Key Points
Question
How are studies that explicitly aim to emulate a target trial reported?
Findings
In this systematic review of 200 studies that explicitly aimed to emulate a target trial, reporting was inconsistent, and studies often did not report all necessary information related to the emulation of the target trial.
Meaning
Inconsistent reporting of studies that explicitly aim to emulate a target trial may impair the appraisal, synthesis, and implementation of study findings.
This systematic review assesses the reporting of observational studies that explicitly aimed to emulate target trials, hypothetical randomized trials that could answer causal questions of interest.
Abstract
Importance
Observational (nonexperimental) studies that aim to emulate a randomized trial (ie, the target trial) are increasingly informing medical and policy decision-making, but it is unclear how these studies are reported in the literature. Consistent reporting is essential for quality appraisal, evidence synthesis, and translation of evidence to policy and practice.
Objective
To assess the reporting of observational studies that explicitly aimed to emulate a target trial.
Evidence Review
We searched Medline, Embase, PsycINFO, and Web of Science for observational studies published between March 2012 and October 2022 that explicitly aimed to emulate a target trial of a health or medical intervention. Two reviewers double-screened and -extracted data on study characteristics, key predefined components of the target trial protocol and its emulation (eligibility criteria, treatment strategies, treatment assignment, outcome[s], follow-up, causal contrast[s], and analysis plan), and other items related to the target trial emulation.
Findings
A total of 200 studies that explicitly aimed to emulate a target trial were included. These studies included 26 subfields of medicine, and 168 (84%) were published from January 2020 to October 2022. The aim to emulate a target trial was explicit in 70 study titles (35%). Forty-three studies (22%) reported use of a published reporting guideline (eg, Strengthening the Reporting of Observational Studies in Epidemiology). Eighty-five studies (43%) did not describe all key items of how the target trial was emulated and 113 (57%) did not describe the protocol of the target trial and its emulation.
Conclusion and Relevance
In this systematic review of 200 studies that explicitly aimed to emulate a target trial, reporting of how the target trial was emulated was inconsistent. A reporting guideline for studies explicitly aiming to emulate a target trial may improve the reporting of the target trial protocols and other aspects of these emulation attempts.
Introduction
Analyses of observational (nonexperimental) data can be used to estimate the causal effect of interventions when randomized clinical trials are unavailable or infeasible. Bias in observational analyses may be limited by conceptualizing them as attempts to emulate target trials, ie, hypothetical randomized trials that would answer causal questions of interest.1,2,3 Hernán and Robins4 have outlined a framework for this approach, which involves first specifying the protocol of the target trial and then emulating the trial as closely as possible using observational data.4,5 The target trial framework may help reduce common biases in observational analyses and enhance transparency regarding design and analytic decisions. Moreover, it facilitates the interpretation of effect estimates and promotes meaningful discourse concerning potentially discrepant findings observed across studies.
Since at least the 1950s, the notion of observational analyses as attempts to approximate the goals of randomized clinical trials has underpinned many comparative studies in health, medicine, and related fields.6,7,8,9,10 The target trial emulation framework, introduced by Hernán and Robins4 in 2016, provided a template for reporting and conducting studies that aim to emulate target trials. The framework outlines items to be reported in the protocol of a target trial and its emulation, including: eligibility criteria, treatment strategies, assignment procedures, follow-up period, outcome(s), causal contrast(s), and analysis plan. Since the introduction of the framework, several articles have been published to assist researchers in conducting these studies and educating clinicians and other end users to interpret their findings.3,5,11,12,13,14 However, there is limited understanding of how researchers have implemented the target trial framework when reporting observational analyses with the explicit aim to emulate a target trial.
This review aimed to (1) describe how studies that explicitly aimed to emulate a target trial were reported and (2) examine whether these used published reporting guidelines. The findings of this review will be used to inform the development of a reporting guideline for studies explicitly aiming to emulate a target trial.15
Methods
This review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.16 The protocol was prospectively registered on the Open Science Framework (OSF).17,18 Protocol deviations are reported in eAppendix 1 in Supplement 1.
Searches
We searched 4 electronic databases of published literature from March 13, 2012, to October 20, 2022, including Medline, Embase, PsycINFO, and Web of Science. Our search included terms such as emulat* trial, target trial, target trial emulat*, real world data, and causal inference. Our complete search strategy is provided in eAppendix 2 in Supplement 1. To supplement the search strategy, we used citationchaser19 to conduct forward citation tracking of 5 selected seminal papers describing the target trial emulation framework.3,4,8,20,21 We also included papers known to the authorship team.
Eligibility Criteria
We included observational studies that explicitly aimed to emulate a target trial of a medical intervention; eAppendix 3 in Supplement 1 provides all terms deemed sufficient for an explicit target trial emulation. We restricted our inclusion of studies published from March 13, 2012 (10 years prior to registration of our protocol18 to capture recent trends in reporting) to October 20, 2022. We excluded studies that did not investigate a medical intervention; did not include human participants; were not written in English; only described the protocol of a study emulating a target trial, ie, a protocol of a planned study without results; or for which the full text was unavailable.
Record Management and Screening
We de-duplicated all records identified through searches in Endnote version 20 and imported into Excel version 2206 (Microsoft Corp). In duplicate, reviewers (H.J.H., A.G.C., M.D.J., and S.R.G.D.) independently performed screening of identified records for eligibility at the level of title and abstract and full text. Disagreements were resolved through discussion.
Data Extraction
Data were extracted in duplicate (H.J.H., M.D.J., S.R.G.D., J.J.D., S.A.W., R.R.N.R., A.G.C. performed this task independently) and compiled into a standardized spreadsheet piloted with 3 included studies. Disagreements were resolved by the lead author (H.J.H.) or through discussion. We did not blind reviewers to the journal article or study authors.
Data Items
We extracted information about the (1) characteristics of the included studies, (2) key protocol components that characterize the target trial approach, and (3) further items that may be important to report in studies emulating a target trial. The complete data extraction spreadsheet and code used are available on OSF.18
Characteristics of the included studies were year of publication, subfield of medicine defined based upon included population, data source (prospective cohort, electronic health records, claims data, registry, randomized clinical trial, or linked data, ie, where data sources were combined), sample size (unique individuals included and analyzed, rather than simulated or duplicated persons, such as in sequential trial designs), primary outcome, and type of treatment strategy being compared. Treatment strategy refers to any health care intervention including treatments, preventative interventions, and no change to current treatments, remaining consistent with the language used by Hernán and Robins.4 If a study investigated prevention of a given outcome in healthy individuals, the subfield of medicine was designated based on the outcome investigated. Each treatment strategy included in an article (eg, ≥2) was counted separately. We classified treatment strategies defined by the authors as no treatment or usual care as no change to current treatment approach(es).
We extracted whether each study reported the eligibility criteria, treatment strategies, assignment procedures, follow-up period, outcome(s), causal contrast(s), and analysis plan of the emulation of the target trial. These items and their definitions were informed by the target trial framework from Hernán and Robins.4 We considered a study to have specified how the target trial was emulated if all the previously listed protocol items were reported; these items are operationalized in eAppendix 4 in the Supplement. We extracted whether the protocol of the target trial or its emulation were presented in a table or in text, with table being prioritized if reported in both table and text format. We also stated whether the protocol of the target trial and how it was emulated were reported. We extracted whether the study reported a baseline in the target trial emulation where eligibility criteria, start of follow-up, and treatment assignment were aligned.
Further details of specific protocol components that may be important to report in studies emulating a target trial were chosen based on expert knowledge and recommendations from methodological papers on the target trial emulation framework.2,3,4 These included:
Treatment strategies: type of treatment strategy (eg, pharmacological, surgical; all studies are expected to include 2 or more treatment strategies), aspects of treatment strategies described (type of treatment, frequency, dose, and duration of treatment strategy).
Analysis plan: method(s) used to emulate randomization, description and selection of potential confounding variables, statistical and causal assumptions that relate to analyses, sensitivity analyses.
Other: study registration, rationale for the target trial emulation, reporting guideline used (referred to as a guideline hereafter). We only included a guideline if it was referenced as guiding the reporting of a study.
We deemed a study to report the assumptions underlying their analyses only when the assumption(s) were described in the text or in a cited reference. When authors reported that no residual confounding was assumed, we took this as equivalent to reporting an assumption of conditional exchangeability. We did not regard practices that may assess a causal assumption (eg, truncation of weights to satisfy the assumption of positivity) as reporting the assumption. We did not assess the appropriateness of authors’ reported assumptions. Items we extracted that were not included in commonly used guidelines are listed in eAppendix 5 in Supplement 1.
Data Analysis
We cleaned and analyzed data in R version 4.2.0 (R Project for Statistical Computing) using tableone, openxlsx, tidyverse, and readxl packages for data management and visualization. We summarized categorical variables using counts and percentages. Continuous variables were summarized using mean and SD or median and interquartile range. Post hoc, we assessed the reporting of how the target trial was emulated stratified by whether a guideline was used.
Results
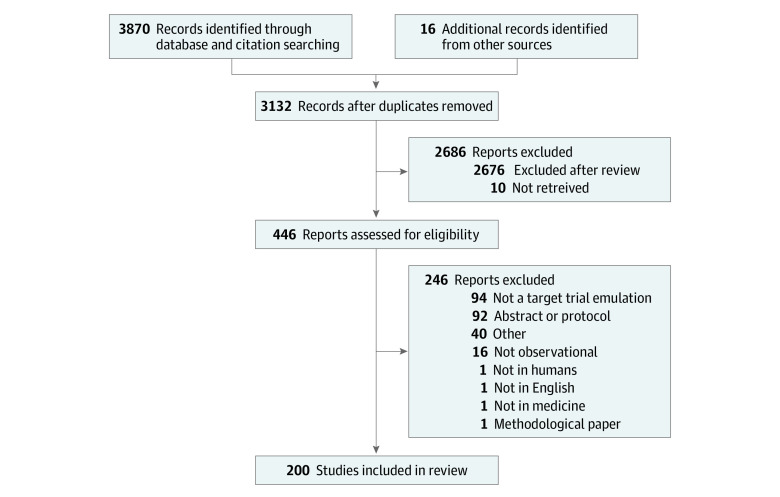
We retrieved 3133 unique records, of which 200 were included in the review (Figure 1).2,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220 All reasons for excluding records after full-text review are given in eAppendix 6 in Supplement 1.
Figure 1. Study Flow Diagram.
Characteristics of Included Studies
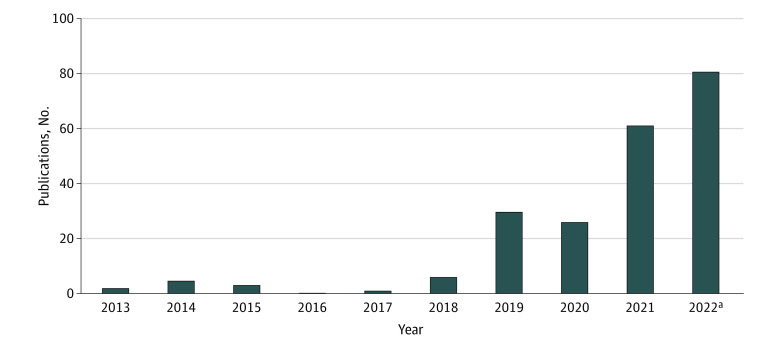
Of the 200 included studies,2,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220 168 (84%) were published from January 2020 to October 2022 (Figure 2).22,23,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,48,49,50,51,53,57,58,60,61,62,63,64,65,66,67,68,72,73,74,75,76,77,79,80,82,83,84,85,86,87,88,89,90,94,95,96,97,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,167,168,169,170,171,172,173,174,175,176,177,178,179,181,183,184,185,186,187,188,189,190,191,192,193,194,195,197,198,199,201,203,205,206,208,210,211,213,214,215,216,217 The included studies spanned 26 fields of medicine, predominately infectious disease (43 [22%]; 27 [14%] on COVID-19),26,27,30,35,36,37,38,39,47,53,54,55,56,59,66,67,68,77,82,90,94,97,99,100,108,109,110,130,136,139,140,141,145,148,157,166,186,197,205,217,218 cardiology (30 [15%]),22,28,31,41,60,70,71,73,85,105,106,116,117,121,122,143,149,150,151,152,161,179,184,187,193,198,206,214 and oncology (30 [15%]).2,32,40,44,45,46,51,76,78,81,89,91,92,93,95,103,104,129,132,138,153,168,173,174,178,182,190,192,204,211 One hundred and thirty-one studies (66%)2,22,23,25,31,32,34,35,36,39,41,42,43,44,45,46,47,51,52,53,58,59,61,62,63,65,67,68,70,71,72,73,74,75,76,77,80,81,85,86,89,90,92,93,94,95,96,100,101,102,103,104,105,106,108,109,111,112,116,117,119,120,121,122,123,124,125,126,127,128,129,130,131,133,134,135,136,137,141,143,144,146,147,148,149,150,151,152,153,154,155,156,157,158,160,161,162,164,167,168,170,171,173,177,178,179,180,181,183,184,185,187,188,189,193,195,199,200,201,205,207,210,212,213,216,217,219,220 used data from electronic health records, claims databases, or linked data sources. The treatment strategies most frequently investigated were pharmacological (228 of 435 [52%]) and no change to current treatment approach(es) (usual care or noninitiation of study treatment; 82 of 435 [19%]). All extracted characteristics of included studies are displayed in Table 1.
Figure 2. Number of Explicit Emulations of a Target Trial Included in Review Published per Year.
Table 1. Characteristics of 200 Included Studies.
| Characteristic | Count, No. (%) |
|---|---|
| Domain | |
| Infectious diseases | 43 (22) |
| Cardiology | 30 (15) |
| Oncology | 30 (15) |
| Nephrology | 14 (7) |
| Endocrinology | 11 (6) |
| Rheumatology | 10 (5) |
| Internal medicine | 9 (5) |
| Neurology | 7 (4) |
| Psychiatry | 6 (3) |
| Other | 40 (20) |
| Data source | |
| Electronic health record data | 49 (25) |
| Linked dataa | 46 (23) |
| Claims data | 36 (18) |
| Registry | 32 (16) |
| Prospective cohortb | 30 (15) |
| Randomized clinical trial | 6 (3) |
| Not reported | 1 (1) |
| Sample size, median (IQR)c | |
| Sample eligible | 11 253 (2157-101 078) |
| Sample analyzed | 9799 (1995-98 718) |
| Primary outcome | |
| Death | 72 (36) |
| Major adverse cardiovascular eventd | 19 (10) |
| Cancer | 8 (4) |
| Other | 101 (51) |
| Treatment strategies compared, No. | |
| 2 | 187 (94) |
| 3 | 5 (3) |
| 4 | 4 (2) |
| 5 | 2 (1) |
| 6 | 2 (1) |
| 10 | 1 (1) |
| Type of treatment strategy, No./total No.(%)e | |
| Pharmacological | 228/435 (52) |
| No change to current treatment | 82/435 (19) |
| Otherf | 61/435 (14) |
| Surgical | 37/435 (9) |
| Vaccine | 19/435 (4) |
| Medical device | 8/435 (2) |
Data in which 2 or more data sources are combined, eg, a registry is linked to a claims database.
Studies could only contribute to 1 data source item; if data collection for a cohort was conducted prospectively, the data source was classified only as a prospective cohort, even if data collection took place in the form of electronic health records or other data source listed.
The sample size includes the number of unique participants.
As major adverse cardiovascular event is often described heterogeneously, myocardial infarction, stroke, or major adverse cardiovascular event as defined by the authors were included; where the primary outcome was death, even if from cardiovascular events, the outcome was classified as death.
A given study may contribute 2 or more treatment strategies, which may be different, ie, 2 pharmacological treatment strategies compared with 2 no change to current treatment strategies.
The other category includes health care consultations, health care programs, organ transplants, and other interventions that would not fall under the other categories listed.
Characteristics of Target Trials and How They Were Emulated
One-hundred and fifteen studies (58%)2,22,23,25,26,30,31,32,34,35,42,45,46,47,48,49,50,51,54,55,56,57,59,61,64,65,66,69,70,71,72,74,75,76,77,78,85,87,88,89,90,91,93,95,96,98,100,101,102,103,104,106,116,127,128,130,132,133,134,135,137,138,139,141,142,143,148,149,151,152,154,156,157,158,160,161,162,167,168,172,174,176,177,178,179,180,182,183,184,187,188,189,190,191,192,195,196,198,199,200,201,203,204,205,206,208,209,210,211,213,214,215,216,219,220 completely reported how the target trial protocol was emulated. Eighty-seven studies (44%)2,24,25,28,30,32,34,42,46,51,52,55,56,57,59,61,62,64,65,66,70,71,72,76,77,78,79,82,85,87,88,89,91,92,93,94,98,100,103,104,107,113,115,116,117,119,120,122,125,126,130,133,134,135,142,143,147,149,150,152,158,162,165,168,170,172,176,179,185,187,188,190,191,192,195,202,203,209,212,213,214,216,219,220 provided both the protocol of the target trial and described how it was emulated (Table 2). The following items of the emulation were frequently reported: eligibility criteria (193 [97%]), treatment strategies (191 [96%]), assignment procedures (173 [87%]), primary outcome (196 [98%]), the follow-up period (186 [93%]), a causal contrast (146 [73%], and an analysis plan (194 [97%]) (Table 2).
Table 2. Characteristics of Target Trials and How They Were Emulated.
| Characteristic | Count, No. (%) |
|---|---|
| How the protocol of the emulated target trial was reported | |
| Not fully described | 85 (42) |
| Only in text | 59 (30) |
| Table | 56 (28) |
| Both target trial protocol and its emulation described explicitly as such | 87 (44) |
| Description of how the target trial was emulateda | |
| Eligibility criteria | 193 (97) |
| Treatment strategies | 191 (96) |
| Assignment procedures | 173 (87) |
| Outcome(s) | 196 (98) |
| Follow-up | 186 (93) |
| Causal contrast(s) | 146 (73) |
| Analysis plan | 194 (97) |
| Specification of time zero (ie, baseline) | 165 (83) |
Operational definitions of target trial protocol items are described in eAppendix 4 in Supplement 1.
Reporting of Further Items That Relate to the Target Trial Emulation
Seventy studies (35%)23,24,25,29,30,32,34,37,43,48,50,51,55,56,57,59,61,62,65,66,70,71,74,75,76,82,86,96,98,100,103,107,109,110,113,116,126,129,130,134,135,139,143,145,146,147,149,153,154,158,159,163,165,167,168,171,172,174,175,177,179,189,199,203,206,207,208,213,216,218 reported in the title that the study aimed to emulate a target trial; 180 (90%)2,22,23,24,25,26,27,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,86,87,88,89,91,92,93,94,95,96,97,99,100,101,102,103,104,106,107,109,110,111,112,113,115,116,117,118,119,121,122,123,124,125,126,127,128,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,146,147,148,149,150,151,152,153,154,156,157,158,160,161,162,164,165,166,167,168,169,170,171,173,174,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,216,217,218,219,220 did so in the Methods section. Twenty studies (10%) 24,25,34,35,43,52,68,80,84,86,117,131,133,147,156,161,169,171,192,216 reported the study was prospectively registered, 16 of these 20 (80%)24,25,34,43,52,68,80,86,117,131,133,156,161,169,171,216 also provided information on how to access the registration. One hundred and twenty-six studies (63%)2,22,23,24,25,26,27,28,29,30,34,35,37,39,40,44,45,46,48,49,50,52,54,57,59,60,62,64,65,66,67,68,69,70,71,72,73,74,75,76,78,79,82,83,86,87,88,89,91,92,93,99,102,103,104,108,109,111,112,113,114,115,117,120,121,122,123,124,125,126,127,130,133,134,135,138,139,141,142,145,146,147,149,150,151,152,153,155,156,158,159,161,162,164,168,169,171,172,173,175,176,179,180,182,184,185,187,190,191,192,194,195,197,198,199,200,201,205,208,209,210,211,213,215,216,217 specified whether a randomized clinical trial could be feasibly conducted; 61 (31%)28,29,34,39,45,48,49,54,57,62,66,67,68,69,73,75,79,83,86,87,88,91,99,102,103,104,108,109,112,115,117,121,122,123,125,126,138,139,146,152,153,156,158,162,171,184,185,187,192,198,199,205,210,211,213,215,216 stated that the randomized clinical trial was possible. Of the studies that stated a randomized clinical trial was possible, uncertainty in the generalizability of available trial findings was the most common reason for the target trial emulation (22 of 61 [36%]).28,45,52,57,68,85,86,103,114,115,117,122,123,152,158,184,187,192,198,210,215 Forty-three studies (22%)2,27,39,45,48,53,61,62,67,75,80,87,99,101,102,104,107,111,112,114,117,118,122,123,128,130,135,144,151,152,155,158,159,160,161,168,183,189,194,195,208,210,215 reported using a guideline, most commonly (29 of 43 [67%]) the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline.221,222 There were no qualitative differences between the reporting of the target trial emulation when studies were stratified by guideline use (eAppendix 7 in Supplement 1).
Most studies (187 [94%])2,22,23,24,25,26,27,28,29,31,32,33,34,35,36,37,38,39,40,42,43,44,45,46,47,48,49,50,51,53,54,55,56,57,58,59,60,61,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,81,82,83,87,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,176,177,178,179,180,181,182,183,184,186,187,188,189,190,191,192,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220 reported the set of variables that authors had decided to adjust for (eg, because they were potential confounders) in analyses, and 77 (39%)22,24,25,26,28,35,41,44,47,48,49,50,51,52,58,61,68,69,70,77,80,81,84,87,95,99,100,104,105,107,110,111,114,124,126,130,135,138,140,141,147,148,149,151,155,156,158,159,160,161,162,165,166,168,170,171,173,175,182,183,185,186,197,199,205,208,212,214,216,217,218 reported how these variables were selected. One hundred and thirty-one studies (66%)2,25,26,28,31,34,36,41,42,43,44,46,47,48,49,52,54,56,57,58,59,61,64,65,66,68,69,70,72,73,75,76,77,78,80,82,83,84,85,86,87,88,90,92,93,94,95,99,100,101,105,106,107,109,112,113,114,115,116,117,119,120,123,125,126,127,128,129,130,131,133,134,135,136,137,138,141,142,143,144,146,152,153,154,157,158,160,161,162,163,164,165,166,168,169,170,171,172,173,174,177,179,180,181,187,188,189,192,194,195,196,198,199,200,201,204,205,206,207,208,210,211,212,213,214,215,216,217,218,219,220 reported conducting a sensitivity analysis for statistical or causal assumptions; the most frequent (42 of 131 [32%]) was the use of a different approach to confounding adjustment (eg, using weighting rather than outcome regression). One hundred and fifty-eight studies (79%)2,22,25,26,27,28,29,30,32,36,38,39,40,41,44,45,46,47,48,49,50,51,52,54,55,56,57,58,59,61,63,64,65,66,67,68,69,70,71,72,75,76,77,78,79,80,81,82,83,84,85,87,88,89,90,93,94,97,99,100,101,103,105,106,107,108,109,111,112,113,114,116,117,118,119,120,121,122,123,126,127,128,129,130,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,149,150,152,153,155,156,158,159,160,161,162,163,165,166,167,168,169,170,171,172,173,174,175,176,177,180,181,182,183,186,187,188,189,190,191,192,194,195,197,198,199,200,201,202,203,204,205,206,207,208,210,211,212,213,214,215,216,219 reported that causal interpretation rests on the assumption that the comparison groups were comparable (ie, exchangeable) given the variables included in the analysis (Table 3). Twenty-six studies (13%)29,42,48,49,56,70,71,82,85,100,119,129,135,153,162,163,165,170,172,175,187,194,200,203,208,213 reported reliance on more than 1 causal assumption.
Table 3. Reporting of Further Items That Relate to the Target Trial Emulation.
| Item | Count, No. (%) |
|---|---|
| Where aim to emulate a target trial was describeda | |
| Title | 70 (35) |
| Abstract | 148 (74) |
| Introduction | 119 (60) |
| Methods | 180 (90) |
| Results | 55 (28) |
| Discussion | 142 (71) |
| Study prospectively registered | 20 (10) |
| Reason given why a randomized clinical trial could not be conducted | |
| Not reported | 74 (37) |
| NA, trial possible | 61 (31) |
| Unethical | 16 (8) |
| Long-term follow-up | 7 (4) |
| Rare outcomes | 7 (4) |
| Too costly | 5 (3) |
| Not timely | 5 (3) |
| Other | 25 (13) |
| When randomized clinical trial was reported as being possible, primary reason given for emulating a target trial, No./total No. (%) | |
| Generalizability of available trial findings | 22/61 (36) |
| Replicate published trial | 14/61 (23) |
| Trial ongoing | 8/61 (13) |
| Comparative effectiveness not previously investigated | 5/61 (8) |
| Previous conflicting results reported | 3/61 (5) |
| Other | 26/61 (43) |
| Data source cited | 125 (63) |
| Reporting guideline reported | 43 (22) |
| Reporting guideline used, No./total No. (%) | |
| STROBE | 29/43 (67) |
| ISPOR Good Research Practices for Comparative Effectiveness Researchb | 5/43 (12) |
| RECORD | 4/43 (9) |
| Nature Research Reporting Summary | 3/43 (7) |
| RECORD-PE | 2/43 (5) |
| TRIPOD | 1/43 (2) |
| Aspects of treatment strategies describeda | |
| Type | 417 (96) |
| Dose | 83 (19) |
| Duration | 57 (13) |
| Frequency | 54 (12) |
| None | 18 (4) |
| Other | 13 (3) |
| Variables adjusted in analyses listed | 187 (94) |
| Potential unmeasured confounders listed | 73 (37) |
| Method for selection of variables adjusted for described | 77 (39) |
| Analytic and causal assumptions stateda | |
| Exchangeability given selected confounders | 158 (79) |
| Positivity | 27 (14) |
| Consistency | 13 (7) |
| Statistical assumptions | 24 (12) |
| Other | 4 (2) |
| None | 35 (18) |
| Sensitivity analyses attempting to assess robustness to analytic or causal assumption(s) violations given | 131 (66) |
| Sensitivity analyses as reported by authors, No./total No. (%) | |
| Different approach to confounding adjustment | 42/131 (32) |
| Negative control | 23/131 (18) |
| Additional adjustment for confounding | 19/131 (15) |
| E-value | 15/131 (11) |
| Different censoring procedure | 10/131 (8) |
| Different approach to handling missing data | 4/131 (3) |
| Other | 24/131 (18) |
| Table describing baseline characteristics of groups presented | 171 (86) |
Abbreviations: ISPOR, International Society of Pharmacoeconomics and Outcomes Research; NA, not applicable; RECORD, Reporting of Studies Conducted Using Observational Routinely Collected Health Data; RECORD-PE, Reporting of studies Conducted Using Observational Routinely Collected Health Data–Statement for Pharmacoepidemiology; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; TRIPOD, Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis.
Total exceeds 100% as multiple characteristics could be included in a single study.
The ISPOR Good Research Practices for Comparative Effectiveness Research are guidelines for the conduct of comparative effectiveness studies, not a reporting guideline, however, were commonly cited as being used for reporting, therefore have been included.
Discussion
This systematic review summarized items reported in observational studies that explicitly aimed to emulate a target trial. We included 200 studies2,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220 published from 2013 to 2022, the majority of which (168 [84%]) were published between January 2020 and October 2022. The studies spanned 26 fields of medicine and mostly used sources of data that were routinely collected, such as electronic health records, health insurance claims data, or these data linked with other data sources. While the publication of studies explicitly aiming to emulate a target trial is increasing, only 58% of included studies completely reported how the target trial protocol was emulated.
Our finding that studies aiming to emulate a target trial inconsistently reported the emulation of the target trial is similar with results of previous systematic reviews of observational studies that did not explicitly aim to emulate a target trial.223 Nguyen et al223 systematically reviewed the risk of bias in observational studies investigating the effectiveness of interventions using the ROBINS-I tool,224 a risk of bias tool informed by the target trial framework. The authors found that only 3% of these observational studies (2 of 77) completely specified all items of the protocol of the (implicit or explicit) target trial. A much larger proportion of our sample of studies that explicitly aimed to emulate a target trial reported how the target trial was emulated; however, many were still incompletely reported. It appears the guidance from Hernán and Robins4 and previous work225,226,227,228 has been used inconsistently or perhaps misinterpreted, leaving key elements of the target trial and its emulation unreported.3
Our review shows there has been an increase in the publication of studies that explicitly aim to emulate a target trial. This trend could be attributed to the growing influence of such studies in shaping policy and regulatory decisions.229,230,231,232 For example, in mid-2022, the UK National Institute of Health and Care Excellence released “Real-World Evidence Framework,”229 which emphasizes the importance of using the framework of a target trial when estimating treatment effects for regulatory decision-making using observational data.229 Considering the emerging role of studies explicitly emulating a target trial within the health care decision-making framework, it is critical these studies are consistently and transparently reported. Once a target trial is emulated, unmeasured confounding may be a primary concern with observational analyses informing decision-making.229,233 We found that only 73 studies (37%) reported potential unmeasured confounders. It is unlikely all confounders would be measured in a given analysis, therefore the robustness of findings from a target trial emulation may be better assessed if potentially important unmeasured confounders are reported.
Guidelines have been developed to address inconsistent reporting,234 and if actively implemented, can improve reporting consistency and completeness.235,236,237 None of the included studies identified specific reporting guidance for studies that aimed to explicitly emulate a target trial, and the authors are not aware of any guidance for studies emulating a target trial published or under development,238 suggesting no formal guidance has been published. Twenty-two percent of studies cited general (eg, STROBE)221,222 and potentially inappropriate guidelines (eg, Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis).239 The use of guidelines was comparatively lower than seen in similar reviews of other types of observational studies, in which observed rates of guideline use ranged from 46% (67 of 147)240 to 47% (68 of 88).241 The lower use of guidelines observed in our review may reflect authors’ uncertainty on the most appropriate guideline when reporting a study that used the target trial framework.
Implications
Despite the growing number of studies using the target trial framework, reporting was inconsistent. Consistent and transparent reports are important for these studies given their emerging role in decision-making. For example, critical appraisal224 of the quality and robustness of findings from a study emulating a target trial is impaired when such analyses are poorly reported, leaving readers unable to understand the quality and conduct of the emulation. Similarly, findings of studies emulating a target trial are frequently compared with those of randomized clinical trials.2,86,242,243,244,245 Differences in effect estimates between target trial emulations and randomized clinical trials may arise due to various factors.246 Transparent reporting of the target trial protocol and how it was emulated may aid in understanding these differences and optimize the usefulness of these studies for decision-making.
No established, consensus-based15 guidelines are available to support authors reporting studies emulating a target trial. Commonly used guidelines (eg, STROBE)221,222 do not include items that relate to the protocol of the target trial4 or key items of the target trial emulation (causal contrast and items that relate to defining time-zero). Reporting of these items was not improved when authors followed guidelines such as STROBE (eAppendix 7 in Supplement 1). A new guideline for studies that explicitly aim to emulate a target trial is needed to provide detailed recommendations for the minimum set of items to be reported for these studies. Improved reporting of studies emulating a target trial may facilitate peer review by helping to ensure publications are complete, accurate, transparent, and reproducible. Improved reporting could also facilitate scientific discourse, support decision-making, reduce research waste, and ultimately improve health care.247,248
Strengths and Limitations
We used a sensitive search strategy to ensure all relevant studies were captured and followed recommended systematic review methods,249 including screening studies and extracting data in duplicate. We prospectively registered this systematic review18 and reported the findings in line with the PRISMA 2020 reporting guideline.16
This study has several limitations. First, we only included studies that explicitly stated that they aimed to emulate a target trial; therefore, our findings may present a more positive view of reporting practices compared with all observational analyses comparing interventions.250,251 Using the target trial framework is neither necessary nor sufficient for obtaining valid causal effect estimates from observational analyses; however, the framework may guide the implementation of sound principles of causal inference and study design. Second, we prespecified the reporting items to be extracted based on published recommendations for the specification of the target trial protocol and its emulation.4 Therefore, our ratings for these items may be skewed toward a particular way of reporting studies explicitly emulating a target trial. Third, we did not assess the appropriateness of the methods of included studies, only their reporting.
Conclusions
In this systematic review, reporting of studies that explicitly emulate a target trial was inconsistent, with several opportunities to improve the reporting of key items. A guideline expanding on the current recommendations may facilitate consistent and transparent reporting, improving the appraisal, synthesis, and implementation of study findings in clinical practice and health policy.
Appendix 1. Methodological Differences to the Protocol
Appendix 2. Complete Search Strategies for All Databases
Appendix 3. List of Phrases Deemed to Indicate Explicit Emulation of a Target Trial
Appendix 4. Operational Definitions for Items of the Emulation of the Target Trial
Appendix 5. Items Extracted That Are Not Included in Commonly Used Reporting Guidelines (STROBE, RECORD, ISPOR)
Appendix 6. Reasons for Study Exclusion at Level of Full Text
Appendix 7. Subgroup Analysis of Reporting of Target Trial Protocol and How It Was Emulated, by Reporting Guideline Use
Data Sharing Statement
References
- 1.Kuehne F, Arvandi M, Hess LM, et al. Causal analyses with target trial emulation for real-world evidence removed large self-inflicted biases: systematic bias assessment of ovarian cancer treatment effectiveness. J Clin Epidemiol. 2022;152:269-280. doi: 10.1016/j.jclinepi.2022.10.005 [DOI] [PubMed] [Google Scholar]
- 2.Dickerman BA, García-Albéniz X, Logan RW, Denaxas S, Hernán MA. Avoidable flaws in observational analyses: an application to statins and cancer. Nat Med. 2019;25(10):1601-1606. doi: 10.1038/s41591-019-0597-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernán MA, Sauer BC, Hernández-Díaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol. 2016;79:70-75. doi: 10.1016/j.jclinepi.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758-764. doi: 10.1093/aje/kwv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernán MA, Wang W, Leaf DE. Target trial emulation: a framework for causal inference from observational data. JAMA. 2022;328(24):2446-2447. doi: 10.1001/jama.2022.21383 [DOI] [PubMed] [Google Scholar]
- 6.Cochran WG. Observational studies. Statistical Papers in Honor of George W Snedecor. Iowa State University Press. 1972:77-90. doi: 10.2307/2346927 [DOI] [Google Scholar]
- 7.Dorn HF. Philosophy of inferences from retrospective studies. Am J Public Health Nations Health. 1953;43(6 Pt 1):677-683. doi: 10.2105/AJPH.43.6_Pt_1.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robins J. A new approach to causal inference in mortality studies with a sustained exposure period—application to control of the healthy worker survivor effect. Math Model. 1986;7(9-12):1393-1512. doi: 10.1016/0270-0255(86)90088-6 [DOI] [Google Scholar]
- 9.Rubin DB. Estimating causal effects of treatments in randomized and nonrandomized studies. J Educ Psychol. 1974;66(5):688. doi: 10.1037/h0037350 [DOI] [Google Scholar]
- 10.Wold H. Causality and econometrics. Econometrica. 1954;22(2):162-177. doi: 10.2307/1907540 [DOI] [Google Scholar]
- 11.Hernán MA. Methods of public health research—strengthening causal inference from observational data. N Engl J Med. 2021;385(15):1345-1348. doi: 10.1056/NEJMp2113319 [DOI] [PubMed] [Google Scholar]
- 12.Kutcher SA, Brophy JM, Banack HR, Kaufman JS, Samuel M. Emulating a andomized controlled trial with observational data: an introduction to the target trial framework. Can J Cardiol. 2021;37(9):1365-1377. doi: 10.1016/j.cjca.2021.05.012 [DOI] [PubMed] [Google Scholar]
- 13.Matthews AA, Danaei G, Islam N, Kurth T. Target trial emulation: applying principles of andomized trials to observational studies. BMJ. 2022;378:e071108. doi: 10.1136/bmj-2022-071108 [DOI] [PubMed] [Google Scholar]
- 14.Gomes M, Latimer N, Soares M, et al. Target trial emulation for transparent and robust estimation of treatment effects for health technology assessment using real-world data: opportunities and challenges. Pharmacoeconomics. 2022;40(6):577-586. doi: 10.1007/s40273-022-01141-x [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Schulz KF, Simera I, Altman DG. Guidance for developers of health research reporting guidelines. PLoS Med. 2010;7(2):e1000217. doi: 10.1371/journal.pmed.1000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster ED, Deardorff A. Open Science Framework (OSF). J Med Libr Assoc. 2017;105(2):203-206. doi: 10.5195/jmla.2017.88 [DOI] [Google Scholar]
- 18.Hansford H, Lee H, Cashin A, et al. What is reported in target trial emulations: a systematic review protocol. March 13, 2022. Accessed August 29, 2023. https://osf.io/uj56m/
- 19.Haddaway H. citationchaser: An R package for forward and backward citations chasing in academic searching. Version 0.0.3. 2021. Accessed August 28, 2023. https://github.com/nealhaddaway/citationchaser
- 20.Hernán MA. How to estimate the effect of treatment duration on survival outcomes using observational data. BMJ. 2018;360:k182. doi: 10.1136/bmj.k182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robins JM, Hernan MJ. Estimation of the causal effects of time-varying exposures. In: Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G, eds. Longitudinal Data Analysis. CRC press; 2008. doi: 10.1201/9781420011579.ch23 [DOI] [Google Scholar]
- 22.Aakjaer M, Werther SK, De Bruin ML, Andersen M. Serious arrhythmia in initiators of citalopram, escitalopram, and other selective serotonin reuptake inhibitors: a population-based cohort study in older adults. Clin Transl Sci. 2022;15(9):2105-2115. doi: 10.1111/cts.13319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abrahami D, Pradhan R, Yin H, Honig P, Baumfeld Andre E, Azoulay L. Use of real-world data to emulate a clinical trial and support regulatory decision making: assessing the impact of temporality, comparator choice, and method of adjustment. Clin Pharmacol Ther. 2021;109(2):452-461. doi: 10.1002/cpt.2012 [DOI] [PubMed] [Google Scholar]
- 24.Admon AJ, Donnelly JP, Casey JD, et al. Emulating a novel clinical trial using existing observational data. predicting results of the PreVent Study. Ann Am Thorac Soc. 2019;16(8):998-1007. doi: 10.1513/AnnalsATS.201903-241OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn N, Nolde M, Günter A, et al. Emulating a target trial of proton pump inhibitors and dementia risk using claims data. Eur J Neurol. 2022;29(5):1335-1343. doi: 10.1111/ene.15284 [DOI] [PubMed] [Google Scholar]
- 26.Al-Samkari H, Gupta S, Leaf RK, et al. ; STOP-COVID Investigators . Thrombosis, bleeding, and the observational effect of early therapeutic anticoagulation on survival in critically ill patients with COVID-19. Ann Intern Med. 2021;174(5):622-632. doi: 10.7326/M20-6739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prats-Uribe A, Tobed M, Villacampa JM, et al. ; TraqueoCOVID SEORL Group . Timing of elective tracheotomy and duration of mechanical ventilation among patients admitted to intensive care with severe COVID-19: a multicenter prospective cohort study. Head Neck. 2021;43(12):3743-3756. doi: 10.1002/hed.26863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Althunian TA, de Boer A, Groenwold RHH, Rengerink KO, Souverein PC, Klungel OH. Rivaroxaban was found to be noninferior to warfarin in routine clinical care: a retrospective noninferiority cohort replication study. Pharmacoepidemiol Drug Saf. 2020;29(10):1263-1272. doi: 10.1002/pds.5065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ankarcrona V, Zhao H, Jacobsson B, Brismar Wendel S. Obstetric anal sphincter injury after episiotomy in vacuum extraction: an epidemiological study using an emulated andomized trial approach. BJOG. 2021;128(10):1663-1671. doi: 10.1111/1471-0528.16663 [DOI] [PubMed] [Google Scholar]
- 30.Atkinson A, Zwahlen M, Barger D, et al. ; Opportunistic Infections Project Working Group of the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) in EuroCoord . Withholding primary pneumocystis pneumonia prophylaxis in virologically suppressed patients with human immunodeficiency virus: an emulation of a pragmatic trial in COHERE. Clin Infect Dis. 2021;73(2):195-202. doi: 10.1093/cid/ciaa615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aubert CE, Sussman JB, Hofer TP, Cushman WC, Ha JK, Min L. Adding a new medication versus maximizing dose to intensify hypertension treatment in older adults : a retrospective observational study. Ann Intern Med. 2021;174(12):1666-1673. doi: 10.7326/M21-1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacic J, Liu T, Thompson RH, et al. Emulating target clinical trials of radical nephrectomy with or without lymph node dissection for renal cell carcinoma. Urology. 2020;140:98-106. doi: 10.1016/j.urology.2020.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bai L, Scott MKD, Steinberg E, et al. Computational drug repositioning of atorvastatin for ulcerative colitis. J Am Med Inform Assoc. 2021;28(11):2325-2335. doi: 10.1093/jamia/ocab165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbulescu A, Askling J, Saevarsdottir S, Kim SC, Frisell T. Combined conventional synthetic disease modifying therapy vs. infliximab for rheumatoid arthritis: emulating a randomized trial in observational data. Clin Pharmacol Ther. 2022;112(4):836-845. doi: 10.1002/cpt.2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078-1090. doi: 10.1056/NEJMoa2110475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398(10316):2093-2100. doi: 10.1016/S0140-6736(21)02249-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becker WC, Li Y, Caniglia EC, et al. Cannabis use, pain interference, and prescription opioid receipt among persons with HIV: a target trial emulation study. AIDS Care. 2022;34(4):469-477. doi: 10.1080/09540121.2021.1944597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bell Gorrod H, Court R, Schomaker M, Maartens G, Murphy RA. Increased mortality with delayed and missed switch to second-line antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr. 2020;84(1):107-113. doi: 10.1097/QAI.0000000000002313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergqvist R, Ahlqvist VH, Lundberg M, et al. HMG-CoA reductase inhibitors and COVID-19 mortality in Stockholm, Sweden: a registry-based cohort study. PLoS Med. 2021;18(10):e1003820. doi: 10.1371/journal.pmed.1003820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bharadwaj M, Kaul S, Fleishman A, et al. Adjuvant chemotherapy versus observation following radical cystectomy for locally advanced urothelial carcinoma of the bladder. Urol Oncol. 2022;40(6):274.e15-274.e23. doi: 10.1016/j.urolonc.2022.02.002 [DOI] [PubMed] [Google Scholar]
- 41.Biscaglia S, Erriquez A, Serenelli M, et al. Complete versus culprit-only strategy in older MI patients with multivessel disease. Catheter Cardiovasc Interv. 2022;99(4):970-978. doi: 10.1002/ccd.30075 [DOI] [PubMed] [Google Scholar]
- 42.Börnhorst C, Reinders T, Rathmann W, et al. Avoiding time-related biases: a feasibility study on antidiabetic drugs and pancreatic cancer applying the parametric g-formula to a large German healthcare database. Clin Epidemiol. 2021;13(13):1027-1038. doi: 10.2147/CLEP.S328342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bosch NA, Law AC, Vail EA, et al. Inhaled nitric oxide vs epoprostenol during acute respiratory failure: an observational target trial emulation. Chest. 2022;162(6):1287-1296. doi: 10.1016/j.chest.2022.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boström P, Hultberg DK, Häggström J, et al. Oncological impact of high vascular tie after surgery for rectal cancer: a nationwide cohort study. Ann Surg. 2021;274(3):e236-e244. doi: 10.1097/SLA.0000000000003663 [DOI] [PubMed] [Google Scholar]
- 45.Boyne DJ, Cheung WY, Hilsden RJ, et al. Association of a shortened duration of adjuvant chemotherapy with overall survival among individuals with stage III colon cancer. JAMA Netw Open. 2021;4(3):e213587. doi: 10.1001/jamanetworkopen.2021.3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braitmaier M, Schwarz S, Kollhorst B, Senore C, Didelez V, Haug U. Screening colonoscopy similarly prevented distal and proximal colorectal cancer: a prospective study among 55-69-year-olds. J Clin Epidemiol. 2022;149:118-126. doi: 10.1016/j.jclinepi.2022.05.024 [DOI] [PubMed] [Google Scholar]
- 47.Brouwer ES, Napravnik S, Eron JJ Jr, et al. Effects of combination antiretroviral therapies on the risk of myocardial infarction among HIV patients. Epidemiology. 2014;25(3):406-417. doi: 10.1097/EDE.0000000000000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruun-Rasmussen P, Andersen PK, Banasik K, Brunak S, Johansson PI. Estimating the effect of donor sex on red blood cell transfused patient mortality: a retrospective cohort study using a targeted learning and emulated trials-based approach. EClinicalMedicine. 2022;51:101628. doi: 10.1016/j.eclinm.2022.101628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruun-Rasmussen P, Kragh Andersen P, Banasik K, Brunak S, Johansson PI. Intervening on the storage time of RBC units and its effects on adverse recipient outcomes using real-world data. Blood. 2022;139(25):3647-3654. doi: 10.1182/blood.2022015892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bujkiewicz S, Singh J, Wheaton L, et al. Bridging disconnected networks of first and second lines of biologic therapies in rheumatoid arthritis with registry data: andomiz evidence synthesis with target trial emulation. J Clin Epidemiol. 2022;150:171-178. doi: 10.1016/j.jclinepi.2022.06.011 [DOI] [PubMed] [Google Scholar]
- 51.Buranupakorn T, Thangsuk P, Patumanond J, Phinyo P. Emulation of a target trial to evaluate the causal effect of palliative care consultation on the survival time of patients with hepatocellular carcinoma. Cancers (Basel). 2021;13(5):992. doi: 10.3390/cancers13050992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burn E, Weaver J, Morales DR, et al. Opioid use, postoperative complications, and implant survival after unicompartmental versus total knee replacement: a population-based network study. Lancet Rheumatol. 2019;1(4):229-236. doi: 10.1016/S2665-9913(19)30075-X [DOI] [PubMed] [Google Scholar]
- 53.Butt AA, Talisa VB, Shaikh OS, Omer SB, Mayr FB. Relative vaccine effectiveness of a severe acute respiratory syndrome coronavirus 2 messenger RNA vaccine booster dose against the Omicron variant. Clin Infect Dis. 2022;75(12):2161-2168. doi: 10.1093/cid/ciac328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cain LE, Hernán MA; HIV-CAUSAL Collaboration . The effect of efavirenz versus nevirapine-containing regimens in the HIV-CAUSAL Collaboration: reply to Llibre and Podzamczer and additional results. AIDS. 2013;27(13):2169-2170. doi: 10.1097/01.aids.0000432446.15061.27 [DOI] [PubMed] [Google Scholar]
- 55.Cain LE, Saag MS, Petersen M, et al. ; Antiretroviral Therapy Cohort Collaboration, the Centers for AIDS Research Network of Integrated Clinical Systems, and the HIV-CAUSAL Collaboration . Using observational data to emulate a randomized trial of dynamic treatment-switching strategies: an application to antiretroviral therapy. Int J Epidemiol. 2016;45(6):2038-2049. doi: 10.1093/ije/dyv295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caniglia EC, Robins JM, Cain LE, et al. Emulating a trial of joint dynamic strategies: an application to monitoring and treatment of HIV-positive individuals. Stat Med. 2019;38(13):2428-2446. doi: 10.1002/sim.8120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caniglia EC, Rojas-Saunero LP, Hilal S, et al. Emulating a target trial of statin use and risk of dementia using cohort data. Neurology. 2020;95(10):e1322-e1332. doi: 10.1212/WNL.0000000000010433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caniglia EC, Stevens ER, Khan M, et al. Does reducing drinking in patients with unhealthy alcohol use improve pain interference, use of other substances, and psychiatric symptoms? Alcohol Clin Exp Res. 2020;44(11):2257-2265. doi: 10.1111/acer.14455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caniglia EC, Zash R, Jacobson DL, et al. Emulating a target trial of antiretroviral therapy regimens started before conception and risk of adverse birth outcomes. AIDS. 2018;32(1):113-120. doi: 10.1097/QAD.0000000000001673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaure-Pardos A, Aguilar-Palacio I, Rabanaque MJ, et al. Effectiveness of statins for primary prevention of cardiovascular disease in low- and medium-risk males: a causal inference approach with observational data. J Pers Med. 2022;12(5):658. doi: 10.3390/jpm12050658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen YB, Liang CS, Wang LJ, et al. Comparative effectiveness of valproic acid in different serum concentrations for maintenance treatment of bipolar disorder: a retrospective cohort study using target trial emulation framework. EClinicalMedicine. 2022;54:101678. doi: 10.1016/j.eclinm.2022.101678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Z, Zhang H, Guo Y, et al. Exploring the feasibility of using real-world data from a large clinical data research network to simulate clinical trials of Alzheimer’s disease. NPJ Digit Med. 2021;4(1):84. doi: 10.1038/s41746-021-00452-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng Y, Zamrini E, Ahmed A, Wu WC, Shao Y, Zeng-Treitler Q. Medication-wide association study plus (MWAS+): a proof of concept study on drug repurposing. Med Sci (Basel). 2022;10(3):48. doi: 10.3390/medsci10030048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiu Y-H, Chavarro JE, Dickerman BA, et al. Estimating the effect of nutritional interventions using observational data: the American Heart Association’s 2020 Dietary Goals and mortality. Am J Clin Nutr. 2021;114(2):690-703. doi: 10.1093/ajcn/nqab100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chiu YH, Yland JJ, Rinaudo P, et al. Effectiveness and safety of intrauterine insemination vs. assisted reproductive technology: emulating a target trial using an observational database of administrative claims. Fertil Steril. 2022;117(5):981-991. doi: 10.1016/j.fertnstert.2022.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cho K, Keithly SC, Kurgansky KE, et al. Early convalescent plasma therapy and mortality among US veterans hospitalized with nonsevere COVID-19: an observational analysis emulating a target trial. J Infect Dis. 2021;224(6):967-975. doi: 10.1093/infdis/jiab330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dagan N, Barda N, Biron-Shental T, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med. 2021;27(10):1693-1695. doi: 10.1038/s41591-021-01490-8 [DOI] [PubMed] [Google Scholar]
- 68.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412-1423. doi: 10.1056/NEJMoa2101765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Danaei G, García Rodríguez LA, Fernandez Cantero O, Hernán MA. Statins and risk of diabetes: an analysis of electronic medical records to evaluate possible bias due to differential survival. Diabetes Care. 2013;36(5):1236-1240. doi: 10.2337/dc12-1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Danaei G, Rodríguez LAG, Cantero OF, Logan R, Hernán MA. Observational data for comparative effectiveness research: an emulation of andomized trials of statins and primary prevention of coronary heart disease. Stat Methods Med Res. 2013;22(1):70-96. doi: 10.1177/0962280211403603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Danaei G, García Rodríguez LA, Cantero OF, Logan RW, Hernán MA. Electronic medical records can be used to emulate target trials of sustained treatment strategies. J Clin Epidemiol. 2018;96:12-22. doi: 10.1016/j.jclinepi.2017.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dave CV, Brittenham GM, Carson JL, Setoguchi S. Risks for anaphylaxis with intravenous iron formulations: a retrospective cohort study. Ann Intern Med. 2022;175(5):656-664. doi: 10.7326/M21-4009 [DOI] [PubMed] [Google Scholar]
- 73.Dawwas GK, Cuker A, Barnes GD, Lewis JD, Hennessy S. Apixaban versus rivaroxaban in patients with atrial fibrillation and valvular heart disease: a population-based study. Ann Intern Med. 2022;175(11):1506-1514. doi: 10.7326/M22-0318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Delapaz NR, Hor WK, Gilbert M, et al. An emulation of randomized trials of administrating antipsychotics in PTSD patients for outcomes of suicide-related events. J Pers Med. 2021;11(3):178. doi: 10.3390/jpm11030178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deng Y, Polley EC, Wallach JD, et al. Emulating the GRADE trial using real world data: retrospective comparative effectiveness study. BMJ. 2022;379:e070717. doi: 10.1136/bmj-2022-070717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dickerman BA, García-Albéniz X, Logan RW, Denaxas S, Hernán MA. Emulating a target trial in case-control designs: an application to statins and colorectal cancer. Int J Epidemiol. 2020;49(5):1637-1646. doi: 10.1093/ije/dyaa144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dickerman BA, Gerlovin H, Madenci AL, et al. Comparative effectiveness of BNT162b2 and mRNA-1273 vaccines in U.S. veterans. N Engl J Med. 2022;386(2):105-115. doi: 10.1056/NEJMoa2115463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dickerman BA, Giovannucci E, Pernar CH, Mucci LA, Hernán MA. Guideline-Based physical activity and survival among US men with nonmetastatic prostate cancer. Am J Epidemiol. 2019;188(3):579-586. doi: 10.1093/aje/kwy261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dickerman BA, Madenci AL, Gerlovin H, et al. Comparative safety of BNT162b2 and mRNA-1273 vaccines in a nationwide cohort of US veterans. JAMA Intern Med. 2022;182(7):739-746. doi: 10.1001/jamainternmed.2022.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Don EE, Mijatovic V, van Eekelen R, Huirne JAF. The effect of myomectomy on reproductive outcomes in patients with uterine fibroids: a retrospective cohort study. Reprod Biomed Online. 2022;45(5):970-978. doi: 10.1016/j.rbmo.2022.05.025 [DOI] [PubMed] [Google Scholar]
- 81.Emilsson L, García-Albéniz X, Logan RW, Caniglia EC, Kalager M, Hernán MA. Examining bias in studies of statin treatment and survival in patients with cancer. JAMA Oncol. 2018;4(1):63-70. doi: 10.1001/jamaoncol.2017.2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Evans RN, Harris J, Rogers CA, MacGowan A. Emulating the MERINO andomized control trial using data from an observational cohort and trial of rapid diagnostic (BSI-FOO). PLoS One. 2022;17(5):e0268807. doi: 10.1371/journal.pone.0268807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Farjat AE, Virdone S, Thomas LE, Kakkar AK, Pieper KS, Piccini JP. The importance of the design of observational studies in comparative effectiveness research: lessons from the GARFIELD-AF and ORBIT-AF registries. Am Heart J. 2022;243:110-121. doi: 10.1016/j.ahj.2021.09.003 [DOI] [PubMed] [Google Scholar]
- 84.Fischer U, Branca M, Bonati LH, et al. ; Investigators of the Swiss Stroke Registry . Magnetic resonance imaging or computed tomography for suspected acute stroke: association of admission image modality with acute recanalization therapies, workflow metrics, and outcomes. Ann Neurol. 2022;92(2):184-194. doi: 10.1002/ana.26413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fotheringham J, Latimer N, Froissart M, et al. Survival on four compared with three times per week haemodialysis in high ultrafiltration patients: an observational study. Clin Kidney J. 2020;14(2):665-672. doi: 10.1093/ckj/sfaa250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Franklin JM, Patorno E, Desai RJ, et al. Emulating randomized clinical trials with nonrandomized real-world evidence studies: first results from the RCT DUPLICATE Initiative. Circulation. 2021;143(10):1002-1013. doi: 10.1161/CIRCULATIONAHA.120.051718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fu EL, Evans M, Carrero JJ, et al. Timing of dialysis initiation to reduce mortality and cardiovascular events in advanced chronic kidney disease: nationwide cohort study. BMJ. 2021;375:e066306. doi: 10.1136/bmj-2021-066306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fu EL, Evans M, Clase CM, et al. Stopping renin-angiotensin system inhibitors in patients with advanced CKD and risk of adverse outcomes: a nationwide study. J Am Soc Nephrol. 2021;32(2):424-435. doi: 10.1681/ASN.2020050682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gaber CE, Shaheen NJ, Edwards JK, et al. Trimodality therapy vs definitive chemoradiation in older adults with locally advanced esophageal cancer. JNCI Cancer Spectr. 2022;6(6):pkac069. doi: 10.1093/jncics/pkac069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gallay L, Tran V-T, Perrodeau E, et al. ; COCO-OLD (Collaborative cOhort COrticoteroids for OLD patients with COvid-19) study Group . Fourteen-day survival among older adults with severe infection with severe acute respiratory syndrome coronavirus 2 treated with corticosteroid: a cohort study. Clin Microbiol Infect. 2021;27(8):1145-1150. doi: 10.1016/j.cmi.2021.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garcia-Albeniz X, Chan JM, Paciorek A, et al. Immediate versus deferred initiation of androgen deprivation therapy in prostate cancer patients with PSA-only relapse: an observational follow-up study. Eur J Cancer. 2015;51(7):817-824. doi: 10.1016/j.ejca.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.García-Albéniz X, Hsu J, Bretthauer M, Hernán MA. Effectiveness of screening colonoscopy to prevent colorectal cancer among Medicare beneficiaries aged 70 to 79 years: a prospective observational study. Ann Intern Med. 2017;166(1):18-26. doi: 10.7326/M16-0758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garcia-Albeniz X, Hernan M, Logan R, Price M, Hsu J. Continuation of annual screening mammograms and breast-cancer mortality in women over 70. Ann Oncol. 2019;30(suppl 5):v675-v676. doi: 10.1093/annonc/mdz263.016 [DOI] [Google Scholar]
- 94.Gazit S, Shlezinger R, Perez G, et al. The incidence of SARS-CoV-2 reinfection in persons with naturally acquired immunity with and without subsequent receipt of a single dose of BNT162b2 vaccine: a retrospective cohort study. Ann Intern Med. 2022;175(5):674-681. doi: 10.7326/M21-4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ghiani M, Maywald U, Wilke T, Heeg B. Bridging the gap between oncology clinical trials and real-world data: evidence on replicability of efficacy results using German claims data. J Comp Eff Res. 2022;11(7):513-521. doi: 10.2217/cer-2021-0224 [DOI] [PubMed] [Google Scholar]
- 96.Gilbert M, Dinh La A, Romulo Delapaz N, et al. An emulation of randomized trials of administrating benzodiazepines in PTSD patients for outcomes of suicide-related events. J Clin Med. 2020;9(11):3492. doi: 10.3390/jcm9113492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Girardi E, Caro-Vega Y, Cozzi-Lepri A, et al. The contribution of late HIV diagnosis on the occurrence of HIV-associated tuberculosis: a 5-year estimate using real-world data. AIDS. 2022;36(14):2005-2013. doi: 10.1097/QAD.0000000000003321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grøn KL, Glintborg B, Nørgaard M, et al. Comparative effectiveness of certolizumab pegol, abatacept, and biosimilar infliximab in patients with rheumatoid arthritis treated in routine care: observational data from the Danish DANBIO registry emulating a randomized trial. Arthritis Rheumatol. 2019;71(12):1997-2004. doi: 10.1002/art.41031 [DOI] [PubMed] [Google Scholar]
- 99.Gupta S, Wang W, Hayek SS, et al. ; STOP-COVID Investigators . Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181(1):41-51. doi: 10.1001/jamainternmed.2020.6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hajage D, Combes A, Guervilly C, et al. ; COVID-ICU Investigators . Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: an emulated target trial analysis. Am J Respir Crit Care Med. 2022;206(3):281-294. doi: 10.1164/rccm.202111-2495OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hassan W, Shrestha P, Sumida K, et al. Association of uric acid-lowering therapy with incident chronic kidney disease. JAMA Netw Open. 2022;5(6):e2215878. doi: 10.1001/jamanetworkopen.2022.15878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hedderson MM, Badon SE, Pimentel N, et al. Association of glyburide and subcutaneous insulin with perinatal complications among women with gestational diabetes. JAMA Netw Open. 2022;5(3):e225026. doi: 10.1001/jamanetworkopen.2022.5026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Heil TC, Verdaasdonk EGG, Maas HAAM, et al. Improved postoperative outcomes after prehabilitation for colorectal cancer surgery in older patients: an emulated target trial. Ann Surg Oncol. 2023;30(1):244-254. doi: 10.1245/s10434-022-12623-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hou J, Zhao R, Cai T, et al. Temporal trends in clinical evidence of 5-year survival within electronic health records among patients with early-stage colon cancer managed with laparoscopy-assisted colectomy vs open colectomy. JAMA Netw Open. 2022;5(6):e2218371. doi: 10.1001/jamanetworkopen.2022.18371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Htoo PT, Buse J, Cavender M, et al. Cardiovascular effectiveness of sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide-1 receptor agonists in older patients in routine clinical care with or without history of atherosclerotic cardiovascular diseases or heart failure. J Am Heart Assoc. 2022;11(4):e022376. doi: 10.1161/JAHA.121.022376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang H-K, Liu PP-S, Lin S-M, et al. Diabetes-related complications and mortality in patients with atrial fibrillation receiving different oral anticoagulants : a nationwide analysis. Ann Intern Med. 2022;175(4):490-498. doi: 10.7326/M21-3498 [DOI] [PubMed] [Google Scholar]
- 107.Huang JY, Cai S, Huang Z, et al. Analyses of child cardiometabolic phenotype following assisted reproductive technologies using a pragmatic trial emulation approach. Nat Commun. 2021;12(1):5613. doi: 10.1038/s41467-021-25899-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hulme WJ, Williamson EJ, Green ACA, et al. Comparative effectiveness of ChAdOx1 versus BNT162b2 COVID-19 vaccines in health and social care workers in England: cohort study using OpenSAFELY. BMJ. 2022;378:e068946. doi: 10.1136/bmj-2021-068946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ioannou GN, Locke ER, Green PK, Berry K. Comparison of Moderna versus Pfizer-BioNTech COVID-19 vaccine outcomes: a target trial emulation study in the U.S. Veterans Affairs healthcare system. EClinicalMedicine. 2022;45:101326. doi: 10.1016/j.eclinm.2022.101326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ioannou GN, Locke ER, O’Hare AM, et al. COVID-19 vaccination effectiveness against infection or death in a national US health care system: a target trial emulation study. Ann Intern Med. 2022;175(3):352-361. doi: 10.7326/M21-3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Izano MA, Lo JC, Adams AL, et al. Bisphosphonate treatment beyond 5 years and hip fracture risk in older women. JAMA Netw Open. 2020;3(12):e2025190. doi: 10.1001/jamanetworkopen.2020.25190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jang HY, Kim IW, Oh JM. Using real-world data for supporting regulatory decision making: comparison of cardiovascular and safety outcomes of an empagliflozin randomized clinical trial versus real-world data. Front Pharmacol. 2022;13:928121. doi: 10.3389/fphar.2022.928121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jin X, Ding C, Hunter DJ, Gallego B. Effectiveness of vitamin D supplementation on knee osteoarthritis: a target trial emulation study using data from the Osteoarthritis Initiative cohort. Osteoarthritis Cartilage. 2022;30(11):1495-1505. doi: 10.1016/j.joca.2022.06.005 [DOI] [PubMed] [Google Scholar]
- 114.Johri M, Sylvestre M-P, Koné GK, Chandra D, Subramanian SV. Effects of improved drinking water quality on early childhood growth in rural Uttar Pradesh, India: a propensity-score analysis. PLoS One. 2019;14(1):e0209054. doi: 10.1371/journal.pone.0209054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Karaboyas A, Morgenstern H, Fleischer NL, Schaubel DE, Robinson BM. Replicating randomized trial results with observational data using the parametric g-formula: an application to intravenous iron treatment in hemodialysis patients. Clin Epidemiol. 2020;12:1249-1260. doi: 10.2147/CLEP.S283321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Katsoulis M, Stavola BD, Diaz-Ordaz K, et al. Weight change and the onset of cardiovascular diseases: emulating trials using electronic health records. Epidemiology. 2021;32(5):744-755. doi: 10.1097/EDE.0000000000001393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kaura A, Sterne JAC, Trickey A, et al. Invasive versus non-invasive management of older patients with non-ST elevation myocardial infarction (SENIOR-NSTEMI): a cohort study based on routine clinical data. Lancet. 2020;396(10251):623-634. doi: 10.1016/S0140-6736(20)30930-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kazda L, McGeechan K, Bell K, Thomas R, Barratt A. Association of attention-deficit/hyperactivity disorder diagnosis with adolescent quality of life. JAMA Netw Open. 2022;5(10):e2236364. doi: 10.1001/jamanetworkopen.2022.36364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Keil AP, Buckley JP, Kalkbrenner AE. Bayesian G-computation for estimating impacts of interventions on exposure mixtures: demonstration with metals from coal-fired power plants and birth weight. Am J Epidemiol. 2021;190(12):2647-2657. doi: 10.1093/aje/kwab053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Keshet A, Rossman H, Shilo S, et al. Estimating the effect of cesarean delivery on long-term childhood health across two countries. PLoS One. 2022;17(10):e0268103. doi: 10.1371/journal.pone.0268103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Keyhani S, Cheng EM, Hoggatt K, et al. Comparative effectiveness of carotid stenting to medical therapy among patients with asymptomatic carotid stenosis. Stroke. 2022;53(4):1157-1166. doi: 10.1161/STROKEAHA.121.036178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Keyhani S, Cheng EM, Hoggatt KJ, et al. Comparative effectiveness of carotid endarterectomy vs initial medical therapy in patients with asymptomatic carotid stenosis. JAMA Neurol. 2020;77(9):1110-1121. doi: 10.1001/jamaneurol.2020.1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Khosrow-Khavar F, Desai RJ, Lee H, Lee SB, Kim SC. Tofacitinib and risk of malignancy: results from the Safety of Tofacitinib in Routine Care Patients With Rheumatoid Arthritis (STAR-RA) Study. Arthritis Rheumatol. 2022;74(10):1648-1659. doi: 10.1002/art.42250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim Y, Zhang K, Savitz SI, Chen L, Schulz PE, Jiang X. Counterfactual analysis of differential comorbidity risk factors in Alzheimer’s disease and related dementias. PLOS Digit Health. 2022;1(3):e0000018. doi: 10.1371/journal.pdig.0000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kirchgesner J, Desai RJ, Beaugerie L, Kim SC, Schneeweiss S. Calibrating real-world evidence studies against randomized trials: treatment effectiveness of infliximab in Crohn’s disease. Clin Pharmacol Ther. 2022;111(1):179-186. doi: 10.1002/cpt.2304 [DOI] [PubMed] [Google Scholar]
- 126.Kirchgesner J, Desai RJ, Schneeweiss MC, Beaugerie L, Kim SC, Schneeweiss S. Emulation of a randomized controlled trial in ulcerative colitis with US and French claims data: infliximab with thiopurines compared to infliximab monotherapy. Pharmacoepidemiol Drug Saf. 2022;31(2):167-175. doi: 10.1002/pds.5356 [DOI] [PubMed] [Google Scholar]
- 127.Kirchgesner J, Desai RJ, Schneeweiss MC, Beaugerie L, Schneeweiss S, Kim SC. Decreased risk of treatment failure with vedolizumab and thiopurines combined compared with vedolizumab monotherapy in Crohn’s disease. Gut. 2022;71(9):1781-1789. doi: 10.1136/gutjnl-2022-327002 [DOI] [PubMed] [Google Scholar]
- 128.Larochelle MR, Lodi S, Yan S, Clothier BA, Goldsmith ES, Bohnert ASB. Comparative effectiveness of opioid tapering or abrupt discontinuation vs no dosage change for opioid overdose or suicide for patients receiving stable long-term opioid therapy. JAMA Netw Open. 2022;5(8):e2226523. doi: 10.1001/jamanetworkopen.2022.26523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lazzati A, Epaud S, Ortala M, Katsahian S, Lanoy E. Effect of bariatric surgery on cancer risk: results from an emulated target trial using population-based data. Br J Surg. 2022;109(5):433-438. doi: 10.1093/bjs/znac003 [DOI] [PubMed] [Google Scholar]
- 130.Lim C, Mo Y, Teparrukkul P, et al. Effect of delays in concordant antibiotic treatment on mortality in patients with hospital-acquired Acinetobacter species bacteremia: emulating a target randomized trial with a 13-year retrospective cohort. Am J Epidemiol. 2021;190(11):2395-2404. doi: 10.1093/aje/kwab158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.LoMartire R, Björk M, Dahlström Ö, et al. The value of interdisciplinary treatment for sickness absence in chronic pain: a nationwide register-based cohort study. Eur J Pain. 2021;25(10):2190-2201. doi: 10.1002/ejp.1832 [DOI] [PubMed] [Google Scholar]
- 132.Lu Y, Gehr AW, Meadows RJ, et al. Timing of adjuvant chemotherapy initiation and mortality among colon cancer patients at a safety-net health system. BMC Cancer. 2022;22(1):593. doi: 10.1186/s12885-022-09688-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lyu H, Yoshida K, Zhao SS, et al. Delayed denosumab injections and fracture risk among patients with osteoporosis: a population-based cohort study. Ann Intern Med. 2020;173(7):516-526. doi: 10.7326/M20-0882 [DOI] [PubMed] [Google Scholar]
- 134.Madenci AL, Wanis KN, Cooper Z, et al. Strengthening health services research using target trial emulation: an application to volume-outcomes studies. Am J Epidemiol. 2021;190(11):2453-2460. doi: 10.1093/aje/kwab170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Madenci AL, Wanis KN, Cooper Z, et al. Comparison of mortality risk with different surgeon and hospital operative volumes among individuals undergoing pancreatectomy by emulating target trials in US Medicare beneficiaries. JAMA Netw Open. 2022;5(3):e221766. doi: 10.1001/jamanetworkopen.2022.1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Magen O, Waxman JG, Makov-Assif M, et al. Fourth dose of BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med. 2022;386(17):1603-1614. doi: 10.1056/NEJMoa2201688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maguen S, Madden E, Holder N, et al. Effectiveness and comparative effectiveness of evidence-based psychotherapies for posttraumatic stress disorder in clinical practice. Psychol Med. 2023;53(2):419-428. doi: 10.1017/S0033291721001628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Martin T, Krishnan A, Yong K, et al. Comparative effectiveness of ciltacabtagene autoleucel in CARTITUDE-1 versus physician’s choice of therapy in the Flatiron Health multiple myeloma cohort registry for the treatment of patients with relapsed or refractory multiple myeloma. EJHaem. 2021;3(1):97-108. doi: 10.1002/jha2.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Martínez-Alés G, Domingo-Relloso A, Quintana-Díaz M, Fernández-Capitán C, Hernán MA; COVID@HULP Group . Thromboprophylaxis with standard-dose vs. flexible-dose heparin for hospitalized COVID-19 patients: a target trial emulation. J Clin Epidemiol. 2022;151:96-103. doi: 10.1016/j.jclinepi.2022.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Martínez-Sanz J, Ron R, Moreno E, et al. Similar CD4/CD8 ratio recovery after initiation of dolutegravir plus lamivudine versus dolutegravir or bictegravir-based three-drug regimens in I adults with HIV. Front Immunol. 2022;13:873408. doi: 10.3389/fimmu.2022.873408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mathews KS, Soh H, Shaefi S, et al. ; STOP-COVID Investigators . Prone positioning and survival in mechanically ventilated patients with coronavirus disease 2019-related respiratory failure. Crit Care Med. 2021;49(7):1026-1037. doi: 10.1097/CCM.0000000000004938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Matthews AA, Dahabreh IJ, Fröbert O, et al. Benchmarking observational analyses before using them to address questions trials do not answer: an application to coronary thrombus aspiration. Am J Epidemiol. 2022;191(9):1652-1665. doi: 10.1093/aje/kwac098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Matthews AA, Szummer K, Dahabreh IJ, et al. Comparing effect estimates in randomized trials and observational studies from the same population: an application to percutaneous coronary intervention. J Am Heart Assoc. 2021;10(11):e020357. doi: 10.1161/JAHA.120.020357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mattishent K, Richardson K, Dhatariya K, Savva GM, Fox C, Loke YK. The effects of hypoglycaemia and dementia on cardiovascular events, falls and fractures and all-cause mortality in older individuals: a retrospective cohort study. Diabetes Obes Metab. 2019;21(9):2076-2085. doi: 10.1111/dom.13769 [DOI] [PubMed] [Google Scholar]
- 145.Mazzotta V, Cozzi-Lepri A, Colavita F, et al. Emulation of a target trial from observational data to compare effectiveness of casirivimab/imdevimab and bamlanivimab/etesevimab for early treatment of non-hospitalized patients with COVID-19. Front Immunol. 2022;13:868020. doi: 10.3389/fimmu.2022.868020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.McDermott G, Fu X, Cook C, et al. The effect of achieving serological remission on subsequent risk of relapse, end-stage renal disease and mortality in ANCA-associated vasculitis: a target trial emulation study. Ann Rheum Dis. 2022;81(10):1438-1444. doi: 10.1136/annrheumdis-2022-222439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McGrath LJ, Nielson C, Saul B, et al. Lessons learned using real-world data to emulate randomized trials: a case study of treatment effectiveness for newly diagnosed immune thrombocytopenia. Clin Pharmacol Ther. 2021;110(6):1570-1578. doi: 10.1002/cpt.2399 [DOI] [PubMed] [Google Scholar]
- 148.Medrinal C, Gillet A, Boujibar F, et al. Role of non-invasive respiratory supports in COVID-19 acute respiratory failure patients with do not intubate orders. J Clin Med. 2021;10(13):2783. doi: 10.3390/jcm10132783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mei H, Wang J, Ma S. An emulated target trial analysis based on Medicare data suggested non-inferiority of dabigatran versus rivaroxaban. J Clin Epidemiol. 2021;139:28-37. doi: 10.1016/j.jclinepi.2021.07.001 [DOI] [PubMed] [Google Scholar]
- 150.Mei H, Xu Y, Wang J, Ma S. Evaluation of survival outcomes of endovascular versus open aortic repair for abdominal aortic aneurysms with a big data approach. Entropy (Basel). 2020;22(12):1349. doi: 10.3390/e22121349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Meid AD, Wirbka L, Groll A, Haefeli WE; ARMIN Study Group . Can machine learning from real-world data support drug treatment decisions? a prediction modeling case for direct oral anticoagulants. Med Decis Making. 2022;42(5):587-598. doi: 10.1177/0272989X211064604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Melgaard L, Overvad TF, Jensen M, et al. Effectiveness and safety of NOAC versus warfarin in patients with atrial fibrillation and aortic stenosis. J Am Heart Assoc. 2021;10(23):e022628. doi: 10.1161/JAHA.121.022628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Merola D, Young J, Schrag D, Lin KJ, Robert N, Schneeweiss S. Oncology drug effectiveness from electronic health record data calibrated against RCT evidence: the PARSIFAL trial emulation. Clin Epidemiol. 2022;14:1135-1144. doi: 10.2147/CLEP.S373291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Meyer A, Neumann A, Drouin J, Weill A, Carbonnel F, Dray-Spira R. Benefits and risks associated with continuation of anti-tumor necrosis factor after 24 weeks of pregnancy in women with inflammatory bowel disease : a nationwide emulation trial. Ann Intern Med. 2022;175(10):1374-1382. doi: 10.7326/M22-0819 [DOI] [PubMed] [Google Scholar]
- 155.Miranda R, Smets T, De Schreye R, et al. Improved quality of care and reduced healthcare costs at the end-of-life among older people with dementia who received palliative home care: a nationwide propensity score-matched decedent cohort study. Palliat Med. 2021;35(9):1701-1712. doi: 10.1177/02692163211019321 [DOI] [PubMed] [Google Scholar]
- 156.Moler-Zapata S, Grieve R, Lugo-Palacios D, et al. Local instrumental variable methods to address confounding and heterogeneity when using electronic health records: an application to emergency surgery. Med Decis Making. 2022;42(8):1010-1026. doi: 10.1177/0272989X221100799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Monge S, Rojas-Benedicto A, Olmedo C, et al. ; IBERCovid . Effectiveness of mRNA vaccine boosters against infection with the SARS-CoV-2 Omicron (B.1.1.529) variant in Spain: a nationwide cohort study. Lancet Infect Dis. 2022;22(9):1313-1320. doi: 10.1016/S1473-3099(22)00292-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Moreno-Betancur M, Lynch JW, Pilkington RM, et al. Emulating a target trial of intensive nurse home visiting in the policy-relevant population using linked administrative data. Int J Epidemiol. 2023;52(1):119-131. doi: 10.1093/ije/dyac092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nabi H, Georgiadis S, Loft AG, et al. Comparative effectiveness of two adalimumab biosimilars in 1318 real-world patients with inflammatory rheumatic disease mandated to switch from originator adalimumab: nationwide observational study emulating a andomized clinical trial. Ann Rheum Dis. 2021;80(11):1400-1409. doi: 10.1136/annrheumdis-2021-219951 [DOI] [PubMed] [Google Scholar]
- 160.Neugebauer R, Schroeder EB, Reynolds K, et al. Comparison of mortality and major cardiovascular events among adults with type 2 diabetes using human vs analogue insulins. JAMA Netw Open. 2020;3(1):e1918554. doi: 10.1001/jamanetworkopen.2019.18554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nolde M, Ahn N, Dreischulte T, et al. The long-term risk for myocardial infarction or stroke after proton pump inhibitor therapy (2008-2018). Aliment Pharmacol Ther. 2021;54(8):1033-1040. doi: 10.1111/apt.16565 [DOI] [PubMed] [Google Scholar]
- 162.O’Donnell A, Pham N, Battisti L, et al. Estimating the causal effect of treatment with direct-acting antivirals on kidney function among individuals with hepatitis C virus infection. PLoS One. 2022;17(5):e0268478. doi: 10.1371/journal.pone.0268478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Olarte Parra C, Waernbaum I, Schön S, Goetghebeur E. Trial emulation and survival analysis for disease incidence registers: a case study on the causal effect of pre-emptive kidney transplantation. Stat Med. 2022;41(21):4176-4199. doi: 10.1002/sim.9503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ozery-Flato M, Goldschmidt Y, Shaham O, Ravid S, Yanover C. Framework for identifying drug repurposing candidates from observational healthcare data. JAMIA Open. 2020;3(4):536-544. doi: 10.1093/jamiaopen/ooaa048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Reijnders TDY, Peters-Sengers H, van Vught LA, et al. ; MARS consortium . Effect of erythromycin on mortality and the host response in critically ill patients with sepsis: a target trial emulation. Crit Care. 2022;26(1):151. doi: 10.1186/s13054-022-04016-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Petersen ML, Tran L, Geng EH, et al. Delayed switch of antiretroviral therapy after virologic failure associated with elevated mortality among HIV-infected adults in Africa. AIDS. 2014;28(14):2097-2107. doi: 10.1097/QAD.0000000000000349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Peterson RG, Xiao R, Katcoff H, Fisher BT, Weiss PF. Effect of first-line biologic initiation on glucocorticoid exposure in children hospitalized with new-onset systemic juvenile idiopathic arthritis: emulation of a pragmatic trial using observational data. Pediatr Rheumatol Online J. 2021;19(1):109. doi: 10.1186/s12969-021-00597-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Petito LC, García-Albéniz X, Logan RW, et al. Estimates of overall survival in patients with cancer receiving different treatment regimens: emulating hypothetical target trials in the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. JAMA Netw Open. 2020;3(3):e200452. doi: 10.1001/jamanetworkopen.2020.0452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Porcher R, Desguerre I, Amthor H, et al. Association between prophylactic angiotensin-converting enzyme inhibitors and overall survival in Duchenne muscular dystrophy-analysis of registry data. Eur Heart J. 2021;42(20):1976-1984. doi: 10.1093/eurheartj/ehab054 [DOI] [PubMed] [Google Scholar]
- 170.Prats-Uribe A, Kolovos S, Berencsi K, et al. Unicompartmental compared with total knee replacement for patients with multimorbidities: a cohort study using propensity score stratification and inverse probability weighting. Health Technol Assess. 2021;25(66):1-126. doi: 10.3310/hta25660 [DOI] [PubMed] [Google Scholar]
- 171.Pufulete M, Harris J, Pouwels K, et al. Real-world bleeding in patients with acute coronary syndrome (ACS) undergoing percutaneous coronary intervention (PCI) and prescribed different combinations of dual antiplatelet therapy (DAPT) in England: a population-based cohort study emulating a ‘target trial’. Open Heart. 2022;9(2):e001999. doi: 10.1136/openhrt-2022-001999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Qiao N, Shen M, He W, et al. Comparative effectiveness of endoscopic versus microscopic transsphenoidal surgery for patients with growth hormone secreting pituitary adenoma: an emulated trial. Clin Neurol Neurosurg. 2021;207:106781. doi: 10.1016/j.clineuro.2021.106781 [DOI] [PubMed] [Google Scholar]
- 173.Rahman S, Thomas B, Maynard N, et al. Impact of postoperative chemotherapy on survival for oesophagogastric adenocarcinoma after preoperative chemotherapy and surgery. Br J Surg. 2022;109(2):227-236. doi: 10.1093/bjs/znab427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Reitblat C, Fleishman A, Kaplan IA, et al. Radical prostatectomy versus external beam radiation therapy for high-grade, clinically localized prostate cancer: emulation of a target clinical trial. Urol Oncol. 2021;39(11):785.e1-785.e10. doi: 10.1016/j.urolonc.2021.03.017 [DOI] [PubMed] [Google Scholar]
- 175.Rogawski McQuade ET, Benjamin-Chung J, Westreich D, Arnold BF. Population intervention effects in observational studies to emulate target trial results: reconciling the effects of improved sanitation on child growth. Int J Epidemiol. 2022;51(1):279-290. doi: 10.1093/ije/dyab070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rojas-Saunero LP, Hilal S, Murray EJ, Logan RW, Ikram MA, Swanson SA. Hypothetical blood-pressure-lowering interventions and risk of stroke and dementia. Eur J Epidemiol. 2021;36(1):69-79. doi: 10.1007/s10654-020-00694-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rossides M, Kullberg S, Di Giuseppe D, et al. Infection risk in sarcoidosis patients treated with methotrexate compared to azathioprine: a retrospective ‘target trial’ emulated with Swedish real-world data. Respirology. 2021;26(5):452-460. doi: 10.1111/resp.14001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sánchez A, Roos VH, Navarro M, et al. Quality of colonoscopy is associated with adenoma detection and postcolonoscopy colorectal cancer prevention in Lynch syndrome. Clin Gastroenterol Hepatol. 2022;20(3):611-621.e9, E9. doi: 10.1016/j.cgh.2020.11.002 [DOI] [PubMed] [Google Scholar]
- 179.Schmidt M, Sørensen HT, Pedersen L. Cardiovascular risks of diclofenac versus other older COX-2 inhibitors (meloxicam and etodolac) and newer COX-2 inhibitors (celecoxib and etoricoxib): a series of nationwide emulated trials. Drug Saf. 2022;45(9):983-994. doi: 10.1007/s40264-022-01211-1 [DOI] [PubMed] [Google Scholar]
- 180.Schmidt M, Sørensen HT, Pedersen L. Diclofenac use and cardiovascular risks: series of nationwide cohort studies. BMJ. 2018;362:k3426. doi: 10.1136/bmj.k3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schneeweiss MC, Kim SC, Wyss R, Schneeweiss S, Merola JF. Dupilumab and the risk of conjunctivitis and serious infection in patients with atopic dermatitis: a propensity score-matched cohort study. J Am Acad Dermatol. 2021;84(2):300-311. doi: 10.1016/j.jaad.2020.09.084 [DOI] [PubMed] [Google Scholar]
- 182.Schoenfeld AJ, Losina E, Ferrone ML, et al. Ambulatory status after surgical and nonsurgical treatment for spinal metastasis. Cancer. 2019;125(15):2631-2637. doi: 10.1002/cncr.32140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schroeder EB, Neugebauer R, Reynolds K, et al. Association of cardiovascular outcomes and mortality with sustained long-acting insulin only vs long-acting plus short-acting insulin treatment. JAMA Netw Open. 2021;4(9):e2126605. doi: 10.1001/jamanetworkopen.2021.26605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Secora AM, Shin JI, Qiao Y, et al. Hyperkalemia and acute kidney injury with spironolactone use among patients with heart failure. Mayo Clin Proc. 2020;95(11):2408-2419. doi: 10.1016/j.mayocp.2020.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Seeger JD, Nunes A, Loughlin AM. Using RWE research to extend clinical trials in diabetes: an example with implications for the future. Diabetes Obes Metab. 2020;22(Suppl 3)(suppl 3):35-44. doi: 10.1111/dom.14021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shaefi S, Brenner SK, Gupta S, et al. ; STOP-COVID Investigators . Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Med. 2021;47(2):208-221. doi: 10.1007/s00134-020-06331-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shin H, Schneeweiss S, Glynn RJ, Patorno E. Cardiovascular outcomes in patients initiating first-line treatment of type 2 diabetes with sodium-glucose cotransporter-2 inhibitors versus metformin: a cohort study. Ann Intern Med. 2022;175(7):927-937. doi: 10.7326/M21-4012 [DOI] [PubMed] [Google Scholar]
- 188.Shin J-I, Fine DM, Sang Y, et al. Association of rosuvastatin use with risk of hematuria and proteinuria. J Am Soc Nephrol. 2022;33(9):1767-1777. doi: 10.1681/ASN.2022020135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Simmonds KP, Burke J, Kozlowski AJ, Andary M, Luo Z, Reeves MJ. Emulating 3 clinical trials that compare stroke rehabilitation at inpatient rehabilitation facilities with skilled nursing facilities. Arch Phys Med Rehabil. 2022;103(7):1311-1319. doi: 10.1016/j.apmr.2021.12.029 [DOI] [PubMed] [Google Scholar]
- 190.Softness K, Kaul S, Fleishman A, et al. Radical cystectomy versus trimodality therapy for muscle-invasive urothelial carcinoma of the bladder. Urol Oncol. 2022;40(6):272.e1-272.e9. doi: 10.1016/j.urolonc.2021.12.015 [DOI] [PubMed] [Google Scholar]
- 191.Solomon DH, Ruppert K, Habel LA, et al. Prescription medications for sleep disturbances among midlife women during 2 years of follow-up: a SWAN retrospective cohort study. BMJ Open. 2021;11(5):e045074. doi: 10.1136/bmjopen-2020-045074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Stattin P, Westerberg M, Lissbrant IF, et al. Real world outcomes in patients with metastatic, castration-resistant prostate cancer treated with radium-223 in routine clinical practice in Sweden. Clin Genitourin Cancer. 2023;21(1):107.e1-107.e9. doi: 10.1016/j.clgc.2022.09.002 [DOI] [PubMed] [Google Scholar]
- 193.Stokes JW, Gannon WD, Sherrill WH, et al. Bleeding, thromboembolism, and clinical outcomes in venovenous extracorporeal membrane oxygenation. Crit Care Explor. 2020;2(11):e0267. doi: 10.1097/CCE.0000000000000267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Strohmaier S, Wallisch C, Kammer M, et al. Survival benefit of first single-organ deceased donor kidney transplantation compared with long-term dialysis across ages in transplant-eligible patients with kidney failure. JAMA Netw Open. 2022;5(10):e2234971. doi: 10.1001/jamanetworkopen.2022.34971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sun JW, Young JG, Sarvet AL, et al. Comparison of rates of type 2 diabetes in adults and children treated with anticonvulsant mood stabilizers. JAMA Netw Open. 2022;5(4):e226484. doi: 10.1001/jamanetworkopen.2022.6484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sung RS, Zhang M, Schaubel DE, Shu X, Magee JC. A reassessment of the survival advantage of simultaneous kidney-pancreas versus kidney-alone transplantation. Transplantation. 2015;99(9):1900-1906. doi: 10.1097/TP.0000000000000663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Swaminathan L, Kaatz S, Chubb H, et al. Impact of early corticosteroids on preventing clinical deterioration in non-critically ill patients hospitalized with COVID-19: a multi-hospital cohort study. Infect Dis Ther. 2022;11(2):887-898. doi: 10.1007/s40121-022-00615-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Szummer K, Montez-Rath ME, Alfredsson J, et al. Comparison between ticagrelor and clopidogrel in elderly patients with an acute coronary syndrome: insights from the SWEDEHEART registry. Circulation. 2020;142(18):1700-1708. doi: 10.1161/CIRCULATIONAHA.120.050645 [DOI] [PubMed] [Google Scholar]
- 199.Takeuchi Y, Kumamaru H, Hagiwara Y, et al. Sodium-glucose cotransporter-2 inhibitors and the risk of urinary tract infection among diabetic patients in Japan: target trial emulation using a nationwide administrative claims database. Diabetes Obes Metab. 2021;23(6):1379-1388. doi: 10.1111/dom.14353 [DOI] [PubMed] [Google Scholar]
- 200.Takeuchi Y, Shinozaki T, Kumamaru H, Hiramatsu T, Matsuyama Y. Analyzing intent-to-treat and per-protocol effects on safety outcomes using a medical information database: an application to the risk assessment of antibiotic-induced liver injury. Expert Opin Drug Saf. 2018;17(11):1071-1079. doi: 10.1080/14740338.2018.1528224 [DOI] [PubMed] [Google Scholar]
- 201.Tapper EB, Zhao Z, Winder GS, Parikh ND. Deprescribing zolpidem reduces falls and fractures in patients with cirrhosis. JHEP Rep. 2022;4(6):100478. doi: 10.1016/j.jhepr.2022.100478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Thamer M, Zhang Y, Kaufman J, Cotter D, Hernán MA. Similar outcomes for two anemia treatment strategies among elderly hemodialysis patients with diabetes. J Endocrinol Diabetes. 2014;1(2):6. doi: 10.15226/2374-6890/1/2/00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Thomas DS, Lee AY, Müller PL, et al. ; UK AMD EMR Users Group . Contextualizing single-arm trials with real-world data: an emulated target trial comparing therapies for neovascular age-related macular degeneration. Clin Transl Sci. 2021;14(3):1166-1175. doi: 10.1111/cts.12974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Thomsen FB, Bosco C, Garmo H, et al. Anti-androgen monotherapy versus gonadotropin-releasing hormone agonists in men with advanced, non-metastatic prostate cancer: a register-based, observational study. Acta Oncol. 2019;58(1):110-118. doi: 10.1080/0284186X.2018.1529427 [DOI] [PubMed] [Google Scholar]
- 205.Tran VT, Mahévas M, Bani-Sadr F, et al. ; COCORICO Collaborative Group . Corticosteroids in patients hospitalized for COVID-19 pneumonia who require oxygen: observational comparative study using routine care data. Clin Microbiol Infect. 2021;27(4):603-610. doi: 10.1016/j.cmi.2020.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Trevisan M, Fu EL, Xu Y, et al. Stopping mineralocorticoid receptor antagonists after hyperkalaemia: trial emulation in data from routine care. Eur J Heart Fail. 2021;23(10):1698-1707. doi: 10.1002/ejhf.2287 [DOI] [PubMed] [Google Scholar]
- 207.Tsilidis KK, Capothanassi D, Allen NE, et al. Metformin does not affect cancer risk: a cohort study in the U.K. Clinical Practice Research Datalink analyzed like an intention-to-treat trial. Diabetes Care. 2014;37(9):2522-2532. doi: 10.2337/dc14-0584 [DOI] [PubMed] [Google Scholar]
- 208.van Santen DK, Boyd A, Matser A, et al. The effect of needle and syringe program and opioid agonist therapy on the risk of HIV, hepatitis B and C virus infection for people who inject drugs in Amsterdam, the Netherlands: findings from an emulated target trial. Addiction. 2021;116(11):3115-3126. doi: 10.1111/add.15503 [DOI] [PubMed] [Google Scholar]
- 209.Wanis KN, Madenci AL, Dokus MK, et al. The meaning of confounding adjustment in the presence of multiple versions of treatment: an application to organ transplantation. Eur J Epidemiol. 2019;34(3):225-233. doi: 10.1007/s10654-019-00484-8 [DOI] [PubMed] [Google Scholar]
- 210.Wei J, Choi HK, Neogi T, et al. Allopurinol initiation and all-cause mortality among patients with gout and concurrent chronic kidney disease: a population-based cohort study. Ann Intern Med. 2022;175(4):461-470. doi: 10.7326/M21-2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Weisel K, Martin T, Krishnan A, et al. Comparative efficacy of ciltacabtagene autoleucel in CARTITUDE-1 vs physician’s choice of therapy in the long-term follow-up of POLLUX, CASTOR, and EQUULEUS clinical trials for the treatment of patients with relapsed or refractory multiple myeloma. Clin Drug Investig. 2022;42(1):29-41. doi: 10.1007/s40261-021-01100-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Xie Y, Bowe B, Yan Y, Xian H, Li T, Al-Aly Z. Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study. BMJ. 2019;365:l1580. doi: 10.1136/bmj.l1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Xie Y, Bowe B, Gibson AK, et al. Comparative effectiveness of SGLT2 inhibitors, GLP-1 receptor agonists, DPP-4 inhibitors, and sulfonylureas on risk of kidney outcomes: emulation of a target trial using health care databases. Diabetes Care. 2020;43(11):2859-2869. doi: 10.2337/dc20-1890 [DOI] [PubMed] [Google Scholar]
- 214.Xu Y, Fu EL, Trevisan M, et al. Stopping renin-angiotensin system inhibitors after hyperkalemia and risk of adverse outcomes. Am Heart J. 2022;243:177-186. doi: 10.1016/j.ahj.2021.09.014 [DOI] [PubMed] [Google Scholar]
- 215.an ZZN, Mason KJ, Hampton PJ, et al. ; BADBIR Study Group . Randomized trial replication using observational data for comparative effectiveness of secukinumab and ustekinumab in psoriasis: a study from the British Association of Dermatologists Biologics and Immunomodulators register. JAMA Dermatol. 2021;157(1):66-73. doi: 10.1001/jamadermatol.2020.4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yland JJ, Chiu YH, Rinaudo P, Hsu J, Hernán MA, Hernández-Díaz S. Emulating a target trial of the comparative effectiveness of clomiphene citrate and letrozole for ovulation induction. Hum Reprod. 2022;37(4):793-805. doi: 10.1093/humrep/deac005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Young J, Wong S, Janjua NZ, Klein MB. Comparing direct acting antivirals for hepatitis C using observational data—why and how? Pharmacol Res Perspect. 2020;8(5):e00650. doi: 10.1002/prp2.650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Young J, Scherrer AU, Calmy A, et al. ; Swiss HIV Cohort Study . The comparative effectiveness of NRTI-sparing dual regimens in emulated trials using observational data from the Swiss HIV Cohort Study. Antivir Ther. 2019;24(5):343-353. doi: 10.3851/IMP3310 [DOI] [PubMed] [Google Scholar]
- 219.Zhang Y, Thamer M, Kaufman J, Cotter D, Hernán MA. Comparative effectiveness of two anemia management strategies for complex elderly dialysis patients. Med Care. 2014;52(0 3)(suppl 3):S132-S139. doi: 10.1097/MLR.0b013e3182a53ca8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang Y, Young JG, Thamer M, Hernán MA. Comparing the effectiveness of dynamic treatment strategies using electronic health records: an application of the parametric g-formula to anemia management strategies. Health Serv Res. 2018;53(3):1900-1918. doi: 10.1111/1475-6773.12718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ. 2007;85(11):867-872. doi: 10.2471/BLT.07.045120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Vandenbroucke JP, von Elm E, Altman DG, et al. ; STROBE Initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. doi: 10.1371/journal.pmed.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Nguyen VT, Engleton M, Davison M, Ravaud P, Porcher R, Boutron I. Risk of bias in observational studies using routinely collected data of comparative effectiveness research: a meta-research study. BMC Med. 2021;19(1):279. doi: 10.1186/s12916-021-02151-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rubin DB. The design versus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Stat Med. 2007;26(1):20-36. doi: 10.1002/sim.2739 [DOI] [PubMed] [Google Scholar]
- 226.Hernán MA. With great data comes great responsibility: publishing comparative effectiveness research in epidemiology. Epidemiology. 2011;22(3):290-291. doi: 10.1097/EDE.0b013e3182114039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hernán MA, Robins JM, García Rodríguez LA. Discussion on ‘Statistical Issues Arising in the Women’s Health Initiative’. Biometrics. 2005;61(4):922-930. doi: 10.1111/j.0006-341X.2005.454_7.x [DOI] [PubMed] [Google Scholar]
- 228.Hernán MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19(6):766-779. doi: 10.1097/EDE.0b013e3181875e61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.National Institute of Health and Care Excellence . NICE real-world evidence framework. June 23, 2022. Accessed August 28, 2023. https://www.nice.org.uk/corporate/ecd9/chapter/overview
- 230.European Medicines Agency. European Medicines Agencies network strategy to 2025. Accessed August 28, 2023. https://www.ema.europa.eu/en/about-us/how-we-work/andomiz-medicines-regulatory-network/andomiz-medicines-agencies-network-strategy
- 231.Government of Canada. Optimizing the Use of Real World Evidence to Inform Regulatory Decision-Making. April 16, 2019, Accessed August 28, 2023. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/announcements/optimizing-real-world-evidence-regulatory-decisions.html
- 232.Therapeutic Goods Administration . Real world evidence and patient reported outcomes in the regulatory context. February 28, 2023. Accessed August 28, 2023. https://www.tga.gov.au/review-real-world-evidence-and-patient-reported-outcomes
- 233.Canada’s Drug and Health Technology Agency . Guidance for Reporting Real-World Evidence. May 2023. Accessed August 28, 2023. https://www.cadth.ca/sites/default/files/RWE/MG0020/MG0020-RWE-Guidance-Report.pdf
- 234.Altman DG, Simera I, Hoey J, Moher D, Schulz K. EQUATOR: reporting guidelines for health research. Lancet. 2008;371(9619):1149-1150. doi: 10.1016/S0140-6736(08)60505-X [DOI] [PubMed] [Google Scholar]
- 235.Moher D, Jones A, Lepage L; CONSORT Group (Consolidated Standards for Reporting of Trials) . Use of the CONSORT statement and quality of reports of randomized trials: a comparative before-and-after evaluation. JAMA. 2001;285(15):1992-1995. doi: 10.1001/jama.285.15.1992 [DOI] [PubMed] [Google Scholar]
- 236.Turner L, Shamseer L, Altman DG, et al. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of andomized controlled trials (RCTs) published in medical journals. Cochrane Database Syst Rev. 2012;11(11):MR000030. doi: 10.1002/14651858.MR000030.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cobo E, Cortés J, Ribera JM, et al. Effect of using reporting guidelines during peer review on quality of final manuscripts submitted to a biomedical journal: masked andomized trial. BMJ. 2011;343:d6783. doi: 10.1136/bmj.d6783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.EQUATOR Network . Reporting guidelines under development for observational studies. Accessed May 29, 2023. https://www.equator-network.org/library/reporting-guidelines-under-development/reporting-guidelines-under-development-for-observational-studies/
- 239.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162(1):55-63. doi: 10.7326/M14-0697 [DOI] [PubMed] [Google Scholar]
- 240.Ziemann S, Paetzolt I, Grüßer L, Coburn M, Rossaint R, Kowark A. Poor reporting quality of observational clinical studies comparing treatments of COVID-19—a retrospective cross-sectional study. BMC Med Res Methodol. 2022;22(1):23. doi: 10.1186/s12874-021-01501-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wiehn E, Ricci C, Alvarez-Perea A, et al. ; Task Force ‘Adherence to reporting guidelines in articles published in EAACI Journals: a systematic review’ of the EAACI Working Group on Epidemiology . Adherence to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist in articles published in EAACI Journals: A bibliographic study. Allergy. 2021;76(12):3581-3588. doi: 10.1111/all.14951 [DOI] [PubMed] [Google Scholar]
- 242.Wang SV, Schneeweiss S, Franklin JM, et al. ; RCT-DUPLICATE Initiative . Emulation of randomized clinical trials with nonrandomized database analyses: results of 32 clinical trials. JAMA. 2023;329(16):1376-1385. doi: 10.1001/jama.2023.4221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Dahabreh IJ, Steingrimsson JA, Robins JM, Hernán MA. Randomized trials and their observational emulations: a framework for benchmarking and joint analysis. arXiv. Preprint posted March 28, 2022. doi: 10.48550/arXiv.2203.14857 [DOI]
- 244.Dahabreh IJ, Robins JM, Hernán MA. Benchmarking observational methods by comparing randomized trials and their emulations. Epidemiology. 2020;31(5):614-619. doi: 10.1097/EDE.0000000000001231 [DOI] [PubMed] [Google Scholar]
- 245.Dahabreh IJ, Matthews A, Steingrimsson JA, Scharfstein DO, Stuart EA. Using trial and observational data to assess effectiveness: trial emulation, transportability, benchmarking, and joint analysis. Epidemiol Rev. 2023;mxac011. doi: 10.1093/epirev/mxac011 [DOI] [PubMed] [Google Scholar]
- 246.Gershman B, Guo DP, Dahabreh IJ. Using observational data for personalized medicine when clinical trial evidence is limited. Fertil Steril. 2018;109(6):946-951. doi: 10.1016/j.fertnstert.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 247.Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet. 2009;374(9683):86-89. doi: 10.1016/S0140-6736(09)60329-9 [DOI] [PubMed] [Google Scholar]
- 248.Glasziou P, Altman DG, Bossuyt P, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet. 2014;383(9913):267-276. doi: 10.1016/S0140-6736(13)62228-X [DOI] [PubMed] [Google Scholar]
- 249.Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019. doi: 10.1002/9781119536604 [DOI] [Google Scholar]
- 250.Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2(4):221-228. doi: 10.1007/s40471-015-0053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915-920. doi: 10.1093/aje/kwg231 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. Methodological Differences to the Protocol
Appendix 2. Complete Search Strategies for All Databases
Appendix 3. List of Phrases Deemed to Indicate Explicit Emulation of a Target Trial
Appendix 4. Operational Definitions for Items of the Emulation of the Target Trial
Appendix 5. Items Extracted That Are Not Included in Commonly Used Reporting Guidelines (STROBE, RECORD, ISPOR)
Appendix 6. Reasons for Study Exclusion at Level of Full Text
Appendix 7. Subgroup Analysis of Reporting of Target Trial Protocol and How It Was Emulated, by Reporting Guideline Use
Data Sharing Statement