Abstract
Mercury is one of the most dangerous contaminants on the planet. In recent years, evidence of mercury contamination in the Amazon has significantly increased, notably due to gold-mining activities. Although mercury contamination in fish has consistently been documented, little is known about the risk associated with fish consumption by populations in urban areas of the Amazon. We sampled 1010 fish sold in public markets in six state capitals and 11 additional cities. Mercury levels were determined for each specimen, and the evaluation of the health risks associated with consuming mercury-contaminated fish was conducted according to the methodology proposed by the World Health Organization (WHO). Our study reveals that more than one-fifth (21.3%) of the fish sold in urban centers had mercury levels above the safe limits (≥0.5 µg/g) established by the Brazilian Health Surveillance Agency (ANVISA). The prevalence of Hg contamination ≥0.5 µg/g was approximately 14 times higher in carnivorous than in noncarnivorous fish. The analysis of the risk attributable to fish consumption reveals that daily mercury intake exceeded the reference dose recommended by the U.S. EPA in all population groups analyzed, reaching up to 7 and 31 times in women of childbearing age and children from 2 to 4 years old, respectively. However, these risks are diverse depending on the type of fish consumed and must be considered to formulate appropriate nutritional guidelines for safe fish consumption by the local community.
Keywords: Amazon, fish, health risk assessment, mercury, mining
1. Introduction
Mercury ranks third in the world for toxicity among the environmental pollutants that are most dangerous to human health. Approximately 19 million people around the world have been estimated to be at risk of becoming sick due to contact with this chemical contaminant. Artisanal gold mining is the largest source of human exposure to mercury in Latin America [1,2]. In response, the United Nations promulgated the Minamata Convention in 2013, which aimed to ban mercury from all industrial processes on the planet and regulate informal mining to control and replace the use of mercury. In Brazil, the Minamata Convention was promulgated by Decree 9470 on 14 August 2018. However, efforts to contain this threat since then have not been sufficient to control the gold-mining boom in the Brazilian Amazon, especially in the last five years.
Over time, metallic mercury used in gold mining accumulates in river sediments, where it is converted into methylmercury (the most dangerous chemical form to human health and the ecosystem) and is quickly incorporated into the organisms that make up the aquatic biota [3,4,5,6]. Much of the danger associated with methylmercury is due to its high neurotoxic potential and its ability to bioaccumulate and biomagnify in aquatic food chains. Fish are directly affected, resulting in serious health damage to humans and various animals that consume these and other contaminated aquatic organisms.
Methylmercury is highly liposoluble, and due to this characteristic, it can cross the blood–brain barrier and reach the central nervous system. The main health damages caused by methylmercury are the following: changes in gait, problems with balance and motor coordination, decreased visual field, and loss of skin sensitivity [7,8]. In pregnant women, contamination is even more serious since methylmercury is capable of crossing the placental barrier and reaching the developing fetus’s brain, causing irreversible damage, including hearing loss, cognitive deficits, developmental delays, and congenital malformations in children exposed during the intrauterine period [9,10,11].
The Amazonian populations have one of the highest rates of per capita fish consumption in the world [12,13,14]. Fish is the animal protein that is most easily accessible in the Amazon, ensuring the food and nutritional security of riverine and urban populations in the region. Fish is a food with high nutritional value due to its high protein content and its inclusion of important vitamins and minerals for maintaining good health [15,16]. Despite numerous pieces of evidence regarding the nutritional quality of fish, the increasing contamination of aquatic systems with environmental pollutants such as pesticides and heavy metals has raised concerns in society and sparked an important debate about the risks and benefits of a diet rich in this type of animal protein.
The contamination of fish in the Amazon Basin via gold mining and other sources has been well documented since the 1960s. In recent years, evidence of increased mercury contamination in fish in the rivers that form the Amazon basin has significantly increased due to the growth of mining activity. This has raised a series of concerns about the health of the population living in the region [6,17,18].
Considering the increase in gold-mining activity in recent years, as well as the severity of health damage that mercury can cause to both the population and the environment, this study evaluated the risk associated with fish consumption by populations in urban areas of the Brazilian Amazon. Based on the findings of this study, we hope to broaden the debate about the deleterious effects caused by gold mining not only for riverside and traditional populations, but also for populations living in urban centers who also have the cultural habit of consuming large amounts of fish from the region.
2. Materials and Methods
2.1. Area and Study Design
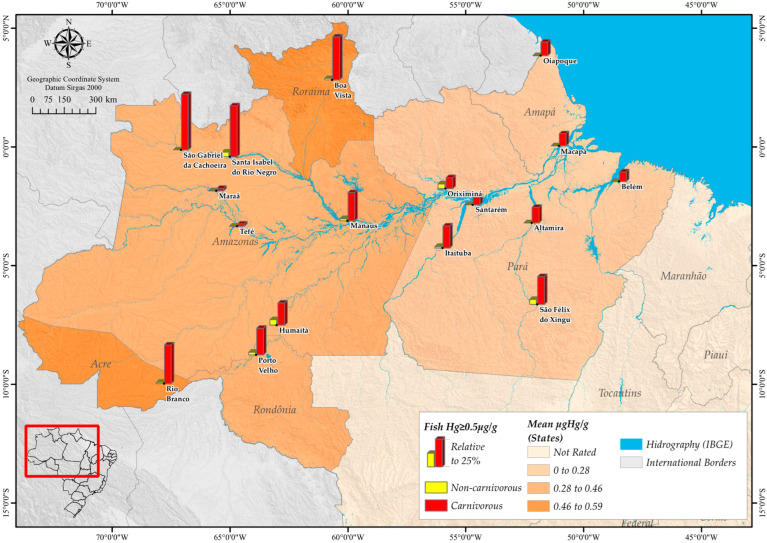
An ecological approach was undertaken to assess the health risks associated with fish consumption in six states of the Brazilian Amazon, including state capitals and 11 additional cities, totaling 17 municipalities: Rio Branco, Macapá, Oiapoque, Humaitá, Manaus, Maraã, Santa Isabel do Rio Negro, São Gabriel da Cachoeira, Tefé, Altamira, Belém, Itaituba, Oriximiná, Santarém, São Félix do Xingú, Porto Velho, and Boa Vista (Figure 1).
Figure 1.
Spatial distribution of average levels of mercury contamination in analyzed fish (average between carnivorous and noncarnivorous fish) by Federative Unit and according to prevalence of contamination ≥0.5 µg/g considering the 17 collection points, Amazon Basin, Brazil, 2021–2022.
2.2. Fish Sampling and Mercury Analysis
The sampled fish were acquired from public markets, open-air markets, or directly from fishermen at the fishing landing points between March 2021 and September 2022. To standardize the sampling, a preliminary list was prepared considering the fish commonly found in open-air markets and markets in the regions [19,20,21,22], their priority feeding habits, and their trophic guild [22,23,24]. We prioritized at least three different species in each trophic guild and at least three individuals of different sizes for each species. The trophic guilds were generally classified as carnivorous, omnivorous, detritivorous, and herbivorous.
After being acquired, the fish were placed in thermal boxes with ice and sent to the characterization/description stage, after which muscle tissue samples were obtained to determine mercury levels. Each of the specimens was photographed, with the following information recorded: common name, scientific name, date, location of fish capture, location of fish purchase, weight (g), and standard length (cm). Each collected fish had its identification confirmed to the lowest possible taxonomic level using specialized literature, dichotomous keys, and consultation with experts.
Subsequently, approximately 20 g of muscle tissue was extracted from the dorsal part of each fish specimen, which was stored in Ziploc plastic bags and properly identified with a code representing the name of the species, location of acquisition, and date of collection. The samples were sent to the Laboratory of Environmental Mercury Speciation of the Mineral Technology Center (LEMA/CETEM) and to the Laboratory of Mercury of The Evandro Chagas Institute, Surveillance Health and Environment Secretariat of the Ministry of Health for total mercury analysis. At the laboratories, aliquots of 3.0 g of wet muscle tissue were precisely weighed, and three replicates were performed per fish sample. The analysis accuracy with the replicates was above 85% (calibration curve R: 0.9949), and the recovery with the reference material IAEA-476 (0.578 mg/kg) was 94%. The analytical technique employed was atomic absorption spectrometry with a graphite furnace (equipment: RA-915+ coupled with Py-ro-915+—Serial number 465). The detection limit (DL) for mercury was 0.0005 mg/kg, and the quantification limit (QL) was 0.009 mg/kg.
2.3. Health Risk Assessment
The evaluation of the health risks associated with consuming mercury-contaminated fish was conducted according to the methodology proposed by the World Health Organization [25] considering the following steps.
2.3.1. Characterization of the Study Population
This stage involved defining the population groups under investigation (i.e., gender and age range) and estimating their respective average weights (in kg) and the average amount of fish consumed daily (in g).
The following population groups were considered: (i) women of childbearing age (from 10 to 49 years); (ii) adult men (≥18 years); (iii) children aged from 5 to 12 years; and (iv) children aged from 2 to 4 years.
The body weight data for each population group were obtained by consulting the Family Budget Survey (POF, 2008), which was organized by the IBGE Automatic Retrieval System (the most recent data available for public consultation). The following body weight averages were used: (i) 50.95 kg for women of childbearing age; (ii) 66.88 kg for adult men; (iii) 27.92 kg for children aged from 5 to 12 years; and (iv) 14.49 kg for children aged from 2 to 4 years.
The estimate of fish consumption by the population of the Amazon was based on a report on fish consumption in the Amazon region of Brazil. The report indicated an average per capita consumption of approximately 100 g of fish per day in urban areas [12].
2.3.2. Estimate of Daily Intake of Mercury
The following assumptions were made: (i) 100% of the mercury detected in the fish samples was in the chemical form of methylmercury (MeHg) and (ii) approximately 80% of the amount of mercury ingested in food was absorbed by the human gastrointestinal tract (absorption rate).
2.3.3. Calculation of the Risk Ratio
The risk ratio (RR) indicated the potential health damage caused by consuming contaminated fish. The calculation was performed by dividing the average amount absorbed by the human body (i.e., 80% of the ingested dose) by the reference dose. For this study, the safe daily intake dose of 0.1 µg MeHg/kg body weight/day proposed by the Environmental Protection Agency (U.S. EPA) [26] was considered as a reference.
When the RR < 1, the absorbed dose of mercury was lower than the reference dose considered. Consequently, the risk of becoming sick was low. On the other hand, when the RR ≥ 1, the absorbed dose of mercury exceeded the reference dose considered, and the risk of becoming sick due to exposure to mercury should be considered. The higher the RR, the greater the potential risk of harm to the health of the population.
2.3.4. Maximum Safe Consumption (MSC) Indication of Fish
In conclusion of the health risk assessment, the maximum safe consumption (MSC) value of fish was defined for the four population groups by multiplying the reference dose by the average body weights and was presented in grams/day. Following this, the product of this multiplication was divided by the average total mercury concentration (µg/g) detected in different fish species. As there were regional preferences in fish consumption, as well as different levels of mercury accumulation depending on the diet of each fish species [27,28], consumption was standardized to an average of 50% carnivorous fish species and 50% noncarnivorous fish species.
2.4. Statistical Analysis
In order to explore factors associated with levels of mercury contamination in fish ≥ 0.5 µg/g in the studied locations, a Poisson regression was performed using the prevalence ratio (PR) as the measure of association and considering a 95% confidence interval. After the initial raw analysis, the variables that demonstrated a level of significance (p-value) < 0.05 remained in the final model. The data were analyzed using Statistical Package for the Social Sciences (SPSS), version 9.0 (SPSS, Chicago Inc.: Chicago, IL, USA).
3. Results
A total of 1010 fish specimens were sampled, belonging to 80 distinct species distributed across four trophic levels: herbivores, detritivores, omnivores, and carnivores. Overall, 159 samples presented mercury levels below the detection limit, and 38 presented mercury levels below the quantification limit, totaling 197 samples (19.5%) in which it was not possible to estimate the levels of mercury contamination.
The concentrations of mercury in fish ranged from 0 to 4.73 μg/g, with an average concentration of 0.34 μg/g (standard deviation of 0.56 and median of 0.13 μg/g) (Table 1). A total of 21.3% presented levels equal to or greater than 0.5 µg/g in sampled fish.
Table 1.
Levels of mercury detected in fish samples acquired from 17 localities in the Amazon Basin, Brazil, 2021–2022.
| State | N | Number of Species | Mean Hg μg/g (D.P *) | Median Hg | Min–Max Hg | Mean Hg μg/g Carnivorous (n) | Mean Hg μg/g Noncarnivorous (n) | % ≥0.5 μg/g |
|---|---|---|---|---|---|---|---|---|
| Acre | 78 | 25 | 0.58 (0.97) | 0.15 | 0.00–4.64 | 1.06 (40) | 0.08 (38) | 36 |
| Amapá | 114 | 27 | 0.18 (0.25) | 0.08 | 0.00–1.24 | 0.27 (74) | 0.02 (40) | 11 |
| Amazonas | 262 | 34 | 0.34 (0.49) | 0.14 | 0.00–3.22 | 0.67 (108) | 0.11 (154) | 22 |
| Pará | 393 | 47 | 0.27 (0.43) | 0.1 | 0.00–3.50 | 0.48 (183) | 0.08 (210) | 16 |
| Rondônia | 88 | 28 | 0.45 (0.80) | 0.16 | 0.00–4.73 | 0.84 (40) | 0.13 (48) | 26 |
| Roraima | 75 | 27 | 0.55 (0.65) | 0.41 | 0.00–3.55 | 0.87 (43) | 0.12 (32) | 40 |
| Amazon Region | 1010 | 80 | 0.34 (0.56) | 0.13 | 0.00–4.73 | 0.60 (488) | 0.09 (522) | 21 |
* Standard deviation.
Analyzing the different trophic levels overall, 110 herbivorous fish, 130 detritivores, 286 omnivores, and 484 carnivores were sampled. The average concentrations of mercury among noncarnivorous fish (i.e., herbivores, detritivores, and omnivores) and carnivorous fish were 0.092 μg/g (n = 526) and 0.603 μg/g (n = 484), respectively (Table 1).
Considering the levels of mercury contamination, the risk ratio, and the maximum safe fish consumption based on the state’s geographic division, the results were as follows. In Acre (AC), 78 fish specimens from 25 different species were sampled. The average concentration of mercury was 0.58 μg/g and the median was 0.15 μg/g, with 35.9% of the samples exceeding the safe limit of 0.5 μg/g (Table 1). The analysis of the risk associated with fish consumption revealed that the daily intake of mercury exceeded the reference dose recommended by the U.S. EPA (0.1 μg/kg bw/day) in all population groups analyzed (Table 2). In summary, the potential intake of mercury ranged from 7 to 31 times higher than the reference dose recommended by the U.S. EPA. Analyzing the population groups most vulnerable to the effects of mercury, women of childbearing age may be ingesting approximately nine times more mercury than the recommended safe dose, whereas children aged from two to four years may be ingesting up to thirty-one times more Hg (Table 2). In the state, the most mercury-contaminated fish were Cachorra (average: 1.45 μg/g), Filhote (average: 2.07 μg/g), and Dourada (average: 3.57 μg/g). On the other hand, Pacú, Pirapitinga, and Tambaqui can be consumed freely by all analyzed population groups since they had mercury levels close to zero (i.e., lower than 0.0005 μg/g and, therefore, undetectable by the analytical method). Furthermore, Tilápia, Jatuarana, Aracú Cabeça Gorda, and Acará can be safely consumed by adult men and women of childbearing age in quantities ranging from 103 to 418 g/day (Table 3).
Table 2.
Attributable risk ratio for consumption of mercury-contaminated fish according to the reference dose recommended by the U.S. EPA by Federal Units and population groups analyzed in the Amazon Basin, Brazil, 2021–2022.
| State | Population Group | Ingested Dose (µg/kg bw/day) | Absorbed Dose ~80% (µg/kg bw/day) | Risk Ratio (U.S. EPA) |
|---|---|---|---|---|
| Acre | Adult men | 0.85 | 0.68 | 7 |
| Women of Childbearing Age | 1.12 | 0.9 | 9 | |
| Children aged from 5 to 12 years | 2.04 | 1.63 | 16 | |
| Children aged from 2 to 4 years | 3.94 | 3.15 | 31 | |
| Adult men | 0.22 | 0.17 | 2 | |
| Amapá | Women of Childbearing Age | 0.28 | 0.23 | 2 |
| Children aged from 5 to 12 years | 0.52 | 0.42 | 4 | |
| Children aged from 2 to 4 years | 1.00 | 0.80 | 8 | |
| Adult men | 0.58 | 0.47 | 5 | |
| Amazonas | Women of Childbearing Age | 0.76 | 0.61 | 6 |
| Children aged from 5 to 12 years | 1.40 | 1.12 | 11 | |
| Children aged from 2 to 4 years | 2.69 | 2.15 | 21 | |
| Adult men | 0.42 | 0.34 | 3 | |
| Pará | Women of Childbearing Age | 0.56 | 0.45 | 4 |
| Children aged from 5 to 12 years | 1.02 | 0.81 | 8 | |
| Children aged from 2 to 4 years | 1.96 | 1.57 | 16 | |
| Adult men | 0.72 | 0.58 | 6 | |
| Rondônia | Women of Childbearing Age | 0.95 | 0.76 | 7 |
| Children aged from 5 to 12 years | 1.73 | 1.39 | 14 | |
| Children aged from 2 to 4 years | 3.34 | 2.67 | 27 | |
| Adult men | 0.74 | 0.59 | 6 | |
| Roraima | Women of Childbearing Age | 0.97 | 0.77 | 8 |
| Children aged from 5 to 12 years | 1.76 | 1.41 | 14 | |
| Children aged from 2 to 4 years | 3.40 | 2.72 | 27 |
Table 3.
Characterization of fish acquired in the state of Acre and calculation of the maximum safe consumption (MSC) in grams in the Amazon Basin, Brazil, 2021–2022.
| Fish Characterization | MSC (g/day) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Common Name | Scientific Name | N | Mean Hg μg/g | Trophic Level | Mean Length (cm) | Mean Weight (g) | Adult Men | Women | Children Aged from 5 to 12 Years | Children Aged from 2 to 4 Years |
| Acará | Cichlidae | 3 | 0.05 | Omnivorous | 21.16 | 213.33 | 136.49 | 104 | 57 | 29 |
| Acari | Loricariidae | 1 | 0.13 | Detritivorous | 39.00 | 465.00 | 53.08 | 40 | 22 | 11 |
| Aracu Cabeça Gorda | Leporinus spp. | 6 | 0.04 | Omnivorous | 36.48 | 848.33 | 171.49 | 130 | 71 | 37 |
| Aracu Flamengo | Leporinus fasciatus | 3 | 0.37 | Omnivorous | 31.50 | 395.00 | 18.08 | 14 | 7 | 4 |
| Bico de Pato | Sorubim lima | 2 | 0.86 | Carnivorous | 36.50 | 295.00 | 7.75 | 6 | 3 | 2 |
| Branquinha | Psectrogaster sp. | 2 | 0.11 | Detritivorous | 24.15 | 165.00 | 61.93 | 47 | 26 | 13 |
| Cachorra | Cynodontidae | 4 | 1.45 | Carnivorous | 42.50 | 730.00 | 4.62 | 3 | 2 | 1 |
| Curimatã | Prochilodus nigricans | 6 | 0.09 | Detritivorous | 51.17 | 1314.17 | 74.31 | 56 | 31 | 16 |
| Dourada | Brachyplatystoma rousseauxii | 3 | 3.57 | Carnivorous | N.D. | N.D. | 1.87 | 1 | 1 | 0 |
| Filhote | Brachyplatystoma filamentosum | 3 | 2.07 | Carnivorous | N.D. | N.D. | 3.24 | 2 | 1 | 1 |
| Jatuarana | Brycon sp. | 3 | 0.02 | Omnivorous | 43.35 | 1395.00 | 393.41 | 300 | 164 | 85 |
| Jundiá | Pimelodiae | 4 | 1.19 | Carnivorous | 74.00 | 1595.00 | 5.62 | 4 | 2 | 1 |
| Mandi | Pimelodus blochii | 1 | 0.17 | Omnivorous | 23.00 | 115.00 | 38.88 | 30 | 16 | 8 |
| Pacú | Myleus sp. | 3 | 0 | Herbivorous | 22.85 | 290.00 | N.R. | N.R. | N.R. | N.R. |
| Pescada | Plagioscion squamossimus | 3 | 0.94 | Carnivorous | 31.00 | 290.00 | 7.15 | 5 | 3 | 1 |
| Piabinha | Characidae | 3 | 0.09 | Omnivorous | 19.33 | 108.33 | 71.91 | 55 | 30 | 15 |
| Pintadinho | Calophysus macropterus | 3 | 1.12 | Carnivorous | N.D. | 593.33 | 5.95 | 4 | 2 | 1 |
| Pirapitinga | Piaractus brachypomus | 1 | 0 | Omnivorous | 60.00 | 3510.00 | N.R. | N.R. | N.R. | N.R. |
| Pirarucu | Arapaima gigas | 3 | 0.69 | Carnivorous | N.D. | N.D. | 9.75 | 7 | 4 | 2 |
| Surubim | Pseudoplatystoma sp. | 6 | 0.64 | Carnivorous | 47.92 | 6290.00 | 10.50 | 8 | 4. | 2 |
| Tambaqui | Colossoma macropomum | 3 | 0 | Omnivorous | 61.33 | 4153.00 | N.R. | N.R. | N.R. | N.R. |
| Tamoatá | Hoplosternum littorale | 3 | 0.20 | Omnivorous | 17.17 | 75.00 | 33 | 25 | 14 | 7 |
| Tilápia | Oreochromis sp. | 3 | 0.02 | Omnivorous | 37.43 | 1050.00 | 418 | 318 | 174 | 90 |
| Traíra | Hoplias malabaricus | 3 | 0.08 | Carnivorous | 39.67 | 608.33 | 82 | 63 | 34 | 18 |
| Tucunaré | Cichla sp. | 3 | 0.21 | Carnivorous | 33.67 | 505.00 | 32 | 24 | 13 | 7 |
N.D.—no date; N.R.—no restriction for consumption.
A total of 114 fish specimens from 27 distinct species were sampled in Amapá (AP). The average concentration of mercury was 0.18 μg/g, the median was 0.08 μg/g, and 11.4% of the samples had mercury levels higher than 0.5 μg/g (Table 1). The analysis of the risk associated with fish consumption revealed that the daily intake of mercury exceeded the reference dose recommended by the U.S. EPA (0.1 μg/kg bw/day) in all population groups. Mercury intake ranged from 1.7 to 8 times more than the reference dose. Among the population groups most vulnerable to the effects of mercury, women of childbearing age ingested approximately four times more mercury than the recommended dose, and children aged from two to four years ingested eight times more (Table 2). The most mercury-contaminated fish were Uéua (average: 0.49 μg/g), Traíra (average: 0.53 μg/g), and Tucunaré (average: 0.84 μg/g). On the other hand, Acari, Aracú Cabeça Gorda, Jatuarana, Pacú, and Pirapitinga can be freely consumed by all population groups analyzed since they had mercury levels close to zero (i.e., below 0.0005 μg/g and, therefore, undetectable by the analytical method). Furthermore, Tambaqui, Aracú, and Pescada Amarela can be safely consumed by all population groups in quantities ranging from 120 to 1,114 g/day (Table 4).
Table 4.
Characterization of fish acquired from the state of Amapá and calculation of the maximum safe consumption (MSC) in grams in the Amazon Basin, Brazil, 2021–2022.
| Fish Characterization | MSC (g/day) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Common Name | Scientific Name | N | Mean Hg μg/g | Trophic Level | Mean Length (cm) | Mean Weight (g) | Adult Men | Women | Children Aged from 5 to 12 Years | Children Aged from 2 to 4 Years |
| Acará | Cichlidae | 3 | 0.04 | Omnivorous | 7.33 | 115 | 167.20 | 127 | 70 | 36 |
| Acari | Loricariidae | 7 | 0 | Detritivorous | 44.8 | 874.71 | N.R. | N.R. | N.R. | N.R. |
| Anujá | Trachelyopterus galeatus | 3 | 0.164 | Omnivorous | 7.33 | 95 | 40.78 | 31 | 17 | 9 |
| Apapá | Pellona sp. | 5 | 0.254 | Carnivorous | 51.8 | 1570.8 | 26.33 | 20 | 11 | 6 |
| Aracu | Schizodon fasciatus | 3 | 0.01 | Herbivorous | 31.5 | 382.66 | 668.80 | 509 | 279 | 145 |
| Aracu Cabeça Gorda | Leporinus spp. | 3 | 0 | Omnivorous | 35.5 | 608 | N.R. | N.R. | N.R. | N.R. |
| Arraia | Potamotrygon sp. | 1 | 0.338 | Carnivorous | N.D. | N.D. | 19.79 | 15 | 8 | 4 |
| Dourada | Brachyplatystoma rousseauxii | 6 | 0.155 | Carnivorous | 82.33 | 3425.16 | 43.15 | 33 | 18 | 9 |
| Filhote | Brachyplatystoma filamentosum | 5 | 0.227 | Carnivorous | 114.33 | 17858 | 29.46 | 22 | 12 | 6 |
| Gurijuba | Sciades parkeri | 3 | 0.029 | Carnivorous | 94.33 | 7012 | 230 | 176 | 96 | 50 |
| Jacundá | Crenicichla sp. | 1 | 0.143 | Carnivorous | 12.5 | 375 | 47 | 35 | 19 | 10 |
| Jatuarana | Brycon sp. | 3 | 0 | Omnivorous | 41 | 1216 | N.R. | N.R. | N.R. | N.R. |
| Jeju | Hoplerythrinus unitaeniatus | 3 | 0.121 | Carnivorous | 10.17 | 236.6 | 55 | 42 | 23 | 12 |
| Mapará | Hypophthalmus sp. | 3 | 0.02 | Herbivorous | 42.67 | 458.66 | 334 | 255 | 139 | 72 |
| Pacu | Myleus sp., Myloplus sp. | 2 | 0 | Herbivorous | 36.5 | 769.5 | N.R. | N.R. | N.R. | N.R. |
| Pescada | Plagioscion squamossimus | 10 | 0.2604 | Carnivorous | 63.35 | 2716.8 | 26 | 19 | 11 | 5 |
| Pescada Amarela | Cynoscion acoupa | 7 | 0.012 | Carnivorous | 70.36 | 2985.14 | 557 | 424 | 233 | 121 |
| Piramutaba | Brachyplatystoma vaillantii | 4 | 0.054 | Carnivorous | 67.5 | 2025 | 124 | 94 | 52 | 27 |
| Piranha | Serrasalmidae | 5 | 0.254 | Carnivorous | 13.9 | 210 | 26 | 20 | 11 | 6 |
| Pirapitinga | Piaractus brachypomus | 4 | 0 | Omnivorous | 63.88 | 4898.5 | N.R. | N.R. | N.R. | N.R. |
| Robalo | Centropomus undecimalis | 3 | 0.04 | Carnivorous | 55.5 | 1396.66 | 167 | 127 | 70 | 36 |
| Surubim | Pseudoplatystoma sp. | 3 | 0.165 | Carnivorous | 68.67 | 2593.33 | 40 | 31 | 17 | 9 |
| Tambaqui | Colossoma macropomum | 6 | 0.006 | Omnivorous | 62.17 | 4436.8 | 1115 | 849 | 465 | 241 |
| Tamoatá | Hoplosternum littorale | 3 | 0.058 | Omnivorous | 8.17 | 151.66 | 115 | 88 | 48 | 25 |
| Traíra | Hoplias malabaricus | 12 | 0.527 | Carnivorous | 58.17 | 3867.58 | 13 | 10 | 5 | 3 |
| Tucunaré | Cichla sp. | 4 | 0.844 | Carnivorous | 54.67 | 2736 | 8 | 6 | 3 | 2 |
| Uéua | Acestrorhynchus falcirostris | 2 | 0.495 | Carnivorous | 10.5 | 157.5 | 13 | 10 | 6 | 3 |
N.D.—no date; N.R.—unrestricted consumption.
A total of 262 fish specimens from 34 different species were analyzed in Amazonas (AM). The average concentration of mercury was 0.34 μg/g, and the median was 0.14 μg/g, with 22.5% of the samples exceeding the safe limit of mercury (Table 1). The analysis of the risk associated with fish consumption revealed that the daily intake of mercury exceeded the reference dose in all analyzed population groups (Table 2). In summary, the intake of mercury ranged from 5 to 21 times higher than the reference dose. Women of childbearing age ingested approximately six times the recommended dose, whereas the group of children aged from two to four years ingested twenty-one times more mercury than the recommended dose. The most mercury-contaminated fish were Apapá (average: 1.49 μg/g), Pirapucu (average: 1.61 μg/g), and Filhote (average: 1.70 μg/g). The fish with the lowest concentrations of mercury were Jundiá, Acari, Pacú, Pirapitinga, and Tambaqui. These species showed average levels of mercury below 0.03 μg/g and, therefore, can be consumed in quantities ranging from 107 to 668 g/day by women of childbearing age, children aged from 5 to 12 years, and adult men (Table 5).
Table 5.
Characterization of fish acquired from the state of Amazonas and calculation of the maximum safe consumption (MSC) in grams in the Amazon Basin, Brazil, 2021–2022.
| Fish Characterization | MSC (g/day) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Common Name | Scientific Name | N | Mean Hg μg/g | Trophic Level | Mean Length (cm) | Mean Weight (g) | Adult Men | Women | Children Aged from 5 to 12 Years | Children Aged from 2 to 4 Years |
| Acará | Cichlidae | 8 | 0.149 | Omnivorous | 21.56 | 187.25 | 44.89 | 34 | 19 | 10 |
| Acará-açu | Astronotus sp. | 3 | 0.069 | Omnivorous | 20.93 | 203.66 | 96.93 | 74 | 40 | 21 |
| Acari | Loricariidae | 9 | 0.012 | Detritivorous | 37.08 | 511.67 | 557.33 | 424 | 233 | 121 |
| Apapá | Pellona sp. | 3 | 1.492 | Carnivorous | 56 | 2166.67 | 4.48 | 3 | 2 | 1 |
| Aracu | Schizodon fasciatus | 5 | 0.078 | Herbivorous | 27.6 | 324 | 85.74 | 65 | 36 | 18 |
| Aracu Cabeça Gorda | Leporinus spp. | 10 | 0.092 | Omnivorous | 34 | 512.86 | 72.70 | 55 | 30 | 16 |
| Aracu Flamengo | Leporinus fasciatus | 5 | 0.099 | Omnivorous | 29.28 | 266 | 67.56 | 51 | 28 | 15 |
| Aruanã | Osteoglossum bicirrhosum | 10 | 0.3713 | Carnivorous | 60.55 | 1453.78 | 18.01 | 14 | 7 | 4 |
| Branquinha | Psectrogaster sp. | 6 | 0.0865 | Detritivorous | 23.38 | 178 | 77.32 | 59 | 32 | 17 |
| Cachorra | Cynodontidae | 5 | 0.6288 | Carnivorous | 48.7 | 877.33 | 10.64 | 8 | 4 | 2 |
| Charuto | Hemiodus sp. | 7 | 0.1681 | Omnivorous | 25.62 | 218.43 | 39.79 | 30 | 16 | 9 |
| Cuiu | Oxydoras niger | 6 | 0.177 | Omnivorous | 51.78 | 1535.67 | 37.79 | 29 | 16 | 8 |
| Curimatã | Prochilodus nigricans | 9 | 0.043 | Detritivorous | 28.78 | 1549.89 | 155.53 | 118 | 65 | 34 |
| Filhote | Brachyplatystoma filamentosum | 4 | 1.702 | Carnivorous | 80 | 6601.67 | 3.93 | 3 | 1 | 1 |
| Jacundá | Crenicichla sp. | 3 | 0.4116 | Carnivorous | 36.33 | 495 | 16.25 | 12 | 7 | 3 |
| Jaraqui | Semaprochilodus sp. | 12 | 0.1 | Detritivorous | 25.04 | 265.33 | 66.88 | 51 | 28 | 14 |
| Jatuarana | Brycon sp. | 19 | 0.071 | Omnivorous | 31.54 | 592.79 | 94.20 | 72 | 39 | 20 |
| Jeju | Hoplerythrinus unitaeniatus | 3 | 0.24 | Carnivorous | 28.67 | 331.33 | 27.87 | 21 | 12 | 6 |
| Jundiá | Pimelodiae | 1 | 0.01 | Carnivorous | 50 | 1255 | 668.80 | 509 | 279 | 145 |
| Mandi | Pimelodus blochii | 1 | 0.783 | Omnivorous | 21 | 500 | 8.54 | 6 | 3 | 2 |
| Mandubé | Auchenipteridae | 1 | 0.783 | Carnivorous | 46 | N.D. | 8.54 | 6 | 3 | 2 |
| Pacú | Myleus sp. Mylossoma sp. | 13 | 0.016 | Herbivorous | 19.75 | 205.09 | 418.00 | 318 | 174 | 9 |
| Pescada | Plagioscion squamossimus | 4 | 0.799 | Carnivorous | 37.5 | 656.67 | 8.37 | 6 | 3 | 2 |
| Pirandira | Hydrolycus scomberoides | 4 | 0.974 | Carnivorous | 37.75 | 648.75 | 6.87 | 5 | 3 | 1 |
| Piranha | Serrasalmidae | 21 | 0.762 | Carnivorous | 24.7 | 433.72 | 8.78 | 7 | 4 | 2 |
| Pirapitinga | Piaractus brachypomus | 7 | 0.0194 | Omnivorous | 39.9 | 1552.14 | 344.74 | 263 | 144 | 75 |
| Pirapucu | Cynodontidae | 3 | 1.609 | Carnivorous | 45 | 477.5 | 4.16 | 3 | 2 | 1 |
| Pirarara | Phractocephalus hemiolipterus | 7 | 0.724 | Omnivorous | 55 | 4247.5 | 9.24 | 7 | 4 | 2 |
| Pirarucu | Arapaima gigas | 4 | 0.287 | Carnivorous | 35.33 | 1233.33 | 23.30 | 18 | 10 | 5 |
| Sardinha | Triportheus sp. | 12 | 0.129 | Omnivorous | 23.75 | 137 | 51.84 | 39 | 22 | 11 |
| Surubim | Pseudoplatystoma sp. | 7 | 0.652 | Carnivorous | 68.42 | 2259 | 10.26 | 8 | 4 | 2 |
| Tambaqui | Colossoma macropomum | 15 | 0.026 | Omnivorous | 52.06 | 4214 | 257.23 | 196 | 107 | 56 |
| Traíra | Hoplias malabaricus | 12 | 0.4215 | Carnivorous | 39.87 | 695.91 | 15.87 | 12 | 7 | 3 |
| Tucunaré | Cichla sp. | 23 | 0.567 | Carnivorous | 37.81 | 900.04 | 11.80 | 9 | 5 | 2 |
A total of 393 fish specimens were collected from 47 distinct species in Pará (PA). The average concentration of mercury was 0.27 μg/g, the median was 0.1 μg/g, and 15.8% of the collected fish had mercury levels above 0.5 μg/g (Table 1). The analysis of the risk associated with fish consumption revealed that mercury intake could be from 3 to 16 times higher than the reference dose. Women of childbearing age ingested approximately four times the recommended dose of mercury, and children aged from two to four years old ingested fifteen times more. The most mercury-contaminated fish were Pirarara (average: 0.92 μg/g), Jaú (average: 0.95 μg/g), and Barbado (average: 1.58 μg/g). The Pacú Branco, Pirapitinga, and Pratiqueira, in turn, can be consumed freely by all population groups analyzed since they had mercury levels close to zero (i.e., lower than 0.0005 μg/g and, therefore, undetectable by the analytical method). Furthermore, the Pacú Manteiga, Tambaqui, Pacú, Tainha, and Aracú can be safely consumed by women of childbearing age, children aged from 5 to 12, and adult men in quantities ranging from 126 to 2229 g/day (Table 6).
Table 6.
Characterization of fish acquired from the state of Pará and calculation of the maximum safe consumption (MSC) in grams in the Amazon Basin, Brazil, 2021–2022.
| Fish Characterization | MSC (g/day) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Common Name | Scientific Name | N | Mean Hg μg/g | Trophic Level | Mean Length (cm) | Mean Weight (g) | Adult Men | Women | Children Aged from 5 to 12 Years | Children Aged from 2 to 4 Years |
| Acará-açu | Astronotus sp. | 3 | 0.067 | Omnivorous | 23.83 | 311.67 | 99.82 | 76 | 42 | 22 |
| Acaratinga | Geophagus sp. | 6 | 0.058 | Omnivorous | 24.72 | 250.50 | 115.31 | 88 | 48 | 25 |
| Acari | Loricariidae | 16 | 0.032 | Detritivorous | 33.08 | 398.63 | 209.00 | 159 | 87 | 45 |
| Apapá | Pellona sp. | 10 | 0.202 | Carnivorous | 39.60 | 476.80 | 33.11 | 25 | 14 | 7 |
| Aracu | Schizodon fasciatus | 12 | 0.022 | Herbivorous | 33.27 | 311.17 | 304.00 | 231 | 127 | 66 |
| Aracu Cabeça Gorda | Leporinus spp. | 6 | 0.054 | Omnivorous | 42.22 | 1134.00 | 123.85 | 94 | 52 | 27 |
| Aracu Flamengo | Leporinus fasciatus | 7 | 0.216 | Omnivorous | 34.42 | 293.57 | 30.96 | 23 | 13 | 7 |
| Arraia | Potamotrygon sp. | 1 | 0.624 | Carnivorous | N.D. | N.D. | 10.72 | 8 | 4 | 2 |
| Barbado | Pinirampus pirinampu | 6 | 1.584 | Carnivorous | 67.87 | 2910.00 | 4.22 | 3 | 2 | 1 |
| Bico de Pato | Sorubim lima | 3 | 0.255 | Carnivorous | 55.67 | 900.00 | 26.23 | 20 | 11 | 6 |
| Branquinha | Psectrogaster sp. | 8 | 0.0503 | Detritivorous | 26.58 | 202.25 | 132.96 | 101 | 55 | 29 |
| Cação | Carcharhinus sp. | 3 | 0.304 | Carnivorous | N.D. | 4800.00 | 22.00 | 17 | 9 | 5 |
| Cachorra | Cynodontidae | 9 | 0.885 | Carnivorous | 59.50 | 2917.78 | 7.56 | 6 | 3 | 2 |
| Charuto | Hemiodus sp. | 8 | 0.032 | Omnivorous | 19.00 | 67.13 | 209.00 | 159 | 87 | 45 |
| Corvina | Sciaenidae | 6 | 0.424 | Carnivorous | 68.67 | 2919.17 | 15.77 | 12 | 6 | 3 |
| Curimatã | Prochilodus nigricans | 16 | 0.071 | Detritivorous | 34.61 | 757.88 | 94.20 | 72 | 39 | 20 |
| Dourada | Brachyplatystoma rousseauxii | 13 | 0.475 | Carnivorous | 83.19 | 4831.92 | 14.08 | 11 | 6 | 3 |
| Fidalgo | Ageneiosus sp. | 4 | 0.457 | Carnivorous | 57.75 | 2280.00 | 14.63 | 11 | 6 | 3 |
| Filhote | Brachyplatystoma filamentosum | 14 | 0.598 | Carnivorous | 91.35 | 13703.21 | 11.18 | 8 | 5 | 2 |
| Gó | Macrodon ancylodon | 3 | 0.032 | Carnivorous | N.D. | 8366.67 | 209.00 | 159 | 87 | 45 |
| Gurijuba | Sciades parkerii | 3 | 0.126 | Carnivorous | N.D. | 8366.67 | 53.08 | 40 | 22 | 11 |
| Jaraqui | Semaprochilodus sp. | 15 | 0.0641 | Detritivorous | 3015.47 | 564.47 | 104.34 | 79 | 43 | 23 |
| Jatuarana | Brycon sp. | 18 | 0.0528 | Omnivorous | 38.73 | 1060.44 | 126.67 | 96 | 53 | 27 |
| Jaú | Zungaro zungaro | 3 | 0.954 | Carnivorous | 82.33 | 6424.33 | 7.01 | 5 | 3 | 1 |
| Jiripoca | Hemisorubim platyrhynchos | 3 | 0.363 | Carnivorous | 51.66 | 1026.67 | 18.42 | 14 | 8 | 4 |
| Mandi | Pimelodus blochii | 1 | 0.635 | Omnivorous | 43.00 | 590.00 | 10.53 | 8 | 4 | 2 |
| Mapará | Hypophthalmus sp. | 20 | 0.227 | Herbivorous | 43.55 | 427.80 | 29.46 | 22 | 12 | 6 |
| Pacu | Myleus sp. Mylossoma sp. | 15 | 0.0104 | Herbivorous | 33.07 | 864.73 | 643.08 | 490 | 268 | 139 |
| Pacu Branco | Myleus sp. | 3 | 0 | Herbivorous | 32.83 | 764.00 | N.R. | N.R. | N.R. | N.R. |
| Pacu Manteiga | Mylossoma duriventre | 11 | 0.003 | Herbivorous | 26.16 | 182.27 | 2229.33 | 1698 | 931 | 48 |
| Pescada | Plagioscion squamossimus | 19 | 0.306 | Carnivorous | 43.54 | 1179.00 | 21.86 | 17 | 9 | 5 |
| Pescada Amarela | Cynoscion acoupa | 3 | 0.158 | Carnivorous | 94.00 | 7196.67 | 42.33 | 32 | 18 | 9 |
| Piramutaba | Brachyplatystoma vaillantii | 4 | 0.1307 | Carnivorous | 62.25 | 2011.25 | 51.17 | 39 | 21 | 11 |
| Piranha | Serrasalmidae | 18 | 0.479 | Carnivorous | 30.37 | 736.06 | 13.96 | 11 | 6 | 3 |
| Pirapema | Megalops atlanticus | 3 | 0.165 | Carnivorous | 96.33 | 6866.67 | 40.53 | 31 | 17 | 9 |
| Pirapitinga | Piaractus brachypomus | 7 | 0 | Omnivorous | 40.93 | 1452.57 | N.R. | N.R. | N.R. | N.R. |
| Pirarara | Phractocephalus hemiolipterus | 5 | 0.921 | Omnivorous | 78.40 | 9918.80 | 7.26 | 5 | 3 | 1 |
| Pirarucu | Arapaima gigas | 4 | 0.391 | Carnivorous | 115.00 | 34767.50 | 17.10 | 13 | 7 | 4 |
| Pratiqueira | Mugil sp. | 3 | 0 | Detritivorous | 31.00 | 366.67 | N.R. | N.R. | N.R. | N.R. |
| Serra | Scomberomorus brasiliensis | 3 | 0.08 | Carnivorous | 66.66 | 1366.67 | 83.60 | 64 | 35 | 18 |
| Surubim | Pseudoplatystoma sp. | 17 | 0.471 | Carnivorous | 63.68 | 2530.35 | 14.20 | 11 | 6 | 3 |
| Tainha | Mugil sp. | 3 | 0.018 | Detritivorous | 50.66 | 1566.67 | 371.56 | 283 | 155 | 80 |
| Tambaqui | Colossoma macropomum | 21 | 0.0098 | Omnivorous | 56.36 | 3548.86 | 682.45 | 520 | 285 | 148 |
| Traíra | Hoplias malabaricus | 5 | 0.3818 | Carnivorous | 54.00 | 2312.00 | 17.52 | 13 | 7 | 4 |
| Tamoatá | Hoplosternum littorale | 6 | 0.0815 | Omnivorous | 19.98 | 145.83 | 82.06 | 62 | 34 | 18 |
| Tucunaré | Cichla sp. | 23 | 0.54 | Carnivorous | 47.24 | 1836.65 | 12.39 | 9 | 5 | 3 |
| Zebra | Pimelodidae | 3 | 0.731 | Carnivorous | 76.66 | 4700 | 9.15 | 7 | 4 | 2 |
N.D.—no date; N.R.—no restriction for consumption.
A total of 88 fish samples from 28 different species were analyzed in Rondônia (RO). The average concentration of mercury was 0.45 μg/g, and the median was 0.16 μg/g, with 26.1% of the fish having mercury levels above 0.5 μg/g (Table 1). The analysis of the risk associated with fish consumption revealed that daily intake of mercury exceeded the reference dose from 6 to 27 times in all population groups analyzed (Table 2). Women of childbearing age ingested approximately eight times more mercury than men, and children aged from two to four years ingest twenty-seven times more mercury than adults. The most mercury-contaminated fish were Dourada (average: 1.81 μg/g), Filhote (average: 1.84 μg/g), and Babão (average: 2.87 μg/g). However, Acará, Bacu, and Pirapitinga can be consumed freely by all analyzed population groups, as they had mercury levels close to zero. Furthermore, Pacú can be safely consumed by women of childbearing age and adult men in quantities ranging from 137 to 180 g/day (Table 7).
Table 7.
Characterization of fish acquired from the state of Rondônia and calculation of the maximum safe consumption (MSC) in grams in the Amazon Basin, Brazil, 2021–2022.
| Fish Characterization | MSC (g/day) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Common Name | Scientific Name | N | Mean Hg μg/g | Trophic Level | Mean Length (cm) | Mean Weight (g) | Adult Men | Women | Children Aged from 5 to 12 Years | Children Aged from 2 to 4 Years |
| Acará | Cichlidae | 3 | 0 | Omnivorous | 19.66 | 153.33 | N.R. | N.R. | N.R. | N.R. |
| Acará-açu | Astronotus sp. | 3 | 0.199 | Omnivorous | 26.33 | 467.66 | 33.61 | 26 | 14 | 7 |
| Aracu | Schizodon fasciatus | 3 | 0.1 | Herbivorous | 31.66 | 301 | 66.88 | 51 | 28 | 14 |
| Aracu Flamengo | Leporinus fasciatus | 3 | 0.32 | Omnivorous | 28.66 | 257 | 20.90 | 16 | 9 | 4 |
| Babão | Goslinia platynema | 3 | 2.87 | Carnivorous | 72.66 | 3533.33 | 2.33 | 18 | 1 | 0 |
| Bacu | Lithodoras dorsalis | 1 | 0 | Omnivorous | N.D. | N.D. | N.R. | N.R. | N.R. | N.R. |
| Branquinha | Psectrogaster sp. | 3 | 0.08 | Detritivorous | 28.33 | 310.33 | 83.60 | 64 | 35 | 18 |
| Cangati | Auchenipteridae | 2 | 0.055 | Carnivorous | 21.5 | 175 | 121.60 | 93 | 51 | 26 |
| Cuiu | Oxydoras niger | 2 | 0.241 | Omnivorous | 82 | 5577.5 | 27.75 | 21 | 12 | 6 |
| Curimatã | Prochilodus nigricans | 3 | 0.019 | Detritivorous | 32.66 | 542.66 | 352.00 | 268 | 147 | 76 |
| Dourada | Brachyplatystoma rousseauxii | 3 | 1.807 | Carnivorous | 88 | 7583 | 3.70 | 3 | 1 | 1 |
| Filhote | Brachyplatystoma filamentosum | 3 | 1.844 | Carnivorous | 112.5 | 24333.33 | 3.63 | 3 | 1 | 1 |
| Jaraqui | Semaprochilodus sp. | 3 | 0.115 | Detritivorous | 27.33 | 337.33 | 58.16 | 44 | 24 | 13 |
| Jatuarana | Brycon sp. | 6 | 0.0751 | Omnivorous | 34.33 | 768.66 | 89.05 | 68 | 37 | 19 |
| Jundiá | Pimelodidae | 1 | 0.309 | Carnivorous | 62 | 2945 | 21.64 | 16 | 9 | 5 |
| Mandi | Pimelodus blochii | 3 | 0.071 | Omnivorous | 23.66 | 120 | 94.20 | 72 | 39 | 20 |
| Pacu | Myleus sp. Mylossoma sp. | 3 | 0.037 | Herbivorous | 22.33 | 346.33 | 180.76 | 138 | 75 | 39 |
| Pintadinho | Calophysus macropterus | 1 | 0.22 | Carnivorous | 37 | 355 | 30.40 | 23 | 13 | 6 |
| Piramutaba | Brachyplatystoma vaillantii | 1 | 0.279 | Carnivorous | 30 | 235 | 23.97 | 18 | 10 | 5 |
| Piranha | Serrasalmidae | 3 | 0.151 | Carnivorous | 23.66 | 461 | 44.29 | 34 | 18 | 10 |
| Pirapitinga | Piaractus brachypomus | 3 | 0 | Omnivorous | 29.33 | 642 | N.R. | N.R. | N.R. | N.R. |
| Pirarara | Phractocephalus hemiolipterus | 1 | 0.816 | Omnivorous | 29.33 | 642 | 8.20 | 6 | 3 | 2 |
| Pirarucu | Arapaima gigas | 5 | 0.323 | Carnivorous | 102 | 7950 | 20.71 | 16 | 9 | 4 |
| Sardinha | Triportheus sp. | 2 | 0.199 | Omnivorous | 17.5 | 121.5 | 33.61 | 26 | 14 | 7 |
| Surubim | Pseudoplatystoma sp. | 13 | 0.778 | Carnivorous | 68.92 | 3716.84 | 8.60 | 6 | 3 | 2 |
| Tambaqui | Colossoma macropomum | 4 | 0.262 | Omnivorous | 60.5 | 4954.25 | 25.53 | 19 | 11 | 5 |
| Traíra | Hoplias malabaricus | 3 | 0.144 | Carnivorous | 36 | 656 | 46.44 | 35 | 19 | 10 |
| Tucunaré | Cichla sp. | 4 | 0.244 | Carnivorous | 38.5 | 911 | 27.41 | 21 | 11 | 6 |
N.D.—no date; N.R.—no restriction for consumption.
A total of 75 fish specimens from 27 different species were collected in Roraima (RR). The average concentration of mercury was 0.55 μg/g, and the median was 0.41 μg/g, with 40% of the fish exceeding the safety limit (Table 1). The analysis of the risk associated with fish consumption revealed that the daily intake of mercury exceeded the reference dose in all population groups analyzed (Table 2), ranging from 6 to 27 times higher. Women of childbearing age ingested approximately eight times and children aged from two to four years ingest twenty-seven times more mercury than the recommended dose. The most mercury-contaminated fish were Pindirá (average: 1.07 μg/g), Filhote (average: 1.14 μg/g), Piracatinga (average: 1.49 μg/g), Barba Chata (average: 2.00 μg/g), and Coroataí (average: 2.13 μg/g). The fish with the lowest mercury concentrations were Pacú Maria Antônia, Aracú Flamengo, Pacú Meião, Pacú, and Jaraqui Escama Grossa. These species showed average mercury levels below 0.05 μg/g and, therefore, can be consumed in quantities ranging from 125 to 418 g/day by women of childbearing age and adult men (Table 8).
Table 8.
Characterization of fish acquired from the state of Roraima and calculation of the maximum safe consumption (MSC) in grams in the Amazon Basin, Brazil, 2021–2022.
| Fish Characterization | MSC (g/day) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Common Name | Scientific Name | N | Mean Hg μg/g | Trophic Level | Mean Length (cm) | Mean Weight (g) | Adult Men | Women | Children Aged from 5 to 12 Years | Children Aged from 2 to 4 Years |
| Acará-açu | Astronotus sp. | 4 | 0.332 | Omnivorous | 33 | 626.25 | 20.14 | 15 | 8 | 4 |
| Aracu Cabeça Gorda | Leporinus sp. | 1 | 0.147 | Omnivorous | 29 | 253 | 45.50 | 35 | 19 | 10 |
| Aracu Flamengo | Leporinus fasciatus | 2 | 0.033 | Omnivorous | 27.75 | 233 | 202.67 | 154 | 84 | 44 |
| Aracu Mandioca | Schizodon fasciatus | 2 | 0.238 | Carnivorous | 32 | 480 | 28.10 | 21 | 11 | 6 |
| Barba Chata | Pirinampus pirinampu | 3 | 1.997 | Carnivorous | 47.3 | 868.33 | 3.35 | 2 | 1 | 1 |
| Coroataí | Platynematichthys notatus | 4 | 2.131 | Detritivorous | 51.75 | 1378 | 3.14 | 2 | 1 | 1 |
| Curimatã | Prochilodus nigricans | 3 | 0.097 | Detritivorous | 28.07 | 358.33 | 68.95 | 52 | 29 | 15 |
| Dourada | Brachyplatystoma rousseauxii | 2 | 0.673 | Carnivorous | 80.5 | 1280 | 9.94 | 7 | 4 | 2 |
| Filhote | Brachyplatystoma filamentosum | 3 | 1.139 | Carnivorous | 99 | 18348.33 | 5.87 | 4 | 2 | 1 |
| Jandiá | Leiarius cf. mamoratus | 2 | 0.094 | Carnivorous | 49.75 | 1111 | 71.15 | 54 | 30 | 15 |
| Jaraqui Escama Grossa | Semaprochilodus insignis | 2 | 0.0405 | Detritivorous | 29 | 380 | 165.14 | 126 | 69 | 36 |
| Liro | Hemisorubim platyrhynchos | 1 | 0.413 | Carnivorous | 38 | 354 | 16.19 | 12 | 7 | 3 |
| Mandi | Pimelodus blochii | 2 | 0.423 | Omnivorous | 21 | 66.5 | 15.81 | 12 | 7 | 3 |
| Mandubé | Ageneiosus inermis | 1 | 0.539 | Carnivorous | 39 | 580 | 12.41 | 9 | 5 | 3 |
| Mantrinxã | Brycon falcatus | 12 | 0.132 | Omnivorous | 27.93 | 357.5 | 50.67 | 38 | 21 | 11 |
| Pacu | Myloplus sp. | 3 | 0.0386 | Herbivorous | 28.67 | 553.33 | 173.26 | 132 | 72 | 37 |
| Pacu Maria Antonia | Myleus sp. | 3 | 0.016 | Herbivorous | 20.33 | 218.33 | 418.00 | 318 | 174 | 90 |
| Pacu Meião | Myleus sp. | 2 | 0.033 | Herbivorous | 20.25 | 178 | 202.67 | 154 | 85 | 44 |
| Pescada | Plagioscion squamosissimus | 3 | 0.721 | Herbivorous | 34.5 | 591 | 9.28 | 7 | 4 | 2 |
| Pescado Branca | Plagioscion squamosissimus | 4 | 0.512 | Herbivorous | 37.8 | 543.75 | 13.06 | 10 | 5 | 3 |
| Piracatinga | Calophysus macropterus | 1 | 1.495 | Carnivorous | 45.6 | 770 | 4.47 | 3 | 2 | 1 |
| Pindirá/Peixe Cachorro | Hydrolycus scomberoides | 1 | 1.072 | Carnivorous | 56.6 | 2025 | 6.24 | 5 | 3 | 1 |
| Piranha Petra | Serrasalmus rhombeus | 2 | 0.406 | Carnivorous | 21.75 | 227 | 16.47 | 12 | 7 | 3 |
| Surubim | Pseudoplatystoma sp. | 7 | 0.649 | Carnivorous | 53.56 | 1134.71 | 10.31 | 8 | 4 | 2 |
| Tucunaré | Cichla sp. | 4 | 0.698 | Carnivorous | 41.02 | 986.25 | 9.58 | 7 | 4 | 2 |
| Tucunaré Borboleta | Cichla monoculus | 1 | 0.645 | Carnivorous | 38.1 | 805 | 10.37 | 8 | 4 | 2 |
Finally, the Poisson regression analysis revealed that the prevalence of mercury contamination ≥ 0.5 µg/g was approximately 14 times higher in carnivorous fish than in noncarnivorous fish (PR 13.8; 95% CI 8.4–22.5). The prevalence of mercury contamination ≥ 0.5 µg/g was approximately four times higher in Roraima (PR 3.9; 95% CI 2.3–6.7) and Acre (PR 3.9; 95% CI 2.3–6.6), three times higher in Rondônia (PR 3.1; 95% CI 1.8–5.6) and Amazonas (PR 2.9; 95% CI 1.7–5.0), and two times higher in Pará (PR 1.9; 95% CI 1.1–3.1) compared to Amapá (Table 9).
Table 9.
Poisson regression considering the response variable as levels of Hg ≥ 0.5 µg/g and the independent variables as trophic level and Federal Unit where fish were acquired in the Brazilian Amazon in 2021–2022.
| Variables | PR * Crude | 95% CI | p-Value | PR Adjusted | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| Trophic Level | ||||||
| Noncarnivorous | 1.0 | |||||
| Carnivorous | 13.3 | 8.1–21.8 | 0.001 | 13.8 | 8.4–22.5 | 0.001 |
| State | ||||||
| AP | 1.0 | |||||
| PA | 1.4 | 0.8–2.4 | 0.256 | 1.9 | 1.1–3.1 | 0.025 |
| AM | 2.0 | 1.1–3.5 | 0.017 | 2.9 | 1.7–5.0 | 0.001 |
| RO | 2.3 | 1.2–4.3 | 0.009 | 3.1 | 1.8–5.6 | 0.001 |
| AC | 3.1 | 1.7–5.7 | 0.001 | 3.9 | 2.3–6.6 | 0.001 |
| RR | 3.5 | 2.0–6.3 | 0.001 | 3.9 | 2.3–6.7 | 0.001 |
* Prevalence ratio.
Additional data on the fish contamination according to the studied municipalities, including the mean and median of mercury levels the trophic level of the sampled species, and the prevalence of exposure above 0.5µg/g can be seen in Table 10.
Table 10.
Mercury levels detected in fish samples from 17 municipalities in the Brazilian Amazon, 2021–2022.
| Municipality (State) | N | No. of Species | Mean Hg μg/g (S.D.) | Median Hg | Min–Max Hg | Mean Hg μg/g Carnivorous (n) | Mean Hg μg/g Noncarnivorous (n) | % ≥0.5 μg/g |
|---|---|---|---|---|---|---|---|---|
| Altamira (PA) | 43 | 13 | 0.30 (0.37) | 0.21 | 0.0–1.55 | 0.46 (25) | 0.08 (18) | 14 |
| Belém (PA) | 70 | 24 | 0.20 (0.33) | 0.08 | 0.0–2.39 | 0.29 (46) | 0.03 (24) | 8 |
| Boa Vista (RR) | 75 | 27 | 0.55 (0.65) | 0.41 | 0.0–3.56 | 0.87 (43) | 0.12 (32) | 40 |
| Humaitá (AM) | 60 | 20 | 0.36 (0.53) | 0.14 | 0.0–2.34 | 0.65 (25) | 0.15 (35) | 25 |
| Itaituba (PA) | 71 | 24 | 0.29 (0.39) | 0.09 | 0.0–1.63 | 0.65 (26) | 0.08 (45) | 21 |
| Macapá (AP) | 73 | 25 | 0.17 (0.24) | 0.09 | 0.0–1.24 | 0.28 (42) | 0.03 (31) | 11 |
| Manaus (AM) | 51 | 18 | 0.42 (0.53) | 0.16 | 0.0–2.18 | 0.85 (21) | 0.12 (30) | 27 |
| Maraã (AM) | 48 | 15 | 0.12 (0.12) | 0.08 | 0.0–0.52 | 0.33 (6) | 0.10 (42) | 2 |
| Oiapoque (AP) | 41 | 12 | 0.19 (0.28) | 0.08 | 0.0–1.13 | 0.25 (32) | 0.0 (9) | 12 |
| Oriximiná (PA) | 71 | 21 | 0.20 (0.30) | 0.06 | 0.0–1.25 | 0.47 (21) | 0.09 (50) | 14 |
| Porto Velho (RO) | 88 | 28 | 0.45 (0.82) | 0.16 | 0.0–4.73 | 0.85 (40) | 0.13 (48) | 26 |
| Rio Branco (AC) | 78 | 25 | 0.58 (0.97) | 0.15 | 0.0–4.64 | 1.06 (40) | 0.08 (38) | 36 |
| Santa Isabel do Rio Negro (AM) | 24 | 16 | 0.70 (0.51) | 0.51 | 0.0–3.22 | 0.95 (16) | 0.19 (8) | 50 |
| Santarém (PA) | 70 | 20 | 0.14 (0.23) | 0.03 | 0.0–1.13 | 0.35 (25) | 0.02 (45) | 7 |
| São Félix do Xingú (PA) | 68 | 22 | 0.50 (0.69) | 0.30 | 0.0–3.5 | 0.70 (40) | 0.22 (28) | 29 |
| São Gabriel da Cachoeira (AM) | 32 | 11 | 0.54 (0.50) | 0.43 | 0.0–2.25 | 0.67 (25) | 0.05 (7) | 50 |
| Tefé (AM) | 47 | 16 | 0.13 (0.15) | 0.05 | 0.0–0.65 | 0.3 (15) | 0.05 (32) | 2 |
S.D.—standard deviation.
4. Discussion
Although many authors have conducted investigations dedicated to analyzing the levels of mercury contamination in different areas of the Amazon at different times, this is the first study that carried out a risk assessment to human health attributed to the consumption of fish contaminated with mercury in urban areas. The samples were collected from places where most of the fish were commercialized in the 17 municipalities assessed in six Brazilian states.
Despite the numerous benefits associated with regular fish consumption, such as reducing blood cholesterol levels, decreasing the risk of myocardial infarction, and improving cognitive development, the increasing contamination of fish with methylmercury represents an important warning signal that authorities should not neglect. Public policies must consider the significance of the fishing industry and its professionals (fishermen), who are also greatly impacted by the increasing contamination. Currently, there are over 350,000 registered professional fishermen in the Secretariat of Aquaculture and Fisheries (2022), with an estimated total fish production of approximately 200,000 tons per year [20]. The estimated economic impact of inland fishing in Brazil is USD 828 million [29], with most of these fisheries occurring in the Amazon region.
Our analysis revealed that more than one-fifth (21.3%) of the fish sold in urban centers, which reach the tables of families in these regions, had mercury levels above the safe limits established by the Food and Agriculture Organization of the United Nations (FAO/WHO) [30] and the Brazilian Health Surveillance Agency (ANVISA) [31] (i.e., ≥ 0.5 µg/g).
The analysis by state revealed that Acre had the highest levels of mercury contamination (average = 0.58 µg/g), whereas the highest prevalence of contamination (i.e., fish with mercury levels ≥0.5 µg/g) was detected in the state of Roraima (40%). On the other hand, Amapá had the lowest levels of contamination (average = 0.18 µg/g), as well as the lowest prevalence of fish with mercury levels above 0.5 µg/g (11.40%).
When considering contamination prevalence ≥0.5 µg/g, the situation becomes slightly different, with Roraima surpassing Acre and taking the first position. The order of contamination prevalence was as follows: Amapá (11%) < Pará (16%) < Amazonas (22%) < Rondônia (26%) < Acre (36%) < Roraima (40%). Our results revealed that the most serious situations of mercury contamination in fish were concentrated in Roraima, Rondônia, and Acre. As widely reported by various authors [28,32,33,34], the increase in illegal gold-mining activities in Roraima and Rondônia is directly related to the high levels of mercury detected in the fish from these regions.
The comparative analysis based on the average levels of mercury in fish samples, as well as the Poisson regression analysis, indicated an increasing contamination in the municipalities that made up the states according to the following ranking: Amapá (0.18 µg/g) < Pará (0.27 µg/g) < Amazonas (0.34 µg/g) < Rondônia (0.45 µg/g) < Roraima (0.55 µg/g) < Acre (0.58 µg/g). It was reported that contamination levels were fourteen times higher in carnivorous fish compared to noncarnivorous fish and approximately four times higher in Roraima and Acre, three times higher in Rondônia and Amazonas, and two times higher in Pará when compared to Amapá.
The results obtained in Acre are intriguing and should be interpreted with caution. Although there are few reports and records of gold-mining activity in the region, other studies [35,36,37,38] have indicated the presence of high levels of mercury in fish samples and other food products. This suggests that the availability of mercury in the region may be influenced by other anthropogenic sources of mercury emissions. Moreover, a significant portion of the fish sold in Rio Branco (the state capital), especially in the Elias Mansou Market, is sourced from the municipalities of Boca do Acre and Porto Velho, which are known to be affected by gold mining.
Despite the differences observed in the average levels of mercury or the prevalence of contamination above 0.5 µg/g, the analysis of the risk attributable to fish consumption according to the state revealed that daily mercury intake exceeded the reference dose recommended by the U.S. EPA (0.1 μg/kg bw/day) in all population groups analyzed and in all states of the Amazon region studied. However, the risks associated with the consumption of contaminated fish are diverse and must be taken into account to formulate appropriate nutritional guidelines for safe fish consumption by the local community. Accordingly, we prioritized the three states with the most concerning results.
For Acre, the ingestion of mercury through consumption of contaminated fish was found to be from 7 to 31 times higher than the recommended safe dose. In the sampled municipalities, Cachorra, Filhote, and Dourada should be avoided or consumed exceptionally. On the other hand, Pacú, Pirapitinga, and Tambaqui can be consumed freely. Moreover, Acará, Aracú Cabeça Gorda, Jatuarana, and Tilápia can be safely consumed by adult men and women of childbearing age in quantities ranging from 103 to 418 g/day. However, these fish are not recommended for children.
In Roraima, mercury intake was found to be from 6 to 27 times higher than the dose recommended by the U.S. EPA. In the sampled municipalities, it is recommended to avoid the consumption of Barba Chata, Coroataí, Pindirá, and Piracatinga for all population groups. On the other hand, Aracú Flamengo, Pacú Maria Antônia, Pacú Meião, Pacú, and Jaraqui Escama Grossa can be consumed by women of childbearing age and adult men in quantities ranging from 125 to 418 g/day. However, children should consume these fish in moderation, not exceeding 44, 36, 38, 91, and 44 g/day, respectively, for the age group from 2 to 4 years and 85, 69, 73, 175, and 86 g/day, respectively, for the age group from 5 to 12 years.
In Rondônia, the results revealed that mercury intake varied at levels from 6 to 27 times higher than the recommended safe dose. In these municipalities, it is recommended to restrict the consumption of Babão, Dourada, and Filhote. On the other hand, Acará, Bacu, and Pirapitinga can be consumed freely by all analyzed population groups. Furthermore, Pacú can be safely consumed by women of childbearing age and adult men in quantities ranging from 137 to 180 g/day.
Without losing sight of the illustrative findings of this investigation, it is important to consider some limitations inherent in the ecological study design. Although 1010 fish specimens, representing 80 distinct species distributed across four trophic levels and originating from at least six river basins in the Brazilian Amazon, were included, the analyzed data do not have the capacity to represent the entire diversity of fish available for human consumption in the region. Another point to be considered is the difficulty of collecting samples during different seasons of the year, considering the rainy and dry periods in the Amazon and their influence on the availability of fish and other food. Therefore, it is possible that, despite conservative estimates, our findings are subject to selection bias and may not reveal the true impact of mercury exposure for the majority of the current population living in urban centers of the Amazon.
The estimated risk factors indicated that strict dietary guidelines are necessary for safe consumption of fish. Comparing the doses of mercury intake among the states, we observed that the risks were varied and higher with the consumption of carnivorous fish species, especially in Acre, Roraima, and Rondônia.
According to safety parameters established by the U.S. Environmental Protection Agency (U.S. EPA), in practically all the locations studied, the risk of becoming sick due to consuming fish contaminated with methylmercury was high, especially among children.
Meanwhile, it is worth acknowledging that the safe intake dose considered by the U.S. EPA was estimated from data produced in longitudinal studies conducted in the Faroe Islands, Denmark. That is, this parameter was estimated based on observations of populations living in another part of the planet with distinct dietary habits and subject to diverse conditions from those experienced in the Amazon region, both from a socioeconomic point of view, as well as from a cultural and access to health services perspective.
The use of this reference parameter may have produced distorted results (with attenuated risk estimates) because other risk factors in the Amazonian ecosystem may increase human exposure to mercury. Some studies have indicated that, in addition to the presence of natural mercury in the soil of the Amazon [27,39,40], the expansion of agribusiness, the construction of dams and hydroelectric plants, burning, and other activities that promote deforestation significantly alter the biogeochemical cycle of mercury in the environment [4]. This alteration favors the entry of methylmercury into the food chain, thus increasing human exposure and the consequent health risks of contact with this environmental contaminant. These anthropogenic activities in combination with illegal gold mining and indiscriminate use of mercury produce a unique risk situation for the local population.
In addition to the study limitations pointed out previously, it is essential to say that other physical, chemical, and biological parameters can also interfere with the mercury bioaccumulation process and, consequently, with the mercury concentration present in fish muscle tissue. On the one hand, the most critical parameter is the amount of different mercury species bioavailable in the aquatic system, which are closely related to mercury-emitting sources, notably those linked to gold-mining activities in the Amazon region and to the water’s physical-chemical characteristics (i.e., pH, temperature, ions dissolved, etc.). On the other hand, parameters related to fish characteristics such as sex, weight, length, growth rate, and age can also explain the mercury concentrations detected in this investigation [41,42]. Unfortunately, we could not consider these parameters in the interpretation of mercury levels detected in fish samples collected for this study; therefore, this can be considered a limitation as well.
It is worth reminding that another important limitation concerns the different mercury species present in the muscle of the studied fish. Although it is reasonable to assume that all mercury present in the analyzed samples is in the methylmercury form, it is important to clarify that about 15% of the mercury present in fish may be in inorganic form, as has been described in several studies [27,43,44,45,46,47]. In order to attenuate this limitation and to prevent bias in the results interpretation, we assumed that only 80% of the mercury available in the fish muscle tissue was absorbed by the human gastrointestinal tract. Finally, unlike methylmercury, ingestion of inorganic forms of mercury seems not to represent a relevant risk to public health.
5. Conclusions
It becomes evident that the development of longitudinal studies involving different population groups in the Amazon (including indigenous peoples, riverine communities, and quilombolas, as well as those living in urban centers) is especially important. Only a long-term study can lead to more accurate estimates of the risks associated with fish consumption, as well as safe doses of mercury intake for the Amazonian population.
Conversely, the strengths of this study include the geographic scope of the fish collection points included in the risk analyses, the prevalence ratios employed in the multivariate analyses, the methodological rigor used in the collection of fish samples, and the analyses of mercury levels being carried out in national reference laboratories, as well as the assumption that only 80% of the amount of mercury ingested in food was absorbed by the human gastrointestinal tract.
Therefore, we believe that, together, our findings establish a solid foundation for the planning of strategic interventions, as they provide relevant information to guide the safe consumption of fish in the study area and contribute robust scientific evidence to clarify a pressing issue in the field of national public health.
Acknowledgments
We acknowledge Marcelo de Oliveira Lima from Evandro Chagas Institute (IEC) of the Brazilian Ministry of Health and Zuleica Carmem Castilhos from the Mineral Technology Center (CETEM) for the mercury analysis of the fish samples. We also thank all teams that helped us to buy fish from the public markets at the studied sites.
Author Contributions
Conceptualization, P.C.B., A.C.S.d.V., G.H., D.Y., D.S.d.A. and M.O.-d.-C.; methodology, A.C.S.d.V., G.H. and P.C.B.; formal analysis P.C.B., A.C.S.d.V., G.H., D.Y., D.S.d.A., and M.O.-d.-C.; investigation, G.H., D.Y., D.S.d.A., C.C.d.S. and M.O.-d.-C.; resources, D.Y., D.S.d.A., C.C.d.S. and M.O.-d.-C.; mapping, D.d.O.d.R.P.; writing—original draft preparation, A.C.S.d.V. and P.C.B.; writing—review and editing, P.C.B., A.C.S.d.V., G.H., D.d.O.d.R.P., D.Y., D.S.d.A., C.C.d.S. and M.O.-d.-C.; project administration, P.C.B., G.H., D.Y., D.S.d.A. and M.O.-d.-C.; funding acquisition, C.C.d.S., D.Y., D.S.d.A. and M.O.-d.-C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study because we used human data available from public databases and because we bought fish directly from public markets at the studied sites.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by Greenpeace Brasil, Iepé—Instituto de Pesquisa e Formação Indígena, the Socioambiental Institute (ISA), and WWF-Brasil.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Coulter M.A. Minamata Convention on Mercury. Int. Leg. Mater. 2016;55:582–616. doi: 10.5305/intelegamate.55.3.0582. [DOI] [Google Scholar]
- 2.Bernhoft R.A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health. 2012;2012:460508. doi: 10.1155/2012/460508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hacon S., Barrocas P.R.G., de Vasconcellos A.C.S., Barcellos C., Wasserman J.C., Campos R.C., Ribeiro C., Azevedo-Carloni F.B. An Overview of Mercury Contamination Research in the Amazon Basin with an Emphasis on Brazil. Cad. Saúde Pública. 2008;24:1479–1492. doi: 10.1590/S0102-311X2008000700003. [DOI] [PubMed] [Google Scholar]
- 4.Crespo-Lopez M.E., Augusto-Oliveira M., Lopes-Araújo A., Santos-Sacramento L., Yuki Takeda P., Macchi B.d.M., do Nascimento J.L.M., Maia C.S.F., Lima R.R., Arrifano G.P. Mercury: What Can We Learn from the Amazon? Environ. Int. 2021;146:106223. doi: 10.1016/j.envint.2020.106223. [DOI] [PubMed] [Google Scholar]
- 5.Hacon S.d.S., Oliveira-da-Costa M., Gama C.d.S., Ferreira R., Basta P.C., Schramm A., Yokota D. Mercury Exposure through Fish Consumption in Traditional Communities in the Brazilian Northern Amazon. Int. J. Environ. Res. Public Health. 2020;17:5269. doi: 10.3390/ijerph17155269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vasconcellos A.C.S., Ferreira S.R.B., de Sousa C.C., de Oliveira M.W., de Oliveira Lima M., Basta P.C. Health Risk Assessment Attributed to Consumption of Fish Contaminated with Mercury in the Rio Branco Basin, Roraima, Amazon, Brazil. Toxics. 2022;10:516. doi: 10.3390/toxics10090516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa Junior J.M.F., Lima A.A.d.S., Rodrigues Junior D., Khoury E.D.T., Souza G.d.S., Silveira L.C.d.L., Pinheiro M.d.C.N. Manifestações Emocionais e Motoras de Ribeirinhos Expostos Ao Mercúrio Na Amazônia. Rev. Bras. Epidemiol. 2017;20:212–224. doi: 10.1590/1980-5497201700020003. [DOI] [PubMed] [Google Scholar]
- 8.Ekino S., Susa M., Ninomiya T., Imamura K., Kitamura T. Minamata Disease Revisited: An Update on the Acute and Chronic Manifestations of Methyl Mercury Poisoning. J. Neurol. Sci. 2007;262:131–144. doi: 10.1016/j.jns.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 9.Dack K., Fell M., Taylor C.M., Havdahl A., Lewis S.J. Prenatal Mercury Exposure and Neurodevelopment up to the Age of 5 Years: A Systematic Review. Int. J. Environ. Res. Public Health. 2022;19:1976. doi: 10.3390/ijerph19041976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petit C., Chevrier C., Durand G., Monfort C., Rouget F., Garlantezec R., Cordier S. Impact on Fetal Growth of Prenatal Exposure to Pesticides Due to Agricultural Activities: A Prospective Cohort Study in Brittany, France. Environ. Health Glob. Access Sci. Source. 2010;9:71. doi: 10.1186/1476-069X-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim B., Shah S., Park H.-S., Hong Y.-C., Ha M., Kim Y., Kim B.-N., Kim Y., Ha E.-H. Adverse Effects of Prenatal Mercury Exposure on Neurodevelopment during the First 3 Years of Life Modified by Early Growth Velocity and Prenatal Maternal Folate Level. Environ. Res. 2020;191:109909. doi: 10.1016/j.envres.2020.109909. [DOI] [PubMed] [Google Scholar]
- 12.Isaac V.J., De Almeida M.C. El Consumo de Pescado En La Amazonía Brasileña. COPESCAL Doc. Ocas. 2011;13:I. [Google Scholar]
- 13.Isaac V.J., Almeida M.C., Giarrizzo T., Deus C.P., Vale R., Klein G., Begossi A. Food Consumption as an Indicator of the Conservation of Natural Resources in Riverine Communities of the Brazilian Amazon. An. Acad. Bras. Ciênc. 2015;87:2229–2242. doi: 10.1590/0001-3765201520140250. [DOI] [PubMed] [Google Scholar]
- 14.Begossi A., Salivonchyk S.V., Hallwass G., Hanazaki N., Lopes P.F.M., Silvano R.A.M., Dumaresq D., Pittock J. Fish Consumption on the Amazon: A Review of Biodiversity, Hydropower and Food Security Issues. Braz. J. Biol. 2018;79:345–357. doi: 10.1590/1519-6984.186572. [DOI] [PubMed] [Google Scholar]
- 15.Kawarazuka N., Béné C. Linking Small-Scale Fisheries and Aquaculture to Household Nutritional Security: An Overview. Food Secur. 2010;2:343–357. doi: 10.1007/s12571-010-0079-y. [DOI] [Google Scholar]
- 16.Hicks C.C., Cohen P.J., Graham N.A., Nash K.L., Allison E.H., D’Lima C., Mills D.J., Roscher M., Thilsted S.H., Thorne-Lyman A.L. Harnessing Global Fisheries to Tackle Micronutrient Deficiencies. Nature. 2019;574:95–98. doi: 10.1038/s41586-019-1592-6. [DOI] [PubMed] [Google Scholar]
- 17.Basta P.C., Viana P.V.d.S., de Vasconcellos A.C.S., Périssé A.R.S., Hofer C.B., Paiva N.S., Kempton J.W., Ciampi de Andrade D., de Oliveira R.A.A., Achatz R.W., et al. Mercury Exposure in Munduruku Indigenous Communities from Brazilian Amazon: Methodological Background and an Overview of the Principal Results. Int. J. Environ. Res. Public Health. 2021;18:9222. doi: 10.3390/ijerph18179222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meneses H.d.N.d.M., Oliveira-da-Costa M., Basta P.C., Morais C.G., Pereira R.J.B., de Souza S.M.S., Hacon S.d.S. Mercury Contamination: A Growing Threat to Riverine and Urban Communities in the Brazilian Amazon. Int. J. Environ. Res. Public Health. 2022;19:2816. doi: 10.3390/ijerph19052816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Mérona B., Juras A.A., dos Santos G.M., Cintra I.H.A. Os Peixes Ea Pesca No Baixo Rio Tocantins: Vinte Anos Depois Da UHE Tecuruí. Eletrobrás; Rio de Janeiro, Brasil: 2010. [Google Scholar]
- 20.Batista V.d.S., Isaac V.J., Fabré N.N., Alonso J.C., Almeida O.T., Rivero S., Júnior J.N.O., Ruffino M.L., Silva C.O., Saint-Paul U. Peixes e Pesca No Solimões-Amazonas: Uma Avaliação Integrada. Ibama/ProVárzea; Brasília, Brazil: 2012. [Google Scholar]
- 21.Silvano R.A. Fish and Fisheries in the Brazilian Amazon: People, Ecology and Conservation in Black and Clear Water Rivers. Springer Nature; Berlin/Heidelberg, Germany: 2020. [Google Scholar]
- 22.De Mérona B., Rankin-de-Mérona J. Food Resource Partitioning in a Fish Community of the Central Amazon Floodplain. Neotrop. Ichthyol. 2004;2:75–84. doi: 10.1590/S1679-62252004000200004. [DOI] [Google Scholar]
- 23.Dos Santos G.M., Juras A.A., de Mérona B., Jégue M. Peixes Do Baixo Rio Tocantins: 20 Anos Depois Da Usina Hidrelétrica Tucuruí. Eletronorte; Rio de Janeiro, Brazil: 2004. [Google Scholar]
- 24.Santos G., Ferreira E., Zuanon J. Peixes Comerciais DeManaus. Ibama/ProVárzea; Manaus, Brazil: 2006. [Google Scholar]
- 25.World Health Organization . Guidance for Identifying Populations at Risk from Mercury Exposure. World Health Organization; Geneva, Switzerland: 2008. [Google Scholar]
- 26.Rice D.C. The US EPA Reference Dose for Methylmercury: Sources of Uncertainty. Environ. Res. 2004;95:406–413. doi: 10.1016/j.envres.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Lino A.S., Kasper D., Guida Y.S., Thomaz J.R., Malm O. Total and Methyl Mercury Distribution in Water, Sediment, Plankton and Fish along the Tapajós River Basin in the Brazilian Amazon. Chemosphere. 2019;235:690–700. doi: 10.1016/j.chemosphere.2019.06.212. [DOI] [PubMed] [Google Scholar]
- 28.Azevedo L.S., Pestana I.A., da Costa Nery A.F., Bastos W.R., Souza C.M.M. Mercury Concentration in Six Fish Guilds from a Floodplain Lake in Western Amazonia: Interaction between Seasonality and Feeding Habits. Ecol. Indic. 2020;111:106056. doi: 10.1016/j.ecolind.2019.106056. [DOI] [Google Scholar]
- 29.Funge-Smith S., Bennett A. A Fresh Look at Inland Fisheries and Their Role in Food Security and Livelihoods. Fish Fish. 2019;20:1176–1195. doi: 10.1111/faf.12403. [DOI] [Google Scholar]
- 30.FAO. WHO . Joint FAO/WHO Expert Committee on Food Additives (JECFA) Report of the Tenth Section; Rotterdam, The Netherlands: 2016. [Google Scholar]
- 31.Ministério da Saúde . Brasil Dispõe Sobre o Regulamento Técnico MERCOSUL Sobre Limites Máximos de Contaminantes Inorgânicos Em Alimentos (Resolução RDC N o 42, de 29 de Agosto de 2013) Ministério da Saúde; Brasília, Brasil: 2013. Diário Of. Repúb. Fed. Bras. [Google Scholar]
- 32.Bastos W.R., de Freitas Fonseca M., Pinto F.N., de Freitas Rebelo M., Silva dos Santos S., Glória da Silveira E., Torres J.P.M., Malm O., Pfeiffer W.C. Mercury Persistence in Indoor Environments in the Amazon Region, Brazil. Environ. Res. 2004;96:235–238. doi: 10.1016/j.envres.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Sing K.A., Hryhorczuk D., Saffirio G., Sinks T., Paschal D.C., Sorensen J., Chen E.H. Organic Mercury Levels among the Yanomama of the Brazilian Amazon Basin. AMBIO J. Hum. Environ. 2003;32:434–439. doi: 10.1579/0044-7447-32.7.434. [DOI] [PubMed] [Google Scholar]
- 34.Vega C., Orellana J., Oliveira M., Hacon S., Basta P. Human Mercury Exposure in Yanomami Indigenous Villages from the Brazilian Amazon. Int. J. Environ. Res. Public Health. 2018;15:1051. doi: 10.3390/ijerph15061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mascarenhas A.F.S., Brabo E.d.S., da Silva A.P., Fayal K.d.F., de Jesus I.M., Santos E.C. Avaliação Da Concentração de Mercúrio Em Sedimentos e Material Particulado No Rio Acre, Estado Do Acre, Brasil. Acta Amaz. 2004;34:61–68. doi: 10.1590/S0044-59672004000100008. [DOI] [Google Scholar]
- 36.De Castro N.S.S., Braga C.M., Trindade P.A.d.A., Giarrizzo T., Lima M.d.O. Mercúrio Em Peixe e Em Sedimento Do Rio Purus, Estado Do Acre, Amazônia. Cad. Saúde Coletiva. 2016;24:294–300. [Google Scholar]
- 37.Santos Serrão de Castro N., de Oliveira Lima M. Hair as a Biomarker of Long Term Mercury Exposure in Brazilian Amazon: A Systematic Review. Int. J. Environ. Res. Public Health. 2018;15:500. doi: 10.3390/ijerph15030500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Oliveira D.F., de Castro B.S., do Nascimento Recktenvald M.C.N., da Costa Júnior W.A., da Silva F.X., de Menezes Alves C.L., Froehlich J.D., Bastos W.R., Ott A.M.T. Mercury in Wild Animals and Fish and Health Risk for Indigenous Amazonians. Food Addit. Contam. Part B Surveill. 2021;14:161–169. doi: 10.1080/19393210.2020.1849410. [DOI] [PubMed] [Google Scholar]
- 39.Malm O. Gold Mining as a Source of Mercury Exposure in the Brazilian Amazon. Environ. Res. 1998;77:73–78. doi: 10.1006/enrs.1998.3828. [DOI] [PubMed] [Google Scholar]
- 40.Roulet M., Lucotte M., Canuel R., Farella N., Courcelles M., Guimarães J.-R.D., Mergler D., Amorim M. Increase in Mercury Contamination Recorded in Lacustrine Sediments Following Deforestation in the Central. Chem. Geol. 2000;165:243–266. doi: 10.1016/S0009-2541(99)00172-2. [DOI] [Google Scholar]
- 41.Liu M., Kakade A., Liu P., Wang P., Tang Y., Li X. Hg2+-binding peptide decreases mercury ion accumulation in fish through a cell surface display system. Sci. Total Environ. 2019;659:540–547. doi: 10.1016/j.scitotenv.2018.12.406. [DOI] [PubMed] [Google Scholar]
- 42.Zupo V., Graber G., Kamel S., Plichta V., Granitzer S., Gundacker C., Wittmann K.J. Mercury accumulation in freshwater and marine fish from the wild and from aquaculture ponds. Environ. Pollut. 2019;255:112975. doi: 10.1016/j.envpol.2019.112975. [DOI] [PubMed] [Google Scholar]
- 43.Ikingura J.R., Akagi H. Total mercury and methylmercury levels in fish from hydroelectric reservoirs in Tanzania. Sci. Total Environ. 2003;304:355–368. doi: 10.1016/S0048-9697(02)00581-8. [DOI] [PubMed] [Google Scholar]
- 44.Kehrig Hdo A., Howard B.M., Malm O. Methylmercury in a predatory fish (Cichla spp.) inhabiting the Brazilian Amazon. Environ. Pollut. 2008;154:68–76. doi: 10.1016/j.envpol.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 45.Bastos W.R., Dórea J.G., Bernardi J.V., Lauthartte L.C., Mussy M.H., Lacerda L.D., Malm O. Mercury in fish of the Madeira river (temporal and spatial assessment), Brazilian Amazon. Environ. Res. 2015;140:191–197. doi: 10.1016/j.envres.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 46.Kasper D., Forsberg B.R., do Amaral Kehrig H., Amaral J.H.F., Bastos W.R., Malm O. Mercury in black-waters of the Amazon. In: Myster R., editor. Igapó (Black-Water Fooded Forests) of the Amazon Basin. Springer; Cham, Switzerland: 2018. [DOI] [Google Scholar]
- 47.Mussy M.H., de Almeida R., de Carvalho D.P., Lauthartte L.C., de Holanda I.B.B., Almeida M.G., de Sousa-Filho I.F., de Rezende C.E., Malm O., Bastos W.R. Evaluating total mercury and methylmercury biomagnification using stable isotopes of carbon and nitrogen in fish from the Madeira River basin, Brazilian Amazon. Environ. Sci. Pollut. Res. Int. 2023;30:33543–33554. doi: 10.1007/s11356-022-24235-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.