Abstract
Respiration protocols have been developed to manipulate mental states, including their use for therapeutic purposes. In this systematic review, we discuss evidence that respiration may play a fundamental role in coordinating neural activity, behavior, and emotion. The main findings are: (1) respiration affects the neural activity of a wide variety of regions in the brain; (2) respiration modulates different frequency ranges in the brain’s dynamics; (3) different respiration protocols (spontaneous, hyperventilation, slow or resonance respiration) yield different neural and mental effects; and (4) the effects of respiration on the brain are related to concurrent modulation of biochemical (oxygen delivery, pH) and physiological (cerebral blood flow, heart rate variability) variables. We conclude that respiration may be an integral rhythm of the brain’s neural activity. This provides an intimate connection of respiration with neuro-mental features like emotion. A respiratory-neuro-mental connection holds the promise for a brain-based therapeutic usage of respiration in mental disorders.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12264-023-01070-5.
Keywords: Respiration, Cognition, Emotion, Heart rate variability, Carbon dioxide
Introduction
Respiration and Brain—Nuisance or Neural?
Respiration, being so closely coupled to heart activity and oxygen supply, is key in maintaining metabolic activity in all organs including the brain. Given the increased efficiency of aerobic over anaerobic conditions in cellular energy dynamics, one might predict that the brain should be optimized to maximize its direct coupling with systems that supply its metabolism [1, 2]. The brain’s metabolic-energetic coupling to respiration is at odds with how neuroscientists methodologically treat respiration. Respiration-related neural activity is typically considered noise, and entire fields are dedicated to stripping it from brain data, particularly in fMRI (e.g., with global signal regression, independent components analysis, and RETROICOR [3–5].
While many researchers do not consider respiration beyond stripping it from their datasets, it is possible that elements of the respiration-coupled neural signal are useful, and can inform us about higher cognitive processing, emotion, and behavior. To test this hypothesis, we have conducted an extensive systematic review of combined brain and respiration studies in both functional magnetic resonance imaging (fMRI) and electroencephalography (EEG). In this analysis, we investigate the topographic and dynamic effects of respiration on the brain’s neural activity. Moreover, to test the strength of the link between neural and respiratory activity, we investigated the impact of different respiration protocols on biochemical, physiological, neural, and mental activity. Our findings should help uncover whether respiration plays an integral role in the brain’s physiology beyond its typical framing as a nuisance variable.
From the Rhythms of Respiration to Brain Rhythms
fMRI studies have shown that respiration frequency and volume are strongly correlated with the blood oxygen level dependent (BOLD) signal [6–10]. In fact, respiration alone has been shown to account for as much as 27% of the variance in the BOLD signal [11]. For this reason, the physiological signals are commonly regressed out of data as noise (for a review see [12]). Emerging evidence, however, suggests that fluctuations in the power of spatially distributed slower neuronal oscillations in the frequency range of respiration (0.01 to 0.3 Hz; [13]) drive the activity of faster frequencies and even mental features like consciousness and self [14–19].
Like fMRI, EEG can be used to assess the respiration-induced modulation of slower neural signals (0.01 to 0.3 Hz [20, 21]. Because of its greater temporal resolution, EEG can be used to investigate the respiration-induced modulation of faster frequencies like theta [22], alpha [23], and gamma [24]. These studies suggest that respiration provides a slow (0.01 to 0.3 Hz) intrinsic rhythm which may be coupled with analogous slower neural rhythms in the same frequency range. Thus, respiration may act as a slow-moving envelope carrying and influencing faster frequencies, which then manifest as a dynamic mixture of respiratory-influenced neural activity.
Respiration and Physiology/Biochemistry
Mammalian respiration is innately connected with other physiology and biochemistry. For example, respiratory sinus arrhythmia (RSA) is a phase relationship between respiration and heart rate. In humans, upon inhalation, the time between heartbeats (measured as R-R intervals or RRIs) tends to decrease in length, which is indicative of heartbeat acceleration. Conversely, upon exhalation, RRIs tend to increase in length, in other words, the heartbeat decelerates [25]. Furthermore, prolonged periods of hyperventilation (>30 s) tend to increase mean heart rate (HR), lower heart rate variability (HRV), reduce the blood gas partial pressure of carbon dioxide (pCO2), and decrease cerebral blood flow [26–33]. Conversely, slow respiration tends to lower mean heart rate, increase HRV, raise pCO2, and increase cerebral blood flow [13, 34–37].
How do these physiological and biochemical factors influence the impact of respiration on the brain’s neural activity? Given that the physiological and biochemical variables reviewed above express similar spatiotemporal influences on the brain’s neural activity, one might expect that these more fundamental biochemical processes might modulate and mediate respiration. This would provide a first insight into the physiological mechanisms for the coupling of the brain’s neural activity to respiration.
Respiration and Mental Activity
Neuropsychiatric conditions such as anxiety and panic disorder are often accompanied by irregularities in respiration patterns (for reviews see [26, 38–40]). In fact, hyperventilation is a trait that researchers argue is self-perpetuating and responsible for worsening neuropsychiatric disorders such as anxiety. The hyperventilation theory [41] states that panic patients often present with chronic hyperventilation. By this account, panic attacks are brought on by cognitive misinterpretations of bodily symptoms such as accelerated heart rate, decreased heart rate variability, dizziness, and tingling in the extremities that often accompany hyperventilation.
In contrast to the hyperventilation theory account [42], hyperventilation can be described as a subconscious preventive measure the body uses to maintain the pCO2 in the blood below the threshold values of peripheral and central chemoreceptors. Klein proposed that over time, chemoreceptors become hypersensitive due to the sustained levels of low pCO2 brought on by chronic hyperventilation. The authors proposed a positive feedback loop, where hypersensitive chemoreceptors promote hyperventilation to avoid triggering the body’s asphyxiation alarm which, in turn, leads to more panic and anxiety. Slow respiration has also been reported to mitigate problematic mental activity and the accompanying biochemistry/physiology brought on by chronic hyperventilation [43]. In fact, multi-session slow respiration protocols have been shown to increase overall positive affect [44–48].
An analysis by Klimesch and colleagues [49] reported on the potential architecture linking brain and body oscillations. The author argues that large reductions in energy demand drive neural systems to express phase and amplitude coupling with physiological rhythms such as respiration at distinct frequencies. Klimesch claims that, as a result, the respiration signal tends to have a set of “preferred” frequencies centered around 0.08, 0.16, and 0.32 Hz. Klimesch hypothesizes that each frequency carries with it distinct neural and mental correlates. This appears to be directly in line with our hypothesis as we suggest that there are distinct neural and mental features that are coupled to breathing at or close to these frequencies, such as in the case of slow (~0.1 Hz), spontaneous (~0.2 Hz), and fast breathing (~0.5 Hz).
The synchronization between respiration and slow neural activity is likely key to understanding the brain-physiology relationship. Higher degrees of coupling between respiration and brain activity likely manifest as the inverse of symptoms of panic disorder (e.g., relaxation, greater attention, and more measured thoughts). Slow rhythms thus provide a link and shared feature of respiration, neural, and mental activity serving as their “common currency” [18, 19]. We, therefore, include in our review studies using different respiration protocols including slow and fast ones in order to show their impact on both neural and mental activity. By establishing a “common currency”, between respiration, brain, and mind, we hope to provide a novel theoretical framework that serves to inspire future research in this area and provide a springboard for possible therapeutic interventions in neuropsychiatric disorders such as anxiety.
Overview of Steps
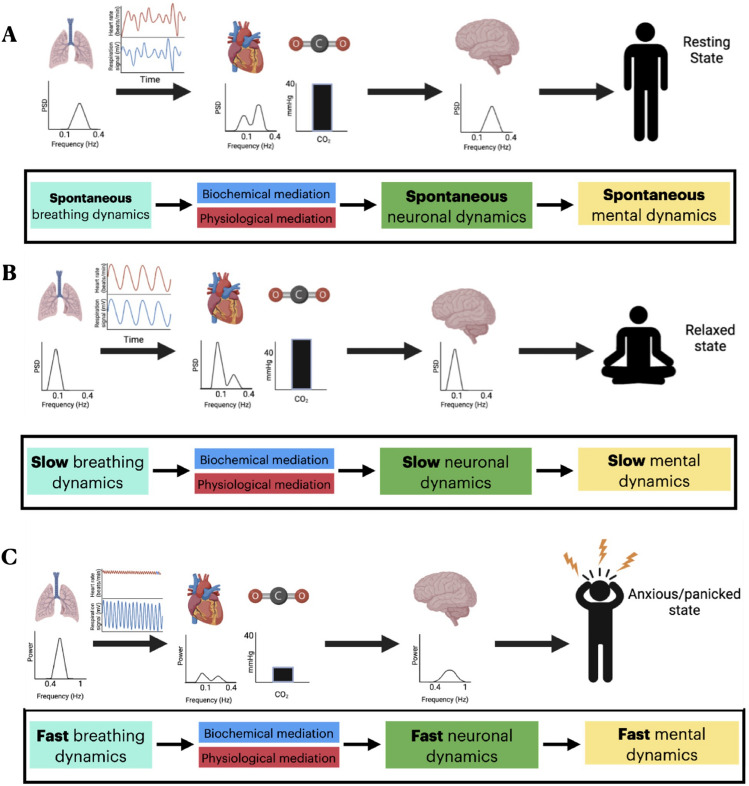
We included studies that reported the pCO2 (via capnography), fMRI, EEG, electrocardiogram (ECG), and subjective questionnaires to piece together the neuronal and mental effects of respiration including their mediation of physiological and biochemical variables. We hypothesized that: (1) respiration affects neural activity in terms of topography and dynamics throughout widespread regions and all frequencies; (2) given the fact that the spontaneous frequency ranges between physiology (heart, pCO2) and respiration overlap, we also expect their to be overlap in the topography and dynamics of neural signals influenced by these processes; and (3) given the existing evidence of respiratory mechanisms that affect pCO2 and HRV, we predict that hyperventilation provokes panic or anxiety, and conversely, we predict that slow respiration promotes emotional well-being and relaxation (Fig. 1).
Fig. 1.
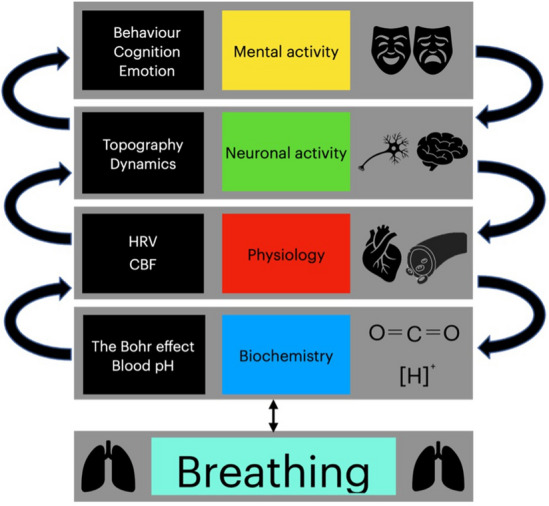
The theoretical framework of the coupling from respiration to mental features through biochemical (blood pH, Bohr effect), physiological (heart rate variability/HRV, cerebral blood flow/CBF), and neuronal (topography, dynamic) variables.
Materials and Methods
Article Search
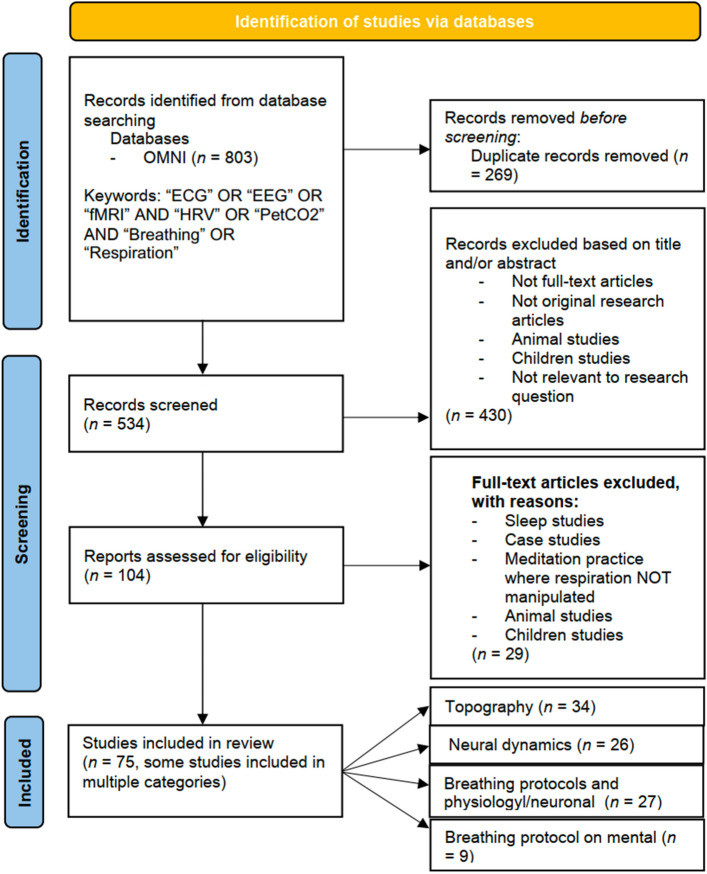
We followed the recommendations of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [50]; its design and flow are shown in Fig. 2). This review focused on the evaluation of research articles obtained from a survey conducted by the primary author in August 2021. The search included terms such as "respiration", "end-tidal carbon dioxide, "heart rate", and "heart rate variability" in combination with "electrocardiogram", "electroencephalogram", and "functional magnetic resonance imaging". To ensure that this study captured recent literature, the results were restricted to studies published between 2000 and 2021.
Fig. 2.
PRISMA scheme. Representation of the reviewal process for studies included in the survey.
Articles were evaluated for inclusion if they included human subjects, were full-text original research articles, and evaluated respiration’s inherent relationships with heart activity, neuronal activity, CO2 levels, or mental activity. Articles were excluded if they focused on animals, children (<18 years of age), sleep, or were case studies. Articles were also excluded if they investigated a meditation practice where slow respiration or hyperventilation was not a primary measured outcome. Twenty-nine articles met these exclusion criteria and were not included in the review.
Our initial search captured relatively few studies focusing on the mental implications of the respiration protocols, particularly hyperventilation. As a result, a secondary search was conducted using the online collaborative database OMNI (https://ocul.on.ca/omni/). Identification, review, and article inclusion were conducted by the first author in January 2022. This search intended to investigate the respiratory pathology of anxiety/panic disorders in hopes of linking it to the information compiled in this review. Search algorithms comprised the terms "anxiety" and "panic disorder" used in combination with "hyperventilation". To ensure this search captured the recent literature, studies were limited to the years between 2000 and 2021. This search yielded 8 full-text research articles.
Organization of the Review
As articles were reviewed, they were placed in the following categories: (1) Topography (spatial relationships), (2) Neuronal Dynamics (temporal relationships), (3) Respiration protocols and physiological or neuronal interactions, and (4) Respiration protocols and mental interactions. Further details on references of individual studies as well as which studies were included in categories 1–4 can be found in Supplementary Tables 2–5. Topographical results included fMRI studies investigating regions associated with respiration, heart activity, and pCO2 fluctuations. ECG, EEG, and fMRI studies that investigated global and regional frequencies associated with respiration, heart activity, and CO2 fluctuations contributed to the Neuronal Dynamics category. Studies that investigated the effects of respiration protocols fell into two categories. The first included studies that investigated the physiological and neuronal interactions of respiration protocols. The second included studies that investigated the impact of respiration protocols on the mental level, that is, on mood and cognition.
The search yielded 75 unique relevant studies. Some studies reported multiple measures (e.g., EEG, fMRI, ECG, pCO2, and questionnaires) and were included in multiple categories. Each section yielded studies [9, 26, 27, 34], respectively. Collectively, these studies attempted to investigate the significant physiological, neuronal, and mental variance correlated to fluctuations in respiration, heart activity, and pCO2.
Results
Topography
Regions Modulated by Respiration
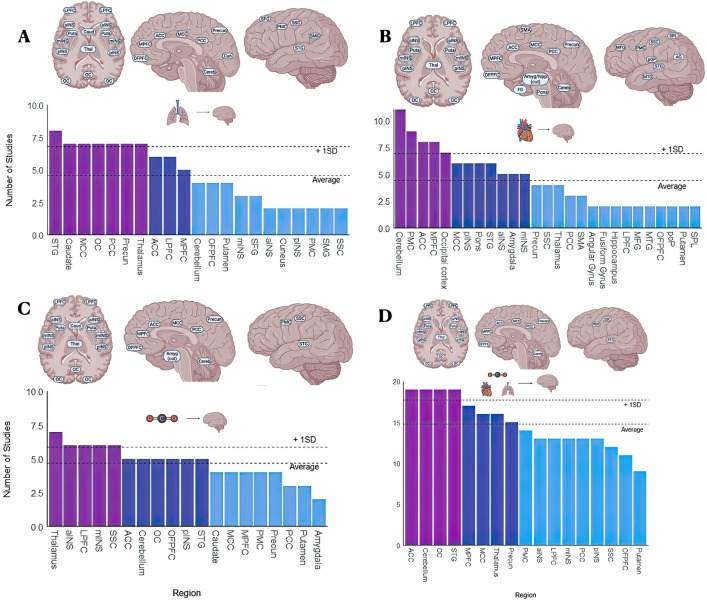
For this review, we compiled 16 studies that collectively attempted to describe the topographical relationships of respiration in the brain under spontaneous conditions. Regions most associated with respiration included frontal [medial and lateral prefrontal cortex (PFC), orbitofrontal PFC, superior frontal gyrus)], temporal [superior temporal gyrus (STG), parietal (somatosensory cortex (SSC), and primary motor cortex (PMC)], occipital [occipital cortex (OC) and supramarginal gyrus SMG)], midline [anterior (ACC), medial (MCC), and posterior cingulate cortex (PCC)/precuneus, and cuneus)], insular [anterior (aINS), medial (mINS), and posterior (pINS)], and cerebellar regions. Subcortical regions (thalamus, caudate, and putamen) also appear to be influenced by respiration. More details on the number of studies and references pertaining to each region are listed in Fig. 3A and Supplementary Table 6, respectively.
Fig. 3.
A–C Regions that have been shown to covary in activity with factors of respiration (A), factors of heart activity (B), and levels of CO2 (C). D Summary of regions that have been shown to covary with all three respiration, heart activity, and CO2. Abbreviations: PCC, posterior cingulate cortex; MCC, middle cingulate cortex; ACC, anterior cingulate cortex; STG, superior temporal gyrus; SSC, somatosensory cortex; SMG, supramarginal gyrus; SMA, supplementary motor area; DLPFC, dorsolateral prefrontal cortex; VLPFC, ventrolateral prefrontal cortex; OFPFC, orbitofrontal prefrontal cortex; PMC, primary motor cortex; SFG, superior frontal gyrus; MFG, middle frontal gyrus; DMPFC, dorsomedial prefrontal cortex; SPL, superior parietal lobule; aINS, anterior insula; mINS, middle insula; pINS, posterior insula; Caud, caudate; Thal, thalamus; LPFC, lateral prefrontal cortex; Cereb, cerebellum; amyg, amygdala; hipp, hippocampus; FG, fusiform gyrus; pOP, parietal operculum; AG, angular gyrus.
Regions Modulated by Heart Activity
For this review, we compiled 19 studies that collectively described the topographical relationships of heart activity under spontaneous conditions. Heart activity is most associated with activity in frontal (OFPFC, MPFC, LPFC, and MFG), temporal (STG, MTG, and fusiform gyrus), parietal (PMC, SSC, SPL, pOP, and SMA) occipital (OC and angular gyrus), midline (ACC, MCC, PCC, precuneus), insular (aINS, mINS, pINS), and cerebellar regions. Subcortical (thalamus, putamen, amygdala) and brainstem (pons) regions have also been shown to be influenced by heart activity. More details on the number of studies and references pertaining to each region can be found in Fig. 3B and Supplementary Table 8, respectively.
Regions Modulated by CO2
For this review, we compiled 10 studies that collectively attempted to describe the topographical relationships of pCO2 under spontaneous conditions. Regions that are most associated with fluctuations in pCO2 include frontal (OFPFC, MPFC, LPFC), temporal (STG), parietal (PMC, SSC), occipital (SMG, OC), midline (ACC, MCC, PCC, precuneus), insular (aINS, mINS, pINS), and cerebellar regions. Activity within subcortical regions (thalamus, putamen, caudate) also appears to fluctuate in activity with pCO2 levels. More details on the number of studies and references pertaining to each region can be found in Fig. 3C and Supplementary Table 7, respectively.
Regions Associated with Physiological Overlap
For this review, we compiled 45 studies (34 unique studies and 11 studies reporting multiple measures) that collectively described the topographical relationships of all the physiology described in this review under spontaneous conditions. Regions associated with overlap of the described physiology include frontal (OFPFC, MPFC, LPFC), temporal (STG), parietal (PMC, SSC), occipital (OC), midline (ACC, MCC, PCC, precuneus), insular (aINS, mINS, pINS), and cerebellar regions. In addition, subcortical regions included the thalamus and the putamen. More details on the number of studies and references pertaining to each region can be found in Fig. 3D and Supplementary Table 9, respectively.
Dynamics
Frequencies Modulated by Respiration
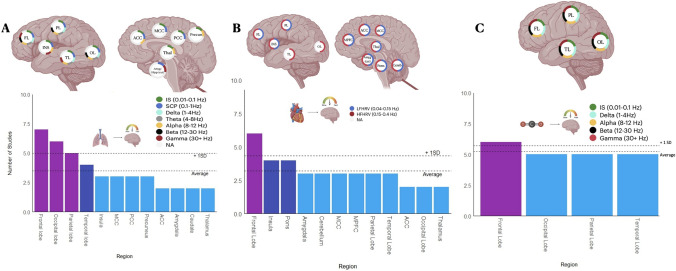
We compiled 11 studies that collectively attempted to describe the dynamic relationship of respiration in the brain under spontaneous conditions. Respiration appears to be coupled to infraslow (IS, 0.01–0.1 Hz), slow cortical potential (SCP, 0.1–1 Hz), delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz), and gamma (>30 Hz) frequencies. The extent of this coupling varied by region. In the frontal lobe, respiration was shown to be coupled to all frequencies. In the insular lobe, respiration was shown to be coupled to IS, SCP, alpha, and gamma frequencies. In the parietal and occipital lobes, respiration appears to be coupled to IS, SCP, delta, theta, alpha, and beta frequencies. In the temporal lobe, respiration was shown to be coupled to IS, SCP, delta, theta, alpha, and gamma frequencies. In the anterior cingulate cortex, respiration was shown to be coupled to IS, SCP, and alpha frequencies. In the middle and posterior cingulate cortices, respiration was shown to be coupled to IS and SCP frequencies. In the precuneus, respiration was shown to be coupled to IS and alpha frequencies. Respiration was also shown to modulate activity in subcortical regions. In the thalamus, respiration was shown to be coupled with IS and alpha frequencies. Finally, in the amygdala and hippocampus, respiration was shown to be coupled to SCP and gamma frequencies. More details on the number of studies and references pertaining to each region can be found in Fig. 4A and Supplementary Table 10, respectively.
Fig. 4.
A–C Neural dynamics linked to respiration (A), cardiac activity (B) and CO2 (C). Structures shown in the left lateral view are bilateral. Abbreviations: FL, frontal lobe; PL, parietal lobe; INS, insula; TL, temporal lobe; OL, occipital lobe; ACC, anterior cingulate cortex; MCC, middle cingulate cortex; PCC, posterior cingulate cortex; Precun, precuneus; Thal, thalamus; Amyg, amygdala; hipp, hippocampus; Cereb, cerebellum; MPFC, medial prefrontal cortex; OC, occipital cortex; LFHRV, low frequency heart rate variability; HFHRV, high frequency heart rate variability; IS, infraslow; SCP, slow cortical potentials.
Frequencies Modulated by Heart Activity
In order to investigate the dynamic relationships of heart activity we compiled 11 studies that collectively attempted to describe the relationships between low-frequency heart rate variability (LFHRV, 0.04–0.15 Hz) and high-frequency heart rate variability (HFHRV, 0.15–0.4 Hz) under spontaneous conditions.
(1) LFHRV
LFHRV appears to be coupled to activity within the frontal, parietal, and insular lobes. In addition, it appears to be coupled with activity within midline (MPFC, MCC), subcortical (thalamus, amygdala), and brainstem (pons) regions. More details on the number of studies and references pertaining to each region can be found in Fig. 4B and Supplementary Table 11, respectively.
(2) HFHRV
HFHRV appears to be coupled to activity within the frontal, parietal, occipital, temporal, and insular lobes. In addition, it appears to be coupled to activity within the cerebellum, midline (MPFC, ACC, MCC), subcortical (thalamus, amygdala), and brainstem (pons) regions. More details on the number of studies and references pertaining to each region can be found in Fig. 4B and Supplementary Table 11, respectively.
Frequencies Modulated by CO2
We compiled 6 studies that collectively attempted to describe the dynamic relationships of fluctuations in the pCO2 under spontaneous conditions. According to the studies included in this review, pCO2 appears to be coupled to IS, delta, alpha, beta, and gamma activity. These coupling patterns were consistent throughout the frontal, parietal, temporal, and occipital lobes. More details on the number of studies and references pertaining to each frequency/region can be found in Fig. 4C and Supplementary Table 12, respectively.
Respiration Protocols and Dynamic and Physiological Interactions
Slow Respiration—Resonance Respiration
We compiled 27 studies that collectively attempted to describe the physiological and dynamic changes induced by resonance respiration. Overall, it appears that resonance respiration causes significant increases in LFHRV, slight increases in pCO2 levels, and an overall shift towards slower neuronal frequencies. According to these data, within the frontal lobe, there appears to be increased IS coherence, increased SCP power, higher levels of inter and intra-hemispheric theta coherence, increased theta power, increased alpha power, increased alpha/high beta ratio, and decreased beta power during resonance respiration compared to spontaneous conditions. Within the temporal lobe, there appears to be increased IS coherence, increased SCP power, increased interhemispheric alpha asymmetry, and increased alpha/high beta ratio during resonance respiration compared to spontaneous conditions. In the occipital lobe, there appears to be increased SCP power, increased inter and intra-hemispheric theta coherence, increased theta power, increased alpha power, and decreased beta power during resonance respiration compared to spontaneous conditions. Within the parietal lobe, there appears to be increased IS coherence, increased SCP power, increased theta power, increased alpha/high beta ratio, increased alpha power, and decreased beta power during resonance respiration compared to spontaneous conditions. Within the cingulate cortex, there appears to be increased activation and increased alpha power during resonance respiration compared to spontaneous conditions. Globally, there appears to be increased IS coherence, increased SCP power, increased delta power, increased alpha power, and increased left brain activation during resonance respiration. Visual representation of the physiological and neuronal changes induced by resonance respiration can be found in Fig. 5B. More details on the main findings of individual references used to construct this figure can be found in Supplementary Table 5.
Fig. 5.
Schematic representation of physiological, neuronal, and mental activity during (A) spontaneous respiration (B) resonance respiration, and (C) hyperventilation.
Fast Respiration—Hyperventilation
We compiled 5 studies that collectively attempted to describe the neuronal dynamic changes induced by hyperventilation. In contrast to resonance respiration, it appears that hyperventilation elicits a decrease in HRV, decreased pCO2, and increases in higher frequencies. Within the frontal lobe, it appears that there is increased theta and beta power during hyperventilation compared to spontaneous conditions. Within the occipital and parietal lobes, it appears that there is increased theta and alpha power during hyperventilation compared to spontaneous conditions. Globally, there appears to be an increase in theta power compared to spontaneous conditions.
In this study, we captured a relatively small number of papers pertaining to the physiological and dynamic changes induced by hyperventilation. As a consequence, these data should be used as an indication of what might occur, but more research is necessary to understand the full scope of the physiological and dynamic implications of hyperventilation. A visual representation of the changes induced by hyperventilation can be found in Fig. 5C. More details on the main findings from individual references used to construct this figure can be found in Supplementary Table 5.
Respiration Protocols—Impact on mental activity
Our search captured 9 studies that collectively attempted to describe the effects that respiration protocols have on mental activity. Four of these studies investigated the effects of resonance respiration protocols (~0.1 Hz) on perceived anxiety/stress levels [44–46, 48]. A common theme among all these studies was an overall decrease in symptoms of negative affect after their respective intervention periods. Two studies investigated the effects of hyperventilation protocols (~0.5 Hz) on mental activity [51, 52]. Overall, these studies reported increased levels of perceived anxiety and stress during fast respiration, however, participants scored lower on overall perceived anxiety/stress after several weeks of interventions.
Three studies only included spontaneous protocols [53–55]. One study investigated interoceptive awareness of respiration and found that decreasing its capacity was correlated with increased levels of activity within the anterior insula and increased levels of anxiety [53]. Another study described a positive association between HRV and heartbeat detection [54]. This study reported that decreases in heartbeat detection were linked with increased levels of perceived stress and anxiety. The third study looked at patients in remission from major depressive disorder [55]. In this study, patients were found to have increased respiration rates and PCC/parahippocampal gyrus activity compared to healthy controls. In addition, this study found patients to experience increased respiration pause variability compared to healthy controls, and this was correlated to symptom severity. Further information on the main findings of individual studies can be found in Supplementary Table 5.
Secondary Search for Articles on Respiration and Anxiety
Our secondary search yielded a total of 8 studies that investigated the respiratory pathology of anxiety and panic disorder patients. Patients in these studies commonly presented with chronic hyperventilation [26, 27, 30, 32, 33, 39, 40, 56], hypocapnia (low pCO2; [26, 27, 39, 56]), and lower levels of HF HRV [30] compared to healthy controls. Researchers suggested that patients tend to experience more significant feelings of discomfort, negative affect, and dyspnea at lower pCO2 threshold values and with smaller increases in pCO2 compared to healthy controls. As a result, patients tend to hyperventilate as a protective mechanism to keep pCO2 levels below chemosensory threshold values. Chronic hypocapnia leads to hypersensitive peripheral and central chemoreceptors, which tend to manifest as a self-perpetuating mechanism for hyperventilation, panic, and anxiety [26, 39, 40, 56]. Other theories represented in this literature [41] suggest that the cognitive misinterpretation of hyperventilation and accompanying bodily symptoms themselves (rather than hypersensitive chemoreception) are responsible for the propagation of anxiety and panic. This assumes that patients tend to disproportionately evaluate feelings of dyspnea, elevated heart rate, and low HRV as deadly. This may lead patients to experience increased levels of anxiety and panic [26, 39, 40, 56]. Further information on the main findings of individual studies can be found in Supplementary Table 14.
Discussion
From Lung to Brain I—Respiration and Topography
We first found the involvement of widespread regions in the brain associated with respiration. Rather than implicating specific regions or networks, respiration seems to impact both medial and lateral cortical regions as well as the anterior and posterior cortex including higher- and lower-order regions. The global role of respiration in the brain signal hinges on physiological and biochemical processes. We show significant topographical overlap between respiration, heart activity, and CO2 fluctuations. Regions associated with this overlap include frontal (OFPFC, MPFC, LPFC), temporal (STG), parietal (PMC, SSC), occipital (OC), midline (ACC, MCC, PCC, precuneus), insular (aINS, mINS, pINS), and cerebellar regions. In addition, subcortical regions included the thalamus and the putamen (for individual references see Supplementary Table 9). Interestingly, many of these regions have been implicated in important networks responsible for the sense of self and cognition such as the default mode and salience networks [57–59]. This, albeit indirectly, provides evidence for the possible connection of respiratory activity with neuro-mental functions like self and consciousness; this is further supported by the differential neuro-mental impact of different slow-fast respiration protocols.
We believe that this global topographic involvement is, at least in part, mediated by the biochemical and physiological dynamics inherently linked to respiration. For example, CO2 has been shown to fluctuate with the respiratory cycle and has been shown to be a potent vasodilator [6–9, 60, 61]. This dilatory property of CO2 facilitates modulations in cerebral blood flow. In fact, there have been studies applying transcranial Doppler ultrasound that show as much as a 5% modulation in blood flow per 1 mmHg change in pCO2 [29]. That considered, one would expect the BOLD signal to be most affected by the respiratory cycle in regions with a high blood supply, which is exactly what we present in this review.
From Lung to Brain Ii—Respiration and Dynamics
It is becoming well established that respiration entrains oscillations at the same frequency as the respiration rate in several regions, at least in the rodent brain [24, 62, 63]. Moreover, these studies particularly implicate the theta and gamma bands in the frontal regions are phase-locked to respiration [24, 62, 63]. Researchers suggest that the respiratory rhythm in rodents, like humans, is propagated and modulated via a central pattern generator (pre-Botzinger complex) buried deep within the brainstem [15, 64, 65].
In mammals, activity within the pre-Botzinger complex is particularly sensitive to changes in blood acidity (pH) levels (for details on the relationship between respiration and pH see the box provided). Central chemoreceptors act in a negative feedback loop with respiratory and cardiac centers to modulate heart and respiration rates accordingly [64, 65]. Given that there is a fundamental connection between respiration and central brain activity, the global frequency involvement of respiration should not come as a surprise.
In line with other studies, our review confirms the involvement of multiple frequencies in the coupling of respiratory and neural activity (for reviews see [43, 66, 67]). Support for the involvement of slower frequencies (0.01 to 0.3 Hz) comes (indirectly) from fMRI where infraslow fluctuations in respiratory volume (0.01 to 0.05 Hz; [6, 12]) or frequency (0.1–0.3 Hz; [10]) modulate neural activity in that frequency range. This is important as the infraslow frequency range of fMRI (0.01 to 0.1 Hz) corresponds to the frequency range of respiration (including its variability; 0.01 to 0.3 Hz).
From Lung to Mind—Respiration and Mental Activity
Although neuroimaging techniques such as fMRI have been used in many studies, about 2% of all examinations had to be aborted due to abnormally high state anxiety related to the scanner [68]. It has been demonstrated that increased state anxiety carries abnormal coupling patterns between breathing and cardiac oscillations. In fact, negative RSA refers to the condition where increased state anxiety induces a complete reversal of the phasic relationship between respiration and cardiac activity where the heart rate slows down during inhalation and speeds up during exhalation [69, 70].
Chronic mental conditions such as anxiety and panic disorder are often accompanied by abnormalities in respiratory patterns [71]. Patients often have a faster respiration rate, higher mean heart rate, diminished HRV amplitude, and a lower level of baseline pCO2 (for reviews see [26, 38–40, 56]). Over time, diminished levels of pCO2 lower the threshold of chemoreceptors to trigger the body's intrinsic asphyxiation alarm. With lower threshold values, slight increases in pCO2 may trigger feelings of dyspnea, discomfort, anxiety, and panic [42]. Interestingly, respiration is an autonomic process that can be somatically modulated. In fact, studies investigating slow respiration techniques have shown it to increase HRV amplitude, raise pCO2, and promote feelings of positive affect [44–46, 71].
Our findings provide a direct link between respiratory, neural, and mental activity. The impact of different respiration protocols on neural dynamics and topography as well as on mental features like emotions suggest a mechanistic link. We propose the topography and slow-fast dynamics shared by respiratory, physiological, neural, and mental activity act as their “common currency” [18, 19]. This is illustrated in Fig. 5 where we draw connections from respiratory dynamics to neural and mental dynamics mediated by the dynamics of physiological and biochemical variables related to respiration. Beyond opening a novel understanding of the body-brain-mind relationship, this can serve as the basis for the brain-based mechanistic development of novel forms of respiration-based therapeutic intervention in mental disorders like anxiety disorders.
Limitations and Future Directions
A major limitation to consider is that in some instances, sample sizes were small, and methodologies between studies varied significantly. Thus, we acknowledge a certain degree of generalizability may be lacking from this work. In addition, although this manuscript clearly describes clinical implications, at the time of writing, there appears to be a lack of large-scale clinical data on this matter. Although we describe some of the existing clinical research in our analysis, more work is needed to establish a clear neuro-respiratory mechanism for mental activity. The future is bright, however. We are hopeful that recent increases in interest in non-pharmacological interventions for individuals suffering from mental illness and other cognitive disorders may bring much-needed funding into the field so that these data can be acquired.
Future research should focus on refining existing paradigms, such as developing a more precise methodology for collecting and analyzing respiration-entrained neuronal data. Large amounts of high-quality neuro-respiratory data may allow researchers to flesh out the mechanistic links between respiration, neuronal activity, and behavior. In addition, large-scale, longitudinal clinical trials should be conducted to assess the efficacy of deliberate breathing protocols in the treatment of neuro-cognitive disorders in at-risk populations (i.e., advanced aging, anxiety, and depression, …). Further, this “big data” approach should focus on the dynamics of neuro-respiratory covariance and how these interactions impact behavior and mental health.
Conclusions
Respiration is a fundamental activity of life as it provides the necessary metabolic ingredients for all organs of our body including the brain. Does respiration contribute useful information to the neural signal rather than just "noise"? Our review suggests this is indeed the case.
We show that respiration affects a widespread set of regions throughout the brain as well as a range of frequencies ranging from slow (0.01 to 0.3 Hz) to faster (1–80 Hz). Our review furthermore demonstrates that physiological (HRV) and biochemical (CO2) variables induce similar neural changes in the brain in both its topography and dynamics. These physiological and biochemical variables likely mediate the coupling of respiratory and neural activity thus providing their direct link. Addressing our initial question, these findings strongly suggest that respiration is not a mere nuisance variable but an integral component of the brain’s neuro-mental activity which is mediated by various physiological and biochemical variables.
Our review also shows the differential neural and even mental effects of slow and fast respiration protocols. The respiratory-neural connection seems to have particularly strong effects on emotions: slow respiration protocols like resonance respiration exert a relaxing and calming effect, while faster respiration tends to induce anxiety states. These observations suggest that slow-fast dynamics may be shared by respiratory, physiological, neural, and mental activity, thus providing a mechanistic (or better yet dynamic) link as their “common currency” [18, 19]. We, therefore, propose that an individual's respiratory rhythm serves a fundamental, intrinsic role that modulates the topography and dynamics of the whole brain. Going beyond respiration-brain coupling, this opens the door for the application of respiration as a therapeutic technique in mental disorders like anxiety disorders and others.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Figures were created using images from Biorender (biorender.com).
Appendix
The following citations were included in the topographic analysis (Supplementary Table 3) but were not referenced in the text [70, 72–95].
The following citations were included in the neural dynamic analysis (Supplementary Table 4) but were not referenced in the text [69, 70, 76, 79, 80, 87, 90–99].
The following citations were included in the effects of breathing protocols on physiological and neuronal dynamics analysis but were not referenced in the text [98, 100–109].
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
References
- 1.Park SM, Jung HY. Respiratory sinus arrhythmia biofeedback alters heart rate variability and default mode network connectivity in major depressive disorder: A preliminary study. Int J Psychophysiol. 2020;158:225–237. doi: 10.1016/j.ijpsycho.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Huang Z, Tumati S, Northoff G. Rest-task modulation of fMRI-derived global signal topography is mediated by transient coactivation patterns. PLoS Biol. 2020;18:e3000733. doi: 10.1371/journal.pbio.3000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch CJ, Silver BM, Dubin MJ, Martin A, Voss HU, Jones RM, et al. Prevalent and sex-biased breathing patterns modify functional connectivity MRI in young adults. Nat Commun. 2020;11:5290. doi: 10.1038/s41467-020-18974-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Power JD, Lynch CJ, Dubin MJ, Silver BM, Martin A, Jones RM. Characteristics of respiratory measures in young adults scanned at rest, including systematic changes and missed deep breaths. NeuroImage. 2020;204:116234. doi: 10.1016/j.neuroimage.2019.116234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Power JD, Lynch CJ, Adeyemo B, Petersen SE. A critical, event-related appraisal of denoising in resting-state fMRI studies. Cereb Cortex. 2020;30:5544–5559. doi: 10.1093/cercor/bhaa139. [DOI] [PubMed] [Google Scholar]
- 6.Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. NeuroImage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 7.Birn RM, Smith MA, Jones TB, Bandettini PA. The respiration response function: The temporal dynamics of fMRI signal fluctuations related to changes in respiration. NeuroImage. 2008;40:644–654. doi: 10.1016/j.neuroimage.2007.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birn RM, Murphy K, Handwerker DA, Bandettini PA. fMRI in the presence of task-correlated breathing variations. Neuroimage. 2009;47:1092–1104. doi: 10.1016/j.neuroimage.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birn RM, Murphy K, Bandettini PA. The effect of respiration variations on independent component analysis results of resting state functional connectivity. Hum Brain Mapp. 2008;29:740–750. doi: 10.1002/hbm.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keller M, Pelz H, Perlitz V, Zweerings J, Röcher E, Baqapuri HI, et al. Neural correlates of fluctuations in the intermediate band for heart rate and respiration are related to interoceptive perception. Psychophysiology. 2020;57:e13594. doi: 10.1111/psyp.13594. [DOI] [PubMed] [Google Scholar]
- 11.Power JD, Plitt M, Laumann TO, Martin A. Sources and implications of whole-brain fMRI signals in humans. Neuroimage. 2017;146:609–625. doi: 10.1016/j.neuroimage.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birn RM. The role of physiological noise in resting-state functional connectivity. Neuroimage. 2012;62:864–870. doi: 10.1016/j.neuroimage.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Critchley HD, Nicotra A, Chiesa PA, Nagai Y, Gray MA, Minati L, et al. Slow breathing and hypoxic challenge: Cardiorespiratory consequences and their central neural substrates. PLoS One. 2015;10:e0127082. doi: 10.1371/journal.pone.0127082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen M, Williams G. Consciousness, plasticity, and connectomics: The role of intersubjectivity in human cognition. Front Psychol. 2011;2:20. doi: 10.3389/fpsyg.2011.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen M, Varga S, Heck DH. Respiratory rhythms of the predictive mind. Psychol Rev. 2022 doi: 10.1037/rev0000391. [DOI] [PubMed] [Google Scholar]
- 16.Zirui Huang. The temporal structure of resting-state brain activity in the medial prefrontal cortex predicts self-consciousness. Neuropsychologia. 2016;82:161–170. doi: 10.1016/j.neuropsychologia.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Northoff G. “Paradox of slow frequencies”—Are slow frequencies in upper cortical layers a neural predisposition of the level/state of consciousness (NPC)? Conscious Cogn. 2017;54:20–35. doi: 10.1016/j.concog.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Northoff G, Wainio-Theberge S, Evers K. Is temporo-spatial dynamics the “common currency” of brain and mind? in Quest of “Spatiotemporal Neuroscience”. Phys Life Rev. 2020;33:34–54. doi: 10.1016/j.plrev.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Northoff G, Wainio-Theberge S, Evers K. Spatiotemporal neuroscience—what is it and why we need it. Phys Life Rev. 2020;33:78–87. doi: 10.1016/j.plrev.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Hinterberger T, Walter N, Doliwa C, Loew T. The brain’s resonance with breathing-decelerated breathing synchronizes heart rate and slow cortical potentials. J Breath Res. 2019;13:046003. doi: 10.1088/1752-7163/ab20b2. [DOI] [PubMed] [Google Scholar]
- 21.Karavaev AS, Kiselev AR, Runnova AE, Zhuravlev MO, Borovkova EI, Prokhorov MD, et al. Synchronization of infra-slow oscillations of brain potentials with respiration. Chaos. 2018;28:081102. doi: 10.1063/1.5046758. [DOI] [PubMed] [Google Scholar]
- 22.Sinha M, Sinha R, Ghate J, Sarnik G. Impact of altered breathing patterns on interaction of EEG and heart rate variability. Ann Neurosci. 2020;27:67–74. doi: 10.1177/0972753120950075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kluger DS, Balestrieri E, Busch NA, Gross J. Respiration aligns perception with neural excitability. Elife. 2021;10:e70907. doi: 10.7554/eLife.70907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heck DH, McAfee SS, Liu Y, Babajani-Feremi A, Rezaie R, Freeman WJ, et al. Breathing as a fundamental rhythm of brain function. Front Neural Circuits. 2016;10:115. doi: 10.3389/fncir.2016.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schäfer A, Kratky KW. Estimation of breathing rate from respiratory sinus arrhythmia: Comparison of various methods. Ann Biomed Eng. 2008;36:476–485. doi: 10.1007/s10439-007-9428-1. [DOI] [PubMed] [Google Scholar]
- 26.Dratcu L. Panic, hyperventilation and perpetuation of anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:1069–1089. doi: 10.1016/S0278-5846(00)00130-5. [DOI] [PubMed] [Google Scholar]
- 27.Friedman SD, Mathis CM, Hayes C, Renshaw P, Dager SR. Brain pH response to hyperventilation in panic disorder: Preliminary evidence for altered acid-base regulation. Am J Psychiatry. 2006;163:710–715. doi: 10.1176/ajp.2006.163.4.710. [DOI] [PubMed] [Google Scholar]
- 28.Holper L, Scholkmann F, Seifritz E. Time-frequency dynamics of the sum of intra- and extracerebral hemodynamic functional connectivity during resting-state and respiratory challenges assessed by multimodal functional near-infrared spectroscopy. Neuroimage. 2016;125:1174. doi: 10.1016/j.neuroimage.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Ide K, Eliasziw M, Poulin MJ. Relationship between middle cerebral artery blood velocity and end-tidal PCO2 in the hypocapnic-hypercapnic range in humans. J Appl Physiol. 1985;2003(95):129–137. doi: 10.1152/japplphysiol.01186.2002. [DOI] [PubMed] [Google Scholar]
- 30.Pittig A, Arch JJ, Lam CW, Craske MG. Heart rate and heart rate variability in panic, social anxiety, obsessive-compulsive, and generalized anxiety disorders at baseline and in response to relaxation and hyperventilation. Int J Psychophysiol. 2013;87:19–27. doi: 10.1016/j.ijpsycho.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Telles S, Singh N, Balkrishna A. Heart rate variability changes during high frequency yoga breathing and breath awareness. Biopsychosoc Med. 2011;5:4. doi: 10.1186/1751-0759-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tunnell NC, Ritz T, Wilhelm FH, Roth WT, Meuret AE. Habituation or normalization? experiential and respiratory recovery from voluntary hyperventilation in treated versus untreated patients with panic disorder. Behav Ther. 2021;52:124–135. doi: 10.1016/j.beth.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wollburg E, Roth WT, Kim S. Effects of breathing training on voluntary hypo- and hyperventilation in patients with panic disorder and episodic anxiety. Appl Psychophysiol Biofeedback. 2011;36:81–91. doi: 10.1007/s10484-011-9150-5. [DOI] [PubMed] [Google Scholar]
- 34.Chalaye P, Goffaux P, Lafrenaye S, Marchand S. Respiratory effects on experimental heat pain and cardiac activity. Pain Med. 2009;10:1334–1340. doi: 10.1111/j.1526-4637.2009.00681.x. [DOI] [PubMed] [Google Scholar]
- 35.Chang CC, Hsu HY, Hsiao TC. The interpretation of very high frequency band of instantaneous pulse rate variability during paced respiration. Biomed Eng Online. 2014;13:46. doi: 10.1186/1475-925X-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zunhammer M, Eichhammer P, Busch V. Do cardiorespiratory variables predict the antinociceptive effects of deep and slow breathing? Pain Med. 2013;14:843–854. doi: 10.1111/pme.12085. [DOI] [PubMed] [Google Scholar]
- 37.Lin IM. Effects of a cardiorespiratory synchronization training mobile application on heart rate variability and electroencephalography in healthy adults. Int J Psychophysiol. 2018;134:168–177. doi: 10.1016/j.ijpsycho.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Giardino ND, Friedman SD, Dager SR. Anxiety, respiration, and cerebral blood flow: Implications for functional brain imaging. Compr Psychiatry. 2007;48:103–112. doi: 10.1016/j.comppsych.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meuret AE, Ritz T, Wilhelm FH, Roth WT. Voluntary hyperventilation in the treatment of panic disorder—functions of hyperventilation, their implications for breathing training, and recommendations for standardization. Clin Psychol Rev. 2005;25:285–306. doi: 10.1016/j.cpr.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Sikter A, Frecska E, Braun IM, Gonda X, Rihmer Z. The role of hyperventilation: Hypocapnia in the pathomechanism of panic disorder. Braz J Psychiatry. 2007;29:375–379. doi: 10.1590/S1516-44462006005000048. [DOI] [PubMed] [Google Scholar]
- 41.Ley R. Blood, breath, and fears: A hyperventilation theory of panic attacks and agoraphobia. Clin Psychol Rev. 1985;5:271–285. doi: 10.1016/0272-7358(85)90008-X. [DOI] [Google Scholar]
- 42.Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch Gen Psychiatry. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- 43.Zaccaro A, Piarulli A, Laurino M, Garbella E, Menicucci D, Neri B, et al. How breath-control can change your life: A systematic review on psycho-physiological correlates of slow breathing. Front Hum Neurosci. 2018;12:353. doi: 10.3389/fnhum.2018.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karavidas MK, Lehrer PM, Vaschillo E, Vaschillo B, Marin H, Buyske S, et al. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl Psychophysiol Biofeedback. 2007;32:19–30. doi: 10.1007/s10484-006-9029-z. [DOI] [PubMed] [Google Scholar]
- 45.Ma X, Yue ZQ, Gong ZQ, Zhang H, Duan NY, Shi YT, et al. The effect of diaphragmatic breathing on attention, negative affect and stress in healthy adults. Front Psychol. 2017;8:874. doi: 10.3389/fpsyg.2017.00874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacKinnon S, Gevirtz R, McCraty R, Brown M. Utilizing heartbeat evoked potentials to identify cardiac regulation of vagal afferents during emotion and resonant breathing. Appl Psychophysiol Biofeedback. 2013;38:241–255. doi: 10.1007/s10484-013-9226-5. [DOI] [PubMed] [Google Scholar]
- 47.Tatschl JM, Hochfellner SM, Schwerdtfeger AR. Implementing mobile HRV biofeedback as adjunctive therapy during inpatient psychiatric rehabilitation facilitates recovery of depressive symptoms and enhances autonomic functioning short-term: A 1-year pre-post-intervention follow-up pilot study. Front Neurosci. 2020;14:738. doi: 10.3389/fnins.2020.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Li S, Wu W. Effects of medical biofeedback trainings on acute stress by hybridizing heart rate variability and brain imaging. Multimed Tools Appl. 2020;79:10141–10155. doi: 10.1007/s11042-019-08004-2. [DOI] [Google Scholar]
- 49.Klimesch W. The frequency architecture of brain and brain body oscillations: An analysis. Eur J Neurosci. 2018;48:2431–2453. doi: 10.1111/ejn.14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chandra S, Sharma G, Sharma M, Jha D, Mittal AP. Workload regulation by Sudarshan Kriya: An EEG and ECG perspective. Brain Inf. 2017;4:13–25. doi: 10.1007/s40708-016-0055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peterson CT, Bauer SM, Chopra D, Mills PJ, Maturi RK. Effects of shambhavi mahamudra kriya, a multicomponent breath-based yogic practice (pranayama), on perceived stress and general well-being. J Evid Based Complementary Altern Med. 2017;22:788–797. doi: 10.1177/2156587217730934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harrison OK, Köchli L, Marino S, Luechinger R, Hennel F, Brand K, et al. Interoception of breathing and its relationship with anxiety. Neuron. 2021;109:4080–4093.e8. doi: 10.1016/j.neuron.2021.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lischke A, Pahnke R, Mau-Moeller A, Weippert M. Heart rate variability modulates interoceptive accuracy. Front Neurosci. 2020;14:612445. doi: 10.3389/fnins.2020.612445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zamoscik VE, Schmidt SNL, Gerchen MF, Samsouris C, Timm C, Kuehner C, et al. Respiration pattern variability and related default mode network connectivity are altered in remitted depression. Psychol Med. 2018;48:2364–2374. doi: 10.1017/S0033291717003890. [DOI] [PubMed] [Google Scholar]
- 56.Meuret AE, Ritz T. Hyperventilation in panic disorder and asthma: Empirical evidence and clinical strategies. Int J Psychophysiol. 2010;78:68–79. doi: 10.1016/j.ijpsycho.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lucherini Angeletti L, Scalabrini A, Ricca V, Northoff G. Topography of the anxious self: Abnormal rest-task modulation in social anxiety disorder. Neuroscientist. 2023;29:221–244. doi: 10.1177/10738584211030497. [DOI] [PubMed] [Google Scholar]
- 58.Melnychuk MC, Dockree PM, O'Connell RG, Murphy PR, Balsters JH, Robertson IH. Coupling of respiration and attention via the locus coeruleus: Effects of meditation and pranayama. Psychophysiology. 2018;55:e13091. doi: 10.1111/psyp.13091. [DOI] [PubMed] [Google Scholar]
- 59.Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 60.Wise RG, Ide K, Poulin MJ, Tracey I. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage. 2004;21:1652–1664. doi: 10.1016/j.neuroimage.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 61.van den Aardweg JG, Karemaker JM. Influence of chemoreflexes on respiratory variability in healthy subjects. Am J Respir Crit Care Med. 2002;165:1041–1047. doi: 10.1164/ajrccm.165.8.2104100. [DOI] [PubMed] [Google Scholar]
- 62.Ito J, Roy S, Liu Y, Cao Y, Fletcher M, Lu L, et al. Whisker barrel cortex delta oscillations and gamma power in the awake mouse are linked to respiration. Nat Commun. 2014;5:3572. doi: 10.1038/ncomms4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tort ABL, Brankačk J, Draguhn A. Respiration-entrained brain rhythms are global but often overlooked. Trends Neurosci. 2018;41:186–197. doi: 10.1016/j.tins.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 64.Feldman JL, Mitchell GS, Nattie EE. Breathing: Rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang CF, Kim EJ, Callaway EM, Feldman JL. Monosynaptic projections to excitatory and inhibitory preBötzinger complex neurons. Front Neuroanat. 2020;14:58. doi: 10.3389/fnana.2020.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mather M, Thayer J. How heart rate variability affects emotion regulation brain networks. Curr Opin Behav Sci. 2018;19:98–104. doi: 10.1016/j.cobeha.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noble DJ, Hochman S. Hypothesis: Pulmonary afferent activity patterns during slow, deep breathing contribute to the neural induction of physiological relaxation. Front Physiol. 2019;10:1176. doi: 10.3389/fphys.2019.01176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Munn Z, Pearson A, Jordan Z, Murphy F, Pilkington D, Anderson A. Addressing the patient experience in a magnetic resonance imaging department: Final results from an action research study. J Med Imaging Radiat Sci. 2016;47:329–336. doi: 10.1016/j.jmir.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 69.Rassler B, Schwerdtfeger A, Aigner CS, Pfurtscheller G. “switch-off” of respiratory sinus arrhythmia can occur in a minority of subjects during functional magnetic resonance imaging (fMRI) Front Physiol. 2018;9:1688. doi: 10.3389/fphys.2018.01688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pfurtscheller G, Rassler B, Schwerdtfeger AR, Klimesch W, Andrade A, Schwarz G, et al. “switch-off” of respiratory sinus arrhythmia may be associated with the activation of an oscillatory source (pacemaker) in the brain stem. Front Physiol. 2019;10:939. doi: 10.3389/fphys.2019.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meuret AE, Ritz T, Wilhelm FH, Roth WT, Rosenfield D. Hypoventilation therapy alleviates panic by repeated induction of dyspnea. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:539–545. doi: 10.1016/j.bpsc.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang C, Cunningham JP, Glover GH. Influence of heart rate on the BOLD signal: The cardiac response function. Neuroimage. 2009;44:857–869. doi: 10.1016/j.neuroimage.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hess A, Yu L, Klein I, De Mazancourt M, Jebrak G, Mal H, et al. Neural mechanisms underlying breathing complexity. PLoS One. 2013;8:e75740. doi: 10.1371/journal.pone.0075740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jack S, Kemp GJ, Bimson WE, Calverley PM, Corfield DR. Patterns of brain activity in response to respiratory stimulation in patients with idiopathic hyperventilation (IHV) Adv Exp Med Biol. 2010;669:341–345. doi: 10.1007/978-1-4419-5692-7_70. [DOI] [PubMed] [Google Scholar]
- 75.Kassinopoulos M, Mitsis GD. Identification of physiological response functions to correct for fluctuations in resting-state fMRI related to heart rate and respiration. Neuroimage. 2019;202:116150. doi: 10.1016/j.neuroimage.2019.116150. [DOI] [PubMed] [Google Scholar]
- 76.Sclocco R, Garcia RG, Kettner NW, Isenburg K, Fisher HP, Hubbard CS, et al. The influence of respiration on brainstem and cardiovagal response to auricular vagus nerve stimulation: A multimodal ultrahigh-field (7T) fMRI study. Brain Stimul. 2019;12:911–921. doi: 10.1016/j.brs.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Golestani AM, Wei LL, Chen JJ. Quantitative mapping of cerebrovascular reactivity using resting-state BOLD fMRI: Validation in healthy adults. NeuroImage. 2016;138:147–163. doi: 10.1016/j.neuroimage.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Golestani AM, Chang C, Kwinta JB, Khatamian YB, Jean Chen J. Mapping the end-tidal CO2 response function in the resting-state BOLD fMRI signal: Spatial specificity, test-retest reliability and effect of fMRI sampling rate. NeuroImage. 2015;104:266–277. doi: 10.1016/j.neuroimage.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 79.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::AID-MRM23>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 80.Yuan H, Zotev V, Phillips R, Bodurka J. Correlated slow fluctuations in respiration, EEG, and BOLD fMRI. NeuroImage. 2013;79:81–93. doi: 10.1016/j.neuroimage.2013.04.068. [DOI] [PubMed] [Google Scholar]
- 81.Falahpour M, Refai H, Bodurka J. Subject specific BOLD fMRI respiratory and cardiac response functions obtained from global signal. Neuroimage. 2013;72:252–264. doi: 10.1016/j.neuroimage.2013.01.050. [DOI] [PubMed] [Google Scholar]
- 82.Bright MG, Bulte DP, Jezzard P, Duyn JH. Characterization of regional heterogeneity in cerebrovascular reactivity dynamics using novel hypocapnia task and BOLD fMRI. Neuroimage. 2009;48:166–175. doi: 10.1016/j.neuroimage.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Faull OK, Cox PJ, Pattinson KTS. Cortical processing of breathing perceptions in the athletic brain. Neuroimage. 2018;179:92–101. doi: 10.1016/j.neuroimage.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 84.Pattinson KT, Mitsis GD, Harvey AK, Jbabdi S, Dirckx S, Mayhew SD, et al. Determination of the human brainstem respiratory control network and its cortical connections in vivo using functional and structural imaging. Neuroimage. 2009;44:295–305. doi: 10.1016/j.neuroimage.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 85.Golestani AM, Kwinta JB, Khatamian YB, Chen JJ. The effect of low-frequency physiological correction on the reproducibility and specificity of resting-state fMRI metrics: Functional connectivity, ALFF, and ReHo. Front Neurosci. 2017;11:546. doi: 10.3389/fnins.2017.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Madjar C, Gauthier CJ, Bellec P, Birn RM, Brooks JC, Hoge RD. Task-related BOLD responses and resting-state functional connectivity during physiological clamping of end-tidal CO(2) Neuroimage. 2012;61:41–49. doi: 10.1016/j.neuroimage.2012.02.080. [DOI] [PubMed] [Google Scholar]
- 87.Chang C, Metzger CD, Glover GH, Duyn JH, Heinze HJ, Walter M. Association between heart rate variability and fluctuations in resting-state functional connectivity. Neuroimage. 2013;68:93–104. doi: 10.1016/j.neuroimage.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: A functional neuroimaging investigation in humans. J Physiol. 2000;523(Pt 1):259–270. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, et al. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 90.Pfurtscheller G, Schwerdtfeger A, Fink D, Brunner C, Aigner CS, Brito J, et al. MRI-related anxiety in healthy individuals, intrinsic BOLD oscillations at 0.1 Hz in precentral gyrus and insula, and heart rate variability in low frequency bands. PLoS One 2018, 13: e0206675. [DOI] [PMC free article] [PubMed]
- 91.Pfurtscheller G, Schwerdtfeger AR, Rassler B, Andrade A, Schwarz G, Klimesch W. Verification of a central pacemaker in brain stem by phase-coupling analysis between HR interval- and BOLD-oscillations in the 0.10–0.15 hz frequency band. Front Neurosci 2020, 14: 922. [DOI] [PMC free article] [PubMed]
- 92.Pfurtscheller G, Schwerdtfeger AR, Seither-Preisler A, Brunner C, Stefan Aigner C, Brito J, et al. Brain-heart communication: Evidence for “central pacemaker” oscillations with a dominant frequency at 0.1Hz in the cingulum. Clin Neurophysiol 2017, 128: 183–193. [DOI] [PubMed]
- 93.Pfurtscheller G, Schwerdtfeger A, Seither-Preisler A, Brunner C, Aigner CS, Calisto J, et al. Synchronization of intrinsic 0.1-Hz blood-oxygen-level-dependent oscillations in amygdala and prefrontal cortex in subjects with increased state anxiety. Eur J Neurosci 2018, 47: 417–426. [DOI] [PMC free article] [PubMed]
- 94.Valenza G, Sclocco R, Duggento A, Passamonti L, Napadow V, Barbieri R, et al. The central autonomic network at rest: Uncovering functional MRI correlates of time-varying autonomic outflow. Neuroimage. 2019;197:383–390. doi: 10.1016/j.neuroimage.2019.04.075. [DOI] [PubMed] [Google Scholar]
- 95.Sakaki M, Yoo HJ, Nga L, Lee TH, Thayer JF, Mather M. Heart rate variability is associated with amygdala functional connectivity with MPFC across younger and older adults. Neuroimage. 2016;139:44–52. doi: 10.1016/j.neuroimage.2016.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Herrero JL, Khuvis S, Yeagle E, Cerf M, Mehta AD. Breathing above the brain stem: Volitional control and attentional modulation in humans. J Neurophysiol. 2018;119:145–159. doi: 10.1152/jn.00551.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morelli MS, Greco A, Valenza G, Giannoni A, Emdin M, Scilingo EP, et al. Analysis of generic coupling between EEG activity and PETCO2 in free breathing and breath-hold tasks using Maximal Information Coefficient (MIC) Sci Rep. 2018;8:4492. doi: 10.1038/s41598-018-22573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Morelli MS, Giannoni A, Passino C, Landini L, Emdin M, Vanello N. A cross-correlational analysis between electroencephalographic and end-tidal carbon dioxide signals: Methodological issues in the presence of missing data and real data results. Sensors (Basel) 2016;16:1828. doi: 10.3390/s16111828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Driver ID, Whittaker JR, Bright MG, Muthukumaraswamy SD, Murphy K. Arterial CO2 fluctuations modulate neuronal rhythmicity: Implications for MEG and fMRI studies of resting-state networks. J Neurosci. 2016;36:8541–8550. doi: 10.1523/JNEUROSCI.4263-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Senthilnathan S, Patel R, Narayanan M, Katholil G, Janawadkar MPR, Radhakrishnan TS, et al. An investigation on the influence of yogic methods on heart rate variability. Ann Noninvasive Electrocardiol. 2019;24:e12584. doi: 10.1111/anec.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Telles S, Sharma SK, Balkrishna A. Blood pressure and heart rate variability during yoga-based alternate nostril breathing practice and breath awareness. Med Sci Monit Basic Res. 2014;20:184–193. doi: 10.12659/MSMBR.892063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang SZ, Li S, Xu XY, Lin GP, Shao L, Zhao Y, et al. Effect of slow abdominal breathing combined with biofeedback on blood pressure and heart rate variability in prehypertension. J Altern Complement Med. 2010;16:1039–1045. doi: 10.1089/acm.2009.0577. [DOI] [PubMed] [Google Scholar]
- 103.Chan AS, Cheung MC, Sze SL, Leung WW, Shi D. Shaolin Dan Tian breathing fosters relaxed and attentive mind: A randomized controlled neuro-electrophysiological study. Evid Based Complement Alternat Med. 2011;2011:180704. doi: 10.1155/2011/180704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cheng KS, Han RPS, Lee PF. Neurophysiological study on the effect of various short durations of deep breathing: A randomized controlled trial. Respir Physiol Neurobiol. 2018;249:23–31. doi: 10.1016/j.resp.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 105.Kozhevnikov M, Elliott J, Shephard J, Gramann K. Neurocognitive and somatic components of temperature increases during g-tummo meditation: Legend and reality. PLoS One. 2013;8:e58244. doi: 10.1371/journal.pone.0058244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Park YJ, Park YB. Clinical utility of paced breathing as a concentration meditation practice. Complement Ther Med. 2012;20:393–399. doi: 10.1016/j.ctim.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 107.Sherlin L, Muench F, Wyckoff S. Respiratory sinus arrhythmia feedback in a stressed population exposed to a brief stressor demonstrated by quantitative EEG and sLORETA. Appl Psychophysiol Biofeedback. 2010;35:219–228. doi: 10.1007/s10484-010-9132-z. [DOI] [PubMed] [Google Scholar]
- 108.Bates ME, Lesnewich LM, Uhouse SG, Gohel S, Buckman JF. Resonance-paced breathing alters neural response to visual cues: Proof-of-concept for a neuroscience-informed adjunct to addiction treatments. Front Psychiatry. 2019;10:624. doi: 10.3389/fpsyt.2019.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huijbers W, Pennartz CM, Beldzik E, Domagalik A, Vinck M, Hofman WF, et al. Respiration phase-locks to fast stimulus presentations: Implications for the interpretation of posterior midline “deactivations”. Hum Brain Mapp. 2014;35:4932–4943. doi: 10.1002/hbm.22523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.