Abstract
The overall prevalence of metabolic diseases such as type 2 diabetes (T2D) and associated co-morbidities have increased at an alarming rate in the United States and worldwide. There is a growing body of epidemiological evidence implicating exposure to persistent organic pollutants (POPs), including legacy organochlorine (OC) pesticides and their bioaccumulative metabolites, in the pathogenesis of metabolic diseases. Therefore, the goal of the present study was to determine if exposure to transnonachlor, a bioaccumulative OC pesticide contaminant, in concert with high fat diet intake induced metabolic dysfunction. Briefly, male Sprague Dawley rats were exposed to trans-nonachlor (.5 or 5 ppm) in either a low fat (LFD) or high fat diet (HFD) for 16 weeks. At 8 weeks of intake, trans-nonachlor decreased serum triglyceride levels in LFD and HFD fed animals and at 16 weeks compared to LFD fed animals. Interestingly, serum glucose levels were decreased by trans-nonachlor (5 ppm) in LFD fed animals at 16 weeks. Serum free fatty acids were increased by trans-nonachlor exposure (5 ppm) in LFD fed animals at 16 weeks. HFD fed animals displayed signs of hepatic steatosis including elevated liver triglycerides, liver enzymes, and liver lipid peroxidation which were not significantly altered by trans-nonachlor exposure. However, there was a trans-nonachlor mediated increase in expression of fatty acid synthase in livers of LFD fed animals and not HFD fed animals. Thus, the present data indicate exposure to trans-nonachlor in conjunction with LFD or HFD intake produces both diet and exposure dependent effects on lipid and glucose metabolism.
Keywords: trans-nonachlor, high fat diet, persistent organic pollutant, diabetes, hepatic steatosis
Introduction
The prevalence of obesity has greatly increased over the past few decades within the United States and the world as a whole. Obesity is widely accepted as a risk factor involved in the development of other diseases, such as dyslipidemia and diabetes. In 2019, 14.7% of adults in the United States, 37.1 million people, were reported to have diabetes, with 90–95% of those cases being type 2 diabetes (T2D).1 This disease costs the healthcare industry an estimated $237 billion a year in the United States, making its increasing prevalence a great concern.2 Known risk factors for this disease are age, poor eating habits, sedentary lifestyle, and genetic predisposition, although these risk factors alone do not account for the growing prevalence of T2D and associated indices of metabolic dysfunction. One potential explanation for this growth may be exposure to persistent organic pollutants (POPs).
Recent epidemiological studies indicate exposure to certain organochlorine (OC) pesticides is positively associated with the development of insulin resistance, diabetes, and metabolic syndrome. Upon analysis of data from the National Health and Nutrition Examination Survey (NHANES) from 1999–2002, Lee et al. (2007) reported that in obese individuals the development of insulin resistance is highly correlated with the level of OC pesticides, namely oxychlordane and transnonachlor, present in the serum.3 However, when exploring the association between diabetes and serum concentration of POPs, a significant association between the prevalence of diabetes and serum concentrations of oxychlordane and dichlorodiphenyldichloroethylene (DDE) was observed.4 Interestingly, in this study, there was no association between obesity and diabetes in subjects with non-detectable levels of POPs. This observation suggests that the elevated serum concentration of POPs and not obesity promotes diabetes in these subjects. Additional studies by this group have indicated low dose exposure to POPs is also predictive of other metabolic abnormalities such as dyslipidemia and this exposure-response relationship appears to be non-monotonic in nature.5 In studies examining the prevalence of diabetes in Swedish men and women, there was a significant correlation between serum DDE and prevalence of diabetes.6,7 In addition to these studies, Turyk et al. (2009) determined that DDE exposure was significantly associated with the incidence of diabetes in a cohort of Great Lakes sport fish consumers who were followed over a period of ten years from a healthy, non-diabetic state to clinical diabetes.8,9 Recent review of the epidemiological studies examining the association between POPs exposure and diabetes by a panel assembled by the National Toxicology Program determined the strongest positive associations between POPs exposures and diabetes existed for OC pesticides, especially DDE and trans-nonachlor, and polychlorinated biphenyls (PCBs).10 To further strengthen this association, a recent meta-analysis performed by Mendes et al. (2021) demonstrated a significant positive association between both oxychlordane and trans-nonachlor and diabetes-related features such as fasting blood glucose, homeostatic model assessment for insulin resistance (HOMA-IR), or hemoglobin A1c (HbA1c).11
Recent animal studies have revealed that exposure to an environmentally relevant mixture of OC compounds, including OC pesticides (DDE, trans-nonachlor, and oxychlordane among others with DDE being the most prevalent) and PCBs, in contaminated salmon oil promotes the development of T2D, including hyperglycemia, hyperinsulinemia, and dyslipidemia, in high fat or western diet fed animals.12,13 This effect of contaminated salmon oil was eliminated upon refinement or decreasing the POPs load in the salmon oil. Additionally, Mulligan et al. (2017) determined chronic exposure to a POPs mixture containing prevalent OC and PCB compounds found in contaminated salmon oil significantly increased hepatic steatosis in male ob/ob mice.14 When examined in isolation, the prevalent OC compound DDE has been shown to significantly increase fasting blood glucose in male mice following subacute administration whereas chronic/subchronic exposure to DDE has been shown to produce a biphasic effect on fasting blood glucose in male mice consuming a high fat diet.15,16 Bondy et al. (2000) determined that the liver was a major target organ of trans-nonachlor in male Sprague Dawley rats as determined by increased liver weight, hepatocyte hypertrophy and fat vacuolization, and hepatic microsomal enzyme induction which was accompanied by an increase in circulating cholesterol following a 28-day exposure via oral gavage.17 Similar patterns of increased liver weight, hepatocyte hypertrophy, and microsomal enzyme induction were recapitulated following a 90-day exposure to trans-nonachlor in feed.18 Recent in vitro studies in immortalized and primary rat hepatocytes have demonstrated that exposure to transnonachlor can promote hepatocyte lipid accumulation which may be mediated in part by increased de novo lipogenesis.19,20 These hepatic effects of trans-nonachlor are significant given the key role of the liver in T2D pathogenesis and that hepatic steatosis is a common pathophysiological alteration in T2D.
Despite this growing amount of evidence for correlations between POPs and T2D, there is a relative lack of empirical evidence for the individual compound effects, underlying mechanisms, and direct relationship between the 2. This study was designed to determine any cause-and-effect relationship between trans-nonachlor and diabetes and related pathophysiological alterations by subchronically exposing male Sprague Dawley rats to different concentrations (0, .5, or 5 ppm) of the organochlorine compound in a high-fat or low-fat diet over the duration of 16 weeks. Both food intake and body weight were measured longitudinally over the course of transnonachlor and experimental diet exposure. At 8 weeks or the end of 16 weeks, key physiological indices of T2D and metabolic syndrome were assessed including fasting glucose, insulin, triglycerides, cholesterol, free fatty acids, and hepatic steatosis as well as potential mechanistic mediators including alterations in genes governing hepatic lipid and glucose homeostasis and indices of hepatic oxidative stress status.
Materials and Methods
Animal Care
Fifty-four male Sprague Dawley (SD) rats were purchased from Envigo (Indianapolis, IN) at 4 weeks of age and housed individually in an AAALAC-approved animal facility. Animals were on 12-h light/dark cycles with access to food and water ad libitum unless being fasted for blood glucose monitoring. One week of acclimation was allowed before the study began. The Mississippi State University Animal Care and Use Committee approved all animal protocols prior to implementation.
Experimental Design
To determine the effect of subchronic exposure to trans-nonachlor on the development of obesity and T2D, male SD rats were placed on either a high-fat diet (HFD) or low-fat diet (LFD) with either no trans-nonachlor (0 ppm), .5 ppm of trans-nonachlor, or 5 ppm of trans-nonachlor incorporated into the feed. After a week of acclimation on normal rodent chow, the animals were randomly divided into 6 experimental groups (n = 9/group): (1) LFD + 0 ppm, (2) .5 ppm trans-nonachlor in LFD, (3) 5 ppm trans-nonachlor inLFD, (4) HFD + 0 ppm, (5) .5ppm trans-nonachlor in HFD, (6) 5 ppm trans-nonachlor in HFD. The LFD (Research Diets, D12450J) consisted of 10% of total kcal from fat (lard), 20% from protein, and 70% from carbohydrates whereas the HFD (Research Diets, D12451) consisted of 60% of total kcal from fat (lard), 20% from protein, and 20% from carbohydrates. Access to diet and water was allowed ad libitum for 16weeks.Animals were weighed each week to track any fluctuation in weight gain. Weekly food intake was monitored by weighing the amount of food prior to addition at the beginning of the week and weighing the leftover food at the end of the week and calculating the difference. Following 8 weeks of diet intake and trans-nonachlor exposure, fasting blood glucose levels were determined by handheld glucometer and blood samples were taken via the tail vein for serum separation to assess serum insulin and triglyceride levels at the midway point of exposure. At the end of the study, the animals were euthanized by CO2 asphyxiation after a six-hour fasting period. Organs including the liver, heart, kidneys, and epididymal adipose tissue were harvested in addition to collection of whole blood for serum isolation. Organ and body weights were obtained at necropsy.
Measurement of Systemic Glucose Homeostasis, Insulin Resistance, and Dyslipidemia
HOMA-IR was utilized to determine the development of insulin resistance in all of the animals following a 6 hour fast and euthanasia. Following euthanasia fasting serum glucose (Glucose Colorimetric Assay Kit; Cayman Chemical) and fasting serum insulin (Ultra Sensitive Rat Insulin ELISA kit; Crystal Chem) levels were measured from the serum via commercially available assays.Glucose measurements at 8 weeks are expressed as fasting blood glucose (mg/dl) due to being measured by handheld glucometer and not from separated serum. HOMA-IR was calculated using the formula: fasting serum insulin (ng/ml) x fasting serum glucose (mg/dl)/22.5, as previously performed.21 In order to determine the effect of exposure to trans-nonachlor on dyslipidemia promotion, commercially available assays were used to measure serum triglyceride (Triglyceride Colorimetric Assay Kit; Cayman Chemical), free fatty acid (EnzyChrom™ Free Fatty Acid Assay Kit; BioAssay Systems), and total cholesterol (EnzyChrom™ Cholesterol Assay Kit; BioAssay Systems) concentrations per the manufacturer’s protocols as previously performed.16
Measurement of NAFLD and Liver Injury
As an index of hepatic steatosis, levels of triglycerides within the liver were measured using commercially available assays as previously performed.14,16 Per the manufacturer’s protocols, hepatic triglyceride was measured using the Triglyceride Colorimetric Assay Kit from Cayman Chemical and normalized to mg of tissue used. To assess hepatic injury which typically accompanies hepatic steatosis/steatohepatitis, serum levels of alkaline phosphatase (ALP) and alanine aminotransferase (ALT) were determined via an ACE Alera Clinical Chemistry analyzer (Alfa Wasserman Diagnostic Technologies).
Hepatic Glucose and Lipid Metabolism Gene Expression
To determine if exposure to trans-nonachlor had an effect on the expression of genes governing gluconeogenesis, phosphoenolpyruvate carboxykinase (Pepck) and glucose-6-phosphatase (G6pase), and genes governing glycogen metabolism, glycogen synthase (Gys) and glycogen phosphorylase L (Pygl), were measured by real time PCR. To determine if this exposure had an effect on the expression of genes involved in lipid metabolism within the liver, genes involved in fatty acid oxidation, carnitine palmitoyltransferase 1α (Cpt-1α) and peroxisomal acyl-CoA oxidase (Acox-1), as well as genes involved in lipogenesis including sterol regulatory element-binding protein 1 (Srebp-1c), fatty acid synthase (Fasn), and stearoyl-CoA desaturase 1 (Scd-1) were measured using real time PCR and the ΔΔCt method of analysis.14 Additionally, expression of inflammatory mediators F4/80, Tnfα, IL-6, and IL-1β were measured to determine monocyte/macrophage infiltration (F4/80) and pro-inflammatory cytokine (Tnfα, IL-6, and IL-1β) expression. Alteration of liver P450 genes Cyp3a1, Cyp2b2, and Cyp2c6 were also measured to assess effects of diet and trans-nonachlor on key mediators of xenobiotic metabolism. Briefly, total RNAwas isolated using the PureLink Total RNA Isolation kit (Ambion), cDNA was synthesized from 1 μg of the obtained total RNA using the Verso cDNA Synthesis kit (Thermo Scientific), and real time PCR was performed using Sybr Green detection (Sybr Select Master Mix; Life Technologies).14,19 Primer-BLAST software was used to design primer pairs and melting curve analysis was performed to detect possible primer-dimers. The data is expressed as fold change from the average of the control for the study, the LFD + 0 ppm fed animals. Primer sequences are provided in Supplementary Table 1.
Liver Oxidative Stress Status
In order to determine liver lipid peroxide levels, thiobarbituric acid reactive substances (TBARS) assay was utilized to determine the hepatic malondialdehyde levels as a measure of oxidative stress as previously performed.14 Liver glutathione content was also determined as an index of the overall status of antioxidants following the exposure to trans-nonachlor as previously performed.14 Briefly, to perform the TBARS assay, a 100 mg/ml homogenate of liver was made in 1.15% KCl then 200 μL of the tissue homogenate was mixed with 200 μL of 10% trichloroacetic acid (TCA) and centrifuged at 14,000 rpm for 10 minutes. Following centrifugation, 300 μL of the supernatant was collected and added to an equal amount of .67% thiobarbituric acid, vortexed, and incubated at 90–95°C for 30 minutes. After cooling on ice, the fluorescence was determined at an excitation of 530 nm and emission of 590 nm. The concentrations were calculated from an MDA standard curve and lipid peroxidation was expressed as nmoles of MDA per mg of protein.
To determine the liver glutathione content, 100 μL of liver homogenate was added to 100 μL of 4% sulfosalicylic acid and 800 μL of .05 M Tris-HCl (pH 7.7). This was incubated on ice for 30 minutes then centrifuged for 10 minutes at 14,000 g and 4°C. After centrifugation, 190 μL of the supernatant was collected and 10 μL of 40% Tris-base was added to in order to neutralize the supernatant. Following neutralization, 10 μL of 5,5’-dithio-2-nitrobenzoic acid (DTNB) was added and mixed. Absorbance was read at 412 nm and concentrations determined from a standard curve of reduced glutathione. The liver glutathione concentrations were expressed as μmoles of glutathione per mg protein.
Serum trans-nonachlor measurement
Serum levels of trans-nonachlor were quantified by methods previously described by our laboratory for other OC pesticide metabolites such as DDE.15,16 Briefly, 500 μl of serum was spiked with 50 μl of the internal standard, 13C-trans-nonachlor in hexane, at a final concentration of .01 μg/ml then samples were vortexed for 1 minute then deproteinized by the addition of 2 mL acetonitrile. Samples were then centrifuged, and the supernatant collected and diluted with 2 mL of deionized water prior to loading onto a solid phase extraction column (DPX Labs). Columns were washed, eluted with 1 mL of 1:1 (v/v) ethyl acetate/hexane 2 times, eluents dried under a stream of N2, and then ultimately resuspended in 50 μl of ethyl acetate/hexane for GC/MS analysis.
Trans-nonachlor concentrations were determined by isotope dilution GC/MS (Agilent Technologies 6890N gas chromatograph connected to a 5975C triple-axis mass spectrometer) via modification of CDC method 6015.01 as previously performed.15,16 Targeted mass analysis was performed in electron ionization (EI+) mode utilizing single ion monitoring (SIM) for trans-nonachlor. The mass spectral ions monitored were 406.78 and 408.78 [M-Cl2];[M + 4-Cl2] for trans-nonachlor and 416.82 and 418.82, [L + 2-Cl];[L + 4-Cl] for the internal standard, 13C-trans-nonachlor. Agilent ChemStation software was used to quantify analyte peaks acquired by SIM. Limits of quantitation were 100 pg/L trans-nonachlor and mean percent recovery for trans-nonachlor was more than 85%. Analyte areas under the curve were converted to ng/mL using a standard curve generated from serum spiked with 5 concentrations of trans-nonachlor.
Statistical Analysis
Data collected from this study are expressed as the mean ± standard error of the mean (SEM). Any statistically significant differences between diet and treatment were determined by two-way analysis of variance (ANOVA) with Tukey’s post hoc test for pairwise comparisons. For real-time PCR based gene expression comparisons, significant differences were calculated using the ΔCt value rather than fold change calculations. Statistically significant differences between groups were found using a P-value of ≤.05.
Results
Effects of Diet and Trans-nonachlor on Body Weight, Food Intake, and Daily Trans-nonachlor Exposure
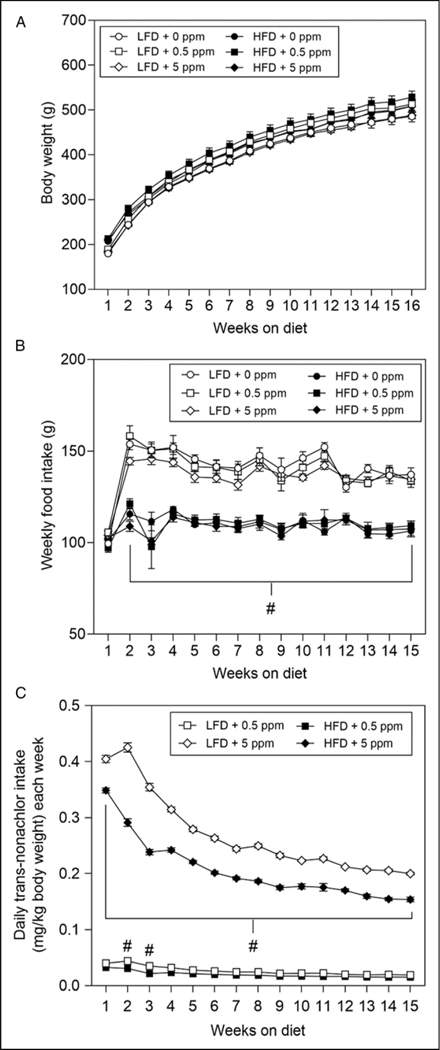
As expected, the body weights of animals on LFD and HFD increased weekly (Figure 1A). Although the body weights of animals on the HFD were slightly higher than those of the animals on LFD, these elevated weights were not significantly higher than the corresponding LFD fed animals. Additionally, there was no effect of trans-nonachlor (.5 ppm or 5 ppm) on body weight over time compared to either the diet matched control or vs the alternative diet exposure groups. While there was no significant alteration in body weights due to diet or trans-nonachlor exposure, there was a significant diet-induced decrease in weekly food intake (Figure 1B). Animals on the HFD had a significantly decreased food intake by approximately 21% compared to their corresponding LFD exposure groups. There was no significant effect of trans-nonachlor exposure on food intake in LFD or HFD fed animals. Interestingly, this reduction in food intake in the HFD groups did not result in a corresponding decrease in body weights indicating a more efficient feed conversion in the HFD fed animals. When daily trans-nonachlor dosing was calculated on a mg/kg body weight basis for each week, there as a higher dosage in the 5 ppm trans-nonachlor LFD and HFD groups compared to the .5 ppm trans-nonachlor LFD and HFD groups as expected. However, the average daily dosing per week was significantly higher in the 5 ppm trans-nonachlor in LFD groups compared to the 5 ppm trans-nonachlor in HFD groups throughout the experiment.
Figure 1.
Effects of trans-nonachlor in LFD or HFD on weight gain, food intake, and average daily trans-nonachlor exposure. Male Sprague Dawley rats were fed either LFD or HFD with 0, .5 or 5 ppm of trans-nonachlor for 16 weeks. Body weights (A), food intake (B), and average daily trans-nonachlor exposure (C) on a mg/kg body weight basis were determined on a weekly basis. Data represents the mean ± SEM of n = 9 animals/group. #P ≤ .05 vs the corresponding dose of trans-nonachlor in LFD.
Alterations in Major Organ Weights Following Trans-nonachlor Exposure in LFD or HFD
Major organ weights and organ/body ratios were determined upon necropsy to evaluate the effects of diets and trans-nonachlor exposures on organ mass (Table 1). Exposure to trans-nonachlor (.5 ppm) in LFD fed animals significantly increased liver weight by 11.6% compared to LFD + 0 ppm. However, when expressed as the liver/body ratio, this effect was not significant. Epididymal adipose tissue weights were significantly increased in HFD (28.8%) and HFD + 5 ppm trans-nonachlor (25.5%) animals compared to their corresponding LFD exposure groups. These increases in adipose tissue weight were also significantly elevated when expressed as the epi/body ratio. Gross kidney weights and kidney/body ratios were significantly decreased in the LFD + 5 ppm trans-nonachlor (12.3% gross weight; 12.7% ratio) and HFD (13% gross weight; 16.7% ratio) groups compared to the LFD + 0 ppm group. In addition, when expressed as the kidney/body ratio, LFD + .5 ppm trans-nonachlor animals had significantly lower ratios (15%) compared to the LFD + 0 ppm group. With regards to heart effects, animals exposed to HFD + .5 ppm trans-nonachlor (11.4%) had significantly higher heart weights compared to LFD + .5 ppm trans-nonachlor animals. However, these effects on the heart were not statistically significant when adjusted for body weight.
Table 1.
Effects of LFD or HFD Intake With trans-nonachlor on Weights of Major Organ Systems.
| Organ weights and organ/body ratios following LFD or HFD intake with or without trans-nonachlor for 16 weeks | ||||||
|---|---|---|---|---|---|---|
| Orqan (g) | LFD + 0 ppm | LFD + 0.5 ppm | LFD + 5 ppm | HFD + 0 ppm | HFD + 0.5 ppm | HFD + 5 ppm |
| Liver | 12.9 (0.4) | 14.4 (0.3) * | 13.7 (0.3) | 12.7 (0.2) | 12.8 (0.5)# | 13.0 (0.5) |
| Epididymal adipose (Epi) | 97 (0.5) | 10.5 (0.9) | 9.0 (0.6) | 12.5 (0.5)# | 11.9 (0.7) | 11.3 (1.2)# |
| Kidney | 1.46 (0.06) | 1.3 (0.03) | 1.28 (0.03) * | 1.27 (0.05)# | 1.4 (0.04) | 1.34 (0.05) |
| Heart | 1.58 (0.05) | 1.49 (0.06) | 1.48 (0.03) | 1.57 (0.06) | 1.66 (0.06) # | 1.61 (0.04) |
| Liver/body | 0.0268 (0.0006) | 0.0283 (0.0002) | 0.0282 (0.0003) | 0.0252 (0.0003) # | 0.0244 (0.0005) # | 0.0257 (0.0008) # |
| Epi/body | 0.0199 (0.0007) | 0.0204 (0.0014) | 0.0185 (0.0010) | 0.0247 (0.0008) # | 0.0226 (0.0012) | 0.0221 (0.0019)# |
| Kidney/body | 0.003 (0.00016) | 0.00255 (0.00004) * | 0.00262 (0.00004) * | 0.0025 (0.00008) # | 0.00267 (0.00005) | 0.00267 (0.00006) |
| Heart/body | 0.00328 (0.00011) | 0.00292 (0.00012) | 0.00304 (0.00007) | 0.00309 (0.00011) | 0.00315 (0.00008) | 0.00318 (0.00006) |
Major Organs Including Liver, Epididymal Adipose Tissue, Kidney, and Heart Were Weighed Upon Necropsy Following 16 weeks of LFD or HFD Intake With 0, .5 or 5 ppm of trans-nonachlor to Assess Alterations in Organ size. Weights are Expressed as Crude Organ Weight and Organ Weight/Body Weight Ratios. Data Represents the Mean ± SEM of 9 Animals/Group.
P ≤ .05 vs 0 ppm Exposed LFD Animals,
P ≤ .05 vs the Corresponding Dose of trans-nonachlor in LFD.
Effects of Diet and Trans-nonachlor on Systemic Glucose and Lipid Metabolism
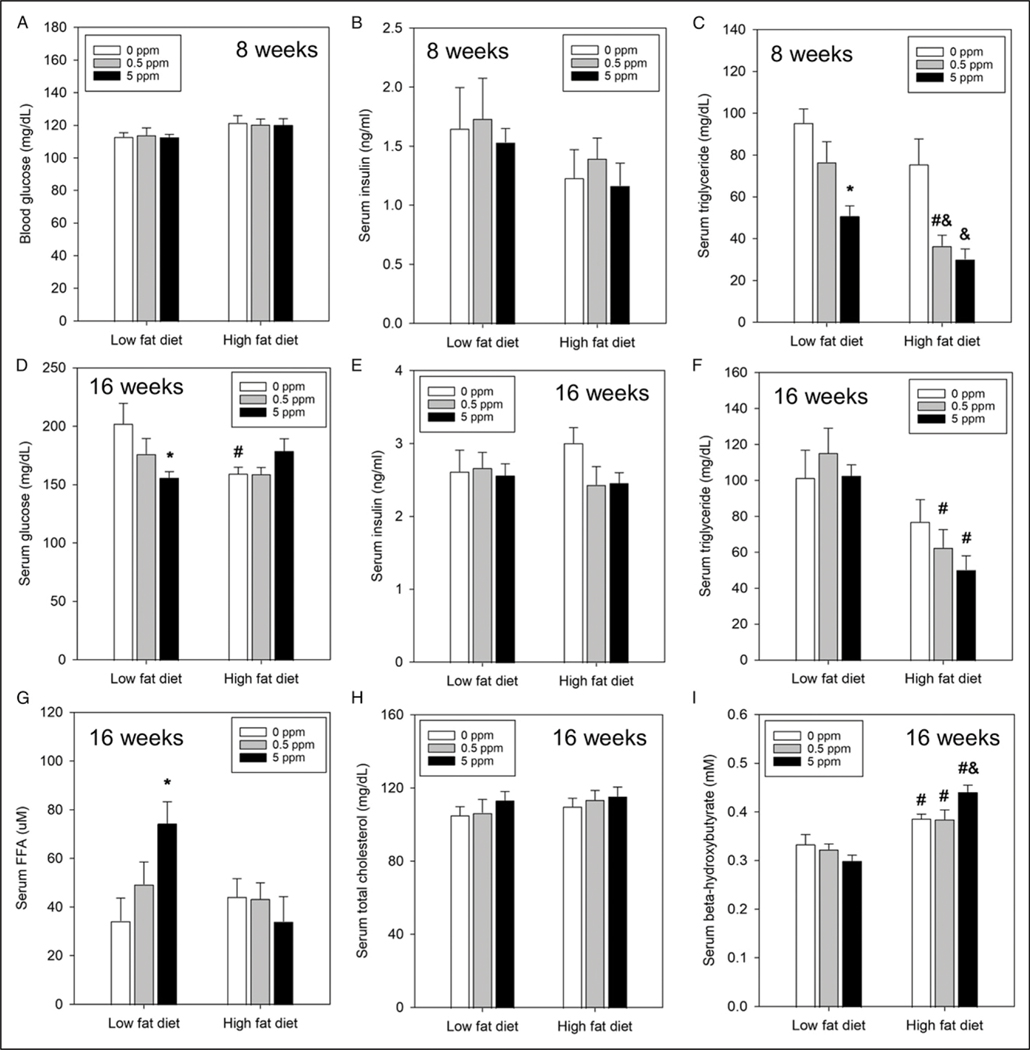
To determine if diet and/or exposure to trans-nonachlor altered systemic glucose or lipid metabolism, key indices of each were measured at 8 or 16 weeks from the start of experimental diet consumption. Following 8 weeks of diet consumption there were no significant alterations in fasting blood glucose (Figure 2A) or serum insulin (Figure 2B) concentrations. However, at 16 weeks of diet consumption, there were significant alterations in fasting serum glucose levels (Figure 2D). Exposure to trans-nonachlor (5 ppm) significantly decreased serum glucose levels in LFD fed animals compared to LFD + 0 ppm fed animals. Additionally, animals fed HFD + 0 ppm had significantly lower serum glucose levels compared to LFD + 0 ppm fed animals. These alterations in serum glucose at 16 weeks were not accompanied by alterations in fasting insulin levels (Figure 2E).
Figure 2.
Alterations in blood/serum biochemical indices of metabolic function by diet and trans-nonachlor. Blood glucose (A), serum insulin (B), and serum triglycerides (C) were measured at 8 weeks of diet intake whereas serum glucose (D), serum insulin (E), serum triglycerides (F), serum free fatty acids (G), serum total cholesterol (H), and beta-hydroxybutyrate (I) were measured upon necropsy at 16 weeks to assess alterations in glucose homeostasis and dyslipidemia. Data represents the mean ± SEM of 7–9 animals/group. *P ≤ .05 vs 0 ppm exposed LFD animals, #P ≤ .05 vs the corresponding dose of trans-nonachlor in LFD, &P ≤ .05 vs 0 ppm exposed HFD animals.
Regarding lipid metabolism, exposure to trans-nonachlor (5 ppm) significantly decreased fasting serum triglyceride levels by ~47% at 8 weeks in LFD fed animals compared to LFD + 0 ppmfed animals and by ~60% inHFD fed animals compared to HFD + 0 ppm fed animals (Figure 2C). Additionally, exposure to trans-nonachlor (.5 ppm) significantly decreased fasting serum triglyceride levels by ~52% at 8 weeks in HFD fed animals compared to HFD + 0 ppm fed animals and by ~52% compared to LFD + trans-nonachlor (.5 ppm) fed animals. At 16 weeks on the experimental diets, the decrease in serum triglyceride in trans-nonachlor (5 ppm) in LFD fed animals was not observed (Figure 2F). However, the significant decrease in serum triglyceride in HFD + trans-nonachlor (.5 ppm; ~46% decrease) fed animals compared to LFD + trans-nonachlor (.5 ppm) fed animals was still present. There was a significant decrease in serum triglyceride following exposure to HFD + trans-nonachlor (5 ppm) fed animals compared to LFD + trans-nonachlor (5 ppm) fed animals at 16 weeks. In addition to alterations in serum triglyceride, exposure to trans-nonachlor significantly increased fasting serum free fatty acids (FFA) in the LFD + trans-nonachlor (5 ppm) fed animals by ~117% compared to LFD + 0 ppm fed animals at 16 weeks of intake (Figure 2G). Serum β-hydroxybutyrate (β-HB), an index of ketosis and fatty acid oxidation, was significantly increased in HFD fed animals compared to their corresponding LFD + trans-nonachlor (0 ppm, .5 ppm,or 5 ppm) fed controls at 16 weeks of intake (Figure 2I). There were no alterations in serum total cholesterol (TC) at 16 weeks (Figure 2H). Serum levels of FFA, β-HB, and TC were unavailable for the 8-week time point due to limited sample volumes.
Effects of Diet and Trans-nonachlor on NAFLD and Indices of Liver Damage
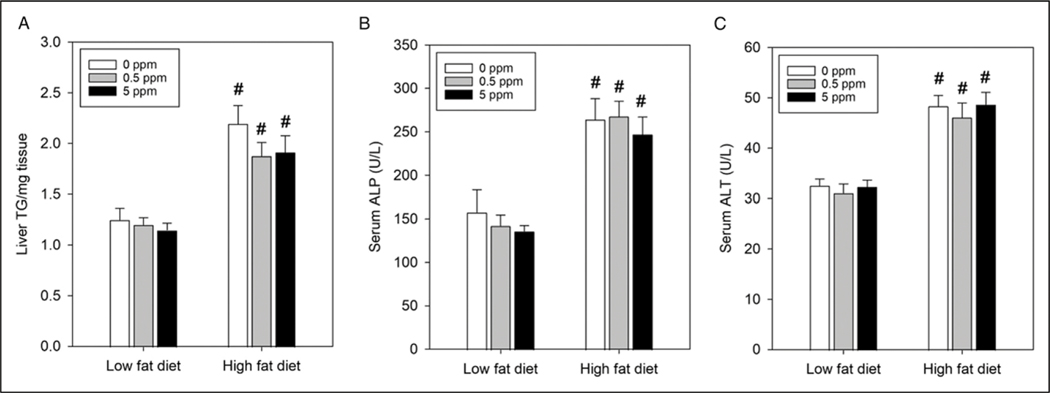
To determine if diet and/or exposure to trans-nonachlor altered hepatic steatosis, liver triglyceride content was measured to determine the degree of steatosis and serum ALP and ALT were measured to determine if diet or exposures were producing liver damage. Overall, animals on HFD exhibited significantly higher levels of hepatic triglyceride content compared to their corresponding LFD groups (Figure 3A). HFD + 0 ppm animals had an overall increase of 76% in hepatic triglyceride compared to LFD + 0 ppm animals whereas HFD + .5 ppm and HFD + 5 ppm trans-nonachlor animals had increases of 56% and 67% compared to LFD + .5 ppm and LFD + 5 ppm trans-nonachlor animals, respectively. These increases in hepatic triglyceride content corresponded with increases in serum ALP and ALT values. HFD + 0 ppm animals had an overall increase of 67% in serum ALP compared to LFD + 0 ppm treated animals (Figure 3(B)). HFD + .5 ppm and HFD + 5 ppm trans-nonachlor animals had increases of 88% and 82% compared to LFD + .5 ppm and LFD + 5 ppm trans-nonachlor animals. A similar trend was observed with serum ALT values where HFD + 0 ppm animals had an overall increase of 48% in serum ALT compared to LFD + 0 ppm animals (Figure 3C). HFD + .5 ppm and HFD + 5 ppm trans-nonachlor animals had increases of 48% and 51% compared to LFD + .5 ppm and LFD + 5 ppm trans-nonachlor animals, respectively. Therefore, these data indicate trans-nonachlor did not exacerbate diet-induced hepatic steatosis or liver damage.
Figure 3.
Effects of diet and trans-nonachlor exposure on hepatic steatosis. Liver triglyceride content (A) was measured as an index of hepatic steatosis and serum ALP (B) and serum ALT (C) were measured as indices of hepatic health/function following 16 weeks of LFD or HFD intake with 0, .5 or 5 ppm of trans-nonachlor. Data represents the mean ± SEM of 8–9 animals/group. #P ≤ .05 vs the corresponding dose of trans-nonachlor in LFD.
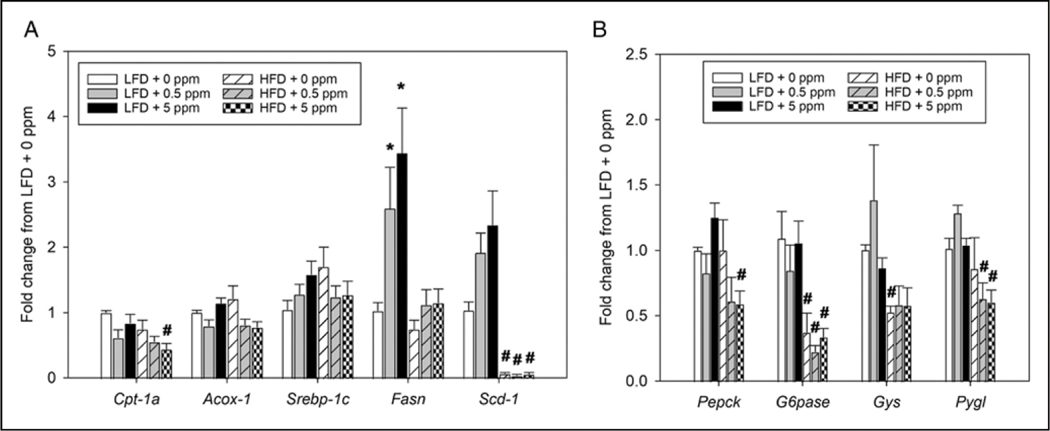
Effects of Diet and Trans-nonachlor on Hepatic Expression of Genes Governing Lipid and Glucose Metabolism
The potential alterations in hepatic expression of genes involved in both lipid and glucose metabolism were explored as potential molecular mediators which may be affected by either diet or trans-nonachlor exposure. Key genes governing both fatty acid oxidation and de novo lipogenesis were measured to assess effects on lipid production (Srebp-1c, Fasn, and Scd-1) and oxidation (Cpt-1α and Acox-1) within the liver and presumably the hepatocyte (Figure 4A). Regarding fatty acid oxidation, exposure to trans-nonachlor in addition to HFD intake (HFD + 5 ppm) significantly decreased expression of Cpt-1α, a major mediator of mitochondrial beta oxidation of long chain fatty acids, compared to animals on LFD + 5 ppm trans-nonachlor. There were no significant effects of diet or trans-nonachlor exposure on expression of Acox-1. In terms of fatty acid lipogenesis, exposure to trans-nonachlor (.5 and 5 ppm) in the LFD fed animals significantly increased the expression of Fasn compared to LFD + 0 ppm animals. There were also increases in expression of Scd-1 in animals exposed to trans-nonachlor (.5 and 5 ppm) in the LFD fed animals compared to LFD + 0 ppm animals, but these were not statistically significant. Interestingly, HFD fed animals displayed significant decreases in Scd-1 compared to their corresponding LFD + trans-nonachlor exposure groups.
Figure 4.
Effects of diet and trans-nonachlor exposure on expression of genes governing hepatic lipid production and hepatic glucose homeostasis. The expression of genes governing hepatic de novo lipogenesis and lipid oxidation (A) as well as hepatic glucose production and glycogen flux (B) were measured following 16 weeks of LFD or HFD intake with 0, .5 or 5 ppm of trans-nonachlor. Data are expressed as the fold change from LFD + 0 ppm and represent the mean ± SEM of 5–6 animals/group. *P ≤ .05 vs 0 ppm exposed LFD animals, #P ≤ .05 vs the corresponding dose of trans-nonachlor in LFD.
To determine effects on hepatic glucose homeostasis, the expression of key genes governing both glucose production (Pepck and G6pase) and glycogen storage (Gys and Pygl) were determined (Figure 4B). With regard to glucose production, exposure to trans-nonachlor (5 ppm) in HFD fed animals significantly decreased the expression of both Pepck and G6pase compared to the corresponding trans-nonachlor (5 ppm) exposure in LFD fed animals. Additionally, expression of G6pase was significantly decreased in HFD + 0 ppm and HFD + trans-nonachlor (.5 ppm) animals compared to their corresponding LFD + trans-nonachlor exposure groups. For alterations in glycogen storage, there was a diet dependent decrease in Gys expression in the HFD + 0 ppm fed group. However, exposure to .5 and 5 ppm trans-nonachlor produced significant decreases in Pygl expression in HFD fed animals compared to their corresponding LFD + trans-nonachlor exposure groups and this effect was lacking in the HFD + 0 ppm exposed group.
Effects of Diet and Trans-nonachlor Exposure on Hepatic Oxidative Stress
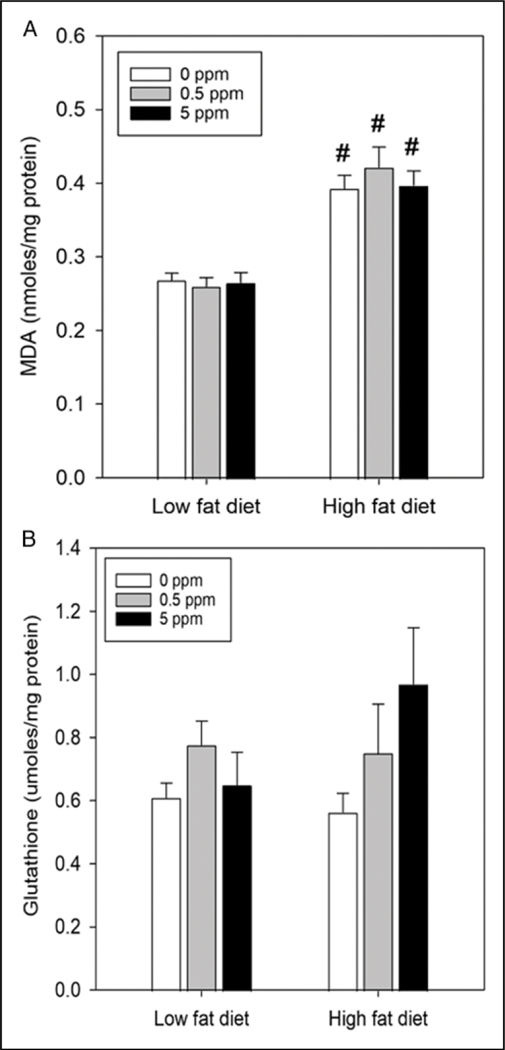
To assess the effects of diet and trans-nonachlor on oxidative stress status, hepatic lipid peroxidation was determined via TBARS assay (Figure 5A) as an index of oxidative stress and hepatic glutathione levels (Figure 5B) were determined as an index of antioxidant capacity. HFD + 0 ppm increased hepatic MDA levels by ~44% compared to LFD + 0 ppm whereas HFD + trans-nonachlor (.5 ppm) and HFD + trans-nonachlor (5 ppm) increased hepatic MDA levels by ~62% and ~48% compared to LFD + trans-nonachlor (.5 ppm) and LFD + trans-nonachlor (5 ppm), respectively. These increases in lipid peroxidation were not accompanied by significant alterations in hepatic glutathione levels. Thus, trans-nonachlor did not alter diet-induced alterations in hepatic lipid peroxidation or glutathione levels.
Figure 5.
Alterations of hepatic oxidative stress status by diet and trans-nonachlor intake. The effects of diet and trans-nonachlor on hepatic lipid peroxidation (A) was determined as an index of hepatic oxidative stress and hepatic glutathione levels (B) were determined as an index of antioxidant capacity following 16 weeks of LFD or HFD intake with 0, .5 or 5 ppm of trans-nonachlor. Data represent the mean ± SEM of 9 animals/group in (A) and 6–9 animals/group in (B). #P ≤ .05 vs the corresponding dose of trans-nonachlor in LFD.
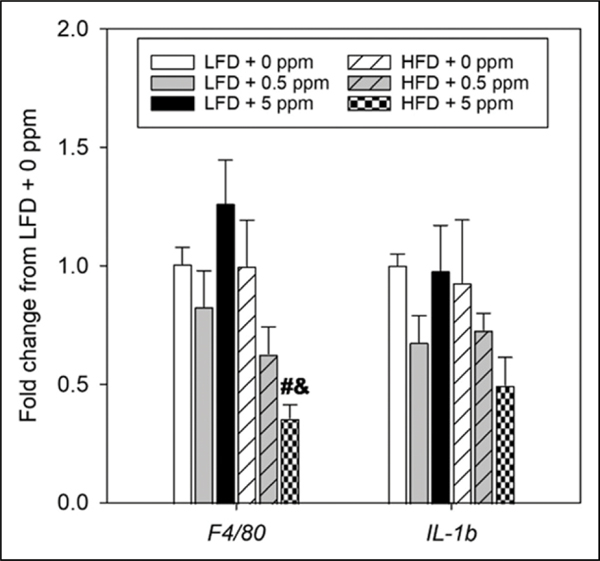
Effects of Diet and Trans-nonachlor on Expression of Hepatic Inflammatory Markers
Expression of F4/80, a monocyte/macrophage marker, and pro-inflammatory cytokines was determined to evaluate the effects of diet and trans-nonachlor exposure on cellular mediators of hepatic inflammation and key cytokines governing hepatic inflammation (Figure 6). Interestingly, expression of F4/80 and the pro-inflammatory cytokine IL-1β was not increased in the HFD fed animals regardless of trans-nonachlor exposure level. Surprisingly, exposure to trans-nonachlor (5 ppm) in the HFD fed animals significantly decreased the expression of F4/80 compared to the corresponding transnonachlor exposure level in LFD fed animals and to HFD + 0 ppm animals. A similar trend was observed for IL-1β expression however this decrease was not statistically significant. It should be noted that expression levels of Tnfα and IL-6 were undetectable in the current real-time PCR based analysis.
Figure 6.
Expression of inflammatory mediators in the livers of trans-nonachlor exposed LFD or HFD fed animals. Hepatic expression of the inflammatory mediators F4/80, a monocyte/ macrophage marker, and IL-1β, a pro-inflammatory cytokine, were measured following 16 weeks of LFD or HFD intake with 0, .5 or 5 ppm of trans-nonachlor to assess hepatic inflammatory status. Data are expressed as the fold change from LFD + 0 ppm and represent the mean ± SEM of 5–6 animals/group. #P ≤ .05 vs the corresponding dose of trans-nonachlor in LFD, &P ≤ .05 vs 0 ppm exposed HFD animals.
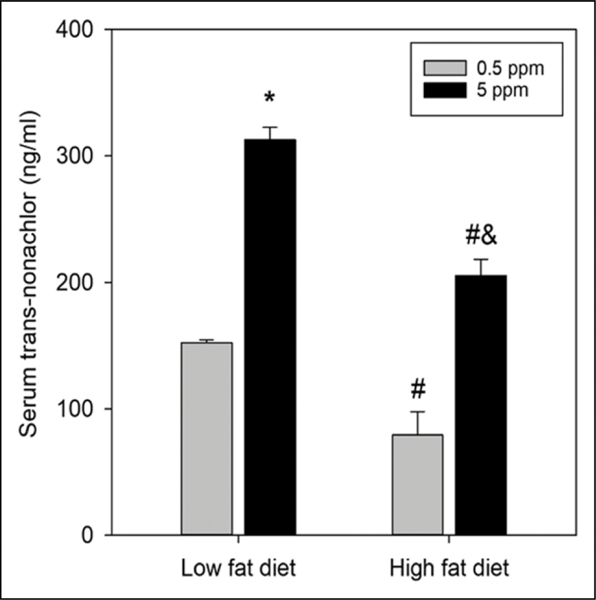
Serum Levels of Trans-nonachlor in LFD and HFD Fed Animals
Serum levels of trans-nonachlor were measured following euthanasia to determine systemic exposure levels to transnonachlor between diets and trans-nonachlor concentrations within each diet (Figure 7). As expected, there was a concentration dependent increase in serum levels of trans-nonachlor within each diet group. Specifically, there was a ~105% increase in serum trans-nonachlor levels in the LFD + trans-nonachlor (5 ppm) animals compared to the LFD + trans-nonachlor (.5 ppm) animals. There was a ~159% increase in serum trans-nonachlor levels in the HFD + trans-nonachlor (5 ppm) animals compared to the HFD + trans-nonachlor (.5 ppm) animals. Interestingly, there were significant decreases in serum trans-nonachlor levels in the HFD fed animals compared to the LFD fed animals. HFD + trans-nonachlor (.5 ppm) animals had decreased serum levels by ~48% compared to the LFD + trans-nonachlor (.5 ppm) animals whereas the HFD + trans-nonachlor (5 ppm) animals had decreased serum levels by ~34% compared to the LFD + trans-nonachlor (5 ppm) animals. Thus, there was a diet dependent effect on serum trans-nonachlor levels which may be related to the overall decrease in food intake in the HFD fed animals compared to the LFD fed animals.
Figure 7.
Determination of serum trans-nonachlor levels in LFD and HFD fed animals. Serum trans-nonachlor levels were determined following 16 weeks of diet intake and trans-nonachlor exposure to assess trans-nonachlor exposure levels. Data represents the mean ± SEM of 6 animals/group. *P ≤ .05 vs .5 ppm trans-nonachlor exposed LFD animals, #P ≤ .05 vs the corresponding dose of trans-nonachlor in LFD, &P ≤ .05 vs .5 ppm trans-nonachlor exposed HFD animals.
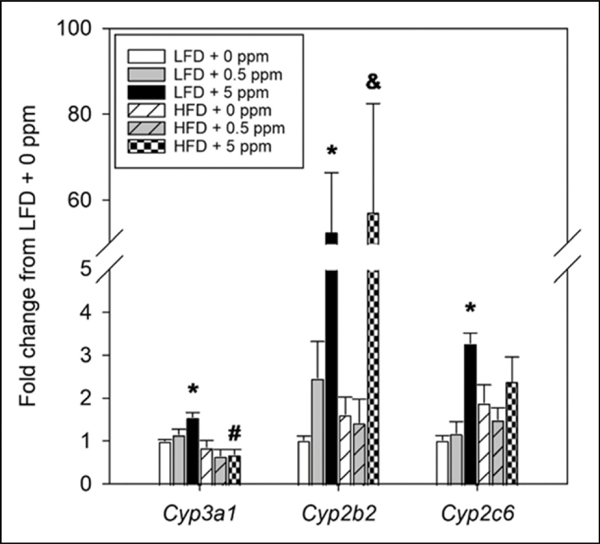
Effects of Diet and Trans-nonachlor on Expression of Hepatic P450 Isoforms
The expression of major P450 isoforms in the liver was assessed to determine if diet or trans-nonachlor exposure may alter xenobiotic metabolism capacity (Figure 8). Exposure to trans-nonachlor (5 ppm) significantly induced expression of Cyp3a1 in LFD fed animals. However, expression of Cyp3a1 was significantly decreased in trans-nonachlor (5 ppm) exposed animals on a HFD compared to their corresponding LFD + trans-nonachlor exposure groups. Expression of Cyp2b2 was significantly induced following exposure to trans-nonachlor (5 ppm)in both the LFD and HFD fed animals compared to LFD + 0 ppm and HFD + 0 ppm animals, respectively. Similar to Cyp3a1 and Cyp2b2, exposure to trans-nonachlor (5 ppm) in LFD fed animals significantly induced the expression of Cyp2c6 compared to LFD + 0 ppm fed animals.
Figure 8.
Effects of diet and trans-nonachlor exposure on hepatic P450 gene expression. The expression of major P450 isoforms was determined in the liver following 16 weeks of LFD or HFD intake with 0, .5 or 5 ppm of trans-nonachlor to assess alterations in major mediators of xenobiotic metabolism. Data are expressed as the fold change from LFD + 0 ppm and represent the mean ± SEM of 5–6 animals/group. *P ≤ .05 vs 0 ppm exposed LFD animals, #P ≤ .05 vs the corresponding dose of trans-nonachlor in LFD, &P ≤ .05 vs 0 ppm exposed HFD animals.
Discussion
As mentioned, recent epidemiological studies have implicated the POP class of environmental pollutants in producing states of metabolic dysfunction such as T2D, metabolic syndrome, and NAFLD as well as being potential obesogenic compounds [for recent reviews see10,22,23]. Within the POP class of pollutants, the OC pesticides or their bioaccumulative metabolites, such as trans-nonachlor, have been routinely associated with these phenomena. However, a cause-and-effect relationship between exposure to trans-nonachlor and T2D, metabolic syndrome, or NAFLD has not been thoroughly examined in animal studies. Thus, in the current study was examined the effects of exposure to trans-nonachlor in conjunction with LFD or HFD intake on production of metabolic dysfunction in male Sprague Dawley rats.
To determine if trans-nonachlor in conjunction with a LFD or HFD altered weight gain as an indicator of obesity, animals were fed trans-nonachlor (.5 or 5 ppm) in each diet with a corresponding no trans-nonachlor control for each diet. Body weights of animals on the HFD were slightly higher than those of animals on the LFD and there was no effect of trans-nonachlor. This lack of effect of HFD or trans-nonachlor on body weight is most likely due to the discrepancies in food intake. Animals on the HFD, regardless of trans-nonachlor exposure, had significantly lower amounts of food intake compared to animals fed LFD (Figure 1B). This decrease in HFD feed intake is in line with the theory that rodents eat for calories and not mass. Previous studies in our lab revealed a significant increase of HFD consumption, which was significantly blunted by DDE exposure, in male C57BL/6 mice which normalized to LFD consumption levels after approximately 6 weeks on HFD.16 Woods et al. (2003) reported a decrease in HFD intake in Long Evans rats over time which declined to a level comparable LFD fed rats.24 Similar observations have been noted in Wistar rats and A/J mice fed a HFD where the HFD fed animals decreased their feed intake to more closely match caloric intake compared to LFD or normal chow fed animals.25–27
High fat fed animals did have significantly elevated levels of hepatic triglyceride content and circulating levels of ALP and ALT indicating the presence of NAFLD (Figure 3). However, exposure to trans-nonachlor did not appear to exacerbate HFD induced hepatic steatosis/ NAFLD as indicated by liver triglyceride content and circulating levels of ALP and ALT. Given hepatic steatosis is due in part to an imbalance between hepatic de novo lipogenesis and fatty acid oxidation, alterations in the molecular mediators of hepatic fatty acid oxidation and de novo lipogenesis were assessed.28,29 While the molecular mediators of hepatic fatty acid oxidation, Cpt-1α and Acox1, did not display a consistent pattern of alteration by either HFD or trans-nonachlor exposure, 2 primary mediators of de novo lipogenesis, Fasn and Scd-1, display trans-nonachlor-induced increases in the LFD fed animals with Fasn being significantly upregulated compared to control (Figure 4). These data are similar to previously published in vitro data indicating exposure to trans-nonachlor has a direct effect on primary rat hepatocytes or rat hepatoma cells to increase either basal or insulin-stimulated de novo lipogenesis with a corresponding increase in insulin-stimulated Fasn production in a cell type specific manner.19,20
While the current animals were fed .5 or 5 ppm of trans-nonachlor in LFD or HFD for 16 weeks, it is difficult to equate intake with body burden given the bioaccumulative nature of this compound.18 Therefore, serum levels of trans-nonachlor were determined at the end of the current study to determine body burden and evaluate how the current exposure levels correspond to known human exposure levels (Figure 7). LFD + .5 ppm trans-nonachlor fed rats had an average serum content of 152 ng/ml (40 μg/g lipid) whereas LFD + 5 ppm trans-nonachlor fed rat had an average serum content of 313 ng/ml (77 μg/g lipid). These values declined in HFD fed animals with HFD + .5 ppm fed animals having an average serum content of 79 ng/ml (21 μg/g lipid) and HFD + 5 ppm fed animals having an average serum content of 205 ng/ml (54 μg/g lipid). Overall, the present serum levels of trans-nonachlor in LFD and HFD fed animals is higher than that observed in the human population by approximately 200–1000 fold based on serum levels reported in the Centers for Disease Control’s Fourth National Report on Human Exposure to Environmental Chemicals where the 50th percentile of the total U.S. population in 2003–2004 was 14.8 ng/g lipid and the 95th percentile was 68.3 ng/g lipid for this time period.30 These serum trans-nonachlor levels from the U.S. population are comparable to those from a recent study examining levels in a cohort of participants in the Tromsø Study based in Norway where diabetic patients’ serum levels were approximately 36 ng/g lipid and non-diabetic patients’ serum levels were approximately 26 ng/g lipid from 2015–2016.31 The discrepancies between the serum concentrations of trans-nonachlor in our LFD and HFD fed animals and those of the general human population are a noted limitation of the current study. However, species related sensitivities to chronic or subchronic trans-nonachlor exposure, especially in terms of metabolic dysfunction that would occur in the metabolic syndrome or T2D, have not been elucidated and warrant future studies to determine the full dose-related effects of trans-nonachlor exposure on these metabolic endpoints. Additionally, potential differences in xenobiotic metabolism between rat and human such as species-specific alterations in cytochrome P450 isoforms and regulation of these enzymes in states of metabolic dysfunction is an additional factor to consider when extrapolating between rat and human.
Exposure to organochlorine POPs typically results in a diverse pattern of hepatic microsomal enzyme induction characterized by increased cytochrome P450 induction and resulting activities. Campbell et al. (1983) determined that exposure to trans-nonachlor induced hepatic drug metabolizing enzymes in a pattern that resembled induction by phenobarbital.32 Bondy and colleagues corroborated these findings by determining exposure to trans-nonachlor induced hepatic microsomal enzymes in a pattern similar to phenobarbital induced enzymatic induction in rat livers.17,18 Phenobarbital is a preferential agonist of the constitutive androstane receptor (CAR) which is a xenobiotic nuclear receptor that has been shown to primarily govern cytochrome 2b10 (Cyp2b10) expression in mice and the homolog, Cyp2b2, in rats.33–36 In our current study, subchronic exposure to trans-nonachlor (5 ppm) significantly induced Cyp2b2 by approximately 53-fold in LFD fed mice and 57-fold in HFD fed mice (Figure 8). Lower degrees of induction for Cyp3a1 (approximately 1.5-fold) and Cyp2c6 (approximately 3.3-fold) were noted in the LFD + trans-nonachlor 5 ppm groups. Thus, these data suggest that trans-nonachlor preferentially induced Cyp2b2 via activation of the CAR and this induction was not significantly altered by dietary fat intake.
In summary, the overall goal of the present study was to examine the effects of trans-nonachlor exposure in concert with LFD and HFD intake to determine if trans-nonachlor altered HFD induced metabolic dysfunction with a focus on hepatic effects. Unexpectedly the HFD fed animals consumed less feed compared to the LFD fed animals which probably accounted for comparable body weights and decreased trans-nonachlor levels in HFD fed animals. While HFD fed animals consumed less feed compared to their LFD counterparts, HFD fed animals did display signs of high fat induced metabolic dysfunction through the development of hepatic steatosis with accompanying alterations in liver enzyme levels and lipid peroxidation. Interestingly, these indices of hepatic steatosis were not altered by trans-nonachlor exposure. Exposure to trans-nonachlor did produce some metabolic alterations in the LFD fed animals noted by decreased blood glucose, increased circulating free fatty acids, and increased expression of fatty acid synthase in the liver. Thus, these data indicate exposure to trans-nonachlor may produce some metabolic alterations in a diet and dose dependent manner which warrant further studies to fully characterize these alterations and mechanisms through which they may occur.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present work was funded (research and salary support) by grant #1R15ES026791-01 to G.E.H from the National Institute of Environmental Health Sciences (NIEHS) and by a pilot project to G.E.H. (salary support) from grant #P20GM103646 from the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH). The contentis solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.CDC. National Diabetes Statistics Report. In: Department of Health and Human Services CfDCaP. Atlanta, GA: CDC; 2022. [Google Scholar]
- 2.Riddle MC, Herman WH. The Cost of Diabetes Care-An Elephant in the Room. Diabetes Care. 2018;41:929–932. doi: 10.2337/dci18-0012. [DOI] [PubMed] [Google Scholar]
- 3.Lee DH, Lee IK, Jin SH, Steffes M, Jacobs DR Jr. Association between serum concentrations of persistent organic pollutants and insulin resistance among nondiabetic adults: results from the National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2007;30:622–628. doi: 10.2337/dc06-2190. [DOI] [PubMed] [Google Scholar]
- 4.Lee DH, Lee IK, Song K, et al. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999–2002. Diabetes Care. 2006;29:1638–1644. doi: 10.2337/dc06-0543.2006/06/28. [DOI] [PubMed] [Google Scholar]
- 5.Lee DH, Steffes MW, Sjodin A, Jones RS, Needham LL, JacobsDR Jr. Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS One. 2011;6:e15977. doi: 10.1371/journal.pone.0015977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rignell-Hydbom A, Rylander L, Hagmar L. Exposure to persistent organochlorine pollutants and type 2 diabetes mellitus. Hum Exp Toxicol. 2007;26:447–452. doi: 10.1177/0960327107076886. [DOI] [PubMed] [Google Scholar]
- 7.Rylander L, Rignell-Hydbom A, Hagmar L. A cross-sectional study of the association between persistent organochlorine pollutants and diabetes. Environ Health. 2005;4:28. doi: 10.1186/1476-069X-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turyk M, Anderson H, Knobeloch L, Imm P, Persky V. Organochlorine exposure and incidence of diabetes in a cohort of Great Lakes sport fish consumers. Environ Health Perspect. 2009;117:1076–1082. doi: 10.1289/ehp.0800281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turyk M, Anderson HA, Knobeloch L, Imm P, Persky VW. Prevalence of diabetes and body burdens of polychlorinated biphenyls, polybrominated diphenyl ethers, and p,p’-diphenyldichloroethene in Great Lakes sport fish consumers. Chemosphere. 2009;75:674–679. doi: 10.1016/j.chemosphere.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 10.Taylor KW, Novak RF, Anderson HA, et al. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a national toxicology program workshop review. Environ Health Perspect. 2013;121: 774–783. doi: 10.1289/ehp.1205502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendes V, Ribeiro C, Delgado I, et al. The association between environmental exposures to chlordanes, adiposity and diabetes-related features: a systematic review and meta-analysis. Sci Rep. 2021;11:14546. doi: 10.1038/s41598-021-93868-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrahim MM, Fjaere E, Lock EJ, et al. Chronic consumption of farmed salmon containing persistent organic pollutants causes insulin resistance and obesity in mice. PLoS One. 2011;6: e25170. doi: 10.1371/journal.pone.0025170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruzzin J, Petersen R, Meugnier E, et al. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ Health Perspect. 2010;118:465–471. doi: 10.1289/ehp.0901321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulligan C, Kondakala S, Yang EJ, et al. Exposure to an environmentally relevant mixture of organochlorine compounds and polychlorinated biphenyls Promotes hepatic steatosis in male Ob/Ob mice. Environ Toxicol. 2017;32:1399–1411. doi: 10.1002/tox.22334. [DOI] [PubMed] [Google Scholar]
- 15.Howell GE 3rd, Meek E, Kilic J, Mohns M, Mulligan C, Chambers JE. Exposure to p,p’-dichlorodiphenyldichloroethylene (DDE) induces fasting hyperglycemia without insulin resistance in male C57BL/6H mice. Toxicology. 2014;320:6–14. doi: 10.1016/j.tox.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howell GE 3rd, Mulligan C, Meek E, Chambers JE. Effect of chronic p,p’-dichlorodiphenyldichloroethylene (DDE) exposure on high fat diet-induced alterations in glucose and lipid metabolism in male C57BL/6H mice. Toxicology. 2015;328:112–122. doi: 10.1016/j.tox.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bondy GS, Newsome WH, Armstrong CL, et al. trans-Nonachlor and cis-nonachlor toxicity in Sprague-Dawley rats: comparison with technical chlordane. Toxicol Sci. 2000;58:386–398. [DOI] [PubMed] [Google Scholar]
- 18.Bondy G, Curran I, Doucet J, et al. Toxicity of trans-nonachlor to Sprague-Dawley rats in a 90-day feeding study. Food Chem Toxicol. 2004;42:1015–1027. doi: 10.1016/j.fct.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Howell GE 3rd, McDevitt E, Henein L, Mulligan C, Young D. Alterations in cellular lipid metabolism produce neutral lipid accumulation following exposure to the organochlorine compound trans-nonachlor in rat primary hepatocytes. Environ Toxicol. 2018;33:962–971. doi: 10.1002/tox.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howell GE 3rd, McDevitt E, Henein L, Mulligan C, Young D. Trans-nonachlor increases extracellular free fatty acid accumulation and de novo lipogenesis to produce hepatic steatosis in McArdle-RH7777 cells. Toxicol Vitro. 2018;50:285–292. doi: 10.1016/j.tiv.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowe JE, Franklin ZJ, Hauge-Evans AC, King AJ, Persaud SJ, Jones PM. Metabolic phenotyping guidelines: assessing glucose homeostasis in rodent models. J Endocrinol. 2014;222: G13–G25. doi: 10.1530/JOE-14-0182. [DOI] [PubMed] [Google Scholar]
- 22.Heindel JJ, Howard S, Agay-Shay K, et al. Obesity II: Establishing causal links between chemical exposures and obesity. Biochem Pharmacol. 2022;199:115015. doi: 10.1016/j.bcp.2022.115015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heindel JJ, Blumberg B, Cave M, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017;68: 3–33. doi: 10.1016/j.reprotox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woods SC, Seeley RJ, Rushing PA, D’Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr. 2003;133:1081–1087. doi: 10.1093/jn/133.4.1081. [DOI] [PubMed] [Google Scholar]
- 25.Bullen JW Jr., Ziotopoulou M, Ungsunan L, et al. Short-term resistance to diet-induced obesity in A/J mice is not associated with regulation of hypothalamic neuropeptides. Am J Physiol Endocrinol Metab. 2004;287:E662–E670. doi: 10.1152/ajpendo.00114.2004. [DOI] [PubMed] [Google Scholar]
- 26.Higuchi T, Mizuno A, Narita K, Ichimaru T, Murata T. Leptin resistance does not induce hyperphagia in the rat. J Physiol Sci. 2012;62:45–51. doi: 10.1007/s12576-011-0184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitra A, Alvers KM, Crump EM, Rowland NE. Effect of highfat diet during gestation, lactation, or postweaning on physiological and behavioral indexes in borderline hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2009;296:R20–R28. doi: 10.1152/ajpregu.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angulo P.Nonalcoholic fatty liver disease. N Engl J Med. 2002; 346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 29.Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50: 1844–1850. [DOI] [PubMed] [Google Scholar]
- 30.CDC. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables. In: Services DoHaH. Atlanta, GA: CDC; 2022. [Google Scholar]
- 31.Charles D, Berg V, Nost TH, et al. Longitudinal changes in concentrations of persistent organic pollutants (1986–2016) and their associations with type 2 diabetes mellitus. Environ Res. 2022;204:112129. doi: 10.1016/j.envres.2021.112129. [DOI] [PubMed] [Google Scholar]
- 32.Campbell MA, Gyorkos J, Leece B, Homonko K, Safe S. The effects of twenty-two organochlorine pesticides as inducers of the hepatic drug-metabolizing enzymes. Gen Pharmacol. 1983; 14:445–454. doi: 10.1016/0306-3623(83)90028-9. [DOI] [PubMed] [Google Scholar]
- 33.Honkakoski P, Negishi M. Regulation of cytochrome P450 (CYP) genes by nuclear receptors. Biochem J. 2000;347: 321–337. doi: 10.1042/0264-6021:3470321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masahiko N, Honkakoski P. Induction of drug metabolism by nuclear receptor CAR: molecular mechanisms and implications for drug research. Eur J Pharm Sci. 2000;11:259–264. doi: 10.1016/s0928-0987(00)00112-3. [DOI] [PubMed] [Google Scholar]
- 35.Honkakoski P, Zelko I, Sueyoshi T, Negishi M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol. 1998;18:5652–5658. doi: 10.1128/MCB.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maglich JM, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. Nuclear pregnane x receptor and constitutive and rostane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol. 2002;62:638–646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.