Abstract
Objective
To systematically review and perform a meta-analysis of radiation associated risks of cardiovascular disease in all groups exposed to radiation with individual radiation dose estimates.
Design
Systematic review and meta-analysis.
Main outcome measures
Excess relative risk per unit dose (Gy), estimated by restricted maximum likelihood methods.
Data sources
PubMed and Medline, Embase, Scopus, Web of Science Core collection databases.
Eligibility criteria for selecting studies
Databases were searched on 6 October 2022, with no limits on date of publication or language. Animal studies and studies without an abstract were excluded.
Results
The meta-analysis yielded 93 relevant studies. Relative risk per Gy increased for all cardiovascular disease (excess relative risk per Gy of 0.11 (95% confidence interval 0.08 to 0.14)) and for the four major subtypes of cardiovascular disease (ischaemic heart disease, other heart disease, cerebrovascular disease, all other cardiovascular disease). However, interstudy heterogeneity was noted (P<0.05 for all endpoints except for other heart disease), possibly resulting from interstudy variation in unmeasured confounders or effect modifiers, which is markedly reduced if attention is restricted to higher quality studies or those at moderate doses (<0.5 Gy) or low dose rates (<5 mGy/h). For ischaemic heart disease and all cardiovascular disease, risks were larger per unit dose for lower dose (inverse dose effect) and for fractionated exposures (inverse dose fractionation effect). Population based excess absolute risks are estimated for a number of national populations (Canada, England and Wales, France, Germany, Japan, USA) and range from 2.33% per Gy (95% confidence interval 1.69% to 2.98%) for England and Wales to 3.66% per Gy (2.65% to 4.68%) for Germany, largely reflecting the underlying rates of cardiovascular disease mortality in these populations. Estimated risk of mortality from cardiovascular disease are generally dominated by cerebrovascular disease (around 0.94-1.26% per Gy), with the next largest contribution from ischaemic heart disease (around 0.30-1.20% per Gy).
Conclusions
Results provide evidence supporting a causal association between radiation exposure and cardiovascular disease at high dose, and to a lesser extent at low dose, with some indications of differences in risk between acute and chronic exposures, which require further investigation. The observed heterogeneity complicates a causal interpretation of these findings, although this heterogeneity is much reduced if only higher quality studies or those at moderate doses or low dose rates are considered. Studies are needed to assess in more detail modifications of radiation effect by lifestyle and medical risk factors.
Systematic review registration
PROSPERO CRD42020202036
Introduction
Cardiovascular diseases are the leading cause of death worldwide.1 2 Cardiovascular disease was the underlying cause of death for about a third of the 2.8 million deaths in the USA in 2018: ischaemic heart disease accounted for 42% and stroke for 17% of all cardiovascular disease deaths.3 Worldwide, ischaemic heart disease ranks first in years of life lost and stroke ranks third. Consistently identified independent risk factors include age, smoking, diabetes mellitus, hypertension, obesity, and increased total and low density lipoprotein or decreased high density lipoprotein cholesterol.4 5 6 A heritable genetic component for coronary heart disease has also been reported.7 8 9 10
Environmental factors might also contribute to cardiovascular disease risk and exposure to ionising radiation during radiotherapy can damage the heart.11 Radiotherapy doses to the heart and other organs or tissues of relevance to the cardiovascular system can be very high, with doses to some regions of the heart exceeding 40 Gy in previous years;12 although doses tend to be lower among groups treated for non-malignant disease than for cancer, and lower among people treated for cancer in more recent years.13 Many older studies of radiotherapy and cardiovascular disease do not have detailed individual radiation organ dosimetry,14 15 16 17 18 or data for concomitant chemotherapy drugs, of which some types (eg, vinca alkaloids including vincristine, and anthracyclines including doxorubicin) are cardiotoxic, irrespective of the administration of concomitant radiotherapy.17 Concomitant chemotherapy is often correlated with radiotherapy dose therefore confounding of the dose response is possible.
The Life Span Study of the Japanese atomic bomb survivors provides evidence of increased risk of cardiovascular disease at lower levels of dose, less than 5 Gy, and with mean doses of much less than 0.5 Gy.19 20 No findings suggested an appreciable non-linear association in the radiation dose-response for cardiovascular disease mortality in the Life Span Study data, although the form of the dose-response relation, particularly at doses less than 0.5 Gy, is uncertain.20 Therefore, the extent of cardiovascular disease risk is uncertain for low doses (<0.1 Gy), which are characteristic of doses from medical diagnostic exposures. Emerging, and still controversial, evidence suggests that exposure to much lower doses and dose rates of radiation, in particular occupational and medical diagnostic exposure,21 might be associated with excess risk of cardiovascular disease. Claims have been made of a no effect dose threshold for cardiovascular disease mortality in the Life Span Study, below which no radiation induced excess risk exists,22 although this finding has been disputed.23 Observational epidemiological studies are likely to have difficulty in detecting increased risk at low dose levels because the main types of cardiovascular disease of concern are very common in the population as a whole and because of the multiple contributory risk factors that are potentially confounding. The International Commission on Radiological Protection has classified cardiovascular disease as a tissue reaction (formerly termed a deterministic effect), with an approximate nominal threshold dose of 0.5 Gy independent of dose rate.24 This level is determined by linear models fitted to epidemiological data that yield less than a 1% lifetime risk. As such, this threshold is a practical one but is not a true no effect dose threshold.
In this systematic review and meta-analysis, we research the risks of radiation associated cardiovascular disease that have been observed in therapeutically or diagnostically exposed cohorts. Risks among groups exposed to generally lower levels of radiation dose (with maximum dose <0.5 Gy) or low dose rate (<5 mGy/h) are also assessed, specifically in the Life Span Study and in groups that are occupationally and environmentally exposed. Attention is concentrated on studies with informative individual organ dosimetry. In contrast to previous systematic reviews,21 25 26 which were published at least 10 years ago, we do not limit our inclusion to the lower dose literature; a previous review and meta-analysis covered literature up to about 2016, but the review was not systematic.27
Methods
Selection of studies
We conducted a systematic review and reported according to PRISMA and registered in PROSPERO (CRD42020202036) on 1 November 2020. Thereafter, we made a few small changes in the course of progress with the screening, which are detailed in PROSPERO. We used PubMed/Medline, Embase, Scopus, and Web of Science’s Core Collection to systematically search the literature, with no limits applied (date, language), on 6 October 2022. Cardiovascular disease is defined as those causes of mortality and incidence with International Classification of Diseases 10th revision (ICD-10) cardiovascular disease codes I00-I99 (or equivalently the ICD 8th revision codes 390-458 or ICD 9th revision (ICD-9) codes 390-459). We excluded animal studies and any study without an abstract. The database search was conducted by AL with input from MPL and NH, and yielded a total of 15 098 articles; these were loaded into Covidence by AL and then subjected to joint review by MPL and NH. In the first stage, we used only title and abstract to determine eligibility. Later stages of the search used much more complete information. Among other things, the reviewers (MPL, NH) independently ascertained if the organ dosimetry was adequate to estimate radiation risk and to determine if they were potentially informative on the desired outcome measure, ie, excess relative risk per unit dose. Two reviewers (MPL, KA) independently coded the information from the final 93 papers into a Microsoft Excel spreadsheet, and various semi-automated procedures were used to prepare the analytical database from these data; the coding was also checked by a third author (NH). Further details, including search criteria and dosimetry ascertainment, are given in supplement S1. Information coded included whether the exposure was chronic (ie, low dose rate exposure (<5 mGy/h)),28 or acute (ie, at dose rates above this level).
Classification of outcome measures
We used four major subtypes of cardiovascular disease determined a priori, which are more or less as used in an older meta-analysis,21 namely:
Ischaemic heart disease (ICD-9 410-414, ICD-10 I20-I25);
Heart disease apart from ischaemic heart disease (ICD-9 390-398, 402, 404, 415-429, ICD-10 I11, I13, I26-I52);
Cerebrovascular disease (ICD-9 430-438, ICD-10 I60-I69); and
All other types of cardiovascular disease (ICD-9 399-401, 403, 405-409, 439-459, ICD-10 I00-I10, I12, I14-I19, I53-59, I70-I99).
As well as these four outcome categories, we also sought information on cardiovascular disease overall. We map study endpoints to these four endpoint groups and to the endpoint for all cardiovascular disease in supplement S3 tables S3.1 and S3.2.
Statistical methods
The basis of all estimations of radiation risk is the value of excess relative risk per unit of effective dose (excess relative risk per Sv) or absorbed dose (excess relative risk per Gy) of radiation exposure. The excess relative risk (ERR) is related to the relative risk (RR) by ERR=RR-1, so that the excess relative risk per unit dose is ERR/D=(RR-1)/D where D represents dose. For absorbed dose, most publications use unweighted absorbed dose (Gy), but some use weighted absorbed dose (Gy) to account for the higher biological effectiveness of neutrons compared with photons (eg, in the Life Span Study20). The basis for this use in most studies is fitting of a model in which the disease or over death rate (cases or deaths per year) in the group with age a, sex s, organ/tissue absorbed dose D (in Gy) is given by: λ(a,s)×[1+αD] for some function λ(a,s) representing the disease/death rate without radiation exposure. The parameter α is the excess relative risk per Gy. We collected additional information on maximum radiation dose, maximum radiation dose rate, age at exposure (a grouped variable), mortality versus incidence for each study endpoint within each study. All survivors of Hodgkin’s lymphoma were deemed young adults for the purposes of analysis by age at exposure. All survivors of other cancers, except when these were treated in childhood, were assumed to be treated in adulthood.
An aggregate estimate of excess relative risk per Gy is computed across subsets of these studies by use of random effects models, using standard statistical methods (ie, meta-regression). In certain fits, adjustment was made for specific factors (ie, dose rate, level of maximum dose, mortality v morbidity, age group, disease endpoint), but in other fits, the main effect of the specific factor was assessed. Random effects models are fitted by restricted maximum likelihood because of the theoretically superior performance, in particular the absence of bias in the estimates of variance.29 30 Ordinary maximum likelihood fits were also used because these facilitate comparison of nested models; in particular, to test against improvement over the null (ie, lack of homogeneity of risk), where homogeneity of risk across categories is the assumed null hypothesis. Random effects models were also fitted by use of the one step approximation of DerSimonian and Laird.31 Residual heterogeneity was assessed using Cochran’s Q statistic. The I2 statistic of Higgins and Thompson32 was computed to assess the contribution of heterogeneity to the aggregate data. These results are expressed as a percentage, so that a value near 0% implies little estimated interstudy heterogeneity relative to the intrastudy variance, and values near 100% that the interstudy heterogeneity dominates the intrastudy variance.32 Confidence intervals on the I2 statistic were derived by use of the method of Knapp and Hartung.33
To assess selection or publication bias, funnel plots were used. Funnel plots are scatterplots of the central estimates (here, of excess relative risk) against estimates of standard error, and as discussed by Egger and colleagues,34 35 are useful qualitative means of detecting various types of selection bias, in particular publication bias. If the funnel plot has the form of an inverted symmetrical funnel, then selection bias is considered to be unlikely.34 35 More formal tests of selection or publication bias were also conducted using the test statistic suggested by Egger and colleagues. 34 We also used the trim-and-fill method of Duval and Tweedie36 to assess the likely extent of the change in excess relative risk that can result from selection bias.
The Risk Of Bias In Non-randomised Studies-of Interventions (ROBINS-I) framework37 was used to assess risk of bias associated with various characteristics of each study. A separate and objectively defined study quality score was also determined (supplement S1). Both scores were used to exclude lower quality studies.
All statistical models were fitted using the metafor package38 39 in R.40 Forest plots were prepared by use of the forestplot package41 in R.40 Results of the meta-analysis were based on the data given in supplement S5.
Estimates of population risks
We used pooled excess relative risk from the meta-analysis to derive population based excess absolute risk estimates according to underlying cause specific mortality rates for each population. Specifically, we used estimates for England and Wales for 200342 and 2021,43 2021 for Japan,44 2017 for France,45 2020 for Germany,46 2020 for USA,47 and 2005-09 for Canada.48 We assumed a five year minimum latency period, after which the excess relative risk was assumed to apply for the remainder of life. For all of these countries listed, we estimated the risk of exposure induced death per Sv, by applying methods previously used to derive comparable estimates for radiation induced cancer.49
Patient and public involvement statement
Two cancer survivors, Josh Mailman and Jacob Adams, both of whom received radiotherapy and diagnostic radiation doses, were consulted about the implications of the findings for diagnostic and therapeutic doses received. Plain language messages about the results will be shared with the appropriate offices at the National Cancer Institute that engage with members of the public (eg, via social media feeds) and advocacy groups. A link to the open access article will be posted on LinkedIn and ResearchGate. Some of the lead researchers (MPL, NH, LBZ) will also contact various advisory and regulatory bodies (International Commission on Radiological Protection, National Council on Radiation Protection and Measurements, United Nations Scientific Committee on the Effects of Atomic Radiation), which they are already part of. Organisations like the National Cancer Institute rely on publications like ours to inform their patient facing materials on health information websites such as cancer.gov.
Results
MPL and NH selected 194 articles from the second stage of the systematic review by consensus. These articles were subject to a more rigorous reading, and they were removed if uninformative or overlapped too much (for more details see supplement S1) with other studies, leaving 93 articles in the final selection (fig 1).
Fig 1.
PRISMA flow diagram showing exclusions made to derive the final set of studies used
We provide a detailed survey of the risks given in each study in supplement S3 tables S4-S6 and in supplement S4. A measure of concordance is noted between the magnitude of excess risk (excess relative risk per Gy) in many different types of study and in medically (therapeutically or diagnostically) exposed groups (supplement S3 table S3.4), or in people exposed to lower levels of radiation (supplement S3 table S3.5). A few therapeutic studies used alternative measures of dose rather than the canonical ones (supplement S3 table S3.6); these alternative dose metrics generally do not suggest very different magnitudes of risk.
Risk modifying factors
In the Life Span Study, excess relative risk associated with radiation for all cardiovascular disease decreases with increasing age at exposure21 and borderline significant trends decrease with attained age.20 21 However, for heart disease in this cohort, risk did not substantially vary by sex or time since exposure;20 21 no modifying effects were reported of sex, age at exposure, or attained age.50 Trends of increasing risk with increasing time since exposure were observed for some non-ischaemic heart disease endpoints in the Life Span Study50 and for heart disease in UK nuclear workers51 (although not for cerebrovascular disease in this group52), and for all cardiovascular disease in the International Agency for Research on Cancer 15-country study.53 However, decreasing trends (for all cardiovascular disease) were documented in people given diagnostic x ray exposures as part of the treatment for tuberculosis54 and in a cohort of people treated for peptic ulcers.13 Patients with peptic ulcers also had slightly (albeit not significantly) lower excess relative risk for women than for men for all cardiovascular disease, ischaemic heart disease, and cardiovascular disease excluding ischaemic heart disease and cerebrovascular disease. However, excess relative risk for women was higher (but not significantly so) than that for men for cerebrovascular disease in this cohort.13 In the INWORKS study, women had significantly higher excess relative risk than did men.55 Age at treatment in the Nordic study56 had no effect but radiation risk with increasing age at treatment had a borderline significant reduction in a Dutch study of breast cancer survivors.57
Few studies assessed the possible modifying effect of lifestyle, medical, and environmental variables, particularly the major risk factors for cardiovascular disease (eg, smoking, obesity, diabetes, hypertension, and hypercholesterolaemia). The Nordic and Dutch studies on breast cancer,56 57 58 and the three Dutch studies on Hodgkin lymphoma,59 60 61 all of which have particularly rich data of this sort (supplement S3 table S3.4), found little evidence of modification of radiation dose response for various heart related endpoints for any of these variables. No modifying effects were reported of smoking or alcohol consumption in a cohort with peptic ulcers.13 Recorded data for lifestyle is extensive in the Life Span Study study20 (supplement S3 table S3.5) but no assessment was made of possible modifications in radiation dose-response associated with any of these risk factors. A case-control study of industrial workers at two UK nuclear plants (Sellafield and Springfield) collected information on numerous lifestyle and environmental risk factors (ie, body mass index, smoking status, diastolic and systolic blood pressure, shift work),62 as did a case-control study of French nuclear fuel cycle workers (supplement S3 table S3.5).63 Neither study assessed modifying effects on the radiation dose response, possibly because adjustments to the background risk did not change risk estimates. Information about lifestyle and environmental risk factors is available from the workers from the Mayak nuclear facility in Russia (supplement S3 table S3.5); however, analyses did not report modifying effects of these variables on the dose-response, again, possibly because adjustments to the baseline risk had little effect.64 65 66 67 68
Only modest information is available of the modifying effects of cardiotoxic treatment on radiation response. No modifying effects were reported in the Nordic and Dutch breast cancer case-control studies,56 57 58 nor were such modifications indicated in Dutch Hodgkin lymphoma studies.59 60 61
Results of meta-analysis
Table 1, table 2, table 3, table 4, table 5, and figure 2 report the results of the meta-analysis. We found (table 3) that radiation exposure was associated with a generally significant meta-excess relative risk per Gy for all cardiovascular disease (0.11 (95% confidence interval 0.08 to 0.14)), ischaemic heart disease (0.07 (0.05 to 0.10)), other heart disease (0.03 (0.02 to 0.05)), cerebrovascular disease (0.19 (0.09 to 0.28)), and other cardiovascular disease (0.17 (−0.03 to 0.37)). Meta excess relative risk per Gy varied significantly between subtypes of disease (table 2). For all cardiovascular disease, a significant meta excess relative risk per Gy was also noted for all levels of maximum radiation dose, even for lower-dose exposures with maximum exposure of 0.5 Gy or less (0.45 per Gy (95% confidence interval 0.06 to 0.84)), and if combined with low dose rate studies, this was also the case for ischaemic heart disease (0.20 (95% confidence interval 0.09 to 0.32); table 3). Significant heterogeneity was noted in relation to maximum radiation dose (P=0.001), with cardiovascular disease risk higher when maximum radiation dose was at 0.5 Gy or less, 0.5-1 Gy, and 1-5 Gy than at more than 5 Gy (table 2). We also observed significant difference in meta excess relative risk per Gy by radiation dose rate (P<0.001), with risk appreciably higher for low dose rate exposure. Table 2 also suggests that risks are significantly higher (P=0.002) for mortality endpoints compared with those of incidence.
Table 1.
Studies considered in systematic review and meta-analysis
| Endpoint | No. of endpoints within studies | No. of studies |
|---|---|---|
| All studies | ||
| Ischaemic heart disease | 38 | 38 |
| Other heart | 27 | 16 |
| Cerebrovascular disease | 32 | 31 |
| Other cardiovascular disease | 12 | 10 |
| All cardiovascular disease* | 105 | 86 |
| All studies/endpoints† | 157 | 93 |
| Studies with mean bias score ≥4 | ||
| Ischaemic heart disease | 17 | 17 |
| Other heart | 13 | 7 |
| Cerebrovascular disease | 14 | 14 |
| Other cardiovascular disease | 8 | 7 |
| All cardiovascular disease* | 48 | 34 |
| All studies/endpoints† | 75 | 34 |
| Studies with mean quality score ≥4 | ||
| Ischaemic heart disease | 4 | 4 |
| Other heart | 1 | 1 |
| Cerebrovascular disease | 4 | 4 |
| Other cardiovascular disease | 4 | 4 |
| All cardiovascular disease* | 10 | 9 |
| All studies/endpoints† | 15 | 9 |
Considering maximal set of all non-overlapping endpoints within a study.
Considering maximal set of all non-overlapping endpoints within a study, as well as all non-overlapping endpoints within each of the four specific cardiovascular disease subtypes (ischaemic heart disease, other heart, cerebrovascular disease, other cardiovascular disease).
Table 2.
Meta-analysis of excess relative risk for cardiovascular diseases as a result of radiation exposure, by disease endpoint
| No. of study endpoints | Meta excess relative risk/Gy (95% CI) | P value for heterogeneity | P value residual heterogeneity | I2 (%) (Hartung-Knapp 95% CI) | |
|---|---|---|---|---|---|
| Adjusting for endpoint | |||||
| Ischaemic heart disease | 38 | 0.110 (0.053 to 0.167) | 0.02 | <0.001 | 80.00 (73.04 to 93.19) |
| Other heart disease * | 27 | 0.054 (–0.006 to 0.113) | — | — | — |
| Cerebrovascular disease | 32 | 0.176 (0.109 to 0.244) | — | — | — |
| Other cardiovascular disease † | 12 | 0.183 (0.074 to 0.292) | — | — | — |
| Adjusting for radiation type | |||||
| Low dose rate ‡ | 55 | 0.223 (0.157 to 0.290) | <0.001 | <0.001 | 76.97 (70.31 to 93.07) |
| Acute moderate/high dose rate § | 12 | 0.143 (0.063 to 0.223) | — | — | — |
| Acute fractionated moderate/high dose rate ¶ | 42 | 0.068 (0.030 to 0.106) | — | — | — |
| Adjusting for maximum dose | |||||
| Maximum dose ≤0.5 Gy | 16 | 0.311 (0.081 to 0.540) | 0.001 | <0.001 | 70.42 (60.84 to 92.14) |
| Maximum dose >0.5 Gy to ≤ 1 Gy | 9 | 0.284 (0.071 to 0.497) | — | — | — |
| Maximum dose >1 Gy to ≤5 Gy | 23 | 0.159 (0.097 to 0.221) | — | — | — |
| Maximum dose >5 Gy | 53 | 0.064 (0.032 to 0.096) | — | — | — |
| Adjusting for incidence v mortality | |||||
| Mortality | 55 | 0.199 (0.134 to 0.264) | 0.002 | <0.001 | 82.17 (74.77 to 93.32) |
| Incidence | 54 | 0.086 (0.047 to 0.125) | — | — | — |
| Adjusting for age at exposure group | |||||
| Childhood and in utero | 18 | 0.083 (0.012 to 0.154) | 0.23 | <0.001 | 84.30 (77.54 to 93.97) |
| Young adult | 4 | 0.059 (–0.079 to 0.197) | — | — | — |
| Older adult and all ages | 87 | 0.138 (0.095 to 0.182) | — | — | — |
| Endpoint analysis (simultaneously adjusted for low dose rate, dose ≤0.5 Gy, mortality) | |||||
| Ischaemic heart disease | 34 | 0.131 (–0.019 to 0.281) | <0.001 | <0.001 | 55.16 (46.74 to 91.49) |
| Other heart disease * | 23 | 0.121 (–0.034 to 0.277) | — | — | — |
| Cerebrovascular disease | 32 | 0.170 (0.019 to 0.321) | — | — | — |
| Other cardiovascular disease† | 12 | 0.203 (0.081 to 0.325) | — | — | — |
All four main endpoints are analysed together. Values used in the analysis are from supplement S3 tables S3.4-S3.5. Random effects models are fitted via restricted maximum likelihood. CI=confidence interval; acute=all people exposed at moderate or high dose rate (discussed further in the methods); young adult=all survivors of Hodgkin lymphoma (discussed further in the methods).
Heart disease other than ischaemic heart disease.
Cardiovascular disease other than heart disease and cerebrovascular disease.
Maximum dose rate <5 mGy/h.
Maximum dose rate ≥5 mGy/h given in single acute dose.
Maximum dose rate ≥5 mGy/h given in multiple fractions.
Table 3.
Meta regression analyses of excess relative risk for each major cardiovascular disease endpoint group, with restriction by dose and dose rate
| Cardiovascular disease endpoint | No. of study endpoints | Meta excess relative risk/Gy (95% CI) | Residual heterogeneity P value | I2 (%) (Knapp-Hartung 95% CI) | Egger selection test P value | Duval-Tweedie trim-fill selection bias corrected meta excess relative risk/Gy (95% CI) |
|---|---|---|---|---|---|---|
| Full data | ||||||
| Ischaemic heart disease | 38 | 0.073 (0.047 to 0.099) | 0.01 | 17.80 (3.52 to 95.64) | 0.002 | 0.073 (0.052 to 0.095) |
| Other heart disease* | 27 | 0.034 (0.020 to 0.049) | 0.49 | 0.00 (0.00 to 84.81) | 0.13 | 0.034 (0.021 to 0.048) |
| Cerebrovascular disease | 32 | 0.188 (0.093 to 0.283) | <0.001 | 83.56 (68.52 to 99.22) | 0.05 | 0.184 (0.099 to 0.269) |
| Other cardiovascular disease† | 12 | 0.172 (–0.029 to 0.373) | <0.001 | 90.27 (68.02 to 98.43) | 0.49 | 0.172 (–0.029 to 0.373) |
| All cardiovascular disease (using maximal cardiovascular disease data per study) | 105 | 0.106 (0.076 to 0.135) | <0.001 | 88.61 (85.58 to 95.99) | <0.001 | 0.102 (0.075 to 0.129) |
| Maximum dose ≤0.5 Gy only | ||||||
| Ischaemic heart disease | 6 | 0.438 (–0.131 to 1.007) | 0.17 | 21.89 (0.00 to 99.74) | 0.68 | 0.438 (–0.131 to 1.007) |
| Other heart disease* | 2 | –0.108 (–0.528 to 0.313) | 0.76 | 0.00 (0.00 to 68.13) | 0.39 | –0.188 (–0.617 to 0.242) |
| Cerebrovascular disease | 7 | 0.542 (–0.281 to 1.366) | 0.24 | 0.00 (0.00 to >97.54) | 0.79 | 0.542 (–0.281 to 1.366) |
| Other cardiovascular disease† | 1 | –1.80 (–11.95 to 8.35) | NA | NA | NA | NA |
| All cardiovascular disease (using maximal cardiovascular disease data per study) | 9 | 0.452 (0.064 to 0.840) | 0.36 | 17.88 (0.00 to 98.38) | 0.26 | 0.452 (0.092 to 0.811) |
| Low dose rate data only | ||||||
| Ischaemic heart disease | 22 | 0.202 (0.085 to 0.319) | 0.13 | 45.34 (0.00 to 86.89) | 0.86 | 0.202 (0.085 to 0.319) |
| Other heart disease* | 6 | –0.207 (–0.456 to 0.042) | 0.98 | 0.00 (0 to >0) | 0.55 | –0.241 (–0.727 to 0.246) |
| Cerebrovascular disease | 21 | 0.298 (0.101 to 0.495) | <0.001 | 61.07 (29.38 to 99.94) | 0.18 | 0.294 (0.130 to 0.458) |
| Other cardiovascular disease† | 6 | 0.166 (–0.069 to 0.401) | 0.17 | 39.95 (0.00 to 99.58) | 0.79 | 0.166 (–0.069 to 0.401) |
| All cardiovascular disease (using maximal cardiovascular disease data per study) | 41 | 0.229 (0.136 to 0.322) | <0.001 | 68.23 (31.40 to 92.81) | 0.09 | 0.224 (0.134 to 0.315) |
| Maximum dose ≤0.5 Gy or low dose rate only | ||||||
| Ischaemic heart disease | 24 | 0.205 (0.092 to 0.318) | 0.07 | 39.39 (0.00 to 93.39) | 0.31 | 0.205 (0.092 to 0.318) |
| Other heart disease* | 8 | –0.168 (–0.429 to 0.094) | 0.90 | 0.00 (0.00 to 55.12) | 0.29 | –0.233 (–0.567 to 0.101) |
| Cerebrovascular disease | 23 | 0.306 (0.127 to 0.485) | <0.001 | 57.05 (22.37 to 99.87) | 0.17 | 0.303 (0.148 to 0.458) |
| Other cardiovascular disease† | 6 | 0.166 (–0.069 to 0.401) | 0.17 | 39.95 (0.00 to 99.58) | 0.79 | 0.166 (–0.069 to 0.401) |
| All cardiovascular disease (using maximal cardiovascular disease data per study) | 44 | 0.231 (0.141 to 0.320) | <0.001 | 65.03 (31.27 to 94.25) | 0.04 | 0.226 (0.141 to 0.311) |
All four main endpoints and all cardiovascular disease are analysed separately. Values used in the analysis are from supplement S3 tables S3.4-S3.5. Random effects models are fitted via restricted maximum likelihood. CI=confidence interval; NA=not available.
Heart disease other than ischaemic heart disease.
Cardiovascular disease other than heart disease and cerebrovascular disease.
Table 4.
Meta-analysis of higher quality estimates of excess relative risk for cardiovascular diseases as a result of radiation exposure, by disease endpoint. All four main endpoints are analysed together
| No of study endpoints | Excess relative risk (meta excess relative risk/Gy) (95% CI) | P value for heterogeneity | P value residual heterogeneity | I2 (%) (Hartung-Knapp 95% CI) | |
|---|---|---|---|---|---|
| Analysis using mean bias score ≥4 | |||||
| Adjusting for endpoint: | |||||
| Ischaemic heart disease | 17 | 0.132 (0.027 to 0.237) | 0.50 | <0.001 | 72.16 (40.34 to 85.62) |
| Other heart disease* | 13 | 0.117 (–0.032 to 0.266) | — | — | — |
| Cerebrovascular disease | 14 | 0.236 (0.102 to 0.370) | — | — | — |
| Other cardiovascular disease† | 8 | 0.208 (0.057 to 0.359) | — | — | — |
| Adjusting for radiation type: | |||||
| Low dose rate‡ | 26 | 0.250 (0.132 to 0.369) | 0.25 | <0.001 | 72.92 (41.84 to 86.38) |
| Acute moderate/high dose rate§ | 12 | 0.148 (0.048 to 0.248) | — | — | — |
| Acute fractionated moderate/high dose rate¶ | 14 | 0.117 (0.005 to 0.229) | — | — | — |
| Adjusting for maximum dose: | |||||
| Maximum dose ≤0.5 Gy | 13 | 0.303 (0.067 to 0.538) | 0.55 | <0.001 | 72.97 (36.47 to 87.89) |
| Maximum dose >0.5 Gy, ≤1 Gy | 4 | 0.158 (–0.163 to 0.479) | — | — | — |
| Maximum dose >1 Gy, ≤5 Gy | 18 | 0.149 (0.059 to 0.239) | — | — | — |
| Maximum dose >5 Gy | 11 | 0.106 (–0.026 to 0.238) | — | — | — |
| Adjusting for incidence v mortality: | |||||
| Mortality | 34 | 0.214 (0.120 to 0.308) | 0.20 | <0.001 | 76.10 (47.80 to 86.95) |
| Incidence | 18 | 0.129 (0.042 to 0.215) | — | — | — |
| Adjusting for age at exposure group | |||||
| Childhood and in utero | 5 | 0.140 (–0.064 to 0.344) | 0.94 | <0.001 | 77.57 (50.29 to 88.28) |
| Young adult | 1 | 0.141 (–0.417 to 0.699) | — | — | — |
| Older adult and all age | 46 | 0.172 (0.102 to 0.243) | — | — | — |
| Endpoint analysis (simultaneously adjusted for low dose rate, dose ≤0.5 Gy, mortality) | |||||
| Ischaemic heart disease | 15 | 0.397 (0.047 to 0.747) | 0.16 | <0.001 | 56.57 (12.64 to 81.54) |
| Other heart disease* | 9 | 0.474 (0.057 to 0.891) | — | — | — |
| Cerebrovascular disease | 14 | 0.539 (0.157 to 0.921) | — | — | — |
| Other cardiovascular disease† | 8 | 0.595 (0.175 to 1.015) | — | — | — |
| Analysis using mean quality score ≥4 | |||||
| Adjusting for endpoint: | |||||
| Ischaemic heart disease | 4 | 0.109 (–0.236 to 0.453) | 0.47 | <0.001 | 92.72 (63.27 to 98.79) |
| Other heart disease* | 1 | 0.038 (–0.451 to 0.526) | — | — | — |
| Cerebrovascular disease | 4 | 0.290 (–0.057 to 0.636) | — | — | — |
| Other cardiovascular disease† | 4 | 0.270 (–0.036 to 0.575) | — | — | — |
| Adjusting for radiation type | |||||
| Low dose rate‡ | 8 | 0.299 (0.039 to 0.558) | 0.36 | <0.001 | 91.15 (65.49 to 97.93) |
| Acute high dose rate§ | 3 | 0.211 (–0.060 to 0.482) | — | — | — |
| Acute fractionated high dose rate¶ | 2 | 0.056 (–0.253 to 0.365) | — | — | — |
| Adjusting for maximum dose | |||||
| Maximum dose ≤0.5 Gy | 5 | 0.364 (–8.955 to 9.684) | 0.72 | <0.001 | 93.23 (63.08 to 99.98) |
| Maximum dose >0.5 Gy, ≤ 1 Gy | 0 | NA | — | — | — |
| Maximum dose >1 Gy, ≤ 5 Gy | 3 | 0.203 (–0.138 to 0.544) | — | — | — |
| Maximum dose >5 Gy | 4 | 0.056 (–0.340 to 0.452) | — | — | — |
| Adjusting for incidence v mortality | |||||
| Mortality | 7 | 0.342 (0.043 to 0.641) | 0.26 | <0.001 | 92.34 (73.54 to 97.75) |
| Incidence | 6 | 0.154 (–0.023 to 0.332) | — | — | — |
| Adjusting for age at exposure group | |||||
| Childhood and in utero | 0 | NA | 0.35 | <0.001 | 92.50 (73.22 to 97.93) |
| Young adult | 1 | 0.038 (–0.390 to 0.466) | — | — | — |
| Older adult and all age | 12 | 0.229 (0.060 to 0.397) | — | — | — |
| Endpoint analysis (simultaneously adjusted for low dose rate, dose ≤0.5 Gy, mortality) | |||||
| Ischaemic heart disease | 3 | 6.84 (–19.03 to 32.72) | 0.003 | 0.67 | 0.00 (0.00 to >27.08) |
| Other heart disease* | 1 | 6.81 (–19.07 to 32.68) | — | — | — |
| Cerebrovascular disease | 4 | –1.36 (–12.85 to 10.14) | — | — | — |
| Other cardiovascular disease† | 4 | –0.90 (–12.39 to 10.60) | — | — | — |
Values for the analysis are from supplement S3 tables S3.4-S3.5. Analysis is restricted to studies with mean bias score ≥4 or mean quality score ≥4 (as given in supplement S3 tables S3.1, S3.2). Random effects models are fitted via restricted maximum likelihood. CI=confidence interval. NA=not available.
Heart disease other than ischaemic heart disease.
Cardiovascular disease other than heart disease and cerebrovascular disease.
Maximum dose rate <5 mGy/h.
Maximum dose rate ≥5 mGy/h given in single acute dose.
Maximum dose rate ≥5 mGy/h given in multiple fractions.
Table 5.
Meta regression analyses of higher quality estimates of excess relative risk for each major cardiovascular disease endpoint group, with restriction by dose and dose rate
| No. of study endpoints | Excess relative risk (meta excess relative risk/Gy) (95% CI) | Residual heterogeneity P value | I2 (%) (Knapp-Hartung 95% CI) | Egger selection test P value | Duval-Tweedie trim-fill selection bias corrected meta excess relative risk/Gy (95% CI) | |
|---|---|---|---|---|---|---|
| Analysis using mean bias score ≥4 | ||||||
| Full data: | ||||||
| Ischaemic heart disease | 17 | 0.099 (0.059 to 0.139) | 0.17 | 0.37 (0.00 to 98.72) | 0.13 | 0.099 (0.066 to 0.132) |
| Other heart disease* | 13 | 0.108 (–0.007 to 0.224) | 0.25 | 36.00 (0.00 to 74.22) | 0.56 | 0.108 (–0.007 to 0.224) |
| Cerebrovascular disease | 14 | 0.237 (0.096 to 0.378) | <0.001 | 72.86 (21.64 to 94.48) | 0.15 | 0.214 (0.074 to 0.353) |
| Other cardiovascular disease† | 8 | 0.203 (–0.067 to 0.474) | <0.001 | 86.31 (56.18 to 98.15) | 0.82 | 0.203 (–0.067 to 0.474) |
| All cardiovascular disease (using maximal cardiovascular disease data per study) | 48 | 0.148 (0.087 to 0.209) | <0.001 | 85.19 (72.47 to 94.61) | 0.007 | 0.136 (0.077 to 0.195) |
| Maximum dose ≤0.5 Gy or low dose rate: | ||||||
| Ischaemic heart disease | 12 | 0.153 (0.080 to 0.227) | 0.23 | 0.00 (0.00 to 99.53) | 0.28 | 0.153 (0.095 to 0.211) |
| Other heart disease* | 7 | –0.107 (–0.471 to 0.257) | 0.85 | 0.00 (0.00 to 45.34) | 0.38 | –0.214 (–0.637 to 0.209) |
| Cerebrovascular disease | 11 | 0.389 (0.191 to 0.588) | 0.52 | 14.48 (0.00 to 81.22) | 0.29 | 0.377 (0.171 to 0.584) |
| Other cardiovascular disease† | 2 | 0.299 (–0.226 to 0.825) | 0.69 | 0.00 (0.00 to >88.17) | NA | NA |
| All cardiovascular disease (using maximal cardiovascular disease data per study) | 22 | 0.214 (0.090 to 0.339) | <0.001 | 72.09 (34.23 to 96.55) | 0.09 | 0.211 (0.095 to 0.327) |
| Analysis using mean quality score ≥4 | ||||||
| Full data: | ||||||
| Ischaemic heart disease | 4 | 0.105 (0.025 to 0.186) | 0.39 | 27.16 (0.00 to >99.99) | 0.63 | 0.105 (0.041 to 0.170) |
| Other heart disease* | 1 | 0.038 (–0.035 to 0.111) | NA | NA | NA | 0.108 (–0.007 to 0.224) |
| Cerebrovascular disease | 4 | 0.289 (–0.147 to 0.726) | <0.001 | 91.14 (48.40 to >99.99) | 0.50 | 0.288 (–0.045 to 0.622) |
| Other cardiovascular disease† | 4 | 0.254 (–0.326 to 0.835) | <0.001 | 88.16 (47.23 to 99.52) | 0.56 | 0.258 (–0.157 to 0.673) |
| All cardiovascular disease (using maximal cardiovascular disease data per study) | 10 | 0.204 (0.039 to 0.369) | <0.001 | 94.71 (84.55 to 98.28) | 0.97 | 0.204 (0.039 to 0.369) |
| Maximum dose ≤0.5 Gy or low dose rate: | ||||||
| Ischaemic heart disease | 3 | 0.140 (0.050 to 0.230) | 0.67 | 0.00 (0.00 to >43.68) | 0.65 | 0.140 (0.050 to 0.230) |
| Other heart disease* | 0 | NA | NA | NA | NA | NA |
| Cerebrovascular disease | 3 | 0.460 (0.317 to 0.603) | 0.62 | 0.00 (0.00 to >16.79) | 0.52 | 0.460 (0.317 to 0.603) |
| Other cardiovascular disease† | 2 | 0.299 (–0.226 to 0.825) | 0.69 | 0.00 (0.00 to >88.17) | NA | NA |
| All cardiovascular disease (using maximal cardiovascular disease data per study) | 5 | 0.298 (0.105 to 0.491) | <0.001 | 82.50 (34.55 to 95.70) | 0.78 | 0.297 (0.101 to 0.494) |
All four main endpoints and all cardiovascular disease are analysed separately. Analysis is restricted to studies with mean bias score ≥4 or mean quality score ≥4 (as given in Supplement S3 tables S3.1, S3.2). Random effects models are fitted via restricted maximum likelihood. CI=confidence interval. NA=not available.
Heart disease other than ischaemic heart disease.
Cardiovascular disease other than heart disease and cerebrovascular disease.
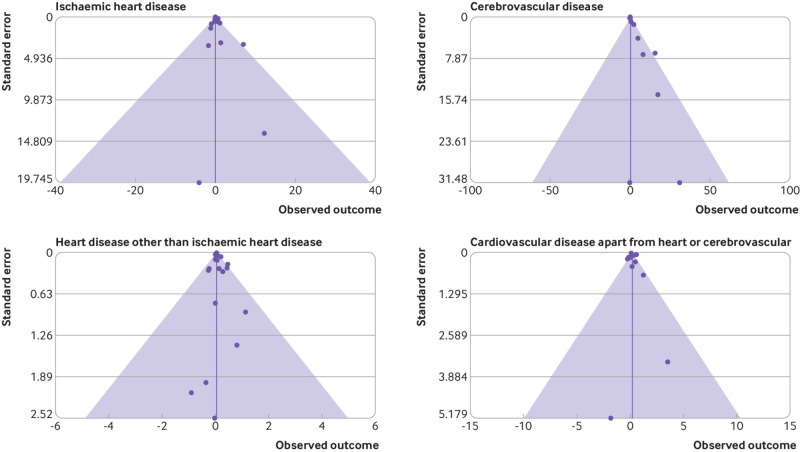
Fig 2.
Funnel plot of risks by four major cardiovascular disease endpoints. A study without appreciable selection bias should have a more or less balanced funnel plot, with points spread more or less equally to left and right of the vertical axis of the funnel
Table 3 suggests that for ischaemic heart disease, cerebrovascular disease, and all cardiovascular disease, meta excess relative risk per Gy was significantly elevated for low dose rate data or low dose rate combined with studies in which maximum dose was 0.5 Gy or less. For these three endpoints, the meta excess relative risk per Gy are higher for maximum dose under 0.5 Gy or for low dose rate (separately or together), and the residual heterogeneity (as measured by the I2 statistic) tended also to be lower.
The funnel plots given in figure 2 do not suggest any material selection or publication bias, and more formal tests of selection suggest presence of such bias for only a few endpoints (eg, ischaemic heart disease using all data (table 3)). The Duval-Tweedie trim-and-fill bias corrected meta excess relative risk per Gy and confidence interval generally differ little from the uncorrected estimates (table 3). Interstudy heterogeneity is substantial, particularly for cerebrovascular disease and all cardiovascular disease, with values of the I2 statistic generally above 50%.
When analysis was restricted to higher quality studies, with a mean bias score of at least 4 or a mean study quality score of at least 4, results were similar (table 4, table 5). A notable feature of the higher quality studies is the weakening evidence of interstudy heterogeneity, which remains significant only (in some cases) for cerebrovascular disease, all other cardiovascular disease, and all cardiovascular disease (table 5). However, with higher quality studies, only the meta excess relative risk per Gy for all cardiovascular disease and the subtypes ischaemic heart disease and cerebrovascular disease generally remained statistically significant; and heterogeneity by maximum dose, dose rate, and mortality versus incidence are no longer significant (P>0.2) (table 4, table 5). Nevertheless, indications suggest that risk is higher at lower doses, and at a lower dose rate (table 4, table 5). We saw a slight tendency for meta excess relative risk per Gy to be higher (although also more uncertain) among higher quality studies, for all four main endpoints (supplement S3 figures S3.1, S3.2).
The alternative fitting methods (maximum likelihood, DerSimonian-Laird one step) yielded very similar estimates and confidence intervals as those of restricted maximum likelihood, the default method used (supplement S3 table S3.3).
Sensitivity analyses in which we excluded Mayak morbidity data, Mayak mortality data or the Canadian data of Zielinski and colleagues69, or in which we added the Los Alamos70 and Rochester thymus71 data to the analysis did not suggest large changes. The most substantial change resulted from removal of the Mayak incidence data, when the meta excess relative risk per Gy for all cardiovascular disease reduced from 0.11 (95% confidence interval 0.08 to 0.14) to 0.09 (95% confidence interval 0.06 to 0.11), but hardly changed when the Mayak mortality data or the Canadian data were removed, or the Los Alamos and Rochester cohorts were added (supplement S3 table S3.7).
Population risks
Population based excess absolute risk estimates for radiation exposure induced death for all cardiovascular disease range from 2.33% per Gy (95% confidence interval 1.69% to 2.38%) for England and Wales (using 2021 rates) to 3.66% per Gy (2.65% to 4.68%) for Germany, largely reflecting the underlying risk of cardiovascular disease mortality (table 6). Estimated mortality risks of cardiovascular disease are generally dominated by cerebrovascular disease, with the next largest contribution from ischaemic heart disease (table 6). If the 2003 England and Wales rates are used, the excess absolute risk is appreciably higher, 3.94% per Gy (2.85% to 5.03%), reflecting the much higher proportion of deaths due to cardiovascular disease in this earlier population (39.88% v 23.57%). Years of life lost per Gy range from 0.190 (0.137 to 0.243) for Japan to 0.373 (0.270 to 0.477) for USA. The markedly higher figures for years of life lost per death induced by radiation for USA compared with other populations are largely artefactual because US mortality rates are not published for any age group older than 80-84 years. Therefore, mortality rates at older ages, which very steeply increase for all causes as well as cardiovascular disease in all other populations, must be assumed to be those given by this age group. If, for example, the data for the 85-89 and 90 and older year groups for the 2021 population in England and Wales were removed, so that effectively rates at ages older than 80 years simply reflected those for ages 80-84, then all circulatory risk of exposure induced death would decrease to 2.08% per Gy (1.51% to 2.66%). Years of life lost per Gy would markedly increase to 0.311 per Gy (0.225 to 0.398), so that years life lost per radiation induced death, which is simply the quotient of these (years of life lost/risk of exposure induced death), would nearly double, to 14.927 (14.925 to 14.929). This value is quite close to the USA figure of 14.746 (14.743 to 14.748). This highlights the effect of age groups older than 80 years on population risks.
Table 6.
Estimated excess absolute risk of radiation exposure induced death (% per Gy), years life lost per Gy, and years life lost per radiation induced death, and 95% confidence intervals for various subtypes of cardiovascular disease by country, using subtype specific restricted maximum likelihood estimates for all data from table 3
| Cardiovascular disease endpoint | Canada (2005-09 rates) | England and Wales (2021 rates) | England and Wales (2003 rates) | France (2017 rates) | Germany (2020 rates) | Japan (2021 rates) | USA (2020 rates) |
|---|---|---|---|---|---|---|---|
| Baseline proportion of deaths due to cardiovascular disease (%) | 33.97 | 23.57 | 39.88 | 26.45 | 37.00 | 25.35 | 25.57 |
| Risk of exposure induced deaths×10–2: | |||||||
| Ischaemic heart disease | 1.20 (0.77 to 1.62) | 0.64 (0.41 to 0.87) | 1.30 (0.84 to 1.75) | 0.39 (0.25 to 0.53) | 0.89 (0.58 to 1.21) | 0.30 (0.19 to 0.40) | 0.73 (0.48 to 0.99) |
| Other heart disease | 0.21 (0.13 to 0.30) | 0.21 (0.12 to 0.29) | 0.18 (0.11 to 0.25) | 0.37 (0.22 to 0.52) | 0.27 (0.16 to 0.38) | 0.37 (0.22 to 0.52) | 0.26 (0.15 to 0.37) |
| Cerebrovascular disease | 1.25 (0.62 to 1.88) | 0.94 (0.46 to 1.41) | 2.04 (1.01 to 3.07) | 0.99 (0.49 to 1.50) | 1.00 (0.49 to 1.51) | 1.26 (0.62 to 1.90) | 0.83 (0.41 to 1.25) |
| Other cardiovascular disease | 0.47 (–0.08 to 1.02) | 0.41 (–0.07 to 0.88) | 0.61 (–0.10 to 1.31) | 0.61 (–0.10 to 1.32) | 1.61 (–0.27 to 3.49) | 0.42 (–0.07 to 0.90) | 0.33 (–0.06 to 0.73) |
| All cardiovascular disease | 3.37 (2.43 to 4.30) | 2.33 (1.69 to 2.98) | 3.94 (2.85 to 5.03) | 2.63 (1.90 to 3.35) | 3.66 (2.65 to 4.68) | 2.52 (1.82 to 3.22) | 2.53 (1.83 to 3.23) |
| Years of life lost per Gy: | |||||||
| Ischaemic heart disease | 0.101 (0.065 to 0.136) | 0.061 (0.040 to 0.083) | 0.112 (0.072 to 0.151) | 0.032 (0.021 to 0.044) | 0.077 (0.050 to 0.105) | 0.027 (0.018 to 0.037) | 0.108 (0.070 to 0.146) |
| Other heart disease | 0.016 (0.010 to 0.023) | 0.016 (0.009 to 0.023) | 0.013 (0.008 to 0.019) | 0.024 (0.014 to 0.034) | 0.021 (0.012 to 0.030) | 0.024 (0.014 to 0.033) | 0.039 (0.023 to 0.055) |
| Cerebrovascular disease | 0.097 (0.048 to 0.145) | 0.075 (0.037 to 0.112) | 0.148 (0.073 to 0.223) | 0.075 (0.037 to 0.113) | 0.086 (0.043 to 0.130) | 0.101 (0.050 to 0.152) | 0.118 (0.058 to 0.178) |
| Other cardiovascular disease | 0.038 (–0.006 to 0.082) | 0.035 (–0.006 to 0.075) | 0.051 (–0.009 to 0.111) | 0.048 (–0.008 to 0.105) | 0.131 (–0.022 to 0.283) | 0.035 (–0.006 to 0.075) | 0.051 (-0.008 to 0.110) |
| All cardiovascular disease | 0.273 (0.197 to 0.349) | 0.201 (0.145 to 0.256) | 0.317 (0.229 to 0.406) | 0.191 (0.138 to 0.245) | 0.305 (0.221 to 0.390) | 0.190 (0.137 to 0.243) | 0.373 (0.270 to 0.477) |
| Years of life lost per radiation induced death: | |||||||
| Ischaemic heart disease | 8.419 (8.418 to 8.421) | 9.557 (9.556 to 9.558) | 8.613 (8.612 to 8.615) | 8.220 (8.220 to 8.221) | 8.668 (8.667 to 8.669) | 9.238 (9.238 to 9.238) | 14.719 (14.718 to 14.720) |
| Other heart disease | 7.697 (7.697 to 7.698) | 7.719 (7.718 to 7.719) | 7.497 (7.496 to 7.497) | 6.476 (6.476 to 6.477) | 7.860 (7.860 to 7.860) | 6.448 (6.448 to 6.449) | 14.931 (14.931 to 14.932) |
| Cerebrovascular disease | 7.744 (7.742 to 7.746) | 7.950 (7.949 to 7.952) | 7.261 (7.258 to 7.264) | 7.544 (7.542 to 7.546) | 8.598 (8.597 to 8.600) | 7.986 (7.984 to 7.988) | 14.295 (14.294 to 14.297) |
| Other cardiovascular disease | 8.114 (8.112 to 8.116) | 8.495 (8.493 to 8.496) | 8.438 (8.436 to 8.441) | 7.912 (7.910 to 7.915) | 8.121 (8.116 to 8.125) | 8.312 (8.311 to 8.314) | 15.098 (15.097 to 15.100) |
| All cardiovascular disease | 8.121 (8.118 to 8.124) | 8.588 (8.586 to 8.591) | 8.056 (8.053 to 8.060) | 7.292 (7.290 to 7.295) | 8.334 (8.331 to 8.336) | 7.551 (7.549 to 7.554) | 14.746 (14.743 to 14.748) |
Calculations use a test dose of 0.1 Gy and are given to populations assumed to be in equilibrium having the overall mortality and cardiovascular disease mortality rates of the given population. The population is assumed to be followed up to age 120 years.
Discussion
Principal findings
Our comprehensive meta-analysis, covering a range of individuals who had been exposed to radiation medically (therapeutically or diagnostically), occupationally, or environmentally, shows a significantly increased excess risk per unit dose for all subtypes of cardiovascular disease. We noted heterogeneity between studies, possibly resulting from variation between studies in unmeasured confounders or effect modifiers, however, this evidence of interstudy heterogeneity is markedly reduced among studies with a maximum dose of less than 0.5 Gy or low dose rate or if consideration is restricted to higher quality studies. For ischaemic heart disease, cerebrovascular disease and all cardiovascular disease, risks were larger per unit dose for lower dose (inverse dose effect) and lower dose rate and fractionated exposures (inverse dose fractionation effect). However, the evidence was weaker if attention was restricted to higher quality studies.
Comparison with other studies
A reduction in mean cumulative dose increased excess relative risk per unit dose, a finding that is consistent with the Life Span Study of atomic bomb survivors. In particular, analysis of Life Span Study data, considering dose error, suggests a substantial downwardly curving dose response for cardiovascular disease,72 which has also been observed for ischaemic heart disease51 and cerebrovascular disease52 55 in workers at nuclear facilities. Our finding that increased fractionation increases cardiovascular disease risk is consistent with what was found in an analysis of the Canadian tuberculosis fluoroscopy cohort73; however, no evidence of such a fractionation effect was reported if latency was more than or less than 10 years. Additionally, no such evidence was found in the pooled analysis of the Massachusetts and Canadian data,54 although the pattern of sparing and enhancing effects of dose protraction is complex depending on irradiation regimens at high dose.74 Excess relative risk reduced with increasing age at exposure for stroke and all cardiovascular disease in the Life Span Study,21 but not for heart disease.50
The excess relative risks that we derived (table 2, table 3) were generally consistent with those of a previous systematic review and meta-analysis, published over a decade ago, of moderate to low dose studies,21 and with those of a subsequent non-systematic review.27 Given the overlap in the moderate to low dose studies considered in our paper and previously, this consistency is perhaps unsurprising. However, as suggested by the results of the meta-regression analysis (table 2, table 3), even if the differences between risks at low and moderate to high dose rate were not substantial, they were nevertheless significant.
Population risks
Ischaemic heart disease and cerebrovascular disease are the most strongly associated with radiation, even if analysis was restricted to less than 0.5 Gy or low dose rate data (table 2). The excess absolute risk coefficients that we derived for a UK population of 2.3% (for a 2021 population) to 3.9% per Gy (for a 2003 population) (table 6) were slightly lower than, but of similar extent to, those estimated for cancer mortality by the United Nations Scientific Committee on the Effects of Atomic Radiation,49 which for a 2003 UK population were in the range 4.4-5.2%. At the population level, the largest risks were for cerebrovascular disease and ischaemic heart disease (table 6). For a 2003 UK population, the years of life lost from all cardiovascular disease per Gy was about 0.3 (table 6), which is slightly lower than but similar to the estimated 0.6-0.7 years of life lost per Gy for all solid cancers, estimated by the United Nations Scientific Committee on the Effects of Atomic Radiation.49 This finding has considerable implications for the system of radiological protection, assuming that the extrapolation is permissible, even, for example, over the restricted dose range 0-0.5 Gy. This added risk would nearly double the low dose detriment. Even the restricted range risks were based on people who were potentially exposed to up to 0.5 Gy of radiation, possibly augmented by the people exposed at low dose rate. This level is not what is normally thought of as low dose, which usually refers to risks at doses of less than 0.1 Gy.75 The available evidence does not suggest that lifestyle, environmental, or medical risk factors appreciably modify the excess relative risk related to radiation. Nevertheless, because most major risk factors for cardiovascular disease (smoking, obesity, diabetes, hypertension, elevated cholesterol, unhealthy diet, physical inactivity, and psychosocial factors) multiply (by factors of two or more) the normal risk of cardiovascular disease,4 5 6 76 77 lifetime radiation risk might greatly increase among people with these extra risk factors. This effect should prompt extra vigilance to control modifiable cardiovascular risk factors in patients who receive substantial doses of ionising radiation as part of their medical care. The risks that we estimated for most types of radiation exposure received by the population were relatively trivial. However, this might not be the case for patients who received radiotherapy, radionuclide treatment, a fluoroscopically guided interventional procedure with high doses of radiation, or numerous medical imaging or fluoroscopically guided procedures. Patients can receive doses of 0.1 Gy to relevant organs when they receive radiotherapy for benign conditions, radionuclide treatment, multiple high dose diagnostic (computed tomography and nuclear medicine), or fluoroscopic procedures. The lifetime risks that we estimated (2.3-3.9% Gy−1 (table 6)), imply that the 10-20 Gy that might be delivered to the heart with some types of treatment (supplement S3 table S3.5) would result in lifetime cardiovascular disease risk that exceeds 50%. However, such risks are to some extent an inevitable consequence of life saving treatment, and the benefits of such therapy in general will outweigh cardiovascular disease risks. Doses from most diagnostic procedures are considerably lower, so that, for example, a typical computed tomography scan might deliver a dose of between 0.0005 and 0.015 Gy to the heart.78 79 This dosage together with the risks in table 6 suggests that a group of 10 000 people in the UK each exposed to 10 procedures of this sort might expect between 0.2-13.0 excess ischaemic heart disease deaths over a lifetime.
Mechanistic information and relevant target tissue
Various reviews suggest candidate biological mechanisms.25 80 81 82 Inflammatory mechanisms are plausible, if not completely understood, means by which high doses of radiation could affect the cardiovascular system81; radiation effects on the immune system might also play a part.83 At lower doses, much less is known. Numerous mechanisms have been proposed, for example, monocyte cell killing in the arterial intima,84 likewise, radiation induced endothelial cell senescence and associated monocyte adhesion85 86 87 88 89; however, these mechanisms remain speculative.
Evidence from the radiotherapy cohorts suggests that radiation dose to the heart could be the most relevant for ischaemic heart disease.56 Doses to the heart and thyroid (surrogate for a carotid dose) might also be relevant for cerebrovascular disease; however, doses to the brain are unlikely to be associated.13 The generally uniform whole body radiation with low linear energy transfer in the lower dose cohorts is uninformative as to specific target tissues. In many occupational studies, effective dose is used, in which absorbed dose to each organ is weighted by appropriate tissue weighting factors; this contrasts with the absorbed organ dose that is used elsewhere. However, these different dose metrics would not be expected to be markedly different for the penetrating ionising radiations with low linear energy transfer considered here, so would not substantially contribute to heterogeneity in radiation risk. The consistency of risks, across a wide range of doses (supplement S3 tables S3.4, S3.5) suggests that target tissues and associated mechanisms might be the same for all levels of dose.
Limitations
A concerning feature of our meta-analysis is that for many endpoints, and in particular for cerebrovascular disease, heterogeneity was significant (P<0.001), and this together with high values (generally >50%) of the I2 statistic imply that a material proportion of the variance was due to interstudy heterogeneity (table 3). This issue makes interpretation of summary measures of risk problematic. However, when we restricted analysis to lower dose or dose rate studies (table 3) or when we restricted attention to the higher quality studies, or both, the evidence of interstudy heterogeneity was for most endpoints greatly reduced (table 3, table 5), and the values of the I2 statistic were also lower. The causes of the heterogeneity are not known, although perhaps the multiplicity of lifestyle and medical risk factors in these studies could have a role. However, little evidence exists for the modifying effect of lifestyle and medical factors on excess relative risk associated with radiation. The different target tissues or their surrogates used in specific studies (eg, whole heart, coronary artery, left anterior descending artery, lung for ischaemic heart disease, carotid, thyroid gland, salivary gland, whole brain, Willis Circle arteries for cerebrovascular disease; supplement S3 tables S3.4, S3.5, S3.6) might have differences in radiosensitivity, and this might also contribute to the heterogeneity.
A limitation of our analysis, as with all meta-analysis, is that the effects of key aspects such as dose, dose rate, or age at exposure were only measured at the level of the study. We adjusted for these and other factors for each study via meta-regression (table 2, table 4). Inevitably such meta-regressions are quite generalised, amounting to a type of ecological analysis, and might not adequately control for the effects of these factors.
Many of the studies of medical exposure (supplement S3 table S3.4) had a substantial amount of information on standard lifestyle and medical risk factors for cardiovascular disease. However, information was more limited in the lower dose occupational or environmental studies (supplement table S3.5). In the lower dose studies of the Japanese atomic bomb survivors,20 Mayak workers,64 65 66 and a few other occupationally90 91 92 93 94 and environmentally exposed95 96 groups, substantial information was available on lifestyle factors (supplement S3 table S3.5). In most groups that were exposed to radiation, lifestyle risk factors had little or no evidence of interacting with cardiovascular disease risk related to radiation.13 19 20 54 56 57 59 60 61 62 63 67 90 91 93 95 97 98 99 The types of chemotherapy that were likely to have been administered are more problematic because some (eg, anthracyclines) are known to be cardiotoxic. As discussed above, the data56 57 58 59 60 61 do not suggest that these modify radiation risk. Nevertheless, such factors could confound the associations observed in relation to radiation exposure, although such confounding is unlikely. In some of these studies such information was collected and used in the analysis, and we give this information in supplement S3 tables S3.4-S3.6.
The heterogeneity in outcomes, and how they are defined and aggregated, is a potential problem in conducting reviews of this sort. We used both incidence and mortality data, in some cases within the same cohort. Our analysis highlights differences in meta excess relative risk per Gy of these factors (table 3). Mortality data could be more reliable because disease diagnosis (by a physician knowing the patient’s history) could vary with dose. Although this issue is unlikely to affect the data relating to the Mayak workers,100 this variability is of more concern in the Russian Chernobyl recovery workers.101 102 103 Conversely, if ascertainment of incidence can be done in a uniform way, incidence data are to be preferred because mortality data are intrinsically less reliable. Arguably the variety of different dose metrics used in each study is problematic (supplement S3 tables S4, S5). Wherever possible, we used the absorbed dose (which generally is unweighted dose) to the relevant organ (heart for ischaemic heart disease, dose to carotid artery, or salivary gland for cerebrovascular disease). However, only effective dose is given in some studies. Supplement S3 table S3.6 shows the risks in some therapeutic studies where alternative measures of dose were used. This variety does not suggest marked variation in risk by dose metric within a study and endpoint; however, this could contribute to interstudy heterogeneity.
We used an adaptation of the ROBINS-I system,37 which we modified to make slightly more quantitative, in relation to the extent of likely bias. However, we regard this adapted ROBINS-I system as still quite subjective. For that reason, we also used the much more algorithmic (and less subjective) assessment of study quality, a slight modification of a system that was previously employed.21 The advantage of these two scoring systems is that they yield semiquantitative scores of study quality. The United Nations Scientific Committee on the Effects of Atomic Radiation has outlined some general principles to be used in assessing study quality104 but these are even less specific than the ROBINS-I system, and would not obviously result in a quantitative score.
Strengths
Our systematic review and meta-analysis provides substantial advances on several earlier reviews, which also tended to concentrate on groups exposed to moderate and low doses of radiation.21 25 26 Other more recent reviews have not been of systematic form.27 105 We used state-of-the-art meta-analysis techniques to highlight possible contributions of dose level and dose fractionation. Another striking feature is that despite variation in quality of the individual studies (supplement S3 tables S3.1, S3.2), the overall inference was not much affected when attention was restricted to higher quality studies (table 4, table 5; supplement S3 fig S3.1, S3.2). Evidence has indicated selection bias, particularly for ischaemic heart disease, although if attention is restricted to lower dose (<0.5 Gy) or fractionated data, evidence of bias was much weaker, as also when using higher quality data only (table 3, table 5). The screening process was conducted independently by two authors (MPL, NH), so that omission of studies is unlikely. The data abstraction process was conducted independently by two authors (MPL, KA) and the results were also checked by a third (NH), so that errors in this process are even less likely.
Conclusions
Our systematic review and meta-analysis supports an association between acute high dose and chronic low dose radiation exposure and most types of cardiovascular disease. Low dose and low dose rate exposure tend to be associated with higher risk per unit dose. Although heterogeneity complicates a causal interpretation of these findings, this heterogeneity is markedly reduced if attention is restricted to higher quality studies or to studies at lower dose or dose rate. Our findings suggest that radiation detriment might have been significantly underestimated, implying that radiation protection and optimisation at low doses should be rethought. The possible mechanisms for risk at low doses and low dose rates are, in contrast to the situation at higher doses and dose rates, relatively poorly understood, thus underscoring a crucial need for further research in this area.106 107 Further research is also needed to assess modifications of radiation effect by other lifestyle and medical risk factors.
What is already known on this topic
Exposure to high dose ionising radiation during radiotherapy can damage the heart
Cardiovascular disease risk in the low dose range (<0.1 Gy), characteristic of doses that patients receive from medical diagnostic exposures or those radiation workers receive from occupational exposures is not well understood
Previous systematic reviews published over a decade ago looked at a much smaller number of studies, mostly with lower dose or lower dose rate exposures
What this study adds
A systematic review of 15 098 studies yielded 93 informative and largely non-overlapping studies and suggest modest but significantly increased excess lifetime risk of 2.3-3.9 deaths per 100 people exposed to one Gy of radiation
These findings have implications for patients who undergo radiation exposure as part of their medical care, as well as policy makers involved in managing radiation risks to radiation workers and the public
The potential increased risk of radiogenic cardiovascular disease should prompt vigilance to control other modifiable cardiovascular risk factors and extra consideration of cardiovascular disease following radiation exposure
Web extra.
Extra material supplied by authors
Web appendix 1: Supplements S1-S4
Web appendix 2: Supplement S5 - Cardiovascular disease datafiles, quality coding, R script files
We are grateful for the detailed and helpful comments of Josh Mailman and Jacob Adams.
Contributors: AL, MPL, and NH conducted the systematic literature review. MPL and KA performed the data abstraction and statistical analysis. All authors wrote the review. MPL is the guarantor. MPL, TVA, DBR, and ST should be regarded as joint first authors; KA, LBZ, AJE, and NH should be regarded as joint last authors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The Intramural Research Program of the National Institutes of Health, the National Cancer Institute, Division of Cancer Epidemiology and Genetics supported the work of MPL and LHSV. The work of LBZ was supported by National Cancer Institute and National Institutes of Health (Grant No. R01CA197422). The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication. NIH funded the work of MPL, LHSV, and LBZ, and approved the study for publication, but otherwise NIH played no role in determining the study design, analysis, or conclusions.
Competing interest: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: AJE has received speaker fees from Ionetix; has received consulting fees from WL Gore & Associates; has received authorship fees from Wolters Kluwer Healthcare–UpToDate; and has received grants to his institution from Attralus, Canon Medical Systems, Eidos Therapeutics, GE Healthcare, Pfizer, Roche Medical Systems, WL Gore & Associates, and XyloCor Therapeutics; none of these are related to the present work. Otherwise no other authors declare any competing interests.
The lead author (MPL) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Ethical approval is not required because all data used are in the public domain.
Data availability statement
All data and R and EpiWin code used in the article is provided in online Supplement S5.
References
- 1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095-128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). World Health Organization Statistical Information System (WHOSIS). Updated 17 November 2015. https://www.who.int/gho/en/. 2015.
- 3.Centers for Disease Control and Prevention (CDC). CDC Wonder Atlanta, GA: Centers for Disease Control; 2020. https://wonder.cdc.gov/.
- 4. Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837-47. 10.1161/01.CIR.97.18.1837 [DOI] [PubMed] [Google Scholar]
- 5. Dhingra R, Vasan RS. Age as a risk factor. Med Clin North Am 2012;96:87-91. 10.1016/j.mcna.2011.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yusuf S, Hawken S, Ounpuu S, et al. INTERHEART Study Investigators . Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937-52. 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 7. Slack J. Risks of ischaemic heart-disease in familial hyperlipoproteinaemic states. Lancet 1969;2:1380-2. 10.1016/S0140-6736(69)90930-1 [DOI] [PubMed] [Google Scholar]
- 8. Rissanen AM, Nikkilä EA. Aggregation of coronary risk factors in families of men with fatal and non-fatal coronary heart disease. Br Heart J 1979;42:373-80. 10.1136/hrt.42.4.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rissanen AM. Familial aggregation of coronary heart disease in a high incidence area (North Karelia, Finland). Br Heart J 1979;42:294-303. 10.1136/hrt.42.3.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rissanen AM. Familial occurrence of coronary heart disease: effect of age at diagnosis. Am J Cardiol 1979;44:60-6. 10.1016/0002-9149(79)90251-0 [DOI] [PubMed] [Google Scholar]
- 11. Adams MJ, Hardenbergh PH, Constine LS, Lipshultz SE. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol 2003;45:55-75. 10.1016/S1040-8428(01)00227-X [DOI] [PubMed] [Google Scholar]
- 12. Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin’s disease. JAMA 1993;270:1949-55. 10.1001/jama.1993.03510160067031 [DOI] [PubMed] [Google Scholar]
- 13. Little MP, Kleinerman RA, Stovall M, Smith SA, Mabuchi K. Analysis of dose response for circulatory disease after radiotherapy for benign disease. Int J Radiat Oncol Biol Phys 2012;84:1101-9. 10.1016/j.ijrobp.2012.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Darby S, McGale P, Peto R, Granath F, Hall P, Ekbom A. Mortality from cardiovascular disease more than 10 years after radiotherapy for breast cancer: nationwide cohort study of 90 000 Swedish women. BMJ 2003;326:256-7. 10.1136/bmj.326.7383.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol 2005;6:557-65. 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 16. Clarke M, Collins R, Darby S, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366:2087-106. 10.1016/S0140-6736(05)67887-7 [DOI] [PubMed] [Google Scholar]
- 17. Swerdlow AJ, Higgins CD, Smith P, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. J Natl Cancer Inst 2007;99:206-14. 10.1093/jnci/djk029. [DOI] [PubMed] [Google Scholar]
- 18. Hancock SL, Donaldson SS, Hoppe RT. Cardiac disease following treatment of Hodgkin’s disease in children and adolescents. J Clin Oncol 1993;11:1208-15. 10.1200/JCO.1993.11.7.1208 [DOI] [PubMed] [Google Scholar]
- 19. Yamada M, Wong FL, Fujiwara S, Akahoshi M, Suzuki G. Noncancer disease incidence in atomic bomb survivors, 1958-1998. Radiat Res 2004;161:622-32. 10.1667/RR3183 [DOI] [PubMed] [Google Scholar]
- 20. Shimizu Y, Kodama K, Nishi N, et al. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950-2003. BMJ 2010;340:b5349. 10.1136/bmj.b5349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Little MP, Azizova TV, Bazyka D, et al. Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks. Environ Health Perspect 2012;120:1503-11. 10.1289/ehp.1204982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schöllnberger H, Kaiser JC, Jacob P, Walsh L. Dose-responses from multi-model inference for the non-cancer disease mortality of atomic bomb survivors. Radiat Environ Biophys 2012;51:165-78. 10.1007/s00411-012-0410-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Little MP, Azizova TV, Bazyka D, et al. Comment on “dose-responses from multi-model inference for the non-cancer disease mortality of atomic bomb survivors” (Radiat. Environ. Biophys (2012) 51:165-178) by Schöllnberger et al. Radiat Environ Biophys 2013;52:157-9. 10.1007/s00411-012-0453-6 [DOI] [PubMed] [Google Scholar]
- 24. International Commission on Radiological Protection . ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs - threshold doses for tissue reactions in a radiation protection context. ICRP publication 118. Ann ICRP 2012;41:1-322. 10.1016/j.icrp.2012.02.001 . [DOI] [PubMed] [Google Scholar]
- 25. Little MP, Tawn EJ, Tzoulaki I, et al. A systematic review of epidemiological associations between low and moderate doses of ionizing radiation and late cardiovascular effects, and their possible mechanisms. Radiat Res 2008;169:99-109. 10.1667/RR1070.1. [DOI] [PubMed] [Google Scholar]
- 26. McGale P, Darby SC. Low doses of ionizing radiation and circulatory diseases: a systematic review of the published epidemiological evidence. Radiat Res 2005;163:247-57. 10.1667/RR3314. [DOI] [PubMed] [Google Scholar]
- 27. Little MP. Radiation and circulatory disease. Mutat Res Rev Mutat Res 2016;770(Pt B):299-318. 10.1016/j.mrrev.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wakeford R, Tawn EJ. The meaning of low dose and low dose-rate. J Radiol Prot 2010;30:1-3. 10.1088/0952-4746/30/1/E02 [DOI] [PubMed] [Google Scholar]
- 29. Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behav Stat 2005;30:261-93 10.3102/10769986030003261 . [DOI] [Google Scholar]
- 30. Bartlett MS, Fowler RH. Properties of sufficiency and statistical tests. Proc Royal Soc London Series A - Math Phys Sci 1937;160:268-82. 10.1098/rspa.1937.0109. [DOI] [Google Scholar]
- 31. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 32. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 33. Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med 2003;22:2693-710. 10.1002/sim.1482 [DOI] [PubMed] [Google Scholar]
- 34. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sterne JAC, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 2001;54:1046-55. 10.1016/S0895-4356(01)00377-8 [DOI] [PubMed] [Google Scholar]
- 36. Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc 2000;95:89-98. [Google Scholar]
- 37. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010;36:1-48 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 39.metafor. Version 2.4-0: CRAN - The Comprehensive R Archive Network 2020.
- 40.R: A language and environment for statistical computing. version 3.6.1 https://www.r-project.org. Vienna, Austria: R Foundation for Statistical Computing, 2019.
- 41.forestplot. Version 1.9: CRAN - The Comprehensive R Archive Network 2019.
- 42. Office for National Statistics (ONS) . Mortality statistics cause. Series DH2 no. 30. Review of the Registrar General on deaths by cause, sex and age. In: England and Wales, 2003. Her Majesty's Stationery Office, 2004: 1-291. [Google Scholar]
- 43.Office of National Statistics. nomis - mortality statistics - underlying cause, sex and age Office for National Statistics; 2022; https://www.nomisweb.co.uk/query/construct/summary.asp?reset=yes&mode=construct&dataset=161&version=0&anal=1&initsel=.
- 44.Ministry of Health Labour and Welfare of Japan (MHLW). Vital Statistics of Japan for 2021: Ministry of Health, Labour, and Welfare of Japan; 2022 https://www.mhlw.go.jp/english/database/db-hh/1-2.html. [Google Scholar]
- 45.Inserm CépiDc (Centre d’épidémiologie sur les causes médicales de décès). Je suis un particulier 2022 https://www.cepidc.inserm.fr/je-suis-un-particulier.
- 46.Statistisches Bundesamt (Federal Statistical Office). Genesis-online Wiesbaden: Statistisches Bundesamt (Federal Statistical Office); 2022 https://www-genesis.destatis.de/genesis/online?language=en&sequenz=statistikTabellen&selectionname=23211#abreadcrumb
- 47.Centers for Disease Control and Prevention (CDC). CDC Wonder Atlanta, GA: Centers for Disease Control; 2022 https://wonder.cdc.gov/ [Google Scholar]
- 48.Surveillance Division - Public Health Agency of Canada (PHAC). Provincial vital registry data (unpublished mortality tabulations). 2015 [Google Scholar]
- 49. United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) . UNSCEAR 2006 Report. Annex A. Epidemiological Studies of Radiation and Cancer. United Nations, 2008: 13-322. [Google Scholar]
- 50. Takahashi I, Shimizu Y, Grant EJ, Cologne J, Ozasa K, Kodama K. Heart Disease Mortality in the Life Span Study, 1950-2008. Radiat Res 2017;187:319-32. 10.1667/RR14347.1 [DOI] [PubMed] [Google Scholar]
- 51. Zhang W, Haylock RGE, Gillies M, Hunter N. Mortality from heart diseases following occupational radiation exposure: analysis of the National Registry for Radiation Workers (NRRW) in the United Kingdom. J Radiol Prot 2019;39:327-53. 10.1088/1361-6498/ab02b2 [DOI] [PubMed] [Google Scholar]
- 52. Hinksman CA, Haylock RGE, Gillies M. Cerebrovascular disease mortality after occupational radiation exposure among the UK National Registry for Radiation Workers Cohort. Radiat Res 2022;197:459-70. 10.1667/RADE-20-00204.1 [DOI] [PubMed] [Google Scholar]
- 53. Vrijheid M, Cardis E, Ashmore P, et al. Mortality from diseases other than cancer following low doses of ionizing radiation: results from the 15-country study of nuclear industry workers. Int J Epidemiol 2007;36:1126-35. 10.1093/ije/dym138 [DOI] [PubMed] [Google Scholar]
- 54. Tran V, Zablotska LB, Brenner AV, Little MP. Radiation-associated circulatory disease mortality in a pooled analysis of 77,275 patients from the Massachusetts and Canadian tuberculosis fluoroscopy cohorts. Sci Rep 2017;7:44147. 10.1038/srep44147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gillies M, Richardson DB, Cardis E, et al. Mortality from circulatory diseases and other non-cancer outcomes among nuclear workers in France, the United Kingdom and the United States (INWORKS). Radiat Res 2017;188:276-90. 10.1667/RR14608.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. 10.1056/NEJMoa1209825 [DOI] [PubMed] [Google Scholar]
- 57. Jacobse JN, Duane FK, Boekel NB, et al. Radiation dose-response for risk of myocardial infarction in breast cancer survivors. Int J Radiat Oncol Biol Phys 2019;103:595-604. 10.1016/j.ijrobp.2018.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Boekel NB, Duane FK, Jacobse JN, et al. Heart failure after treatment for breast cancer. Eur J Heart Fail 2020;22:366-74. 10.1002/ejhf.1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cutter DJ, Schaapveld M, Darby SC, et al. Risk of valvular heart disease after treatment for Hodgkin lymphoma. J Natl Cancer Inst 2015;107:djv008. 10.1093/jnci/djv008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van Nimwegen FA, Ntentas G, Darby SC, et al. Risk of heart failure in survivors of Hodgkin lymphoma: effects of cardiac exposure to radiation and anthracyclines. Blood 2017;129:2257-65. 10.1182/blood-2016-09-740332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van Nimwegen FA, Schaapveld M, Cutter DJ, et al. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol 2016;34:235-43. 10.1200/JCO.2015.63.4444 [DOI] [PubMed] [Google Scholar]
- 62. de Vocht F, Hidajat M, Martin RM, Agius R, Wakeford R. Ischemic heart disease mortality and occupational radiation exposure in a nested matched case-control study of British Nuclear Fuel cycle workers: investigation of confounding by lifestyle, physiological traits and occupational exposures. Radiat Res 2020;194:431-44. 10.1667/RADE-19-00007.1 [DOI] [PubMed] [Google Scholar]
- 63. Bouet S, Davesne E, Samson E, et al. Analysis of the association between ionizing radiation and mortality in uranium workers from five plants involved in the nuclear fuel production cycle in France. Int Arch Occup Environ Health 2019;92:249-62. 10.1007/s00420-018-1375-7 [DOI] [PubMed] [Google Scholar]
- 64. Azizova TV, Haylock RGE, Moseeva MB, Bannikova MV, Grigoryeva ES. Cerebrovascular diseases incidence and mortality in an extended Mayak Worker Cohort 1948-1982. Radiat Res 2014;182:529-44. 10.1667/RR13680.1 [DOI] [PubMed] [Google Scholar]
- 65. Azizova TV, Moseeva MB, Grigoryeva ES, Hamada N. Incidence risks for cerebrovascular diseases and types of stroke in a cohort of Mayak PA workers. Radiat Environ Biophys 2022;61:5-16. 10.1007/s00411-022-00966-6 [DOI] [PubMed] [Google Scholar]
- 66. Azizova TV, Bannikova MV, Grigoryeva ES, Briks KV, Hamada N. Mortality from various diseases of the circulatory system in the Russian Mayak nuclear worker cohort: 1948-2018. J Radiol Prot 2022;42. 10.1088/1361-6498/ac4ae3 [DOI] [PubMed] [Google Scholar]
- 67. Azizova TV, Grigoryeva ES, Haylock RGE, Pikulina MV, Moseeva MB. Ischaemic heart disease incidence and mortality in an extended cohort of Mayak workers first employed in 1948-1982. Br J Radiol 2015;88:20150169. 10.1259/bjr.20150169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Azizova TV, Grigorieva ES, Hunter N, Pikulina MV, Moseeva MB. Risk of mortality from circulatory diseases in Mayak workers cohort following occupational radiation exposure. J Radiol Prot 2015;35:517-38. 10.1088/0952-4746/35/3/517 [DOI] [PubMed] [Google Scholar]
- 69. Zielinski JM, Ashmore PJ, Band PR, et al. Low dose ionizing radiation exposure and cardiovascular disease mortality: cohort study based on Canadian national dose registry of radiation workers. Int J Occup Med Environ Health 2009;22:27-33. 10.2478/v10001-009-0001-z [DOI] [PubMed] [Google Scholar]
- 70. Boice JD, Jr, Cohen SS, Mumma MT, et al. Mortality among workers at the Los Alamos National Laboratory, 1943-2017. Int J Radiat Biol 2022;98:722-49. 10.1080/09553002.2021.1917784 [DOI] [PubMed] [Google Scholar]
- 71. Adams MJ, Fisher SG, Lipshultz SE, et al. Risk of coronary events 55 years after thymic irradiation in the hempelmann cohort. Cardiooncology 2018;4:1. 10.1186/s40959-018-0027-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Little MP, Pawel D, Misumi M, et al. Lifetime mortality risk from cancer and circulatory disease predicted from the Japanese atomic bomb survivor Life Span Study data taking account of dose measurement error. Radiat Res 2020;194:259-76. 10.1667/RR15571.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zablotska LB, Little MP, Cornett RJ. Potential increased risk of ischemic heart disease mortality with significant dose fractionation in the Canadian Fluoroscopy Cohort Study. Am J Epidemiol 2014;179:120-31. 10.1093/aje/kwt244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hamada N, Kawano KI, Nomura T, et al. Vascular damage in the aorta of wild-type mice exposed to ionizing radiation: sparing and enhancing effects of dose protraction. Cancers (Basel) 2021;13:5344. 10.3390/cancers13215344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. International Commission on Radiological Protection (ICRP) . The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 2007;37:1-332. 10.1016/j.icrp.2007.10.003 [DOI] [PubMed] [Google Scholar]
- 76. Burns DM. Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis 2003;46:11-29. 10.1016/S0033-0620(03)00079-3 [DOI] [PubMed] [Google Scholar]
- 77. Hajifathalian K, Ueda P, Lu Y, et al. A novel risk score to predict cardiovascular disease risk in national populations (Globorisk): a pooled analysis of prospective cohorts and health examination surveys. Lancet Diabetes Endocrinol 2015;3:339-55. 10.1016/S2213-8587(15)00081-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lee C, Yeom YS, Folio L. CT organ dose calculator size adaptive for pediatric and adult patients. Biomed Phys Eng Express 2022;8:065020. 10.1088/2057-1976/ac9845 [DOI] [PubMed] [Google Scholar]
- 79. Valentin J, International Commission on Radiation Protection . Managing patient dose in multi-detector computed tomography(MDCT). ICRP Publication 102 [iii.]. Ann ICRP 2007;37:1-79, iii. [DOI] [PubMed] [Google Scholar]
- 80.McMillan TJ, Bennett MR, Bridges BA, et al. Circulatory disease risk. Report of the independent Advisory Group on Ionising Radiation, Health Protection Agency, Holborn Gate, 330 High Holborn, London, 2010:1-116. [Google Scholar]
- 81. Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys 2007;67:10-8. 10.1016/j.ijrobp.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 82. Azimzadeh O, Moertl S, Ramadan R, et al. Application of radiation omics in the development of adverse outcome pathway networks: an example of radiation-induced cardiovascular disease. Int J Radiat Biol 2022;98:1722-51. 10.1080/09553002.2022.2110325 [DOI] [PubMed] [Google Scholar]
- 83. Fernández-Ruiz I. Immune system and cardiovascular disease. Nat Rev Cardiol 2016;13:503-03. 10.1038/nrcardio.2016.127 [DOI] [PubMed] [Google Scholar]
- 84. Little MP, Gola A, Tzoulaki I. A model of cardiovascular disease giving a plausible mechanism for the effect of fractionated low-dose ionizing radiation exposure [doi]. PLoS Comput Biol 2009;5:e1000539. 10.1371/journal.pcbi.1000539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lowe D, Raj K. Premature aging induced by radiation exhibits pro-atherosclerotic effects mediated by epigenetic activation of CD44 expression. Aging Cell 2014;13:900-10. 10.1111/acel.12253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rombouts C, Aerts A, Quintens R, et al. Transcriptomic profiling suggests a role for IGFBP5 in premature senescence of endothelial cells after chronic low dose rate irradiation. Int J Radiat Biol 2014;90:560-74. 10.3109/09553002.2014.905724 [DOI] [PubMed] [Google Scholar]
- 87. Yentrapalli R, Azimzadeh O, Barjaktarovic Z, et al. Quantitative proteomic analysis reveals induction of premature senescence in human umbilical vein endothelial cells exposed to chronic low-dose rate gamma radiation. Proteomics 2013;13:1096-107. 10.1002/pmic.201200463 [DOI] [PubMed] [Google Scholar]
- 88. Yentrapalli R, Azimzadeh O, Sriharshan A, et al. The PI3K/Akt/mTOR pathway is implicated in the premature senescence of primary human endothelial cells exposed to chronic radiation. PLoS One 2013;8:e70024. 10.1371/journal.pone.0070024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Azimzadeh O, Sievert W, Sarioglu H, et al. Integrative proteomics and targeted transcriptomics analyses in cardiac endothelial cells unravel mechanisms of long-term radiation-induced vascular dysfunction. J Proteome Res 2015;14:1203-19. 10.1021/pr501141b [DOI] [PubMed] [Google Scholar]
- 90. Drubay D, Caër-Lorho S, Laroche P, Laurier D, Rage E. Mortality from circulatory system diseases among French Uranium miners: a nested case-control study. Radiat Res 2015;183:550-62. 10.1667/RR13834.1 [DOI] [PubMed] [Google Scholar]
- 91. Kreuzer M, Dufey F, Sogl M, Schnelzer M, Walsh L. External gamma radiation and mortality from cardiovascular diseases in the German WISMUT uranium miners cohort study, 1946-2008. Radiat Environ Biophys 2013;52:37-46. 10.1007/s00411-012-0446-5 [DOI] [PubMed] [Google Scholar]
- 92. Krasnikova LI, Buzunov VO, Solonovitch SI. Radiation and non-radiation factors impact on development of cerebrovascular diseases in the Chornobyl clean-up workers. The epidemiological study results. Probl Radiac Med Radiobiol 2013;(18):89-101. [PubMed] [Google Scholar]
- 93. Cha ES, Zablotska LB, Bang YJ, Lee WJ. Occupational radiation exposure and morbidity of circulatory disease among diagnostic medical radiation workers in South Korea. Occup Environ Med 2020;77:752-60. 10.1136/oemed-2019-106326 [DOI] [PubMed] [Google Scholar]
- 94. Park S, Lee DN, Jin YW, et al. Non-cancer disease prevalence and association with occupational radiation exposure among Korean radiation workers. Sci Rep 2021;11:22415. 10.1038/s41598-021-01875-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Markabayeva A, Bauer S, Pivina L, et al. Increased prevalence of essential hypertension in areas previously exposed to fallout due to nuclear weapons testing at the Semipalatinsk Test Site, Kazakhstan. Environ Res 2018;167:129-35. 10.1016/j.envres.2018.07.016 [DOI] [PubMed] [Google Scholar]
- 96. Semenova Y, Rakhimova I, Nurpeissov T, et al. Epidemiology of stroke and transient ischemic attacks in the population of the territories adjacent to the former Semipalatinsk Nuclear Test Site, Kazakhstan. Radiat Environ Biophys 2022;61:17-28. 10.1007/s00411-021-00955-1 [DOI] [PubMed] [Google Scholar]
- 97. Maraldo MV, Giusti F, Vogelius IR, et al. European Organisation for Research and Treatment of Cancer (EORTC) Lymphoma Group . Cardiovascular disease after treatment for Hodgkin’s lymphoma: an analysis of nine collaborative EORTC-LYSA trials. Lancet Haematol 2015;2:e492-502. 10.1016/S2352-3026(15)00153-2 [DOI] [PubMed] [Google Scholar]
- 98. Moseeva MB, Azizova TV, Grigoryeva ES, Haylock R. Risks of circulatory diseases among Mayak PA workers with radiation doses estimated using the improved Mayak Worker Dosimetry System 2008. Radiat Environ Biophys 2014;53:469-77. 10.1007/s00411-014-0517-x [DOI] [PubMed] [Google Scholar]
- 99. Azizova TV, Bannikova MV, Grigorieva ES, Bagaeva YP, Azizova EV. Risk of lower extremity arterial disease in a cohort of workers occupationally exposed to ionizing radiation over a prolonged period. Radiat Environ Biophys 2016;55:147-59. 10.1007/s00411-016-0645-6 [DOI] [PubMed] [Google Scholar]
- 100. Little MP, Azizova TV, Hamada N. Low- and moderate-dose non-cancer effects of ionizing radiation in directly exposed individuals, especially circulatory and ocular diseases: a review of the epidemiology. Int J Radiat Biol 2021;97:782-803. 10.1080/09553002.2021.1876955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ivanov VK, Maksioutov MA, Chekin SY, et al. The risk of radiation-induced cerebrovascular disease in Chernobyl emergency workers. Health Phys 2006;90:199-207. 10.1097/01.HP.0000175835.31663.ea. [DOI] [PubMed] [Google Scholar]
- 102. Kashcheev VV, Chekin SY, Maksioutov MA, et al. Radiation-epidemiological study of cerebrovascular diseases in the cohort of Russian recovery operation workers of the Chernobyl accident. Health Phys 2016;111:192-7. 10.1097/HP.0000000000000523 [DOI] [PubMed] [Google Scholar]
- 103. Kashcheev VV, Chekin SY, Karpenko SV, et al. Radiation risk of cardiovascular diseases in the cohort of Russian emergency workers of the Chernobyl accident. Health Phys 2017;113:23-9. 10.1097/HP.0000000000000670 [DOI] [PubMed] [Google Scholar]
- 104. United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) . Sources, effects and risks of ionizing radiation. UNSCEAR 2017 report to the General Assembly. Scientific annex A. Principles and criteria for ensuring the quality of the Committee’s reviews of epidemiological studies of radiation exposure. United Nations, 2017: 19-64. [Google Scholar]
- 105. Little MP, Lipshultz SE. Low dose radiation and circulatory diseases: a brief narrative review. Cardiooncology 2015;1:4. 10.1186/s40959-015-0007-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kreuzer M, Auvinen A, Cardis E, et al. Low-dose ionising radiation and cardiovascular diseases--Strategies for molecular epidemiological studies in Europe. Mutat Res Rev Mutat Res 2015;764:90-100. 10.1016/j.mrrev.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 107. Tapio S, Little MP, Kaiser JC, et al. Ionizing radiation-induced circulatory and metabolic diseases. Environ Int 2021;146:106235. 10.1016/j.envint.2020.106235 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix 1: Supplements S1-S4
Web appendix 2: Supplement S5 - Cardiovascular disease datafiles, quality coding, R script files
Data Availability Statement
All data and R and EpiWin code used in the article is provided in online Supplement S5.