreation center density quartile were assessed by χ2 or analysis of variance tests for categorical and continuous variables, respectively. Participant population density and recreation center density quartiles were graphed by census tract.
To minimize potential selection bias due to excluding participants who did not complete an assessment or completed by proxy we applied an inverse probability weighting approach to increase the contribution of participants in the study who were similar to those excluded.5,30 We applied chained multiple imputation to retain participants with partial missing data.5,30 Missing values were imputed using data from study populations for each outcome (Table S1). Only IQCODE was missing for >5% of participants (17–19%).
We conducted a series of generalized linear regression models with the gamma family, log link function, and exchangeable working correlation structure to predict each outcome, using generalized estimating equations to account for clustering within census tract. Recreation center density was centered at the median (1.60) and rescaled by the IQR (0.41, 3.06). We assessed model fit by plotting raw and Pearson residuals against fitted values. We assessed the functional form of continuous variables and selected linear, quadratic, or categorized terms as appropriate. Covariates were sequentially added to models to observe their impact on the effect of the exposure, as specified herein: 1) unadjusted, 2) age, sex, and race/ethnicity, 3) educational attainment and insurance status, 4) pre-stroke: modified Rankin scale, IQCODE, depression, comorbidity score, , and smoking status, 5) marital status and social support score, 6) neighborhood affluence and disadvantage score, 7) stroke type and severity, and 8) interaction of the exposure and stroke severity. We tested for multi-collinearity between covariates by considering the variance inflation factors of the covariates. For ease of interpretation, estimated model parameters are exponentiated. These represent predicted proportional difference in the outcome between neighborhoods with high versus low density of recreation centers (75th versus 25th percentile). For model 8, parameters are computed for each stratum of stroke severity using the obtained coefficients for the exposure and interaction terms. To assess significance, we considered the magnitude of the coefficient for recreation center density, 95% confidence intervals (CI), and p-values. A priori, a value of p=0.05 was set for main effects and p=0.15 for effect modification.5,31 We did not adjust for multiple comparisons as this study was restricted to four planned outcomes and interpretation was not based solely on statistical testing.32 To consider the effect size of each outcome regardless of scale, we computed Hedges’ g, a standardized mean difference between two groups.
We conducted sensitivity analysis to determine robustness of results. 1) To consider potential exposure misclassification, we restricted the study population to those who reported they had not moved at follow-up. This information was available for survivors from 2014–2020. 2) To control for urbanicity we excluded persons residing in rural census tracts (≤25% of the tract population resided in an urbanized area – area containing ≥2,500 people) 3) We repeated analyses for only those with complete data. 4) We considered interactions between race/ethnicity and sex with recreation center density. 5) We considered the effect of confounders within stroke severity strata for outcomes where effect modification was identified by repeating the analysis after stratifying by stroke severity.
We conducted statistical analyses and created figures with SAS 9.4 (SAS, Institute, Inc, Cary, NC). This manuscript follows the STROBE reporting guideline.33
Results
From 2009–2020, 4,331 incident strokes were identified. The final analytic sample included 1,392 stroke survivors from 78 census tracts (MSE: 1,299, SS-QoL: 1,293, and PHQ-8: 1,188; figure 1). Figure 2 maps the relative population density of stroke survivors and quartiles of recreation center density by census tract. Table 1 provides study population characteristics overall and by quartile of recreation center density. Overall, median recreation center density was 1.60 per square mile (IQR= 0.41–3.06). Figure S1 displays the recreation centers by type (health and fitness centers was the most common identified type). On average, participants were 65 years old (standard deviation=11.1), 53% male, and 63% Mexican American. In general, the population within neighborhoods with the greatest density of recreation centers was older, had a higher proportion of non-Hispanic white stroke survivors, higher educational attainment and neighborhood affluence and lower neighborhood disadvantage. The median post-stroke outcomes for the overall population were ADL/IADL:1.77 (IQR:1.23–2.48), 3MSE:81 (IQR:73–85), SS-QoL:3.75 (IQR:2.83–4.50), and PHQ-8:5 (IQR:1–11). In general, neighborhoods with the highest density of recreation centers had more favorable scores (Table 1).
Figure 2.
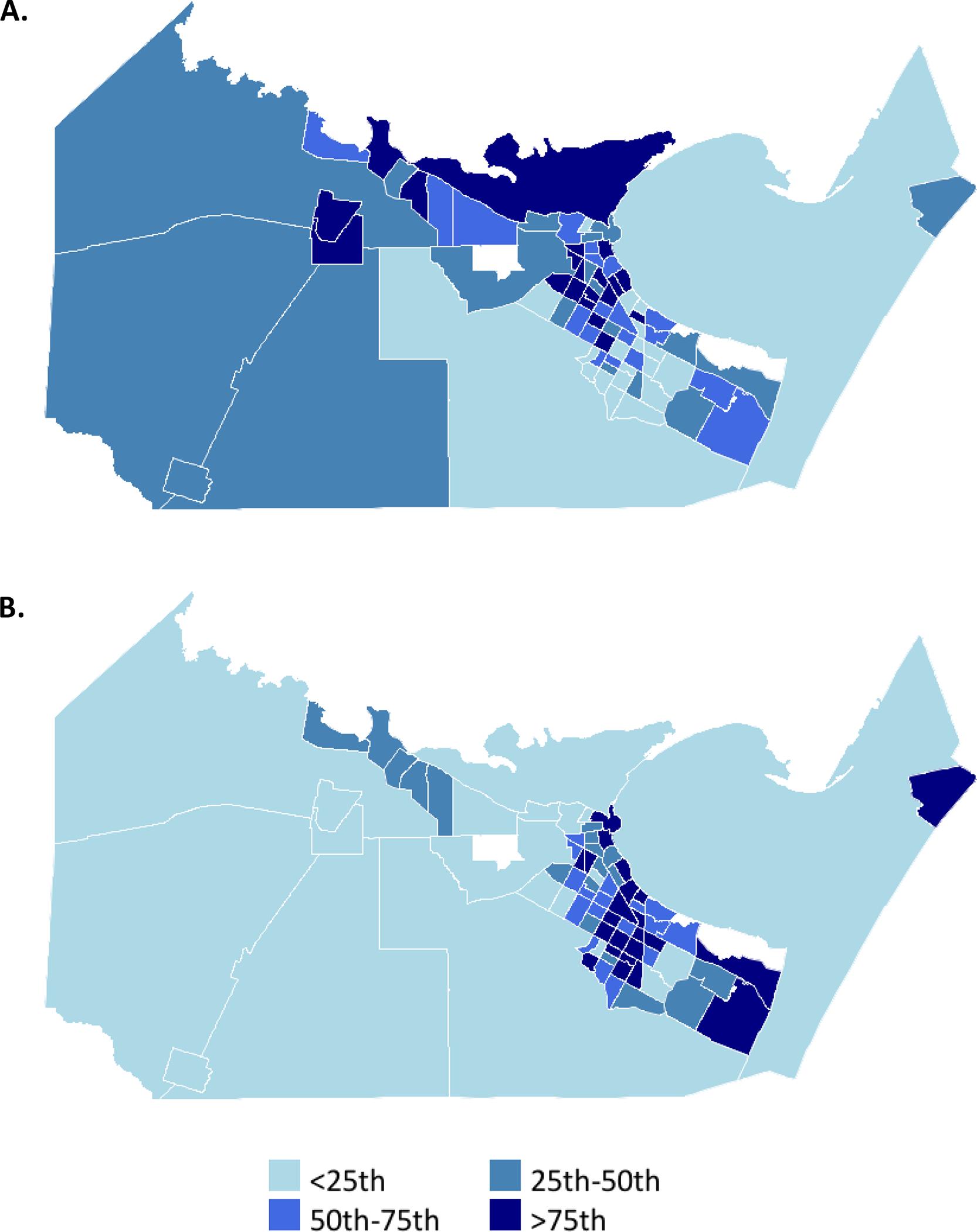
Maps 78 census tracts within Nueces County, Texas. (A) Relative stroke case population density by quartile. (B) Relative recreation center density by quartile.
Table 1.
Participant characteristics by quartiles of recreation center density among stroke survivors (N=1,392)
| Neighborhood recreation center density |
||||||
|---|---|---|---|---|---|---|
| Characteristics | Overall Sample | < 0.41 (n=346) | 0.41 to 1.60 (n=353) | 1.60 to 3.06 (n=349) | >3.06 (n=344) | P-value* |
|
| ||||||
| Recreation Center Density †, Median (IQR)‡ | 1.60 (0.41, 3.06) | 0.02 (0.00, 0.19) | 1.08 (0.70, 1.49) | 2.37 (1.84, 2.89) | 5.04 (4.13, 5.99) | <0.0001 |
| Age in years †, mean (SD) ‡ | 64.85 (11.07) | 64.47 (11.03) | 64.21 (10.52) | 64.43 (10.99) | 66.33 (11.61) | 0.0399 |
| Age Quartiles †, No. (%)‡ | ||||||
| < 56 years | 307 (22.05%) | 80 (23.12%) | 74 (20.96%) | 88 (25.21%) | 65 (18.90%) | |
| 56 to 64 years | 348 (25.00%) | 84 (24.28%) | 109 (30.88%) | 74 (21.20%) | 81 (23.55%) | |
| 64 years to 73 years | 384 (27.59%) | 99 (28.61%) | 90 (25.50%) | 102 (29.23%) | 93 (27.03%) | |
| ≥ 73 years | 353 (25.36%) | 83 (23.99%) | 80 (22.66%) | 85 (24.36%) | 105 (30.52%) | 0.0510 |
| Sex, No. (%)‡ | ||||||
| Male | 743 (53.38%) | 180 (52.02%) | 210 (59.49%) | 169 (48.42%) | 184 (53.49%) | |
| Female | 649 (46.62%) | 166 (47.98%) | 143 (40.51%) | 180 (51.58%) | 160 (46.51%) | 0.0293 |
| Race/Ethnicity, No. (%)‡ | ||||||
| Mexican American | 872 (62.64%) | 263 (76.01%) | 205 (58.07%) | 226 (64.76%) | 178 (51.74%) | |
| Non-Hispanic White | 520 (37.36%) | 83 (23.99%) | 148 (41.93%) | 123 (35.24%) | 166 (48.26%) | <0.0001 |
| Education Attainment †, No. (%)‡ | ||||||
| < High School | 396 (28.45%) | 132 (38.15%) | 88 (24.93%) | 90 (25.79%) | 86 (21.72%) | |
| High School | 413 (29.67%) | 104 (30.06%) | 116 (32.86%) | 106 (30.37%) | 87 (25.29%) | |
| > High School | 583 (41.88%) | 110 (31.79%) | 149 (25.56%) | 153 (43.84%) | 171 (29.33%) | <0.0001 |
| Health Insurance Status †,§, No. (%)‡ | ||||||
| Insured | 1141 (83.16%) | 280 (81.87%) | 295 (85.76%) | 285 (82.37%) | 281 (82.65%) | |
| No Insurance | 231 (16.84%) | 62 (26.84%) | 49 (14.24%) | 61 (17.63%) | 59 (17.35%) | 0.5165 |
| Modified Rankin Score (pre-stroke) §,∥, No. (%)‡ | ||||||
| No disability | 488 (35.44%) | 119 (34.90%) | 128 (36.78%) | 126 (36.31%) | 115 (33.72%) | |
| No significant disability | 259 (18.81%) | 51 (14.96%) | 72 (20.63%) | 70 (20.17%) | 66 (19.35%) | |
| Slight disability | 446 (32.39%) | 114 (33.43%) | 109 (31.32%) | 109 (31.41%) | 114 (33.43%) | |
| Moderate disability | 120 (8.71%) | 43 (12.61%) | 21 (6.03%) | 29 (8.36%) | 27 (7.92%) | |
| Moderately-severe to severe disability | 64 (4.65%) | 14 (4.11%) | 18 (5.17%) | 13 (3.75%) | 19 (5.57%) | 0.2143 |
| IQCODE (pre-stroke)§,∥,‡, median (IQR) ‡ | 3.00 (3.00, 3.19) | 3.00 (3.00, 3.13) | 3.00 (3.00, 3.13) | 3.00 (3.00, 3.19) | 3.00 (3.00, 3.25) | 0.2973 |
| Depression (pre-stroke) §,∥,, No. (%)‡ | ||||||
| No Depression | 907 (65.72%) | 217 (63.08%) | 236 (67.62%) | 232 (67.25%) | 222 (64.91%) | |
| Depression or Antidepressant Use | 473 (34.28%) | 127 (36.92%) | 113 (32.38%) | 113 (32.75%) | 120 (35.09%) | 0.5561 |
| Comorbid Score (pre-stroke) ∥, median (IQR) ‡ | 2.00 (1.00, 3.00) | 3.00 (2.00, 3.00) | 2.00 (1.00, 4.00) | 2.00 (1.00, 3.00) | 2.00 (1.00, 4.00) | 0.1509 |
| Smoking Status †,§, No. (%)‡ | ||||||
| Never smoked | 794 (57.12%) | 204 (58.96%) | 192 (54.55%) | 192 (55.01%) | 206 (60.06%) | |
| Smoker | 596 (42.88%) | 142 (41.04%) | 160 (45.45%) | 157 (44.99%) | 137 (39.94%) | 0.3516 |
| Marital Status †, No. (%)‡ | ||||||
| Single/Never Married | 125 (8.98%) | 32 (9.25%) | 30 (8.50%) | 34 (9.74%) | 29 (8.43%) | |
| Married/Living with Someone | 711 (51.08%) | 177 (51.16%) | 193 (54.64%) | 167 (47.85%) | 174 (50.58%) | |
| Widowed | 240 (17.24%) | 58 (16.76%) | 46 (13.03%) | 64 (18.34%) | 72 (20.93%) | |
| Divorced/Separated | 316 (22.70%) | 79 (22.83%) | 84 (23.80%) | 84 (24.07%) | 69 (20.06%) | 0.3305 |
| Social Support Scale †,§, mean (SD)‡ | 2.19 (0.53) | 2.17 (0.54) | 2.21 (0.53) | 2.20 (0.54) | 2.19 (0.53) | 0.7900 |
| Neighborhood Affluence Score †, mean (SD) ‡ | 0.26 (0.14) | 0.23 (0.12) | 0.28 (0.15) | 0.25 (0.13) | 0.29 (0.15) | <0.0001 |
| Neighborhood Disadvantage Score †, mean (SD) ‡ | 0.14 (0.07) | 0.17 (0.08) | 0.12 (0.06) | 0.14 (0.06) | 0.13 (0.07) | <0.0001 |
| Stroke Type †, No. (%)‡ | ||||||
| Ischemic | 1263 (90.73%) | 313 (90.46%) | 325 (92.07%) | 314 (89.97%) | 311 (90.41%) | |
| Intracerebral hemorrhage | 129 (9.27%) | 33 (9.54%) | 28 (7.93%) | 35 (10.03%) | 33 (9.59%) | 0.7861 |
| Stroke Severity (NIHSS) †,‡, No. (%)‡ | ||||||
| Mild (<5) | 1,075 (77.67%) | 272 (79.30%) | 278 (78.98%) | 265 (75.93%) | 260 (76.47%) | |
| Moderate-Severe (≥5) | 309 (22.33%) | 71 (20.70%) | 74 (21.02%) | 84 (24.07%) | 80 (23.53%) | 0.6230 |
| ADL/IADL score §,#,‡, median (IQR) ‡ | 1.77 (1.23, 2.48) | 1.91 (1.32, 2.68) | 1.59 (1.18, 2.38) | 1.82 (1.20, 2.46) | 1.73 (1.18, 2.36) | 0.0023 |
| 3MSE score §,#,‡,**median (IQR) ‡ | 81.00 (73.00, 85.00) | 79.00 (70.00, 84.00) | 81.50 (74.50, 86.00) | 81.00 (69.00, 85.00) | 82.00 (74.00, 86.00) | 0.0006 |
| SS-QOL score §,#,‡, ††, median (IQR) ‡ | 3.75 (2.83, 4.50) | 3.50 (2.58, 4.33) | 3.83 (3.00, 4.58) | 3.67 (2.83, 4.50) | 4.00 (3.00, 4.50) | 0.0002 |
| PHQ-8 score §,#,‡, **, ‡‡, median (IQR) ‡ | 5.00 (1.00, 11.00) | 6.00 (2.00, 11.00) | 4.00 (1.00, 10.00) | 5.00 (2.00, 11.00) | 4.00 (1.00, 10.00) | 0.1133 |
χ2 or analysis of variance test were used to test for differences between quartiles.
Representative of participant at time of stroke.
Abbreviations: IQR – Interquartile Range; SD – Standard Deviation; No. (%) – Number (Percentage); IQCODE - Informant Questionnaire on Cognitive Decline in the. Elderly; NIHSS – NIH Stroke Scale; ADL/IADL – Activities of Daily Living/ Instrumental Activities of Daily Living; 3MSE – Modified Mini-Mental State Examination; PHQ-8 – Patient Health Questionnaire Eight; SS-QOL - Stroke Specific Quality of Life Scale
Missing data for some participants.
Representative of participant pre-stroke.
Assessed at 90-day post-stroke follow-up interview.
2009–2019 stroke survivors (N=1,299) and recreation center density median=1.70, IQR=0.41–3.19.
2010–2020 stroke survivors (N=1,293) and recreation center density median=1.60, IQR=0.41–3.06.
2011–2020 stroke survivors (N=1,188) and recreation center density median=1.59, IQR=0.39–3.06.
The sequentially adjusted associations between recreation center density and post-stroke outcomes are shown in Table 2. In unadjusted analyses (model 1), we observed non-significant associations between density of recreation centers and all post stroke outcomes except for depression (Table 2, PHQ-8: 0.951, 95% CI: 0.907–0.998). These were attenuated after adjusting for confounders (Table 2, model 7, PHQ-8: 0.974, 95% CI: 0.930–1.020). Stroke severity modified the effect of neighborhood recreation center density on post-stroke function and quality of life (ADL/IADL: p=0.044; SS-QoL: p=0.137 3MSE: p=0.702; PHQ-8: p=0.698). Residing in neighborhoods with greater density of recreation centers was associated with improved function and possibly quality of life among those with moderate-severe stroke (Table 2, figures 3 and S2; −4.8% change in ADL/IADL, 95% CI: −0.11 to −9.27%; 3.7% change in SS-QoL, 95% CI: −0.7% to 8.2%). We observed small effect sizes for function, cognition, and quality of life (Hedges’ g > 0.2, Table S2).34
Table 2.
Association between neighborhood recreation center density and 3-month post-stroke outcomes after accounting for missing data and differential loss to follow-up.
| ADL/IADL* (N=1,392) | 3MSE * (N=1,299) | PHQ-8 * (N=1,198) | SS-QOL * (N=1,293) | |||||
|---|---|---|---|---|---|---|---|---|
| Model† | IQR* Proportional Difference (95% CI*) | p-value | IQR* Proportional Difference (95% CI*) | p-value | IQR* Proportional Difference (95% CI*) | p-value | IQR* Proportional Difference (95% CI*) | p-value |
|
| ||||||||
| 1 | 0.987 (0.963, 1.011) | 0.286 | 1.004 (0.997, 1.011) | 0.240 | 0.951 (0.907, 0.998) | 0.040 | 1.013 (0.996, 1.030) | 0.132 |
| 2 | 0.994 (0.969, 1.019) | 0.643 | 1.003 (0.996, 1.010) | 0.456 | 0.969 (0.921, 1.019) | 0.224 | 1.009 (0.994, 1.025) | 0.240 |
| 3 | 0.997 (0.973, 1.021) | 0.781 | 1.001 (0.995, 1.008) | 0.631 | 0.976 (0.927, 1.027) | 0.350 | 1.008 (0.994, 1.023) | 0.265 |
| 4 | 0.999 (0.975, 1.022) | 0.903 | 1.002 (0.996, 1.008) | 0.480 | 0.973 (0.928, 1.019) | 0.245 | 1.006 (0.992, 1.021) | 0.367 |
| 5 | 0.997 (0.973, 1.020) | 0.775 | 1.003 (0.997, 1.009) | 0.361 | 0.972 (0.927, 1.020) | 0.239 | 1.007 (0.994, 1.021) | 0.302 |
| 6 | 0.998 (0.974, 1.022) | 0.870 | 1.003 (0.997, 1.009) | 0.334 | 0.975 (0.930, 1.021) | 0.281 | 1.006 (0.992, 1.020) | 0.383 |
| 7 | 0.998 (0.973, 1.022) | 0.899 | 1.003 (0.97, 1.009) | 0.339 | 0.974 (0.930, 1.020) | 0.263 | 1.007 (0.992, 1.022) | 0.358 |
| 8 | 0.044‡ | 0.702‡ | 0.698‡ | 0.137‡ | ||||
| NIHSS* score ≤5§ | 1.007 (0.980, 1.035) | 0.608 | 1.002 (0.997, 1.008) | 0.430 | 0.977 (0.935, 1.021) | 0.294 | 1.002 (0.988, 1.016) | 0.801 |
| NIHSS* score >5§ | 0.952 (0.907, 0.999) | 0.045 | 1.007 (0.985, 1.029) | 0.546 | 0.957 (0.856, 1.069) | 0.435 | 1.037 (0.993, 1.082) | 0.101 |
Abbreviations: Activities of Daily Living/ Instrumental Activities of Daily Living; 3MSE – Modified Mini-Mental State Examination; PHQ-8 – Patient Health Questionnaire Eight; SS-QOL - Stroke Specific Quality of Life Scale; IQR – Interquartile Range; CI – Confidence Interval; NIHSS – NIH Stroke Scale
Generalized linear model using Gamma family with Log Link function, exponentiated estimates provided. Models 1) unadjusted, 2) age, sex, and race/ethnicity, 3) model 2 + education and insurance status, 4) model 3 + pre-stroke: function, cognition, depression, and health, 5) model 4 + marital status and social support, 6) model 5 + neighborhood disadavantage and affluence 7) model 6 + stroke type and severity, and 8) model 7 + interaction term of recreational center density and stroke severity
P-value associated with interaction term.
Among ADL/IADL: NIHSS ≤ 5 n=1,081 and NIHSS >5 n=311; among 3MSE: NIHSS ≤ 5 n=998 and NIHSS >5 n=301; among SS-QOL: NIHSS ≤ 5 n= 1,024 and NIHSS >5 n=269; among PHQ-8: NIHSS ≤ 5 n=944 and NIHSS >5 n=244. Proportional differences are computed for each stratum of stroke severity using the obtained coefficients for the exposure and interaction term.
Figure 3.
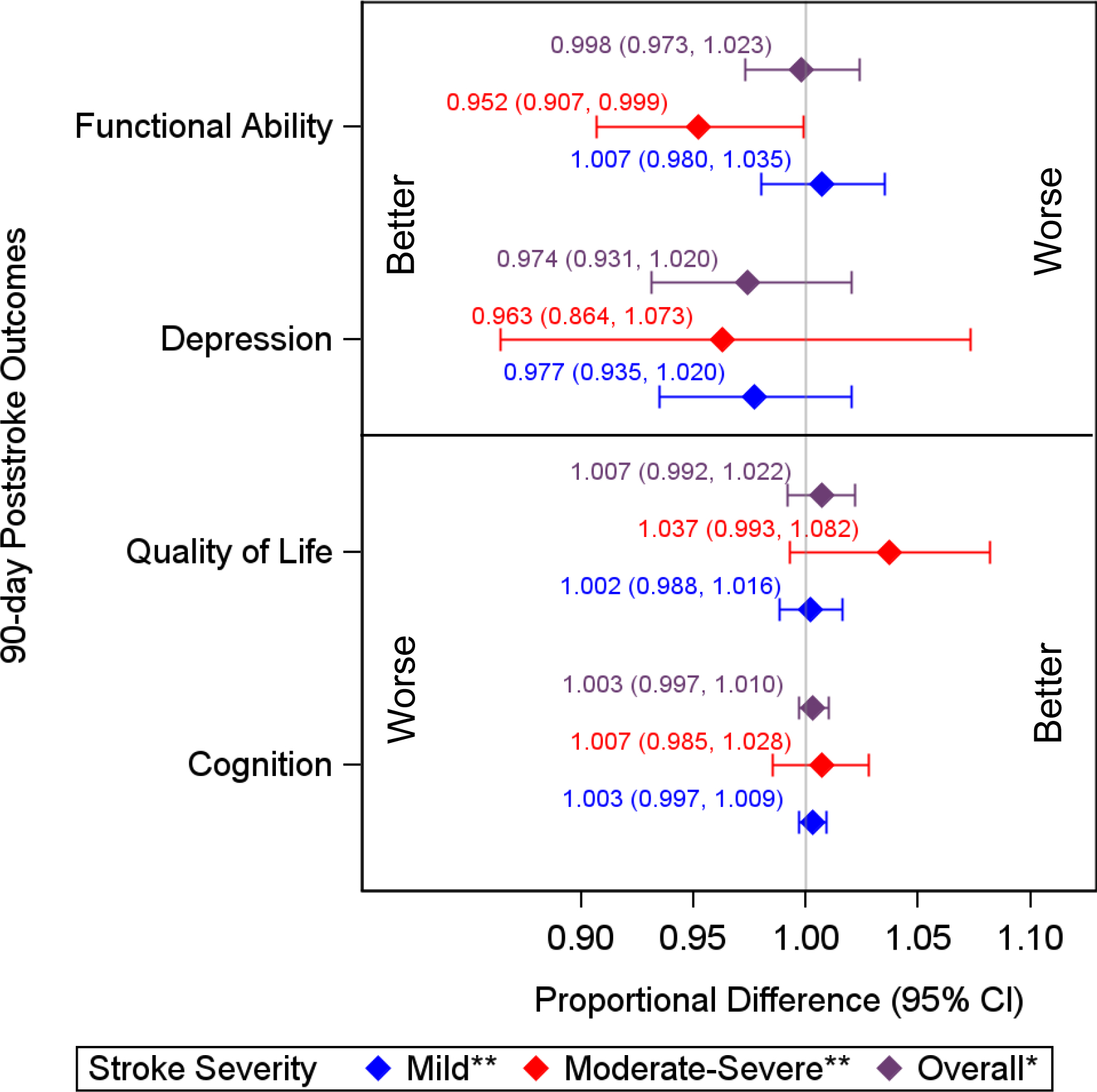
Proportional difference of 90-day post-stroke outcomes associated with IQR increase in neighborhood recreation center density by stroke severity.
*Adjusted for demographics (age, sex, race/ethnicity), individual socioeconomic status (education, health insurance), pre-stroke health (comorbidities, function, cognition, depression, smoking), interpersonal factors (marital status, social support), neighborhood socioeconomic status (neighborhood disadvantage, neighborhood affluence), stroke type and severity.
**Additionally adjusted for interaction between religious organization density and stroke severity.
In sensitivity analyses, we obtained similar conclusions. We observed possible proportional differences for ADL/IADL and SS-QoL among survivors of moderate-severe stroke in 2014–2020 and after restricting to those who did not move (figures S3–4). Excluding persons from the primary study population who resided in rural areas (n=55) yielded comparable results to the primary analyses (figure S5). Table S3 presents results from the complete case analyses, which were similar to the primary analyses. Race/ethnicity and sex did not modify the associations of interest overall or within severity strata, except for a stronger observed association with cognition among Mexican Americans with moderate-severe stroke and function among females with moderate-severe stroke (Tables S4–6). Table S7 portrays the results of stratified analyses for ADL/IADL and SS-QoL. Among moderate-severe stroke, we observed varying small impacts of identified confounders by outcome and level (individual, interpersonal, and neighborhood).
Discussion
We did not observe an association between recreation center density and post-stroke outcomes in the overall population of stroke survivors; however, we observed effect modification of the association by stroke severity for function and quality of life. Among those with moderate-severe stroke, recreation center density was associated with a small increase in function and quality of life. This study extends previous research by considering the outcomes of function, cognition, depression, and health-related quality of life, considering stroke severity as an effect modifier, using methods to minimize potential selection bias due to differential attrition, and controlling for confounding on multiple levels.
Two previous studies investigating the effect of neighborhood recreation centers on physical activity among stroke survivors limited their study population to stroke survivors capable of walking.12,13 Kanai et al. further restricted their study population to those without dementia, moderate-severe aphasia, or moderate-severe disability.12 These restrictions meant the study populations may not be generalizable to the overall stroke population.12,13 Both may have selection bias due to the inclusion of prevalent stroke survivors and differential participation potentially related to health/disability. In this study, we observed no to minimal effects among those with mild stroke, consistent with these two previous studies.12 Furthermore, the associations we observed may be due to other mechanisms such as increased socialization, rather than physical activity.
The current study findings of an interaction between stroke severity and neighborhood recreation centers support the World Health Organization International Classification of Functioning, Disability, and Health (ICF) model which posits that individual-level and environmental-level factors interact to influence disability.35 Stulberg et al. suggested neighborhood effects on post-stroke outcomes may be greater among those with more severe stroke due to greater dependence on their immediate surroundings and more room for improvement.5 The current results further support this hypothesis.
This study has some limitations to consider. The BASIC project was conducted in Nueces County, Texas and findings may not be generalizable to other regions with different racial/ethnic distributions or more rural environments. Furthermore, these results may not be generalizable to non-Hispanic Black, Asian, or American Indian survivors in the study community as these individuals had to be excluded due to small numbers. We do not know if participants used the neighborhood recreation centers or their physical/social activity levels. We chose not to adjust for multiple comparison because of pre-planned analyses and consideration of more than p-values. It’s still possible that our results are due to chance. We may have had differential participation related to stroke severity; however, we used inverse probability weighting to account for this. We used initial stroke severity as a proxy for severity at discharge. While stroke severity at admission is a predictor of disability at discharge, the capacity of participants to benefit from recreational centers may have been misclassified. We considered effect modification by initial stroke severity, sex, and race/ethnicity based on existing evidence in the literature; it’s possible other factors may modify these effects, (e.g., pre-existing depression or cognitive impairment). There is potential misclassification of our exposure due to use of census tract to define neighborhood, the use of neighborhood level data from 2017 for strokes occurring in 2018–2020, and inclusion of participants who were discharged to short-term care before arriving home. These sources of misclassification are likely non-differential and would be expected to bias our results towards the null.
There is also potential uncontrolled confounding and index event bias. Neighborhood factors such as racial/ethnic segregation, sidewalks/accommodations, and safety in the neighborhood have been associated with post-stroke outcomes and may be associated with recreation center availability.36,37 Individual characteristics may influence exposure due to self-selection into the neighborhood and may also be associated with post-stroke outcomes resulting in confounding.38,39 Furthermore, residual confounding may be present due to assessing pre-existing depression by self-report and comorbidities by medical record. Index event bias may be present due to restricting our study population to stroke survivors.40 Our comprehensive control of many common risk factors of post-stroke outcomes (models 1–7) should reduce these potential biases.
Neighborhood availability of recreation centers may be associated with post-stroke function and quality of life among those with moderate-severe stroke. Further prospective studies are needed to confirm these findings and to explore potential mechanisms by which availability of recreation centers may improve post-stroke outcomes. If availability of recreation centers is determined beneficial, this knowledge may inform discharge planning since most stroke survivors in this community and nationally are discharged directly home or to short-term rehabilitation and then home.6,7 Furthermore, rehabilitation efforts may be targeted to take advantage of or address a lack of recreation centers.
Supplementary Material
Acknowledgements
This study was performed in the Corpus Christi Medical Center and CHRISTUS Spohn hospitals, CHRISTUS Health system, in Corpus Christi, Texas.
Funding Sources
This research is supported by investigator-initiated grants (R01NS038916, Lynda Lisabeth and Lewis Morgenstern, MPIs and R01HL126700, Devin Brown and Lynda Lisabeth, MPIs) funded by the National Institute of Neurologic Disorders and Stroke (NINDS) and National Heart, Lung, and Blood Institute (NHLBI) of the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS/NHLBI/NIH.
Disclosures
Leanna Delhey reports grants from NINDS/NIH (StrokeNet, U24NS107214; R01NS038916), NHLBI/NIH (R01HL126700) and from American Heart Association (AHA, 23POST1026064). Erin Case reports grants from NIH (R01NS038916 and R01HL126700). Mellanie Springer reports employment by the Department of Neurology, University of Michigan, and grants from NINDS/NIH (K01NS117555).
Non-standard Abbreviations and Acronyms
- 3MSE
Modified Mini-Mental State Exam
- ADL
Activities of Daily Living
- AHA
American Heart Association
- ALS
Amyotrophic Lateral Sclerosis
- BASIC
Brain Attack Surveillance In Corpus Christi
- IADL
Instrumental Activities of Daily Living
- ICF
International Classification of Functioning, disability, and health
- IQCODE
Informant Questionnaire on Cognitive Decline in the Elderly
- IQR
Interquartile Range
- NaNDA
National Neighborhood Data Archive
- NHLBI
National Heart, Lung, and Blood Institute
- NIH
National Institute of Health
- NIHSS
National Institute of Health Stroke Scale
- NINDS
National Institute of Neurological Disorders and Stroke
- PHQ-8
Patient Health Questionnaire Eight
- SES
Socioeconomic Status
- SS-QoL
abbreviated Stroke Specific Quality of Life scale
- STROBE
Strengthening the Reporting of Observational studies in Epidemiology
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 2.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 3.Guo Y, Zhang Z, Lin B, Mei Y, Liu Q, Zhang L, Wang W, Li Y, Fu Z. The Unmet Needs of Community-Dwelling Stroke Survivors: A Systematic Review of Qualitative Studies. Int J Environ Res Public Health. 2021;18. doi: 10.3390/ijerph18042140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Organization WH. International Classification of Functioning, Disability, and Health: Children & Youth Version: ICF-CY. World Health Organization; 2007. [Google Scholar]
- 5.Stulberg EL, Twardzik E, Kim S, Hsu CW, Xu Y, Clarke P, Morgenstern LB, Lisabeth LD. Association of Neighborhood Socioeconomic Status With Outcomes in Patients Surviving Stroke. Neurology. 2021;96:e2599–e2610. doi: 10.1212/WNL.0000000000011988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan SU, Khan MZ, Khan MU, Khan MS, Mamas MA, Rashid M, Blankstein R, Virani SS, Johansen MC, Shapiro MD, et al. Clinical and Economic Burden of Stroke Among Young, Midlife, and Older Adults in the United States, 2002–2017. Mayo Clin Proc Innov Qual Outcomes. 2021;5:431–441. doi: 10.1016/j.mayocpiqo.2021.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgenstern LB, Sais E, Fuentes M, Ifejika NL, Jiang X, Horn SD, Case E, Lisabeth LD. Mexican Americans Receive Less Intensive Stroke Rehabilitation Than Non-Hispanic Whites. Stroke. 2017;48:1685–1687. doi: 10.1161/STROKEAHA.117.016931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egan M, Kubina LA, Dubouloz CJ, Kessler D, Kristjansson E, Sawada M. Very low neighbourhood income limits participation post stroke: preliminary evidence from a cohort study. BMC Public Health. 2015;15:528. doi: 10.1186/s12889-015-1872-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Twardzik E, Clarke P, Elliott MR, Haley WE, Judd S, Colabianchi N. Neighborhood Socioeconomic Status and Trajectories of Physical Health-Related Quality of Life Among Stroke Survivors. Stroke. 2019;50:3191–3197. doi: 10.1161/STROKEAHA.119.025874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finlay J, Esposito M, Li M, Colabianchi N, Zhou HJ, Judd S, Clarke P. Neighborhood active aging infrastructure and cognitive function: A mixed-methods study of older Americans. Prev Med. 2021;150. doi: 10.1016/j.ypmed.2021.106669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Debora Pacheco B, Guimaraes Caetano LC, Amorim Samora G, Sant’Ana R, Fuscaldi Teixeira-Salmela L, Scianni AA. Perceived barriers to exercise reported by individuals with stroke, who are able to walk in the community. Disabil Rehabil. 2021;43:331–337. doi: 10.1080/09638288.2019.1624396 [DOI] [PubMed] [Google Scholar]
- 12.Kanai M, Izawa KP, Kubo H, Nozoe M, Mase K, Koohsari MJ, Oka K, Shimada S. Association of Perceived Built Environment Attributes with Objectively Measured Physical Activity in Community-Dwelling Ambulatory Patients with Stroke. Int J Environ Res Public Health. 2019;16. doi: 10.3390/ijerph16203908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Twardzik E, Clarke PJ, Lisabeth LL, Brown SH, Hooker SP, Judd SE, Colabianchi N. The Relationship Between Environmental Exposures and Post-Stroke Physical Activity. Am J Prev Med. 2022. doi: 10.1016/j.amepre.2022.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espernberger KR, Fini NA, Peiris CL. Personal and social factors that influence physical activity levels in community-dwelling stroke survivors: A systematic review of qualitative literature. Clin Rehabil. 2021;35:1044–1055. doi: 10.1177/0269215521993690 [DOI] [PubMed] [Google Scholar]
- 15.Jellema S, van Hees S, Zajec J, van der Sande R, Nijhuis-van der Sanden MW, Steultjens EM. What environmental factors influence resumption of valued activities post stroke: a systematic review of qualitative and quantitative findings. Clin Rehabil. 2017;31:936–947. doi: 10.1177/0269215516671013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lisabeth LD, Sanchez BN, Baek J, Skolarus LE, Smith MA, Garcia N, Brown DL, Morgenstern LB. Neurological, functional, and cognitive stroke outcomes in Mexican Americans. Stroke. 2014;45:1096–1101. doi: 10.1161/STROKEAHA.113.003912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Census-Bureau U Quick facts: Nueces County, Texas. https://www.census.gov/quickfacts/nuecescountytexas. 2021. Accessed 11/29/2021.
- 18.Finlay J, Li Mao, Esposito Michael, Gomez-Lopez Iris, Khan Anam, Clarke Philippa, and Chenoweth Megan [database online]. Ann Arbor, MI: Inter-university Consortium for Political and Social Research; 2020. [Google Scholar]
- 19.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–173. doi: 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- 20.Post MW, Boosman H, van Zandvoort MM, Passier PE, Rinkel GJ, Visser-Meily JM. Development and validation of a short version of the Stroke Specific Quality of Life Scale. J Neurol Neurosurg Psychiatry. 2011;82:283–286. doi: 10.1136/jnnp.2009.196394 [DOI] [PubMed] [Google Scholar]
- 21.Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol. 2016;45:1887–1894. doi: 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- 22.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8:70. doi: 10.1186/1471-2288-8-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38:1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6 [DOI] [PubMed] [Google Scholar]
- 24.Quinn TJ, Fearon P, Noel-Storr AH, Young C, McShane R, Stott DJ. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the diagnosis of dementia within community dwelling populations. Cochrane Database Syst Rev. 2014:CD010079. doi: 10.1002/14651858.CD010079.pub2 [DOI] [PubMed] [Google Scholar]
- 25.Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke. 2000;31:858–862. doi: 10.1161/01.str.31.4.858 [DOI] [PubMed] [Google Scholar]
- 26.Marsh EB, Lawrence E, Gottesman RF, Llinas RH. The NIH Stroke Scale Has Limited Utility in Accurate Daily Monitoring of Neurologic Status. Neurohospitalist. 2016;6:97–101. doi: 10.1177/1941874415619964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlegel D, Kolb SJ, Luciano JM, Tovar JM, Cucchiara BL, Liebeskind DS, Kasner SE. Utility of the NIH Stroke Scale as a predictor of hospital disposition. Stroke. 2003;34:134–137. doi: 10.1161/01.str.0000048217.44714.02 [DOI] [PubMed] [Google Scholar]
- 28.Melendez RC P; Khan A; Gomez-Lopez I; Li M; Chenoweth M [database online]. Ann Arbor, MI: Inter-university Consortium for Political and Social Research; 2020. [Google Scholar]
- 29.Clarke P, Morenoff J, Debbink M, Golberstein E, Elliott MR, Lantz PM. Cumulative exposure to neighborhood context: consequences for health transitions over the adult life course. Res Aging. 2014;36:115–142. doi: 10.1177/0164027512470702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong L, Sanchez BN, Skolarus LE, Morgenstern LB, Lisabeth LD. Ethnic Differences in Prevalence of Post-stroke Depression. Circ Cardiovasc Qual Outcomes. 2018;11:e004222. doi: 10.1161/CIRCOUTCOMES.117.004222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiese MS, Ronna B, Ott U. P value interpretations and considerations. J Thorac Dis. 2016;8:E928–E931. doi: 10.21037/jtd.2016.08.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulz KF, Grimes DA. Multiplicity in randomised trials I: endpoints and treatments. Lancet. 2005;365:1591–1595. doi: 10.1016/S0140-6736(05)66461-6 [DOI] [PubMed] [Google Scholar]
- 33.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;18:800–804. doi: 10.1097/EDE.0b013e3181577654 [DOI] [PubMed] [Google Scholar]
- 34.Durlak JA. How to select, calculate, and interpret effect sizes. J Pediatr Psychol. 2009;34:917–928. doi: 10.1093/jpepsy/jsp004 [DOI] [PubMed] [Google Scholar]
- 35.Organization WH. How to use the ICF: A practical manual for using the International Classification of Functioning, Disability and Health (ICF). Exposure draft for comment October 2013. In: Geneva: Geneva: WHO; 2013. [Google Scholar]
- 36.Twardzik E, Clarke P, Judd S, Colabianchi N. Neighborhood Participation Is Less Likely among Older Adults with Sidewalk Problems. J Aging Health. 2021;33:101–113. doi: 10.1177/0898264320960966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz LD, Brown M, Li Y, Boots EA, Barnes LL, Jason L, Zenk S, Clarke P, Lamar M. Neighborhood Socioeconomic Resources and Crime-Related Psychosocial Hazards, Stroke Risk, and Cognition in Older Adults. Int J Env Res Pub He. 2021;18. doi: 10.3390/ijerph18105122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakao M, Izumi S, Yokoshima Y, Matsuba Y, Maeno Y. Prediction of life-space mobility in patients with stroke 2 months after discharge from rehabilitation: a retrospective cohort study. Disabil Rehabil. 2020;42:2035–2042. doi: 10.1080/09638288.2018.1550533 [DOI] [PubMed] [Google Scholar]
- 39.Zang P, Lu Y, Ma J, Xie B, Wang R, Liu Y. Disentangling residential self-selection from impacts of built environment characteristics on travel behaviors for older adults. Soc Sci Med. 2019;238:112515. doi: 10.1016/j.socscimed.2019.112515 [DOI] [PubMed] [Google Scholar]
- 40.Dahabreh IJ, Kent DM. Index event bias as an explanation for the paradoxes of recurrence risk research. JAMA. 2011;305:822–823. doi: 10.1001/jama.2011.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


