Abstract
Growth differentiation factor 15 (GDF15), a member of the transforming growth factor-β family, is a stress-induced cytokine. Under normal circumstances, the expression of GDF15 is low in most tissues. It is highly expressed during tissue injury, inflammation, oxidative stress and cancer. GDF15 has been established as a biomarker in patients with cancer, and is associated with cancer cachexia (CC) and poor survival. CC is a multifactorial metabolic disorder characterized by severe muscle and adipose tissue atrophy, loss of appetite, anemia and bone loss. Cachexia leads to reductions in quality of life and tolerance to anticancer therapy, and results in a poor prognosis in cancer patients. Dysregulated GDF15 levels have been discovered in patients with CC and animal models, where they have been found to be involved in anorexia and weight loss. Although studies have suggested that GDF15 mediates anorexia and weight loss in CC through its neuroreceptor, glial cell-lineage neurotrophic factor family receptor α-like, the effects of GDF15 on CC and the potential regulatory mechanisms require further elucidation. In the present review, the characteristics of GDF15 and its roles and molecular mechanisms in CC are elaborated. The targeting of GDF15 as a potential therapeutic strategy for CC is also discussed.
Keywords: growth differentiation factor 15, cancer cachexia, anorexia, muscle atrophy, fat loss, bone loss, anemia
1. Introduction
Cachexia is a fatal disease that is associated with several conditions, including acquired immunodeficiency syndrome, multiple sclerosis, chronic obstructive pulmonary disease (COPD), tuberculosis, congestive heart failure, chronic kidney disease and cancer (1,2). Cancer-associated cachexia is a serious wasting syndrome characterized by a continuous reduction in skeletal muscle mass, with or without the loss of fat mass. Distinct from hunger and nutritional deficiencies, cancer cachexia (CC) cannot be reversed by food supplements, and leads to progressive functional impairment (3,4). It is prevalent in 50–80% of patients with advanced cancer (5). Unfortunately, cancer treatments such as chemotherapy and radiotherapy aggravate cachexia (6). CC can have a negative impact on physical function, tolerance to anticancer treatment, overall survival and well-being in patients with cancer (7). Moreover, it can also increase psychological stress and the financial burden on patients and their families (2,8).
In 2011, an international panel of experts identified a weight loss of >5% over 6 months, any degree of weight loss >2% in an individual with a body mass index <20 kg/m2, or sarcopenia, defined as a skeletal muscle index <7.26 kg/m2 in men and <5.45 kg/m2 in women, as diagnostic criteria for CC (3). CC can be divided into three clinical stages according to the degree of weight loss and metabolic changes, namely pre-cachexia, cachexia and refractory cachexia (3). The main clinical symptoms of CC include anorexia, asthenia, fever, anemia, edema and wasting (7,9). The occurrence of CC has been attributed to systemic inflammation generated by tumor-host interactions and tumor-derived catabolic factors such as proteolysis-inducing factor, zinc-α2-glycoprotein (ZAG), parathyroid hormone-related protein and microRNAs (miRNAs) (6,7). Systemic inflammation is characterized by increased circulating levels of cytokines, including tumor necrosis factor (TNF)-α, TNF-like weak inducer of apoptosis, interleukin (IL)-1, IL-6, IL-8, IL-20, interferon-γ, leukemia inhibitory factor, myostatin, activin and growth differentiation factor 15 (GDF15) (10–13). These factors drive metabolic disorders in multiple tissues and organs during CC, including the muscles (10,11,13,14), adipose tissue (10–12,15,16), heart (17), brain (10,11), liver (10,18–21), gallbladder (19), bone (22), pancreas (21), spleen (18), intestines (23), gonads (24) and blood (18,22,25). Table I summarizes the cytokines involved in the damage of various organs or tissues associated with CC. In addition, other factors such as cancer type, stage, tumor size, inter-individual genetics and sex can also influence the development and progression of CC (3,26,27). The current treatment protocol advocated for CC is a comprehensive treatment system based on drug therapy, including anti-inflammatory drugs, and measures to increase metabolism, inhibit catabolism and stimulate appetite, supplemented by nutrition, exercise and psychological support (28). However, the effectiveness of these treatment options for CC is unclear. Thus, an in-depth understanding of the key factors associated with CC is crucial for the early identification and development of novel therapeutic options.
Table I.
Cytokines involved in organ or tissue damage during CC.
| Organ or tissue | Alterations in CC | Relevant cytokines | (Refs.) |
|---|---|---|---|
| Muscle | Increased muscle proteolysis, increased myocyte apoptosis, reduced muscle synthesis, decreased regeneration, impaired mitochondrial metabolism | TNF-α, TWEAK, IL-6, LIF, IL-1-β, IFN-γ, GDF15, IL-8, myostatin, activin | (10,11,13,14) |
| Adipose | Increased lipolysis, reduced synthesis, white adipose tissue browning | IL-6, LIF, TNF-α, IFN-γ, IL-1-β, IL-8, GDF15, IL-20 | (10–12,15,16) |
| Heart | Atrophy, mitochondrial dysfunction, heart failure | IL-6, TNF-α, TWEAK | (17) |
| Brain | Anorexia | TNF-α, IL-1β, IL-6, IFN-γ, LIF, GDF15 | (10,11) |
| Liver | Increased acute phase response, increased gluconeogenesis, increased bile acid metabolism, mitochondrial dysfunction | IL-6, myostatin, activin, TNF-α | (10,18–21) |
| Gallbladder | Cholestasis | IL-6 | (19) |
| Bone | Osteoclast activation, bone loss, hypercalcemia | IL-6 | (6,22) |
| Pancreas | Insulin resistance | TNF-α | (13,21) |
| Spleen | Splenomegaly | Myostatin, activin | (18) |
| Intestine | Intestinal barrier dysfunction, increase in Enterobacteriaceae | IL-6 | (23) |
| Gonad | Decreased testis size, hypogonadism | IL-6 | (24) |
| Blood system | Decreased hemoglobin, increased platelets | IL-6, TNF-α, myostatin, activin | (18,22,25) |
CC, cancer cachexia; TNF-α, tumor necrosis factor-α; TWEAK, TNF-like weak inducer of apoptosis; IL, interleukin; IFN-γ, interferon-γ; LIF, leukemia inhibitory factor; GDF15, growth differentiation factor 15.
GDF15 is a stress-induced cytokine that regulates food intake, energy metabolism and body weight (29). Its levels have been shown to be upregulated in patients with CC, as well as in animal models of pancreatic, colon, head and neck, breast and prostate cancers (30–33). Elevated circulating levels of GDF15 may lead to anorexia, weight loss and decreased survival in cases of CC (32). Notably, GDF15 plasma levels have been reported to be significantly higher in patients with pre-cachexia than in those with cachexia and refractory cachexia (30). These studies imply that GDF15 is closely associated with CC. The present review aims to clarify the role and molecular mechanisms of GDF15 in CC.
2. Characteristics of GDF15
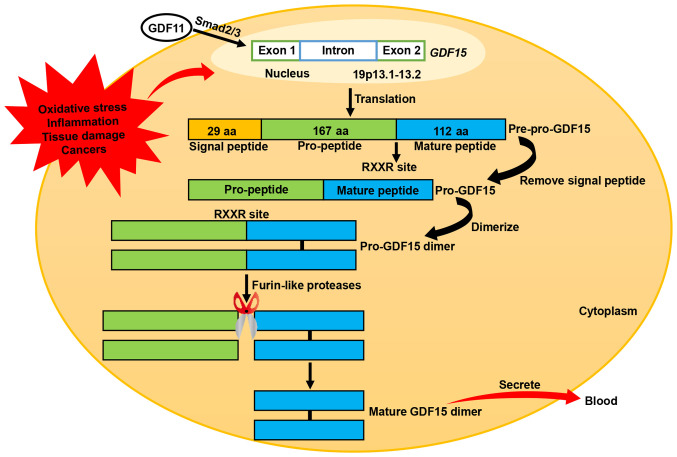
GDF15 is a novel transforming growth factor (TGF)-β superfamily member that was first identified in activated macrophages (34). It is also known as macrophage inhibitory cytokine-1, nonsteroidal anti-inflammatory drug activated gene-1, prostate-derived factor, placental TGF-β and placental bone morphogenetic protein (29,35). The human GDF15 gene is located on chromosome 19p13.1–13.2 and consists of two exons separated by an intron (36). GDF15 is synthesized as an inactive precursor protein consisting of a chain of 308 amino acids, including a signal peptide comprising 29 amino acids, a pro-peptide comprising 167 amino acids and a mature peptide comprising 112 amino acids (29,35). Following removal of the signal peptide, the remaining GDF15 pre-peptide dimerizes in the endoplasmic reticulum through specific disulfide bonding to form a pro-GDF15 dimer precursor. This precursor is subsequently cleaved by furin-like proteases at the RXXR site (amino acid 196), thereby releasing the C-terminal dimeric mature homodimer GDF15. Mature GDF15 eventually diffuses into the circulation as a 25-kD dimer (Fig. 1) (29,35).
Figure 1.
Synthesis process of GDF15. The GDF15 gene is located on chromosome 19p 13.1–13.2 and consists of two exons and an intron. In response to oxidative stress, inflammation, tissue damage and cancer, GDF15 is synthesized into pre-pro-GDF15, an inactive precursor protein, by intracellular translation. Pre-pro-GDF15 is a 308-aa peptide that comprises a 29-aa signal peptide, 167-aa pro-peptide and 112-aa mature peptide. After removal of the signal peptide, the residual pro-GDF15 is dimerized, cleaved by furin-like proteases at an RXXR site and secreted into the circulation as a mature GDF15 dimer. Another member of the transforming growth factor superfamily, GDF11, promotes GDF15 synthesis by inducing Smad2/3 to bind to the GDF15 promoter. GDF15, growth differentiation factor 15; GDF11, growth differentiation factor 11; aa, amino acid.
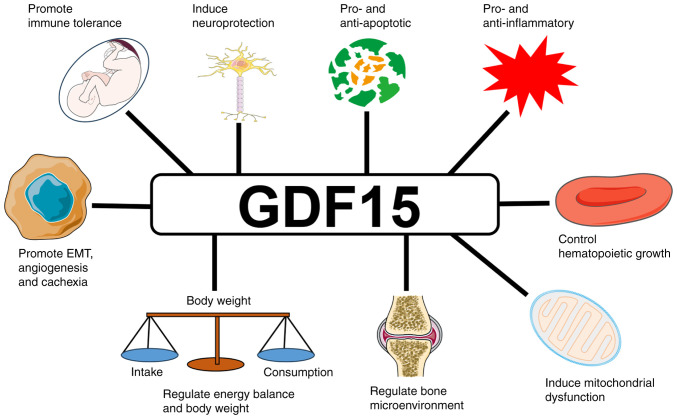
GDF15 is widely expressed in body tissues at different levels under normal conditions, with high expression in the placenta, medium expression in the prostate and bladder, and low expression in the kidney, liver, colon, pancreas, stomach, gallbladder, breast, lung and endometrium (37–39). It has multiple biological functions (Fig. 2). The circulating concentrations of GDF15 range between 0.2 and 1.2 ng/ml in healthy individuals (38). These levels increase with age, pregnancy, exercise, smoking and obesity, and are also influenced by genetic and environmental factors (29,40). GDF15 is highly expressed in vascular smooth muscle cells, cardiomyocytes, endothelial cells, macrophages and adipocytes during oxidative stress, inflammation, tissue damage and cancer (41,42). GDF15 is elevated in a variety of cancers, including those of the prostate, colon, pancreas and breast (31,39). In one study, the mean value of serum GDF15 was almost two-fold higher in cancer patients compared with that in healthy controls (32). GDF15 plays a number of roles in tumorigenesis. In the initial stages of cancer, it induces tumor cell apoptosis and inhibits cancer progression (43). In later stages of cancer, it promotes tumor cell proliferation and metastasis (44,45). GDF15 has been recognized as a tumor biomarker that is closely implicated in tumor progression, cachexia and reduced survival (31,46).
Figure 2.
Biological functions of GDF15. GDF15, growth differentiation factor 15; EMT, epithelial-mesenchymal transition.
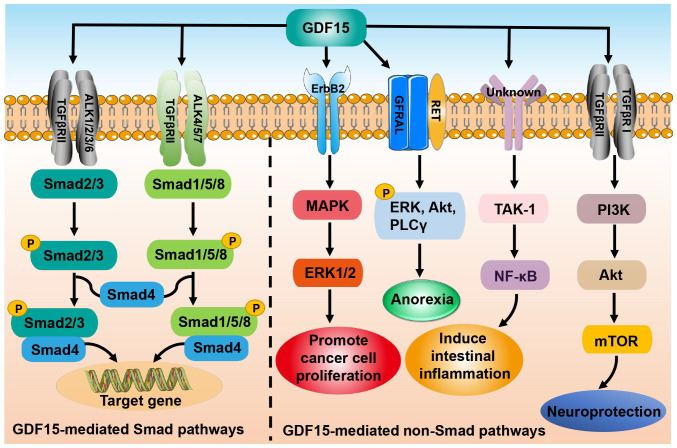
As a member of the TGF-β superfamily, GDF15 signals through both Smad and non-Smad pathways. In the former pathway, GDF15 binds to the type II TGF-β receptor (TGFβRII) and activates the type I TGF-β receptor (TGFβRI), also known as activin receptor-like kinase (47,48). Subsequently, TGFβRII and TGFβRI form a heteromeric complex that induces the phosphorylation of Smad2/3 and Smad1/5/8. The phosphorylated Smad2/3 and Smad1/5/8 are then able to bind to co-Smad (Smad4) and enter the nucleus to regulate gene expression (47,48). In addition, GDF15 exerts its biological functions through non-Smad-dependent pathways such as phosphoinositide 3-kinase/Akt/mammalian target of rapamycin and TGF-β-activated kinase-1 (TAK-1)/nuclear factor-κB (NF-κB), or through other receptors such as glial cell-derived neurotrophic factor receptor α-like (GFRAL) and epidermal growth factor receptor 2 (Fig. 3) (16,45,49,50).
Figure 3.
GDF15-mediates Smad and non-Smad signaling pathways. In the Smad pathways (left), GDF15 binding to TGFβRII activates ALK1/2/3/6 and ALK4/5/7, leading to the phosphorylation of Smad2/3 and Smad1/5/8. The phosphorylated Smads form complexes with Smad4 and thereby regulate gene transcription. In the non-Smad pathways (right), GDF15 exerts neuroprotective effects via PI3K/Akt/mTOR pathway and promotes intestinal inflammation through the TAK-1/NF-κB pathway. In addition, GFRAL interacts with RET receptors after binding to GDF15 to initiate ERK, Akt and PLCγ phosphorylation which induces anorexia. GDF15 also interacts with the receptor ErbB2 to activate the MAPK/ERK1/2 pathway and promote cancer cell proliferation. GDF15, growth differentiation factor 15; TGFβRII, type II transforming growth factor-β receptor; ALK, activin receptor-like kinase; PI3K, phosphoinositide 3-kinase; mTOR, mammalian target of rapamycin; TAK-1, transforming growth factor-β-activating kinase-1; NF-κB, nuclear factor-κB; GFRAL, glial cell-derived neurotrophic factor receptor α-like; RET, ret proto-oncogene; ERK, extracellular signal-regulated kinase; PLCγ, phospholipase C γ; ErbB2, epidermal growth factor receptor 2; MAPK, mitogen-activated protein kinase; p, phosphorylation.
3. GDF15 and anorexia in CC
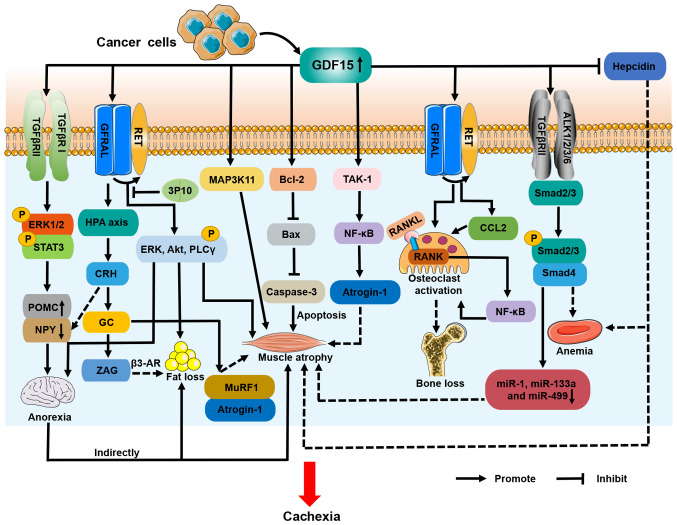
A clinical study evaluated the association between serum levels of GDF15 and anorexia in patients with cancer and reported that GDF15 levels were significantly higher in anorexic patients than in non-anorexic ones (51). This implies that high levels of GDF15 in patients with cancer are associated with anorexia. There is a body of evidence suggesting that GDF15 induces anorexia in patients with CC (16,33,51). Johnen et al (33) originally described the role of GDF15 in CC and anorexia. They observed that food intake was reduced in tumor-bearing mice that were transgenically modified to overexpress GDF15. Lower food intake indirectly resulted in fat loss, tibial and gastrocnemius muscle atrophy and 28% weight loss in tumor-bearing mice (33). These effects were blocked by the administration of a GDF15 monoclonal antibody and reproduced by the injection of recombinant GDF15 (33). Johnen et al (33) further demonstrated that GDF15 promoted anorexia by interacting with TGFβRII to induce the phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 and transducer and activator of transcription 3 (STAT3) in the hypothalamus. This process ultimately inhibited orexigenic neuropeptide Y (NPY) neurons and stimulated anorexigenic pro-opiomelanocortin (POMC) neurons (Fig. 4). GFRAL is a specific receptor for GDF15 that is uniquely expressed in the hindbrain area postrema and nucleus tractus solitarius (52). The binding of GDF15 to GFRAL induces the activation of its co-receptor Ret proto-oncogene (RET), which further promotes the phosphorylation of ERK, Akt and phospholipase C γ, resulting in decreased appetite in cachectic mice (Fig. 4) (16). The GDF15/GFRAL/RET pathway therefore is considered a novel therapeutic target for anorexia and weight loss in CC (16).
Figure 4.
Roles and mechanisms of GDF15 in CC. Solid lines indicate demonstrated mechanisms and dashed lines indicate possible mechanisms. GDF15 initiates the phosphorylation of ERK, Akt and PLCγ via GFRAL/RET signaling to mediate anorexia, muscle atrophy and fat loss in CC; the antibody 3P10 can inhibit these processes. Binding of GDF15 to TGFβRII and TGFβRI leads to the phosphorylation of ERK1/2 and STAT3. This pathway downregulates NPY and upregulates POMC to induce anorexia. Reduced food intake indirectly contributes to muscle wasting and fat depletion in cancer cachexia. Activation of MAP3K11/GDF15 and Bcl-2/caspase-3 apoptotic pathways is responsible for CC muscle atrophy, but their specific receptors are unknown. GDF15 may also reduce muscle mass through the TAK-1/NF-κB signaling pathway, and downregulates the expression of miR-1, miR-133a and miR-499 in muscle. In addition, GDF15 may promote muscle atrophy and fat loss by stimulating the HPA axis. Moreover, the GDF15/Smad2/3 pathway may be involved in CC-induced anemia. High levels of GDF15 directly inhibit the expression of hepcidin, which could further trigger anemia and muscle wasting in CC. GDF15, growth differentiation factor 15; CC, cancer cachexia; ERK, extracellular signal-regulated kinase; PLCγ, phospholipase C γ; GFRAL, glial cell-derived neurotrophic factor receptor α-like; RET, ret proto-oncogene; TGFβRII, type II transforming growth factor-β receptor; TGFβRI, type I transforming growth factor-β receptor; ERK1/2, extracellular signal-regulated kinase1/2; STAT3, signal transducer and activator of transcription 3; NPY, neuropeptide Y; POMC, pro-opiomelanocortin; MAP3K11, mitogen-activated protein kinase 11; Bcl-2, B cell lymphoma-2; TAK-1, transforming growth factor-β-activating kinase-1; NF-κB, nuclear factor-κB; miR, microRNA; HPA, hypothalamus-pituitary-adrenal; CRH, corticotropin-releasing hormone; GC, glucocorticoids; ZAG, zinc-α2-glycoprotein; β3-AR, β3-adrenergic receptor; MuRF1, muscle ring finger 1; Bax, Bcl-2 associated X protein; RANK, receptor activation of NF-κB; RANKL, RANK ligand; CCL2, CC motif chemokine ligand 2; p, phosphorylation.
GDF15 may also influence CC anorexia through other mechanisms. It has been shown that GDF15 activates the hypothalamic-pituitary-adrenal (HPA) axis in a GFRAL-dependent manner, leading to the secretion of corticotropin-releasing hormone (CRH) and glucocorticoids (53). CRH has been found to promote anorexia in CC, due to the inhibition of NPY neurons (Fig. 4) (54).
4. GDF15 and muscle atrophy in CC
As GDF15 is a myokine, its circulating levels are negatively correlated with muscle mass in numerous diseases, including COPD, intensive care unit-acquired weakness (ICUAW), Crohn's disease, pulmonary hypertension (PH) and CC (30,55–58). Muscle atrophy, including that of skeletal, chest, diaphragm and cardiac muscle, is a hallmark of CC and a major cause of mortality in patients with cancer (4,59).
One study indicated that GDF15 indirectly induced muscle atrophy in CC via the inhibition of feeding centers in the hypothalamus (33). However, in other in vitro studies, GDF15 increased the expression of muscle ring finger 1 (MuRF1) and muscle atrophy F-box (MAFbx)/atrogin-1 and decreased myotube diameter (55,57). Researchers also found that the expression of GDF15 and MAFbx/atrogin-1 was elevated in the atrophied rectus abdominis muscle of patients with ICUAW (55). The muscle-specific E3 ubiquitin ligases MuRF1and MAFbx/atrogin-1 are primary factors that drive muscle protein degradation in CC (2). These studies indicate that GDF15 may contribute to muscle atrophy in CC, independently of food intake. It has been reported that GDF15/GFRAL/RET directly induces muscle atrophy in CC (16). In addition, GDF15 has been shown to directly regulate muscle mass in CC through other mechanisms. Lerner et al (30) demonstrated that the activation of mitogen-activated protein kinase 11 (MAP3K11) by GDF15 promoted gastrocnemius and flounder muscle reduction in a genetically engineered mouse model of cachexia. These cachectic mice exhibited weight gain and the retention of skeletal muscle when treated with anti-GDF15 antibody (30). Subsequently, Zhang et al (60) showed that elevated GDF15 levels in the serum exosomes of mice with colon cancer caused the loss of gastrocnemius muscle mass via the B cell lymphoma-2/caspase-3 pathway (Fig. 4).
Despite these studies, the mechanism by which GDF15 facilitates the loss of skeletal muscle mass in CC remains unclear. It has been reported that GDF15 reduces muscle mass in patients with PH and ICUAW through TAK-1/NF-κB/atrogin-1 signaling, and downregulates the expression of various miRNAs, including miR-1, miR-133a and miR-499, in muscle (55,57). In one study, the upregulated phosphorylation of Smad2/3 was observed in the muscles of patients with ICUAW, suggesting that GDF15 may mediate muscle atrophy via the classical Smad pathway; however, this was not observed in C2C12 murine cell-based myotubes exposed to GDF15 in vitro (55). In addition, high levels of GDF15 are known to inhibit the expression of hepcidin and lead to iron overload (61). A recent study revealed that iron overload induced muscle atrophy in patients with gastric cancer and cachexia (62). Moreover, GDF15/GFRAL signaling has been shown to activate the HPA axis and trigger the release of glucocorticoids (53). Animal models of colon, Lewis lung carcinoma (LLC) and pancreatic CC have shown that dysregulated glucocorticoid levels increase the expression of MuRF1 and MAFbx/atrogin-1 in skeletal muscle, driving the breakdown of muscle protein (63). Additional studies are warranted to identify whether GDF15 regulates muscle atrophy in CC through these mechanisms (Fig. 4).
5. GDF15 and adipose tissue depletion in CC
GDF15 is expressed in adipose tissue and secreted by adipocytes (64). Physiological concentrations of GDF15 trigger lipolysis in human adipose tissue (64). Although skeletal muscle is the main tissue that is affected by CC, a study found that fat loss occurs rapidly and earlier than muscle tissue depletion in CC (65).
The molecular mechanisms of GDF15-induced fat loss in CC have not been well studied, and pre-clinical models have mainly been used. In one study, for example, mice with prostate CC and high GDF15 expression lost all retroperitoneal fat and exhibited a reduction of fat in the groin and epididymis of 54 and 89%, respectively, due to decreased food intake (33). In addition, in another study GDF15 increased the expression of differentiation and thermogenic genes in brown adipocytes; the upregulated expression of iodothyronine deiodinase 2, β3-adrenergic receptor (β3-AR), and very long chain fatty acid-3 through GFRAL/RET signaling in cachectic mouse adipose tissue led to a loss of fat and body weight (Fig. 4) (16). The therapeutic monoclonal antibody 3P10, which is a GFRAL antagonist and RET signaling inhibitor, has been reported to reverse lipid hyperoxidation and prevent CC in mice (16). Furthermore, ZAG is known to be a lipid-mobilizing factor and has been demonstrated to stimulate lipolysis via β3-AR during CC (66). Glucocorticoids have been shown to increase ZAG expression and thereby promote lipolysis (67), suggesting that GDF15 may increase adipose tissue depletion in CC via the HPA axis (Fig. 4).
6. GDF15 and bone loss in CC
Bone loss is caused by an imbalance between bone-resorbing osteoclasts and bone-forming osteoblasts, which can result in decreased bone mineral density (BMD), bone mass and bone strength (68). Abnormal activation of osteoclasts is the cause of various bone diseases, including osteoporosis, rheumatoid arthritis, multiple myeloma and metastatic cancers (69). A link between CC and bone loss has been established in patients with CC and in animal models (5,70–72). Elevated levels of C-telopeptide of type I collagen (CTX-1) in serum have been demonstrated to be indicative of increased bone resorption and accelerated bone loss (73). Also, studies in humans have shown that serum CTX-1 is significantly elevated in patients with ovarian, lung and gastrointestinal CC compared with non-cachectic controls (70–72). In a retrospective study, patients with pancreatic CC were found to have significantly lower BMD than control patients who underwent benign gallbladder surgery, and those patients with pancreatic CC with osteopenia exhibited lower median and 2-year postoperative survival times than those without osteopenia (74). In an animal model of LLC-induced CC, Yu et al (5) observed a reduction in bone trabecular volume and BMD, and an increase in osteoclast activation. This result was consistent with the findings of a previous study regarding cachectic mice with colon cancer (71). The researchers further established that the bone loss was induced via the Janus kinase/STAT3 pathway, with the involvement of glucocorticoids (5). Surprisingly, bone loss preceded the onset of muscle and fat loss in the LLC-induced model of cachexia (5). These studies all demonstrate that bone loss is closely associated with CC.
It has been demonstrated that GDF15 increases osteoclast differentiation and inhibits osteoblast differentiation in vivo and in vitro, leading to disturbances in bone metabolism and bone loss (75,76). Wakchoure et al (77) injected a Du-145 human prostate cancer cell line overexpressing GDF15 into the tibias of C57/B6 mice, and found that increased GDF15 was associated with osteoclast activation and cachexia. This result implies that GDF15 is involved in bone loss in CC, but the biological mechanism underlying this has not yet been identified. In a hypoxic mouse model induced by right femoral artery ligation, the upregulation of GDF15 expression in osteoblasts was observed, which stimulated the receptor activator of NF-κB ligand (RANKL)-induced NF-κB signaling pathway and promoted osteoclast activation in the mice, resulting in decreased bone mass (76). An anti-GDF15 antibody inhibited this process and the associated bone loss (76). Moreover, Siddiqui et al (78) demonstrated that elevated GDF15 in prostate cancer upregulated the expression of C-C motif chemokine ligand 2 and RANKL through the GFRAL/RET pathway, thereby activating osteoclasts and leading to decreased bone mass. Other studies have reported that the GDF15/GFRAL/RET receptor signaling complex in the brain mediates anorexia in CC (16,79). GFRAL and RET receptors have also been shown to be expressed in the tibiae (Fig. 4) (78). These findings indicate that GDF15 may activate GFRAL and RET receptors expressed on osteocytes, thereby inducing bone loss in CC.
7. GDF15 and anemia in CC
A recent retrospective cohort study conducted across multiple centers indicated that patients with CC had lower hemoglobin levels than those without cachexia (80). The study demonstrated that patients with CC developed anemia. However, the molecular mechanisms underlying the CC-induced anemia remain unclear. A study in mice with lung CC found that TGF-β activated the Smad2/3 signaling pathway and inhibited hematopoietic stem cell and erythropoietic cell production. The mice exhibited a significant reduction in hemoglobin and erythrocyte levels in the peripheral blood (81). Additionally, GDF15 is produced by erythroid precursor cells, and high levels of GDF15 are known to inhibit effective erythropoiesis and the expression of hepcidin, leading to anemia and iron overload (61). Hepcidin is a hepatic peptide hormone that coordinates the systemic homeostasis of iron. Jiang et al (82) showed that increased serum levels of GDF15 in patients with cancer are associated with downregulated hepcidin levels and cancer-related anemia. According to these findings, we hypothesize that GDF15 may play a role in CC-associated anemia (Fig. 4).
8. GDF15 and other TGF-β superfamily factors in CC
The TGF-β superfamily is a class of secreted peptide cytokines with multiple members that are involved in the development of CC (83). They are categorized into four main subfamilies based on sequence similarity, namely bone morphogenetic proteins/GDFs, activins/inhibins/nodal, TGF-βs and others (84). GDF8, which is also known as myostatin, and activin A have been reported to bind to activin receptor type 2B on skeletal muscle to promote the phosphorylation of Smad2/3 and inhibit Akt phosphorylation, leading to activation of the ubiquitin-proteasome system that eventually leads to muscle atrophy (54). Greco et al (85) demonstrated that blocking TGF-β reduces skeletal muscle catabolism and weight loss in mouse models of pancreatic CC, and decreases phosphorylated Smad2/3 signaling in muscle tissues. This suggests that TGF-β is also involved in skeletal muscle atrophy in CC, via activation of the Smad2/3 signaling pathway. Another member of the TGF-β superfamily, GDF11, is highly homologous to GDF8 (86). GDF11 directly increases the expression of MuRF1 and MAFbx/atrogin-1 in skeletal muscle, leading to muscle atrophy and cachexia (87). In addition, Zimmers et al (88) demonstrated that elevated circulating levels of GDF11 are associated with cardiac atrophy.
CC is accompanied by a complex pro-inflammatory environment in the body (Table I). Therefore, it is possible that proteins of the TGF-β superfamily may coordinate with one another to promote the development and progression of CC. One preclinical study found that GDF11 induced the upregulation of GDF15 expression by activating the binding of Smad2/3 to the GDF15 promoter (Fig. 1) (87). The upregulated GDF15 further suppressed appetite and indirectly induced weight loss (87). However, no further studies have identified the relationships between GDF15 and other TGF-β family members that may be involved in cachexia, such as GDF8, activin A and TGF-β. It would be of great significance to investigate the associations and roles of these proteins in CC in future clinical studies.
9. Targeting GDF15 in CC
CC is a complex syndrome of multiple organ and tissue depletion (6). Despite advances in cancer treatment, there are no effective therapies for CC. To improve the quality of life of patients, it is currently advocated to take a multimodal approach to treatment, based on medication supplemented by nutrition, exercise and psychological counseling (28). As the basis for the treatment of CC, pharmacotherapeutic strategies can stimulate appetite, reduce inflammation, increase anabolism and decrease catabolism (54). During conferences on cachexia, researchers have also reported some novel therapeutic targets for the treatment of CC, such as ZRT/IRT-like protein14, fibroblast growth factor-inducible receptor 14, serum amyloid A1, MuRF1 and GDF15 (89,90).
Several clinical trials have been conducted to investigate the role of GDF15 antibodies in CC. Ponsegromab is a human monoclonal antibody that targets GDF15 (91). It binds to GDF15 and prevents it from binding to GFRAL, thereby blocking GDF15/GFRAL-mediated signaling. A recent phase-1b clinical trial (NCT04299048) conducted by Pfizer evaluated the safety and efficacy of ponsegromab in patients with non-small cell lung, colorectal and pancreatic cancers accompanied by cachexia. In addition to standard anticancer treatments, patients with cachexia received ponsegromab subcutaneously every 3 weeks for a total of 12 weeks (92). The results showed that the median circulating GDF15 levels in patients treated with ponsegromab were lower than the those in healthy controls (92). Moreover, ponsegromab treatment significantly increased weight, physical activity and appetite in patients with CC (92). Clinical studies of ponsegromab in CC remain ongoing and are currently in phase II (91). Table II summarizes clinical trials regarding GDF15 that have been conducted in patients with cancer and CC (https://clinicaltrials.gov/). The information obtained from these studies may ultimately enable patients with CC to benefit from these treatments, and also guide future studies.
Table II.
Clinical trials targeting GDF15 in patients with cancer and CC.
| Clinical trial identifier | Start year | Compound/dr ug | Participants, n | Disease | Phase | Status | Results | Locations | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|
| NCT05865535 | 2023 | AV-380 | 30 (estimated) | Colorectal and pancreatic CC | I | Recruiting | NA | USA | NA |
| NCT05546476 | 2022 | Ponsegromab | 168 (estimated) | Non-small cell, pancreatic and colorectal CC | II | Recruiting | NA | Spain, Taiwan, USA, Australia, Bulgaria, Canada, China, Czechia, Hungary, Japan, Poland, Slovakia | NA |
| NCT04803305 | 2021 | Ponsegromab | 18 (actual) | Non-small cell lung, pancreatic, colorectal, prostate, breast and ovarian CC | I | Completed | NA | Canada, USA | NA |
| NCT04299048 | 2020 | Ponsegromab | 11 (actual) | Non-small cell lung, pancreatic and colorectal CC | Ib | Completed | Compared with controls, patients treated with ponsegromab had lower circulating GDF-15 levels and increased body weight, physical activity and appetite | USA | (92) |
| NCT04725474 | 2020 | CTL-002 | 155 (estimated) | Adult solid tumor | I/II | Recruiting | NA | Germany, Spain, Switzerland | NA |
| NCT05397171 | 2022 | AZD8853 | 16 (actual) | Bladder, colorectal and non-small cell lung cancers | II | Terminated | NA | Canada, USA | NA |
Data in the table are from ClinicalTrials.gov. CC, cancer cachexia; GDF15, growth differentiation factor 15; NCT, National Clinical Trial; NA, not available.
Animal experiments further illustrate that the inhibition of GDF15 is an effective strategy for the treatment of CC (30,33,93). In several animal models, including prostate, ovarian, colon and breast cancer, leukemia and fibrosarcoma models, cachectic mice treated with GDF15 antibodies exhibited increased food intake, muscle mass and adipose tissue, ultimately leading to weight gain (30,33,93). These studies illustrate that targeting GDF15 alleviates CC via the prevention of anorexia, and the loss of muscle and fat. In addition, Hinoi et al (76) demonstrated that an anti-GDF15 antibody inhibited bone loss and osteoclast activation in the tibias of hypoxic mice. The inhibition of GDF15 has also been observed to inhibit ineffective erythropoiesis and improve anemia in patients with cancer (94). Thus, GDF15 may be a potential target for the treatment of bone loss and anemia in CC. Moreover, one study revealed that the combination of an anti-GDF15 antibody with the angiogenesis inhibitor tivozanib significantly increased body weight and survival in mice with CC, when compared with tivozanib alone (30). This implies that the inhibition of tumor growth and amelioration of CC may prolong the lifespans of patients with this condition. The strategy represents a new therapeutic prospect, but requires translation into clinical studies.
10. Discussion and future prospects
In the present review, the roles and mechanisms of GDF15 in CC are summarized. Studies have indicated that the effects of GDF15 on CC are associated with inhibition of the feeding center. However, since GFRAL was identified as an exclusive receptor for GDF15, further studies have demonstrated that GDF15 also induces metabolic effects that are independent of food-intake behavior. It has been established that GDF15 interacts with GFRAL in the brainstem to suppress appetite, promote fat loss and reduce muscle mass in CC. GDF15 also directly promotes muscle atrophy in CC via the apoptotic pathway and MAP3K11, but the receptors involved in these processes are currently unknown. It also appears that GDF15 may have a role in bone loss and anemia in CC. However, it remains uncertain whether other GDF15 receptors or pathways, such as the GDF15/GFRAL/HPA axis, promote the development of CC. Therefore, the roles and potential molecular mechanisms of GDF15 in CC, particularly its receptors and downstream signaling pathways, require further investigation. In addition, GDF15 is a potential therapeutic target for CC, and clinical trials are being conducted to study the safety and therapeutic value of GDF15 antibodies in patients with CC. Notably, since CC is attributed to complex interactions between tumor cells and the host, it may be necessary to combine GDF15-targeting antibodies with anticancer therapies such as immunotherapy or targeted therapy to improve the outcomes of patients with CC.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
TL and JZ were responsible for drafting and revising the manuscript, and creating the figures. FD and LM contributed to manuscript conception. Data authentication is not applicable. All authors have read and approved the final manuscript.
Ethical approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, et al. Cachexia: A new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Baazim H, Antonio-Herrera L, Bergthaler A. The interplay of immunology and cachexia in infection and cancer. Nat Rev Immunol. 2022;22:309–321. doi: 10.1038/s41577-021-00624-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 4.Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. 2016;5:e200. doi: 10.1038/oncsis.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J, Choi S, Park A, Do J, Nam D, Kim Y, Noh J, Lee KY, Maeng CH, Park KS. Bone marrow homeostasis is impaired via JAK/STAT and glucocorticoid signaling in cancer cachexia model. Cancers (Basel) 2021;13:1059. doi: 10.3390/cancers13051059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Argilés JM, Stemmler B, López-Soriano FJ, Busquets S. Inter-tissue communication in cancer cachexia. Nat Rev Endocrinol. 2018;15:9–20. doi: 10.1038/s41574-018-0123-0. [DOI] [PubMed] [Google Scholar]
- 7.Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:17105. doi: 10.1038/nrdp.2017.105. [DOI] [PubMed] [Google Scholar]
- 8.Gupta A, Nshuti L, Grewal US, Sedhom R, Check DK, Parsons HM, Blaes AH, Virnig BA, Lustberg MB, Subbiah IM, et al. Financial burden of drugs prescribed for cancer-associated symptoms. JCO Oncol Pract. 2022;18:140–147. doi: 10.1200/OP.21.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu CA, Zhang Q, Ruan GT, Shen LY, Xie HL, Liu T, Tang M, Zhang X, Yang M, Hu CL, et al. Novel diagnostic and prognostic tools for lung cancer cachexia: Based on nutritional and inflammatory status. Front Oncol. 2022;12:890745. doi: 10.3389/fonc.2022.890745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malla J, Zahra A, Venugopal S, Selvamani TY, Shoukrie SI, Selvaraj R, Dhanoa RK, Hamouda RK, Mostafa J. What role do inflammatory cytokines play in cancer cachexia? Cureus. 2022;14:e26798. doi: 10.7759/cureus.26798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng R, Tong C, Xiong X. The molecular basis and therapeutic potential of leukemia inhibitory factor in cancer cachexia. Cancers (Basel) 2022;14:2955. doi: 10.3390/cancers14122955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu SW, Pan HC, Hsu YH, Chang KC, Wu LW, Chen WY, Chang MS. IL-20 antagonist suppresses PD-L1 expression and prolongs survival in pancreatic cancer models. Nat Commun. 2020;11:4611. doi: 10.1038/s41467-020-18244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Girolamo D, Tajbakhsh S. Pathological features of tissues and cell populations during cancer cachexia. Cell Regen. 2022;11:15. doi: 10.1186/s13619-022-00108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callaway CS, Delitto AE, Patel R, Nosacka RL, D'Lugos AC, Delitto D, Deyhle MR, Trevino JG, Judge SM, Judge AR. IL-8 released from human pancreatic cancer and tumor-associated stromal cells signals through a CXCR2-ERK1/2 axis to induce muscle atrophy. Cancers (Basel) 2019;11:1863. doi: 10.3390/cancers11121863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong H, Ye J, Xie K, Hu W, Xu N, Yang H. Exosomal IL-8 derived from Lung Cancer and Colon Cancer cells induced adipocyte atrophy via NF-κB signaling pathway. Lipids Health Dis. 2022;21:147. doi: 10.1186/s12944-022-01755-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suriben R, Chen M, Higbee J, Oeffinger J, Ventura R, Li B, Mondal K, Gao Z, Ayupova D, Taskar P, et al. Antibody-mediated inhibition of GDF15-GFRAL activity reverses cancer cachexia in mice. Nat Med. 2020;26:1264–1270. doi: 10.1038/s41591-020-0945-x. [DOI] [PubMed] [Google Scholar]
- 17.Belloum Y, Rannou-Bekono F, Favier FB. Cancer-induced cardiac cachexia: Pathogenesis and impact of physical activity (Review) Oncol Rep. 2017;37:2543–2552. doi: 10.3892/or.2017.5542. [DOI] [PubMed] [Google Scholar]
- 18.Nissinen TA, Hentilä J, Penna F, Lampinen A, Lautaoja JH, Fachada V, Holopainen T, Ritvos O, Kivelä R, Hulmi JJ. Treating cachexia using soluble ACVR2B improves survival, alters mTOR localization, and attenuates liver and spleen responses. J Cachexia Sarcopenia Muscle. 2018;9:514–529. doi: 10.1002/jcsm.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thibaut MM, Sboarina M, Roumain M, Pötgens SA, Neyrinck AM, Destrée F, Gillard J, Leclercq IA, Dachy G, Demoulin JB, et al. Inflammation-induced cholestasis in cancer cachexia. J Cachexia Sarcopenia Muscle. 2021;12:70–90. doi: 10.1002/jcsm.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peyta L, Jarnouen K, Pinault M, Coulouarn C, Guimaraes C, Goupille C, de Barros JP, Chevalier S, Dumas JF, Maillot F, et al. Regulation of hepatic cardiolipin metabolism by TNFα: Implication in cancer cachexia. Biochim Biophys Acta. 2015;1851:1490–1500. doi: 10.1016/j.bbalip.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Patel HJ, Patel BM. TNF-α and cancer cachexia: Molecular insights and clinical implications. Life Sci. 2017;170:56–63. doi: 10.1016/j.lfs.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 22.Black K, Garrett IR, Mundy GR. Chinese hamster ovarian cells transfected with the murine interleukin-6 gene cause hypercalcemia as well as cachexia, leukocytosis and thrombocytosis in tumor-bearing nude mice. Endocrinology. 1991;128:2657–2659. doi: 10.1210/endo-128-5-2657. [DOI] [PubMed] [Google Scholar]
- 23.Bindels LB, Neyrinck AM, Loumaye A, Catry E, Walgrave H, Cherbuy C, Leclercq S, Van Hul M, Plovier H, Pachikian B, et al. Increased gut permeability in cancer cachexia: mechanisms and clinical relevance. Oncotarget. 2018;9:18224–18238. doi: 10.18632/oncotarget.24804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White JP, Puppa MJ, Narsale A, Carson JA. Characterization of the male ApcMin/+ mouse as a hypogonadism model related to cancer cachexia. Biol Open. 2013;2:1346–1353. doi: 10.1242/bio.20136544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tracey KJ, Wei H, Manogue KR, Fong Y, Hesse DG, Nguyen HT, Kuo GC, Beutler B, Cotran RS, Cerami A, et al. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. J Exp Med. 1988;167:1211–1227. doi: 10.1084/jem.167.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johns N, Stretch C, Tan BH, Solheim TS, Sørhaug S, Stephens NA, Gioulbasanis I, Skipworth RJ, Deans DA, Vigano A, et al. New genetic signatures associated with cancer cachexia as defined by low skeletal muscle index and weight loss. J Cachexia Sarcopenia Muscle. 2017;8:122–130. doi: 10.1002/jcsm.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong X, Narasimhan A, Silverman LM, Young AR, Shahda S, Liu S, Wan J, Liu Y, Koniaris LG, Zimmers TA. Sex specificity of pancreatic cancer cachexia phenotypes, mechanisms, and treatment in mice and humans: Role of Activin. J Cachexia Sarcopenia Muscle. 2022;13:2146–2161. doi: 10.1002/jcsm.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avancini A, Trestini I, Tregnago D, Lanza M, Menis J, Belluomini L, Milella M, Pilotto S. A multimodal approach to cancer-related cachexia: from theory to practice. Expert Rev Anticancer Ther. 2021;21:819–826. doi: 10.1080/14737140.2021.1927720. [DOI] [PubMed] [Google Scholar]
- 29.Lockhart SM, Saudek V, O'Rahilly S. GDF15: A hormone conveying somatic distress to the brain. Endocr Rev. 2020;41:bnaa007. doi: 10.1210/endrev/bnaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lerner L, Tao J, Liu Q, Nicoletti R, Feng B, Krieger B, Mazsa E, Siddiquee Z, Wang R, Huang L, et al. MAP3K11/GDF15 axis is a critical driver of cancer cachexia. J Cachexia Sarcopenia Muscle. 2016;7:467–482. doi: 10.1002/jcsm.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki H, Mitsunaga S, Ikeda M, Aoyama T, Yoshizawa K, Yoshimatsu H, Kawai N, Masuda M, Miura T, Ochiai A. Clinical and tumor characteristics of patients with high serum levels of growth differentiation factor 15 in advanced pancreatic cancer. Cancers (Basel) 2021;13:4842. doi: 10.3390/cancers13194842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lerner L, Hayes TG, Tao N, Krieger B, Feng B, Wu Z, Nicoletti R, Chiu MI, Gyuris J, Garcia JM. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J Cachexia Sarcopenia Muscle. 2015;6:317–324. doi: 10.1002/jcsm.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnen H, Lin S, Kuffner T, Brown DA, Tsai VW, Bauskin AR, Wu L, Pankhurst G, Jiang L, Junankar S, et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat Med. 2007;13:1333–1340. doi: 10.1038/nm1677. [DOI] [PubMed] [Google Scholar]
- 34.Li P, Lv H, Zhang B, Duan R, Zhang X, Lin P, Song C, Liu Y. Growth differentiation factor 15 protects SH-SY5Y cells from rotenone-induced toxicity by suppressing mitochondrial apoptosis. Front Aging Neurosci. 2022;14:869558. doi: 10.3389/fnagi.2022.869558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asrih M, Wei S, Nguyen TT, Yi HS, Ryu D, Gariani K. Overview of growth differentiation factor 15 in metabolic syndrome. J Cell Mol Med. 2023;27:1157–1167. doi: 10.1111/jcmm.17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wan Y, Fu J. GDF15 as a key disease target and biomarker: Linking chronic lung diseases and ageing. Mol Cell Biochem. 2023 Apr 24; doi: 10.1007/s11010-023-04743-x. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Assadi A, Zahabi A, Hart RA. GDF15, an update of the physiological and pathological roles it plays: A review. Pflugers Arch. 2020;472:1535–1546. doi: 10.1007/s00424-020-02459-1. [DOI] [PubMed] [Google Scholar]
- 38.Johann K, Kleinert M, Klaus S. The Role of GDF15 as a Myomitokine. Cells. 2021;10:2990. doi: 10.3390/cells10112990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wischhusen J, Melero I, Fridman WH. Growth/Differentiation Factor-15 (GDF-15): From biomarker to novel targetable immune checkpoint. Front Immunol. 2020;11:951. doi: 10.3389/fimmu.2020.00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai VW, Brown DA, Breit SN. Targeting the divergent TGFβ superfamily cytokine MIC-1/GDF15 for therapy of anorexia/cachexia syndromes. Curr Opin Support Palliat Care. 2018;12:404–409. doi: 10.1097/SPC.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 41.Siddiqui JA, Pothuraju R, Khan P, Sharma G, Muniyan S, Seshacharyulu P, Jain M, Nasser MW, Batra SK. Pathophysiological role of growth differentiation factor 15 (GDF15) in obesity, cancer, and cachexia. Cytokine Growth Factor Rev. 2022;64:71–83. doi: 10.1016/j.cytogfr.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niu Y, Zhang W, Shi J, Liu Y, Zhang H, Lin N, Li X, Qin L, Yang Z, Su Q. The relationship between circulating growth differentiation factor 15 levels and diabetic retinopathy in patients with type 2 diabetes. Front Endocrinol (Lausanne) 2021;12:627395. doi: 10.3389/fendo.2021.627395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsui KH, Hsu SY, Chung LC, Lin YH, Feng TH, Lee TY, Chang PL, Juang HH. Growth differentiation factor-15: A p53- and demethylation-upregulating gene represses cell proliferation, invasion, and tumorigenesis in bladder carcinoma cells. Sci Rep. 2015;5:12870. doi: 10.1038/srep12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joo M, Kim D, Lee MW, Lee HJ, Kim JM. GDF15 promotes cell growth, migration, and invasion in gastric cancer by inducing STAT3 activation. Int J Mol Sci. 2023;24:2925. doi: 10.3390/ijms24032925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li S, Ma YM, Zheng PS, Zhang P. GDF15 promotes the proliferation of cervical cancer cells by phosphorylating AKT1 and Erk1/2 through the receptor ErbB2. J Exp Clin Cancer Res. 2018;37:80. doi: 10.1186/s13046-018-0744-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spanopoulou A, Gkretsi V. Growth differentiation factor 15 (GDF15) in cancer cell metastasis: From the cells to the patients. Clin Exp Metastasis. 2020;37:451–464. doi: 10.1007/s10585-020-10041-3. [DOI] [PubMed] [Google Scholar]
- 47.Rochette L, Dogon G, Zeller M, Cottin Y, Vergely C. GDF15 and cardiac cells: Current concepts and new insights. Int J Mol Sci. 2021;22:8889. doi: 10.3390/ijms22168889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li L, Zhang R, Yang H, Zhang D, Liu J, Li J, Guo B. GDF15 knockdown suppresses cervical cancer cell migration in vitro through the TGF-β/Smad2/3/Snail1 pathway. FEBS Open Bio. 2020;10:2750–2760. doi: 10.1002/2211-5463.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang CY, Huang AQ, Zhou MH, Mei YA. GDF15 regulates Kv2.1-mediated outward K+ current through the Akt/mTOR signalling pathway in rat cerebellar granule cells. Biochem J. 2014;460:35–47. doi: 10.1042/BJ20140155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park SH, Yu M, Kim J, Moon Y. C/EBP homologous protein promotes NSAID-activated gene 1-linked pro-inflammatory signals and enterocyte invasion by enteropathogenic Escherichia coli. Microbes Infect. 2017;19:110–121. doi: 10.1016/j.micinf.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Molfino A, Amabile MI, Imbimbo G, Rizzo V, Pediconi F, Catalano C, Emiliani A, Belli R, Ramaccini C, Parisi C, et al. Association between growth differentiation factor-15 (GDF-15) serum levels, anorexia and low muscle mass among cancer patients. Cancers (Basel) 2020;13:99. doi: 10.3390/cancers13010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabatini PV, Frikke-Schmidt H, Arthurs J, Gordian D, Patel A, Rupp AC, Adams JM, Wang J, Beck Jørgensen S, Olson DP, et al. GFRAL-expressing neurons suppress food intake via aversive pathways. Proc Natl Acad Sci USA. 2021;118:e2021357118. doi: 10.1073/pnas.2021357118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cimino I, Kim H, Tung YCL, Pedersen K, Rimmington D, Tadross JA, Kohnke SN, Neves-Costa A, Barros A, Joaquim S, et al. Activation of the hypothalamic-pituitary-adrenal axis by exogenous and endogenous GDF15. Proc Natl Acad Sci USA. 2021;118:e2106868118. doi: 10.1073/pnas.2106868118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Setiawan T, Sari IN, Wijaya YT, Julianto NM, Muhammad JA, Lee H, Chae JH, Kwon HY. Cancer cachexia: Molecular mechanisms and treatment strategies. J Hematol Oncol. 2023;16:54. doi: 10.1186/s13045-023-01454-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bloch SA, Lee JY, Syburra T, Rosendahl U, Griffiths MJ, Kemp PR, Polkey MI. Increased expression of GDF-15 may mediate ICU-acquired weakness by down-regulating muscle microRNAs. Thorax. 2015;70:219–228. doi: 10.1136/thoraxjnl-2014-206225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamamoto H, Takeshima F, Haraguchi M, Akazawa Y, Matsushima K, Kitayama M, Ogihara K, Tabuchi M, Hashiguchi K, Yamaguchi N, et al. High serum concentrations of growth differentiation factor-15 and their association with Crohn's disease and a low skeletal muscle index. Sci Rep. 2022;12:6591. doi: 10.1038/s41598-022-10587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garfield BE, Crosby A, Shao D, Yang P, Read C, Sawiak S, Moore S, Parfitt L, Harries C, Rice M, et al. Growth/differentiation factor 15 causes TGFβ-activated kinase 1-dependent muscle atrophy in pulmonary arterial hypertension. Thorax. 2019;74:164–176. doi: 10.1136/thoraxjnl-2017-211440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deng M, Bian Y, Zhang Q, Zhou X, Hou G. Growth differentiation factor-15 as a biomarker for sarcopenia in patients with chronic obstructive pulmonary disease. Front Nutr. 2022;9:897097. doi: 10.3389/fnut.2022.897097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song M, Zhang Q, Tang M, Zhang X, Ruan G, Zhang X, Zhang K, Ge Y, Yang M, Li Q, et al. Associations of low hand grip strength with 1 year mortality of cancer cachexia: A multicentre observational study. J Cachexia Sarcopenia Muscle. 2021;12:1489–1500. doi: 10.1002/jcsm.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang W, Sun W, Gu X, Miao C, Feng L, Shen Q, Liu X, Zhang X. GDF-15 in tumor-derived exosomes promotes muscle atrophy via Bcl-2/caspase-3 pathway. Cell Death Discov. 2022;8:162. doi: 10.1038/s41420-022-00972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanno T, Bhanu NV, Oneal PA, Goh SH, Staker P, Lee YT, Moroney JW, Reed CH, Luban NL, Wang RH, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13:1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 62.Zhou D, Zhang Y, Mamtawla G, Wan S, Gao X, Zhang L, Li G, Wang X. Iron overload is related to muscle wasting in patients with cachexia of gastric cancer: using quantitative proteome analysis. Med Oncol. 2020;37:113. doi: 10.1007/s12032-020-01439-w. [DOI] [PubMed] [Google Scholar]
- 63.Martin A, Castells J, Allibert V, Emerit A, Zolotoff C, Cardot-Ruffino V, Gallot YS, Vernus B, Chauvet V, Bartholin L, et al. Hypothalamic-pituitary-adrenal axis activation and glucocorticoid-responsive gene expression in skeletal muscle and liver of Apc mice. J Cachexia Sarcopenia Muscle. 2022;13:1686–1703. doi: 10.1002/jcsm.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laurens C, Parmar A, Murphy E, Carper D, Lair B, Maes P, Vion J, Boulet N, Fontaine C, Marquès M, et al. Growth and differentiation factor 15 is secreted by skeletal muscle during exercise and promotes lipolysis in humans. JCI Insight. 2020;5:e131870. doi: 10.1172/jci.insight.131870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fouladiun M, Körner U, Bosaeus I, Daneryd P, Hyltander A, Lundholm KG. Body composition and time course changes in regional distribution of fat and lean tissue in unselected cancer patients on palliative care-correlations with food intake, metabolism, exercise capacity, and hormones. Cancer. 2005;103:2189–2198. doi: 10.1002/cncr.21013. [DOI] [PubMed] [Google Scholar]
- 66.Elattar S, Dimri M, Satyanarayana A. The tumor secretory factor ZAG promotes white adipose tissue browning and energy wasting. FASEB J. 2018;32:4727–4743. doi: 10.1096/fj.201701465RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weber BZC, Arabaci DH, Kir S. Metabolic reprogramming in adipose tissue during cancer cachexia. Front Oncol. 2022;12:848394. doi: 10.3389/fonc.2022.848394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Plotkin LI, Sanz N, Brun LR. Messages from the Mineral: How bone cells communicate with other tissues. Calcif Tissue Int. 2023;113:39–47. doi: 10.1007/s00223-023-01091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y, Wang H, Zhu G, Qian A, Chen W. F2r negatively regulates osteoclastogenesis through inhibiting the Akt and NFκB signaling pathways. Int J Biol Sci. 2020;16:1629–1639. doi: 10.7150/ijbs.41867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zwickl H, Zwickl-Traxler E, Haushofer A, Seier J, Podar K, Weber M, Hackner K, Jacobi N, Pecherstorfer M, Vallet S. Effect of cachexia on bone turnover in cancer patients: A case-control study. BMC Cancer. 2021;21:744. doi: 10.1186/s12885-021-08518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bonetto A, Kays JK, Parker VA, Matthews RR, Barreto R, Puppa MJ, Kang KS, Carson JA, Guise TA, Mohammad KS, et al. Differential bone loss in mouse models of colon cancer cachexia. Front Physiol. 2016;7:679. doi: 10.3389/fphys.2016.00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pin F, Jones AJ, Huot JR, Narasimhan A, Zimmers TA, Bonewald LF, Bonetto A. RANKL blockade reduces cachexia and bone loss induced by non-metastatic ovarian cancer in mice. J Bone Miner Res. 2022;37:381–396. doi: 10.1002/jbmr.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adesina OO, Jenkins IC, Wu QV, Fung EB, Narla RR, Lipkin EW, Mahajan K, Konkle BA, Kruse-Jarres R. Urinary cross-linked carboxyterminal telopeptide, a bone resorption marker, decreases after vaso-occlusive crises in adults with sickle cell disease. Blood Cells Mol Dis. 2020;80:102369. doi: 10.1016/j.bcmd.2019.102369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cameron ME, Underwood PW, Williams IE, George TJ, Judge SM, Yarrow JF, Trevino JG, Judge AR. Osteopenia is associated with wasting in pancreatic adenocarcinoma and predicts survival after surgery. Cancer Med. 2022;11:50–60. doi: 10.1002/cam4.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Westhrin M, Moen SH, Holien T, Mylin AK, Heickendorff L, Olsen OE, Sundan A, Turesson I, Gimsing P, Waage A, Standal T. Growth differentiation factor 15 (GDF15) promotes osteoclast differentiation and inhibits osteoblast differentiation and high serum GDF15 levels are associated with multiple myeloma bone disease. Haematologica. 2015;100:e511–e514. doi: 10.3324/haematol.2015.124511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hinoi E, Ochi H, Takarada T, Nakatani E, Iezaki T, Nakajima H, Fujita H, Takahata Y, Hidano S, Kobayashi T, et al. Positive regulation of osteoclastic differentiation by growth differentiation factor 15 upregulated in osteocytic cells under hypoxia. J Bone Miner Res. 2012;27:938–949. doi: 10.1002/jbmr.1538. [DOI] [PubMed] [Google Scholar]
- 77.Wakchoure S, Swain TM, Hentunen TA, Bauskin AR, Brown DA, Breit SN, Vuopala KS, Harris KW, Selander KS. Expression of macrophage inhibitory cytokine-1 in prostate cancer bone metastases induces osteoclast activation and weight loss. Prostate. 2009;69:652–661. doi: 10.1002/pros.20913. [DOI] [PubMed] [Google Scholar]
- 78.Siddiqui JA, Seshacharyulu P, Muniyan S, Pothuraju R, Khan P, Vengoji R, Chaudhary S, Maurya SK, Lele SM, Jain M, et al. GDF15 promotes prostate cancer bone metastasis and colonization through osteoblastic CCL2 and RANKL activation. Bone Res. 2022;10:6. doi: 10.1038/s41413-021-00178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ahmed DS, Isnard S, Lin J, Routy B, Routy JP. GDF15/GFRAL pathway as a metabolic signature for cachexia in patients with cancer. J Cancer. 2021;12:1125–1132. doi: 10.7150/jca.50376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang XW, Zhang Q, Song MM, Zhang KP, Zhang X, Ruan GT, Yang M, Ge YZ, Tang M, Li XR, et al. The prognostic effect of hemoglobin on patients with cancer cachexia: A multicenter retrospective cohort study. Support Care Cancer. 2022;30:875–885. doi: 10.1007/s00520-021-06486-1. [DOI] [PubMed] [Google Scholar]
- 81.Wang B, Wang Y, Chen H, Yao S, Lai X, Qiu Y, Cai J, Huang Y, Wei X, Guan Y, et al. Inhibition of TGFβ improves hematopoietic stem cell niche and ameliorates cancer-related anemia. Stem Cell Res Ther. 2021;12:65. doi: 10.1186/s13287-020-02120-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang F, Yu WJ, Wang XH, Tang YT, Guo L, Jiao XY. Regulation of hepcidin through GDF-15 in cancer-related anemia. Clin Chim Acta. 2014;428:14–19. doi: 10.1016/j.cca.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 83.Balsano R, Kruize Z, Lunardi M, Comandatore A, Barone M, Cavazzoni A, Re Cecconi AD, Morelli L, Wilmink H, Tiseo M, et al. Transforming growth factor-beta signaling in cancer-induced cachexia: From molecular pathways to the clinics. Cells. 2022;11:2671. doi: 10.3390/cells11172671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu S, Ren J, Ten Dijke P. Targeting TGFβ signal transduction for cancer therapy. Signal Transduct Target Ther. 2021;6:8. doi: 10.1038/s41392-020-00436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Greco SH, Tomkötter L, Vahle AK, Rokosh R, Avanzi A, Mahmood SK, Deutsch M, Alothman S, Alqunaibit D, Ochi A, et al. TGF-β blockade reduces mortality and metabolic changes in a validated murine model of pancreatic cancer cachexia. PLoS One. 2015;10:e0132786. doi: 10.1371/journal.pone.0132786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Z, Jiang P, Liu F, Du X, Ma L, Ye S, Cao H, Sun P, Su N, Lin F, et al. GDF11 Regulates PC12 neural stem cells via ALK5-Dependent PI3K-Akt signaling pathway. Int J Mol Sci. 2022;23:12279. doi: 10.3390/ijms232012279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jones JE, Cadena SM, Gong C, Wang X, Chen Z, Wang SX, Vickers C, Chen H, Lach-Trifilieff E, Hadcock JR, Glass DJ. Supraphysiologic administration of GDF11 induces cachexia in part by upregulating GDF15. Cell Rep. 2018;22:1522–1530. doi: 10.1016/j.celrep.2018.01.044. [DOI] [PubMed] [Google Scholar]
- 88.Zimmers TA, Jiang Y, Wang M, Liang TW, Rupert JE, Au ED, Marino FE, Couch ME, Koniaris LG. Exogenous GDF11 induces cardiac and skeletal muscle dysfunction and wasting. Basic Res Cardiol. 2017;112:48. doi: 10.1007/s00395-017-0639-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ebner N, Anker SD, von Haehling S. Recent developments in the field of cachexia, sarcopenia, and muscle wasting: highlights from the 11th Cachexia Conference. J Cachexia Sarcopenia Muscle. 2019;10:218–225. doi: 10.1002/jcsm.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ebner N, Anker SD, von Haehling S. Recent developments in the field of cachexia, sarcopenia, and muscle wasting: Highlights from the 12th Cachexia Conference. J Cachexia Sarcopenia Muscle. 2020;11:274–285. doi: 10.1002/jcsm.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.da Fonseca GWP, Sato R, de Nazaré Nunes Alves MJ, von Haehling S. Current advancements in pharmacotherapy for cancer cachexia. Expert Opin Pharmacother. 2023;24:629–639. doi: 10.1080/14656566.2023.2194489. [DOI] [PubMed] [Google Scholar]
- 92.Crawford J, Calle RA, Collins SM, Weng Y, Lubaczewski SL, Buckeridge C, Wang EQ, Harrington MA, Tarachandani A, Rossulek MI, et al. CT108/16-First-in-patient study of the GDF-15 inhibitor ponsegromab in patients with cancer and cachexia: Safety, tolerability, and exploratory measures of efficacy. https://www.abstractsonline.com/pp8/#!/10828/presentation/10304 Proceedings of the 114th Annual Meeting of the American Association for Cancer Research. 2023:14–19. [Google Scholar]
- 93.Kim-Muller JY, Song L, LaCarubba Paulhus B, Pashos E, Li X, Rinaldi A, Joaquim S, Stansfield JC, Zhang J, Robertson A, et al. GDF15 neutralization restores muscle function and physical performance in a mouse model of cancer cachexia. Cell Rep. 2023;42:111947. doi: 10.1016/j.celrep.2022.111947. [DOI] [PubMed] [Google Scholar]
- 94.Vignjević Petrinović S, Jauković A, Milošević M, Bugarski D, Budeč M. Targeting stress erythropoiesis pathways in cancer. Front Physiol. 2022;13:844042. doi: 10.3389/fphys.2022.844042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.