Abstract
Hair graying is a common and visible sign of aging resulting from decreased or absence of melanogenesis. Although it has been established that gray hair greatly impacts people's mental health and social life, there is no effective countermeasure other than hair dyes. It has long been thought that reversal of gray hair on a large scale is rare. However, a recent study reported that individual gray hair darkening is a common phenomenon, suggesting the possibility of large-scale reversal of gray hair. In this article, we summarize the regulation mechanism of melanogenesis and review existing cases of hair repigmentation caused by several factors, including monoclonal antibodies drugs, tyrosine kinase inhibitors (TKIs), immunomodulators, other drugs, micro-injury, and tumors, and speculate on the mechanisms behind them. This review offers some insights for further research into the modulation of melanogenesis and presents a novel perspective on the development of clinical therapies, with emphasis on topical treatments.
Keywords: hair repigmentation, hair pigmentation, melanogenesis, targeting drugs, topical treatments
Introduction
Hair graying is a common, visible, and early marker of human aging 1, 2. It is now understood that human hair and its pigmentation can greatly affect societal perception, emotional well-being, and psychological state 3. Considering the potential health risks posed by hair dyes 4, new strategies for hair color change are warranted.
Hair graying has long been thought of as an irreversible age-related process. Nonetheless, recent research has revealed that restoring the color of a single gray hair to its original pigmentation is a general phenomenon regardless of age, gender, ethnicity, and corporeal regions but only appears in a single anagen of rare HFs 5. Although there is great heterogeneity between hair follicles (HF), the similarities between the processes of graying and repigmentation imply the potential for systemic behavioral factors (such as life stress) to simultaneously regulate the pigmentation of multiple HFs. Meanwhile, proteomics and computational simulation have proven the theoretical possibility of reversing gray hair temporarily 5. Based on the evidence presented, it becomes evident that the prevention or reversal of hair graying holds significant promise for the future. Therefore, in this review, we summarize the regulation of melanogenesis and focus on cases of hair repigmentation and the mechanisms behind them, trying to inspire future research on the regulation of melanogenesis and therapy development.
Overview of hair pigmentation and hair graying
Melanocytes in human HF are classified into several sub-populations according to function, differentiation status, and location. Within anagen hair follicles, melanocytes responsible for hair pigmentation primarily reside in the hair matrix surrounding the mid to upper dermal papilla. These bulbar melanocytes express active tyrosinase and the melanogenic intermediate dihydroxyphenylalanine (DOPA) and are considered a component of hair follicle pigment unit (HFPU) 1, 6. Melanogenesis occurs in specialized lysosomal-related organelles termed melanosomes. The melanin-containing melanosomes are then transferred to the keratinocytes of hair shaft through dendritic and filopodial processes 7. Melanocyte stem cells (McSCs) are located in the bulge and the sub-bulge area of the outer root sheath (ORS). These cells are immature and poorly or un-pigmented 6. Recent studies have indicated that the majority of melanocyte stem cells possess a unique and unexpected mechanism for self-renewal and melanogenic melanocyte production. These McSCs exhibit a distinctive ability to switch between transit-amplifying and stem cell stages, which fundamentally distinguishes them from other self-renewing systems 8. According to live imaging and single-cell RNA sequencing, McSCs move between the transit-amplifying and hair follicle stem cell compartments through dedifferentiation, reversibly entering multiple differentiation stages controlled by the local microenvironment. Long-term lineage tracing studies have provided compelling evidence that the sustained melanocyte stem cell system is supported by reverted McSCs that dedifferentiate from transit-amplifying stage rather than reserved population of stem cells that inherently maintained in an undifferentiated state.
Indeed, hair pigmentation and the hair cycle are inextricably linked. The hair cycle consists of three distinct stages: anagen, catagen, and telogen. Hair pigmentation only happens during anagen because the melanogenic HFPU exists in this period 9-11. Most differentiated melanocytes experience apoptosis in the catagen phase, while bulge McSCs survive in the secondary hair germ 12-14. As a new anagen phase is initiated, the surviving McSCs differentiate into melanogenic melanocytes to rebuild the HFPU 13, 15.
Current evidence suggests that multiple factors can influence the process of hair graying 6. However, the root cause of hair graying is the dysfunction and cell death of melanogenic melanocytes in the HFPU. It is widely thought that during a single anagen phase, the HFPU is self-maintained and does not require replenishment from McSCs 1. Therefore, the initial onset of hair graying is not necessarily related to the depletion of McSCs. However, as individuals age, stranded McSCs accumulate over time and do not contribute to the production of mature melanocytes 8, 16, 17. The preservation of McSCs is crucial for the reconstruction of the HFPU and provides the possibility for the reversal of grey hair. Once McSCs are exhausted, hair graying becomes irreversible 1.
Epithelial stem cells (EpSCs) in the hair follicle are crucial in providing a functional niche for melanocyte stem cells 18, 19. The offspring of EpSCs in the HF bulge and hair germ develop into outer root sheath (ORS) and transit-amplifying cells (TACs) in the HF matrix, which support HF regeneration. The TACs differentiate into several lineages that eventually give rise to the hair shaft and its supporting components 20. Recently, single-cell transcriptomics revealed that P53 pathway activation-induced specific depletion of matrix TAC, but not HFSCs, is associated with early-stage human hair graying 20. Therefore, the effects of regulatory factors on cells within the HF, other than melanocytes, are also considered in this context.
It is crucial to note that even visually colorless scalp HFs may still have a few hair bulb melanocytes. Some may even continue to produce melanin, although they lack dendritic morphology and melanin transmission to the hair shaft 21. Thus, it is theoretically possible that a special therapy that reverses hair graying before all the hair bulb melanocytes and McSCs disappear can be developed in the future.
Signaling pathways in the regulation of melanogenesis
Wnt/β-catenin signaling pathway
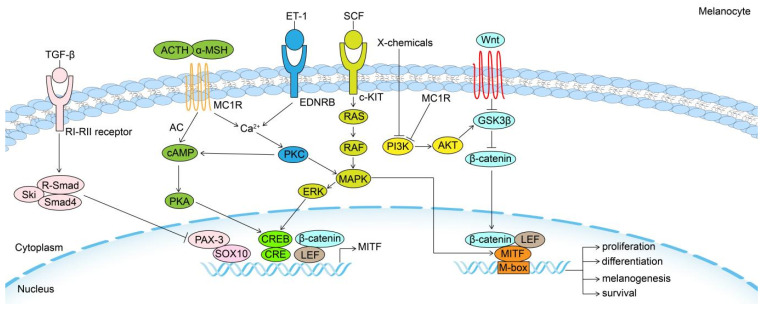
When Wnt molecules bind to their receptors, β-catenin is activated, increasing Melanocyte Inducing Transcription Factor (MITF) transcription in McSCs. Glycogen synthase kinase 3β (GSK3β) phosphorylates β-catenin without Wnt signaling, which causes it to break down through a proteasome-dependent mechanism. The activation of Wnt signaling also increases Endothelin Receptor Type B (EDNRB) signaling 22, 23. These effects of Wnt signaling synergistically promote MsSCs' migration, proliferation, differentiation, and melanogenesis. Moreover, simultaneous activation of Wnt/β-catenin signaling in EpSCs and McSCs initiates pigmented hair regeneration 24.
MC1R
As one of the central regulators of pigmentation, Melanocortin 1 Receptor (MC1R) signaling promotes melanogenesis and melanosome transfer in melanocytes. Interestingly, it has been shown that ultraviolet B (UVB) induces keratinocytes to express pro-opiomelanocortin (POMC), which is cleaved to release α-melanocyte-stimulating hormone (α-MSH) and adrenocorticotropic hormone (ACTH), both ligands of MC1R 25. MC1R activates adenylyl cyclase and then increases cyclic adenosine monophosphate(cAMP). One of the numerous effects of cAMP, mediated by cAMP-dependent protein kinase A (PKA), is the phosphorylation of cAMP-responsive element-binding protein (CREB), which stimulates MITF transcription 26.
Store‐operated Ca2+ entry (SOCE) is initiated upon endoplasmic reticulum (ER) Ca2+ release. The Ca2+ binding ER membrane protein stromal interaction molecule 1 (STIM1) senses the Ca2+ store depletion and then oligomerizes and interacts with plasma membrane store-operated Ca2+ (SOC) channel Orai1, leading to Ca2+ influx 27. α-MSH-induced cAMP stimulates ER Ca2+ release through phospholipase C (PLC)/ inositol triphosphate (IP3) signaling. Subsequently, STIM1 activates the plasma membrane-localized adenylyl cyclase 6 (ADCY6), which acts independently of Orai1, to increase cAMP. This positive feedback loop controlled by cAMP‐Ca2+ crosstalk further promotes the effects of α-MSH 28.
In addition to promoting melanogenesis in melanocytes, the activation of MC1R also enhances the repair of Ultraviolet radiation (UVR)-induced DNA damage 29. Besides, it has been reported that injury or UVB-induced epidermal migration of McSCs also relies on MC1R signaling 30.
SCF/C-KIT
The stem cell factor (SCF)/tyrosine kinase receptor (KIT) signaling system plays a significant role in melanogenesis. When SCF binds to its receptor c-KIT, it triggers the receptor's tyrosine kinase activity, which results in receptor phosphorylation. As a result of the phosphorylation of c-KIT, mitogen-activated protein kinase (MAPK) is stimulated and then triggers the phosphorylation of CREB, activating MITF 31. An extracellular signal-regulated kinase (ERK) can be activated by c-KIT signaling. While ERK signaling activates CREB to promote melanogenesis, it has also been shown to phosphorylate MITF, leading to its ubiquitination and subsequent degradation, thus forming a feedback loop in melanin regulation 32. However, it cannot be ignored that the presence of MITF alone is not sufficient for Tyr expression and that KIT signaling is needed not only for the proliferation and survival of melanoblasts but also for Tyr induction and the transition of melanoblasts to mature melanocytes 33.
EDN/EDNRB
Endothelin receptor B (EDNRB) has been identified as playing indispensable roles in the maintenance, proliferation, differentiation, and migration of McSCs 22. Besides, EDNRB signaling activates PLC/IP3/ signaling and ER Ca2+ release 27. Following SOCE, MITF expression significantly increases via the Ca2+/PKC/MAPK/p90 ribosomal S6 kinase (RSK) pathway, the Mitogen- and stress-activated kinase 1 (MSK1)/CREB pathway, and the PKA/CREB pathway 34-36. The expression of EDNRB is regulated by MITF, suggesting EDNRB signaling and MITF expression form a self-reinforcing positive feedback loop that promotes melanocyte proliferation and melanogenesis 25. After wounding or UVR, Endothelin 1 (EDN1)/EDNRB signaling enhances the proliferation and differentiation of McSC and increases the regeneration of epidermal melanocytes 22, 35.
PI3K/AKT
It is well-established that the phosphoinositide 3-kinase (PI3K) signaling pathway activates the serine/threonine-specific protein kinase (AKT) to increase GSK3β enzyme activity and prevent melanogenesis 37. MC1R can activate PI3K/AKT signaling to produce a negative feedback effect on melanogenesis and stimulate the extracellular release of melanin, preventing oxidative stress, DNA damage, and reduced survival 38. Although SCF/c-KIT is an upstream signal for PI3K/AKT activation in melanocyte and melanoma cells 39, 40, whether c-KIT has such a negative feedback loop remains to be demonstrated experimentally.
TGF-β
Transforming growth factor-β (TGF-β) is the major regulator of McSCs maintenance, causing cell cycle arrest, downregulation of MITF and melanogenic genes, which ultimately keeps McSCs in a state of immaturity and quiescence 41. MITF and its downstream genes are the main targets of TGF-β in inhibiting melanogenesis 42-45. The TGF-β/Smads pathway negatively regulates the paired-box homeotic gene 3 (PAX3), which works synergistically with the SRY-Box Transcription Factor 10 (SOX10) to upregulate MITF, dependent on a cAMP-response element (CRE) 46, 47. Both TGF-β1 and TGF-β3 also downregulate MITF by activating ERK signaling to decrease melanogenesis 48, 49. Lastly, but importantly, TGF-β1 and TGF-β2 are recognized as key catagen-inducing growth factors of HF 50, 51. It has been demonstrated in melanoma cell lines that the direct transcriptional target of TGF-β1-the Kruppel-like transcription factor GLI2 not only suppresses MITF through PKA/cAMP signaling 52 but also directly downregulates tyrosinase-related protein 2 (TRP2) by competitive inhibition of CREB 53.
MITF
MITF is a critical transcriptional regulator of melanogenesis and McSC maintenance and differentiation 26, 54. MITF positively regulates pigmentation-associated genes to promote differentiation-associated function and directly transactivates promoters of three primary melanogenesis enzymes, tyrosinase (TYR), tyrosine-related protein-1 (TYRP-1), and dopachrome tautomerase, also known as tyrosine-related protein-2, TYRP-2 (-2) 55. MITF also upregulates genes contributing to melanosome function, such as G Protein-Coupled Receptor 143 (GPR143), SILV, and melanoma-associated antigens recognized by T cells (MART-1) or melanosome transport such as Rab27 and Myosin5a (MYO5a) 55, 56.
Furthermore, MITF plays a role in melanocyte proliferation. The capacity of MITF to increase cyclin-dependent kinase 2 (CDK2) expression highlights its functions as a pro-proliferative factor 57. MITF also has been known to activate the transcription of T-box transcription factor 2 (TBX2) 58, which inhibits senescence by repression of p21 and p19 and participates in melanocyte growth and invasion 59, 60. Furthermore, MITF directly upregulates cell cyclin-related genes cyclin B1 (CCNB1) and cyclin D1 (CCND1) and mitotic genes such as polo-like kinase 1 (PLK1) 61.
Several downstream genes of MITF promote cell survival. MITF is the positive regulator of anti-apoptotic factors B-cell-lymphoma 2 (BCL2) and baculoviral IAP repeat containing 7 (BIRC7) 62, 63 and is involved in the regulation of the oncogenic hepatocyte growth factor receptor MET and the type III ribonuclease DICER, a necessary regulator of microRNA processing, thereby performing its anti-apoptotic effects 64, 65. It is widely thought that MITF mitigates DNA damage by increasing a group of repair genes, including DNA ligase I (LIG1), telomerase reverse transcriptase (TERT), essential meiotic endonuclease 1 homolog 1 (EME1), Breast Cancer 1 protein (BRCA1), and Fanconi anemia protein A (FANCA) 66. MITF transcriptionally regulates General transcription factor IIH subunit 1 (GTF2H1), which encodes the core component of Transcription Factor IIH (TFIIH), and CDK7, which encodes TFIIH kinase to promote the rapid recovery of nucleotide excision repair 67. In response to reactive oxygen species (ROS), MITF positively regulates apurinic-apyrimidinic endonuclease 1 (APE1), hypoxia-inducible factor 1 (HIF1α), and Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) to improve survival capacity under oxidative stress 68-70.
It has recently been revealed that MITF enhances STIM1 expression transcriptionally 71, indicating the presence of a positive feedback loop between STIM1 and MITF to promote the MITF-inducing effect of MC1R or EDNRB signaling.
MITF can be regarded as a final common pathway in modulating the biological behavior of McSC and melanocyte to some extent, as several external and internal factors converge on it through different intracellular signaling pathways, and their effects depend on downstream genes (Fig. 1).
Figure 1.
Signaling pathways in the regulation of melanogenesis. Melanogenesis and melanocytes proliferation, differentiation and survival are regulated by the MITF transcription factor, which is regulated by a number of important signaling pathways, including Wnt/β-catenin, KIT/SCF, ET-1/EDNRB, α-MSH/MC1R and TGF-β pathways. MITF: Melanocyte Inducing Transcription Factor; GSK3β: Glycogen synthase kinase 3β; AKT: serine/threonine-specific protein kinase; MC1R: Melanocortin 1 Receptor; TGF-β: Transforming growth factor-β; PI3K: phosphoinositide 3-kinase; SCF: stem cell factor; c-KIT: tyrosine kinase receptor; MAPK: mitogen-activated protein kinase; ERK: Extracellular signal-regulated kinase; ET-1: Endothelin 1; EDNRB: Endothelin receptor B; PKC: Protein kinase C; α-MSH: α-melanocyte-stimulating hormone; cAMP: cyclic adenosine monophosphate; PKA: cAMP-dependent protein kinase A; CREB: cAMP response element-binding protein; ACTH: adrenocorticotropic hormone; AC: adenylate cyclase; PAX3: paired-box homeotic gene 3; SOX10: SRY-Box Transcription Factor 10; CRE: cAMP-response element.
Novel mechanisms of hair pigmentation regulation
Sympathetic nerves and sensory nerves
It is widely acknowledged that HFs are innervated by sympathetic and sensory nerves 72. Under stressful conditions, these sympathetic nerves are hyperactivated and release noradrenaline in bursts, resulting in rapid McSC proliferation possibly mediated by β2 adrenergic receptors/AC/cAMP/PKA pathway. It has been shown that these sensory nerves can provide Sonic Hedgehog (SHH) signaling 73, which is required for the normal proliferation of melanocytes 74. Then these McSC differentiate ectopically and migrate out of the hair bulge, eventually causing the permanent depletion of McSC and irreversible grey hair 75.
Neurotransmitters
Multiple neuropeptides released from intra-epidermal sensory nerves, such as calcitonin gene-related peptide (CGRP), substance P (SP), and vasoactive intestinal peptide (VIP), have been reported to regulate melanogenesis by acting on melanocytes directly or indirectly through immune and inflammatory responses 76, 77. In this context, we will primarily focus on their direct effects on HFs and melanocytes.
CGRP exerts catagen-inducing effects on human HFs and can maintain and restore hair follicle immune privilege (IP) via repression of MHC class I antigen 78, 79. Melanogenesis and melanocyte dendricity is enhanced by certain CGRP-induced keratinocyte-derived melanotrophic factors ex vivo 80. In mice, CGRP from sensory neurons increases insulin-like growth factor-I (IGF-I) production in the HF dermal papilla cells, thereby promoting hair growth and melanogenesis 81. However, not all studies support the conclusion that CGRP promotes melanin production. In this regard, it has been shown that in B16F10 cells, CGRP cooperates with SP to inhibit melanogenesis and induce cell apoptosis 82.
SP is released from sensory nerve endings induced by psychoemotional stress and regulates immune cells or HF mainly through neurokinin-1 receptors (NK1R) 83, 84. In organ-cultured HFs, SP upregulates nerve growth factor (NGF) and its apoptosis- and catagen-inducing receptor (p75NTR), while it downregulates the growth-inducing NGF receptor neurotrophic tyrosine kinase receptor type 1 (TrkA). Furthermore, MHC class I and β2-microglobulin are upregulated, suggesting that SP impairs immune privilege (IP) 84. Animal models have also shown that stress-induced SP not only inhibits HF keratinocyte proliferation and induces apoptosis but also promotes mast cell degranulation, causing the production of ROS and neurogenic inflammation, and then inhibits hair growth and induces the catagen phase in the hair cycle 83, 85, 86.
Studies have shown that in B10F16 melanoma cells, SP not only exerts a synergistic effect with CGRP, but also induces apoptosis in B10F16 cells and downregulates 5-hydroxytryptamine (5-HT)1A receptor and 5-HT2A receptor, both of which promote melanogenesis. This effect is mediated by binding to the NK1R receptor and activating S6 kinase 1 (S6K1) while inhibiting the MAPK signaling pathway 87-89. Moreover, excess SP induced by mental stress decreases melanogenesis through keratinocytes. During this process, the hypothalamic-pituitary-adrenocortical (HPA) axis is key in mediating the effects of SP signaling 90. However, contrasting studies revealed that SP promotes EDN1 secretion via endothelin-converting enzyme 1 and upregulates Wnt/ β-catenin signaling by downregulating the Wnt inhibitor Dickkopf-1 (DKK1) to increase melanogenesis in normal human melanocyte ex vivo 91, 92. Interestingly, the results from animal models present a paradoxical outcome. These contradictory results may be due to heterogeneity between cells and different concentrations of SP.
It is well-established that VIP, an immunoinhibitory neuropeptide secreted from perifollicular sensory nerve endings, prevents the HFs from IP collapse 93 and promotes melanogenesis in the B16F10 cell line and normal human melanocytes basically by activating the PKA/CREB/MITF signaling pathway 94.
Adipose tissue
It has been shown that dermal white adipose tissue (dWAT) is located underneath and partially integrated into the reticular dermis, surrounding HFs 95. Interestingly, there is a strong interaction between HFs and dWAT 96. HFs drive a cycle of dWAT remodeling, where HFs secrete adipogenic activators at the beginning of new hair growth, and at the end of hair growth, activator secretion decreases or adipogenesis inhibitor secretion increases, leading to lipolysis 95, 97. dWAT is rich in growth factors that signal reciprocally to HF and regulates the activation state of their stem cells and the rate of hair regeneration 95.
Importantly, mature dWAT can inhibit hair growth. During early telogen, HFs enter a refractory phase to growth signaling, partly mediated by bone morphogenetic protein 2 (BMP2) expressed by adjacent dermal adipocytes 97. A large area of dWAT expresses BMP2, which highly maintains the quiescence of HFSC to prevent excessive hair production 95.
In contrast, dWAT in anagen positively affects hair growth. Adipose progenitor cell is essential for HF to enter a new anagen and is widely thought to stimulate telogen HFs through high levels of platelet‐derived growth factor alpha (PDGFA) 95. Hepatocyte growth factor (HGF), secreted by anagen perifollicular dWAT, stimulates Wnt/β-catenin signaling in the hair matrix by inhibiting Wnt antagonist frizzled-related protein 1 (SFRP1) as well as upregulating WNT10B, thereby promoting melanocyte maturation and pigmentation 98.
Current evidence suggests that dWAT plays significant roles in the aging process of HF 99. Aging dWAT represses Wnt signaling by upregulating Wnt inhibitors dickkopf-related protein (DDK) and SFRP4. Additionally, there is an increase in the expression of fibroblast growth factor (FGF)5 and BMP2, which inhibit hair growth, while the secretion of hair growth-promoting factors such as FGF10 and FGF7 is reduced. Compared with young dWAT, senescent dWAT in telogen expresses abundant abnormal inflammatory factors. In aging dWAT during anagen, the inflammatory process is largely suppressed, but collagen production, angiogenesis, and melanogenesis are impaired.
Given that melanogenesis and hair growth share common signaling pathways, it is highly conceivable that dWAT regulates hair graying, although further research is warranted to validate this hypothesis and explore the underlying mechanisms.
Adiponectin, an adipocyte hormone, is specifically and abundantly expressed in WAT. Adiponectin or activation of adiponectin receptor 1 (AdipoR1) can upregulate multiple hair growth factors through AMP-activated protein kinase (AMPK) in human follicular DPC, including IGF-1, vascular endothelial growth factor (VEGF), IGF, HGF, PDGFA, and FGF 7, and downregulate TGFβ1, thereby inducing the anagen and promoting hair growth ex vivo and in vivo 100-103. In contrast, ex vivo studies revealed that adiponectin oligomer downregulates pigmentation genes in HF and important factors such as Wnt10B and the HGF receptor c-Met within the hair matrix and DP 103. Meanwhile, neutralizing adiponectin isoforms within HF and dWAT promotes melanocyte proliferation, melanogenesis, and tyrosinase activity but produces fewer melanocytes and dendrites. Nevertheless, hair matrix keratinocyte proliferation and hair pigmentation were not altered by adiponectin oligomer within 48h ex vivo.
Adiponectin exists in two forms in circulation, a full-length protein and a fragment containing the globular domain of adiponectin (gAd). The full-length adiponectin induces depigmentation via AMPK/ CREB-regulated transcription coactivators (CRTCs)/CREB signaling pathway 104, but the gAd promotes melanogenesis by activation of the AMPK-p38 MAPK-CREB pathway 105. Hence, the proportion of adiponectin oligomer to globular adiponectin or HGF to adiponectin could determine the quantity of synthesized melanin.
Adipose-derived stem cells (ADSCs) are an important type of stem cell that can be isolated from adipose tissue. They are characterized by their ability to differentiate into multiple cell types, ease of availability, high proliferative capacity, and self-renewal potential 106. ADSCs exert their multiple regulatory roles mainly through autocrine and paracrine pathways 107.
Besides producing various cytokines that promote hair growth, such as VEGF, PDGF, and HGF 108, ADSCs also secrete exosomes to regulate HF. An increasing body of evidence suggests that adipose-derived stem cell exosome (ADSC-Exo) enhance DPC proliferation and survival and promote hair regeneration, mediated in part by inhibiting TGF-β/SMAD3 signaling by miR-122-5p carried in ADSC-Exo 109-111. Moreover, ADSC-induced amphiregulin promotes hair regeneration of skin-derived precursors (SKPs), a multipotent precursor cell population from the dermis capable of differentiating into several lineages through activation of PI3K and MAPK pathways 112. Stromal vascular fraction (SVF), the regenerative cell cocktail obtained mainly from ADSCs, has also been shown to treat alopecia areata effectively and safely 113.
In addition to promoting hair growth, ADSCs are also a regulator of melanogenesis. Interestingly, ADSCs can inhibit the proliferation and melanogenesis of epidermal melanocytes through an interleukin-6 (IL-6)-mediated mechanism 114 and through upregulation of TGF-β1 43. UVB-induced skin pigmentation is reduced in the area of the skin where ADSCs have been injected, possibly related to the fact that α-MSH/MCIR/cAMP signaling is suppressed by basic fibroblast growth factor (bFGF) secreted from ADSCs 115-117. It also has been reported that SVF can inhibit UVB-induced pigmentation in guinea pig skin 118.
Intriguingly, ADSCs have shown the potential to promote pigmentation in the context of vitiligo treatment. ADSCs not only promote the proliferation and migration of co-cultured melanocytes and reduce their differentiation 119 but also improve the effectiveness of melanocyte transplantation for vitiligo, likely because ADSCs upregulate bFGF and SCF and then increases the expression of integrins in melanocyte 120, 121. Ex vivo, adipose tissue extracellular fraction (AT-Ex) induces melanocyte intracellular antioxidant enzymes via acting on nuclear factor (erythroid-derived 2) -like (Nrf-2) to counteract oxidative stress, promotes cell proliferation, and inhibits GSK3β to activate Wnt/β-catenin signaling 122. Similarly, mice models revealed that NB UVB/ADSCs transplantation combination therapy could improve oxidative stress and calcium homeostasis by stimulating Nfr2/ heme oxygenase (HO -1) signaling, causing vitiligo repigmentation 123.
While there are some inconsistencies in the studies mentioned above, it is plausible to speculate that ADSCs may promote the proliferation and survival of melanocytes by reducing inflammation and maintaining melanocyte quiescence.
Cases of gray hair repigmentation and possible mechanisms
Therapeutic monoclonal antibodies
Monoclonal antibodies (mAbs), which are immunoglobulins, can target a specific epitope on an antigen and have emerged as an important class of therapeutic drugs. To date, numerous mAbs have received marketing approval 124.
A cell surface receptor called programmed death-1 (PD-1) acts as a T cell checkpoint and is critical in controlling T cell exhaustion. When PD-1 binds to its ligand programmed death ligand 1 (PD-L1), this triggers a downstream signaling pathway that suppresses T cell activation.
Tumor immune evasion is mediated by abnormally elevated PD-L1 expression on tumor cells and antigen-presenting cells in the tumor microenvironment 125. Anti-PD-1/PD-L1 antibodies, one of the immune checkpoint inhibitors (ICIs), restore the immune response to cancer cells by rescuing T cells from an exhausted state, which have been approved for treating multiple malignancies 126.
A series of 14 patients undergoing anti-PD1/anti-PD-L1 therapy for lung cancer demonstrated hair repigmentation, suggesting it is a promising indicator of positive treatment response 127. Besides, hair repigmentation of the entire body was reported in a male patient with concomitant advanced colorectal cancer and Hodgkin lymphoma who underwent nivolumab treatment, an anti-PD-1 antibody 128. It is widely thought that PD-1/PD-L1 immunotherapy and cytotoxic tumor destruction cause an inflammatory state, which results in the collapse of the immune privilege of HF and hair repigmentation 129. It has also been reported that melanogenesis-related genes and melanin production in B16F10 cells was downregulated by PD-L1 from polyinosinic-polycytidylic-treated HaCaT cell 130, which suggests the direct relationship between PD-1/PD-L1 and melanogenesis. However, vitiligo is a common immune-related adverse event in ICIs treatment for melanoma patients 131, possibly due to the induction of an anti-melanocyte response, which has not been observed in lung cancer patients 132. Moreover, positive staining of anti-PD-L1 antibodies was found in a canities subita patient who experienced extreme trauma 133. Considering these contradictory evidences, more research must be done to clarify the confusing relationship between melanogenesis and PD-1/PD-L1.
Dupilumab, a monoclonal antibody for interleukin 4 (IL-4) receptor alpha subunit, blocks the IL-4/IL-13/IL-4R axis and reduces T helper 2 (Th2) cell response effectively 134. Hair repigmentation has been reported in an atopic dermatitis patient treated with dupilumab 135. It has been revealed that IL-4 suppresses the expression of MITF, TYRP-1, and DCT through the Janus Kinase 2 (JAK2)/ Signal Transducer And Activator Of Transcription 6 (STAT6) signaling pathway and then inhibits melanogenesis in human normal melanocytes (HNMs) 136.
As a tumor necrosis factor (TNF) inhibitor, adalimumab is indicated to treat inflammatory disorders, including psoriasis, rheumatoid arthritis, and inflammatory bowel disease. There has been a reported case of hair repigmentation in a rheumatoid arthritis patient treated with adalimumab 137.
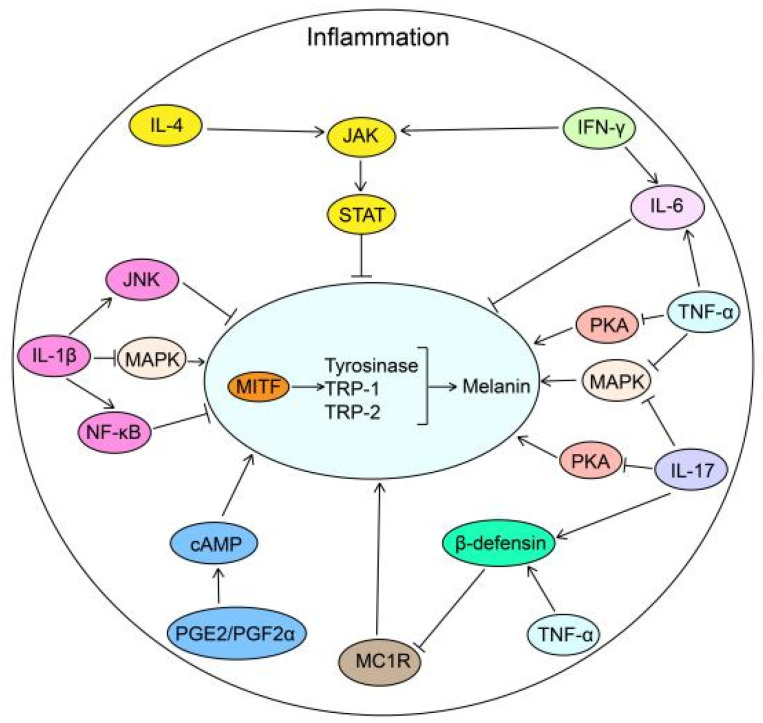
TNF inhibits melanogenesis and the viability of melanocytes through multiple pathways and is intricately connected with the pathogenesis of vitiligo 138. It has been revealed that both TNF and IL-17 treatment of melanocytes downregulated c-KIT, MC1R, MITF, DCT, and other melanogenesis-related genes, and the levels of tyrosinase and melanin significantly decreased 139. The combined action of TNF and IL-17 has been shown to inhibit melanogenesis through the PKA and MAPK signaling pathways 140. TNF and IL-17 have been found to induce the expression of β-defensin 3 in cultured keratinocytes. Acting as an antagonist for MC1R, β-defensin 3 can inhibit the activation of adenylate cyclase and tyrosinase induced by α-MSH 139. Other in vitro studies reveal that TNF-α could reduce melanocyte-stimulating hormones receptor (MSH-R) binding activity, MC1R expression, and the expression of a melanosomal protein gp87, promoters of melanogenesis 141, 142. IL-6, an inhibitor of melanogenesis, was significantly elevated in human normal human melanocytes treated with TNF-α, IL-17, and interferon-gamma (IFN-γ) 136, 138. In vitro, IL-6 decreases melanogenesis by reducing the transcription of MITF in melanocytes 143, 144 and blocking the paracrine function of keratinocytes and fibroblasts through the IL-6 / STAT3 / FGF2 pathway 145.
In vitro, the expression of intracellular adhesion molecule-1 (ICAM-1) in melanocytes can be upregulated by TNF-α, which may promote T cell/melanocyte adherence and immunologic cytotoxic damage, resulting in vitiligo 138, 146. Ample evidence suggests that TNF-α treatment increases ROS in melanocytes and leads to melanocyte toxicity 138, 146.
However, due to the high complexity of the body, TNF may have dual effects on melanogenesis. There is an increasing consensus that TNF-α can stimulate endothelins (EDNs) and SCF secretion from melanocytes and keratinocytes to cause skin hyperpigmentation 147-149. Additionally, TNF-α-induced production of reactive oxygen species (ROS) contributes to melanogenesis 150. It is widely thought that Adalimumab may mitigate the high level of TNF-α in rheumatoid arthritis patients, resulting in hair repigmentation, but the underlying mechanism warrants further research (Fig. 2).
Figure 2.
The biological roles of inflammatory factors in melanogenesis. Inflammatory factors including PGE2 and PGF2α promote melanogenesis by stimulating cAMP pathway. While IL-4 and IFN-γ inhibit melanogenesis through JAK-STAT pathway. TNF-α inhibits melanogenesis by suppressing the PKA, MAPK and MC1R pathways in combination with IL-17. IL-1β inhibits melanogenesis through NF-κB, JNK and MAPK pathways. IL-6, elevated via TNF-α and IFN-γ, decreases melanogenesis by reducing the transcription of MITF in melanocytes. IL-4: interleukin 4; JAK: Janus Kinase; STAT: Signal Transducer And Activator Of Transcription; IFN-γ: Interferon gamma; IL-6: interleukin 6; PKA: cAMP-dependent protein kinase A; MAPK: mitogen-activated protein kinase; IL-17: interleukin 17; TNF-α: tumor necrosis factor alpha; MC1R: Melanocortin 1 Receptor; PGF2α: Prostaglandin F2α; PGE2: prostaglandin E2; cAMP: cyclic adenosine monophosphate; NF-κB: nuclear factor-κB; JNK: c-Jun N-terminal kinase; IL-1β: interleukin-1β; MITF: Melanocyte Inducing Transcription Factor; TRP-1: Tyrosinase related protein-1; TRP-2: Tyrosinase related protein-2.
Hair pigmentation was observed in a patient with plaque psoriasis undergoing treatment with secukinumab 151. Secukinumab is a fully human monoclonal antibody that targets interleukin-17A and is utilized in the treatment of various autoimmune diseases.
In addition to its synergistic effects with TNF, IL-17 suppresses melanogenesis through ROS-dependent autophagic melanocyte apoptosis and stimulates keratinocytes to secrete IL-1β through the nuclear factor-κB (NF-κB)/ROS/ NLR Family Pyrin Domain Containing 3 (NLRP3)/caspase 1 pathway 152. Similarly, the production of TNF-α and IL-6 in keratinocytes and fibroblasts is increased dramatically by IL-17A ex vivo 153. Further investigation is needed to determine the actual impact of IL-1β on melanogenesis when it directly interacts with melanocytes. In melanoma cell lines, IL-1β has been shown to downregulate MITF expression through the activation of NF-κB, c-Jun N-terminal kinases (JNKs), and microRNA-155 154, 155. Additionally, IL-1β has the ability to induce apoptosis in melanocytes 156. However, contrasting findings have also been observed. It has been discovered that IL-1β can upregulate MC1R expression in normal human melanocytes 142. Furthermore, recent research has indicated that IL-1β partially mediates UVB-induced melanogenesis by upregulating the expression of TYR and TRP1 in melanoma B16 cells 157. Nevertheless, it is unquestionable that both IL-17 and IL-1β are positively associated with the progression and extent of vitiligo, likely due to autoimmune melanocyte death 158-161.
Interestingly, an increase in melanocyte abundance and a concomitant decline in pigmentation signaling was observed in psoriasis lesions known to overexpress IL-17 and TNF 139. It has been reported that speckled lentigines appeared in resolved psoriatic plaques after treatment with biological agents, such as scukinumab162, 163. The occurrence of lentigines may be attributed to the removal of inhibition on melanogenesis when TNF-α and IL-17 are blocked. When these inhibitory factors are suppressed, melanocytes in resolved lesions may produce excessive melanin, leading to the formation of lentigines. The mechanism of hair repigmentation following treatment may share similarities with the development of skin lentigines.
Ustekinumab, an anti-interleukin IL-12/23 p40 monoclonal antibody, induced hair repigmentation in a psoriasis vulgaris patient 164. It is widely believed that activation of the TH17 pathway by IL-23 is the predominant mechanism involved in the pathogenesis of psoriasis. The survival and proliferation of TH17 and TH22 cells depend on IL-23. IL-17, IL-22, and TNF-α are produced by TH17 cells, and IL-22 is produced by TH22 cells. Naive T cells are converted into TH1 cells by IL-12, which secretes IFN-γ and TNF-α 165. Ustekinumab may lead to hair pigmentation by decreasing these inhibitors of melanogenesis (IL-17 and TNF-α).
Brentuximab vedotin is an antibody-drug combination commonly used to treat CD30+ lymphomas. This conjugation binds to the CD30 antigen on the surface of cells expressing CD30. Upon absorption by the cell, the compound undergoes proteolytic cleavage, releasing monomethyl auristatin E. Interestingly, a patient who underwent allogeneic hematopoietic stem cell transplantation and received brentuximab vedotin as a trial for refractory chronic graft-versus-host disease (cGVHD) experienced hair repigmentation 166. CD30 signaling enhances the activation of TH1 and TH17 cells, leading to increased production of INF-γ and IL-17A. Additionally, CD30 signaling promotes the secretion of TNF-α and IL-6 through the activation of NF-κB 167, 168. Importantly, these cytokines (INF-γ, IL-17A, TNF-α, and IL-6) are known to negatively regulate melanogenesis. The observed hair repigmentation induced by brentuximab may be attributed to the elimination of these proinflammatory cytokines, which allows for the restoration of normal melanogenesis processes.
Tyrosine kinase inhibitors
In a retrospective cohort study involving 133 chronic myeloid leukemia (CML) patients treated with imatinib, 9 cases of hair repigmentation were reported 169. Hair repigmentation has also been observed in a nilotinib-treated CML patient 170. Several cases of hair pigmentation following sorafenib and erlotinib treatment for lung adenocarcinoma have been documented 171, 172. Indeed, all these drugs belong to the class of tyrosine kinase inhibitors.
As the first protein tyrosine kinase inhibitor, imatinib inhibits Bcr-Abl, platelet-derived growth factor receptor (PDGFR), and c-Kit. It has been approved for treating Philadelphia-chromosome-positive CML and gastrointestinal stromal tumors (GIST) 173. Nilotinib and dasatinib are second-generation inhibitors developed to address imatinib resistance in CML 174. In recent years, alternative mechanisms of nilotinib have been discovered, such inactivation of p38 MAPK in microglial/astroglial cells and a myoblast cell line 175, 176, inhibition of the discoidin domain receptor (DDR) in metastatic colorectal cancer cells 177, and prevention of NF-κB activation in microglial cells 178 indicating there are more target points of nilotinib. It has been reported that nilotinib and dasatinib promote melanogenesis in vitro. In HM3KO melanoma cells, nilotinib was found to upregulate MITF and its downstream genes by activation of the cAMP/PKA/CREB signaling pathway and decreasing the phosphorylation of AKT, which repressed the pigmentation process by inhibition of GSK3β 179. In B16F0 mouse melanoma cells, nilotinib was found to increase ROS levels and ROS-induced JNK activation, thereby inducing TYR, TRP1, and TRP2 180. Dasatinib has also been discovered to promote melanogenesis in human normal melanocytes through ERK/CREB/MITF signaling and possibly through phosphorylation of p38 MAPK and JNK 181.
Sorafenib is a multiple-target tyrosine kinase inhibitor, inhibiting Raf1, VEGF receptors, platelet-derived growth factor (PDGF) receptors, and several other targets 182. It has been shown that Sorafenib also upregulates MITF and melanogenesis in the HM3KO melanoma cell line by repression of AKT and ERK pathway and increase of β-catenin via reduction enzyme activity of GSK3β 183.
It has been established that erlotinib selectively inhibits epidermal growth factor receptor (EGFR) and can be used to treat several solid tumors 184. Although EGFR signaling negatively affects UVR-induced melanogenesis 185, 186, it is highly conceivable that post-inflammatory hyperpigmentation is caused by erlotinib-induced follicle inflammation 171.
It has been established that tyrosine kinase inhibitors (TKIs) have various cutaneous adverse effects, with skin and hair depigmentation being relatively common but hair repigmentation occurring less frequently 187-189. The exact mechanism by which TKIs promote hair repigmentation is not yet fully understood, although it is believed to be due in part to their ability to enhance melanogenesis.
Immunomodulatory drugs
Two cases have been reported in which multiple myeloma (MM) patients experienced hair repigmentation after receiving lenalidomide or thalidomide treatment 190, 191. These drugs, which are immunomodulatory drugs (IMiDs), are approved for specific types of hematological cancers and autoimmune diseases. Protein cereblon (CBRN) is a member of the Lon protease family that plays a crucial role in mediating the anti-myeloma effects and teratogenicity of this class of IMiDs 192. IMiDs have been shown to decrease the production of TNF-α in human peripheral blood mononuclear cells (PBMCs) by promoting the degradation of TNF-α mRNA, potentially through CBRN 192. Additionally, IMiDs have been demonstrated to reduce the expression and secretion of IL-6, which is known to be upregulated by TNF-α 193. IMiDs also produce therapeutic effects on several diseases by inhibiting TGF-β 194-198. It has also been reported that IMiDs reduce IL-1 and IL-1β in plasma 199. What's more, IMiDs inhibit the activation of NF-κB by blocking the degradation of inhibitor of NF-κB (IκB) proteins 200.
TNF-α, IL-6, TGF-β, and IL-1β are all recognized as inhibitors of melanogenesis 201. The impact of TNF-α and IL-6 on melanogenesis was previously examined in the context of "Adalimumab," while the influence of IL-1β was discussed in relation to "Secukinumab."
Moreover, it has been established that IL-10, which activates the STAT-3 and PI3K/AKT/NF-κB signaling pathways to protect primary melanocytes 202, is induced by IMiDs 192, 203.
NF-κB signaling participates in both the stimulation and the suppression of melanogenesis. It was reported that TNF-α, tumor necrosis factor superfamily member 14 (TNFSF14), and IL-1β induce melanogenesis by activating NF-κB 204, 205. However, other studies suggest that activation of NF-κB mediates the facilitation of melanogenic activity from multiple sources, such as IL-18, Toll-like receptor 9 agonists, and UVR-induced-oxidative stress, indicating that the target genes involved in regulating melanogenesis may not be identical when NF-κB signaling is activated 206-208.
Interestingly, in multiple myeloma patients, IMiDs increase the production of IFN-γ (an inhibitor of melanogenesis) from T cells, and IMiDs downregulate VEGF, bFGF, and granulocyte macrophage-colony stimulating factor (GM-CSF), all of which have promotive effects on melanogenesis 193, 201, 209-213. The hair repigmentation effects of IMiDs are primarily attributed to their ability to inhibit the elevated levels of melanogenic inhibitors observed in multiple myeloma patients.
Cyclosporine A (CsA)
Cyclosporine A, the first reported immunosuppressive drug to selectively inhibit T cells, mainly targets Th cells to achieve its therapeutic effect 214. CsA has been shown to induce hair repigmentation in psoriasis patients 215-217. HF is an immediate target of CsA, and hypertrichosis may be the most intriguing and most common adverse effect of CsA 218. In vitro, CsA downregulates SFRP1 in DP, an inhibitor of the Wnt ligand, which activates the Wnt/β-catenin pathway in HF and then induces hair growth 219. Therefore, cyclosporine may promote melanogenesis by activating the WNT pathway in melanocytes. However, it is possible that CsA's immunosuppressive properties and its ability to reduce cytokine levels could contribute to hair repigmentation by inhibiting melanogenesis.
Other drugs
A study revealed that hair repigmentation occurred in two patients (one case is myxedema coma, and the other is iatrogenic hyperthyroidism) after receiving high-dose thyroxine treatment 220. In organ-cultured normal human scalp HFs, TH enhances the proliferation of hair matrix keratinocytes, inhibits their apoptosis, and induces and prolongs the anagen phase through the downregulation of TGF-β2, a key catagen-promoting growth factor 220, 221.
It has been reported that T4 downregulates the intrafollicular expression of clock genes (BMAL1 and PER1) after 24h, both of which inhibit anagen/prolong catagen and inhibit HF pigmentation 222. Both T3 and T4 significantly promote melanogenesis of organ-cultured HF, the mechanism of which possibly is independent of the hair cycle 221. Thyroid hormone signaling is related to many pigmented dermatoses and hair disorders, such as vitiligo, melanocytic nevi, and alopecia areata 223, 224, indicating that thyroxine plays an important role in hair pigmentation and maintaining the homeostasis of melanocytes.
Hair repigmentation was induced in a glaucoma patient treated with latanoprost, a prostaglandin F2α (PGF2α) analog 225. It is now understood that iris pigmentation, eyelashes hypertrichosis, and hyperpigmentation are common adverse events 226. It has been revealed that PGF2α and prostaglandin E2 (PGE2) promote melanocyte dendrites formation and the activation of tyrosinase through cAMP/PLC signaling 227-230. Moreover, ex vivo, PGE2 promotes the delivery of filopodia and quantities of shedding spheroid granules in melanocytes (MCs) but does not influence the morphology of keratinocytes 231. Current evidence suggests that local application of latanoprost activates HFs and encourages hair growth, and bimatoprost, another PGF2α analog, has been approved for treating eyelash hypotrichosis 232, 233. A murine model validated the stimulatory effect of PGF2α and latanoprost on follicular melanogenesis and hair regrowth 234.
A retrospective study reported that hair repigmentation occurred in 24 of the 62 Alzheimer's patients receiving prolonged cholinesterase inhibitor therapy 235. Solar light not only induces skin keratinocytes to secrete acetylcholine (ACh), which represses light-induced melanogenesis probably by inhibiting cAMP/CREB/MITF signaling in melanocytes 236, 237 but also promotes expression of AChE in keratinocytes through transcription factor activator protein 1 (AP1) ex vivo 238. What's more, during melanin production in melanocytes and B16F10 melanoma cells, AChE is downregulated by increased cAMP/CREB signaling 237. The above findings suggest that ACh and AChE inhibitors are local negative regulators of melanogenesis, and there is a negative feedback loop of ACh-melanogenesis-AChE among melanocytes and keratinocytes to maintain melanin homeostasis in the skin.
Nevertheless, it was recently reported that phagocytosis mediated by the α7 nicotinic acetylcholine receptor causes the skin keratinocyte to take up melanosomes in response to UV exposure in vitro 239. Moreover, M4 receptor-KO mice exhibit poor hair growth with no HF melanogenesis 240, indicating that cholinergic signaling is indispensable for pigmentation.
It has been established that these AChE inhibitors work by increasing ACh in the nervous system to treat patients with Alzheimer's disease. Several reports revealed that ACh enhances the release of α-MSH from pituitary melanotropes and nerve-induced pigmentation in lower vertebrates 241. In a similar vein, it is also possible that ACh causes hair repigmentation in a neuroendocrine-dependent manner. Meanwhile, considering cholinergic signaling has multiple effects on neural and intestinal stem cells 242, ACh likely plays a role in McSC homeostasis.
A case report has documented that hair repigmentation occurred in a breast cancer patient who received treatment with tamoxifen, the first selective estrogen receptor modulator, for 2.5 years 243. Although there are conflicting reports, estrogen is considered to have ER-mediated promotive effects on melanogenesis in melanocyte ex vivo, 244 possibly by activation of the cAMP/PKA/MITF pathway 245, 246. Estrogen also induces melanogenesis indirectly via keratinocytes 244. In vivo, high estrogen levels enhance melanogenesis and cause skin hyperpigmentation, such as melasma 247. However, in vitro, tamoxifen was found to stimulate synthesis and extrusion of melanin, with decreased cAMP but upregulated catalase expression in normal human melanocytes, suggesting tamoxifen has a ROS-mediated promelanogenic effect on melanocytes 248. In practical terms, one of the established anti-cancer mechanisms of tamoxifen involves increasing apoptosis through the generation of reactive oxygen species 249.
L-DOPA has been reported to induce hair pigmentation in 3 patients with Parkinson's disease 250. In addition to being the intermediate of melanogenesis, L-DOPA is also a bioregulatory molecule that positively regulates melanogenesis by upregulating TYR and MC1R and regulating several cellular processes, such as cellular metabolism 251. These can be mediated by interacting with certain receptors or by non-receptor mechanisms.
Nevertheless, a study revealed that dopamine directly induced catagen in human scalp HFs ex vivo 252. Thus, L-DOPA may not be a good choice for beauty lovers who desire pigmented hair.
Cerebrolysin is a low-molecular-weight neuropeptide obtained from the porcine brain and has neuroprotective and neurotrophic effects similar to neurotrophic growth factors. It has been reported that cerebrolysin causes hair repigmentation linked to Melan-A, also known as MART-1 reactivation, in 5 neurological patients 253. Besides, some neuropeptides, such as CGRP, SP, and VIP, enhance melanocyte proliferation and melanogenesis 80, 91, 94. In addition, the p75 neurotrophin receptor (p75NTR) regulates apoptosis in the external root sheath of the HF 254. Therefore, cerebrolysin may cause hair repigmentation as a neurotrophin factor. Meanwhile, given that melanocytes are derived from the neural crest, the neuroprotective effects of cerebrolysin, which include preventing nerve cell apoptosis and promoting differentiation and migration, may also be beneficial for melanocytes 253.
As second-generation retinoids, etretinate and acitretin exert their effects by binding to retinoic acid receptors and retinoid-X receptors. Interestingly, in a study involving four patients, both etretinate and acitretin were found to induce hair repigmentation and curling 255-258. It has been demonstrated that an increased level of retinoic acid (RA) upregulates the C-KIT receptor and then sensitizes McSCs to KIT-ligand, eventually leading to ectopic McSCs differentiation in the niche in vivo 19. Although the mechanism behind hair color change induced by etretinate and acitretin is not completely understood, it is possible that their impact on retinoic acid metabolism could be an alternate mechanism. However, current research only suggests that etretinate and acitretin increase the telogen phase of the hair cycle and inhibit melanocyte proliferation through retinoid-X receptor signaling 259, 260.
According to some reports, some plant extracts have ability to prevent hair graying. Eriodictyon angustifolium (Ea) is a plant that grows on the west coast of North America and has been used for many years as a traditional medicinal herb by the indigenous population. Abundant flavonoids contained in Ea extract, such as sterubin and hydroxygenkwanin, seem to be active ingredients to prevent and reduce hair graying 261, 262. Although the target is not molecularly clear, sterubin appears to function by activating Wnt signal and reducing ROS 263, while hydroxygenkwanin relies on KIT signaling 264. Polygonum multiflorum(PM) is a tranditonal Chinese medicine that has been experiencely used to treat early graying hair for a long time. PM extract has been proven to potentiate melanin synthesis by targeting on α-MSH and MC1R and reducing ROS production 265-267. Besides Ea and PM, Pueraria lobata extract and its active compound, puerarin, also have been reported to prevent hair graying via the cAMP/MITF signaling pathway 268, 269.
Micro-injury
Hair repigmentation was observed in an 84-year-old woman after Mohs micrographic surgery and secondary intention wound healing 270. Physical therapy, such as phototherapy and microneedle, has been used to treat pigmentation disorders and hair loss for years 271-273. In response to injury or UVB, HF-McSCs migrate to the epidermis, depending on MC1R signaling. Then they differentiate to produce protective pigmentation against UV 30. Except for the absence of pigmentation, de novo regenerated hair follicles can't be distinguished from regularly growing hair follicles. However, when mice are injured during the anagen, de novo regeneration of pigmented hair is seen. This phenomenon may be caused by the fact that, stimulated by increased Wnt7a of the keratinocytes, McSCs that have been induced to migrate to the interfollicular epidermis by injury are integrated into de novo regenerated hair follicles 274. Moreover, activation of the β-Catenin signaling pathway, at least in part, in melanocyte stem cells located in the hair follicle bulge area is responsible for the narrowband UVB (NBUVB)-induced repigmentation of vitiligo 275. Recent studies have also reported that the Wnt/β-Catenin pathway is involved in hair regeneration and vitiligo repigmentation following micro-injury 276.
As a form of injury, epilation activates McSCs to regenerate follicular and epidermal melanocytes through induced endothelin 3/endothelin type-B receptor (EDN3/EDNRB) signaling, leading to skin and hair hyperpigmentation 277.
Overall, the mechanism of micro-injury-induced hair repigmentation is closely related to the Wnt/β-Catenin and EDN3/EDNRB pathways.
Tumor
Roughly ten cases have been reported documenting focal hair repigmentation occurring on and around the area of scalp melanoma 278-287. A hypothesized model for this phenomenon involves two key cascades. The first cascade involves infiltrating melanoma cells, directly delivering melanin to follicular keratinocytes 283, 287. However, histopathological examination revealed that many hair follicles remain uninvaded by melanoma cells but still exhibit repigmented hair. This leads to the second cascade, where benign bulbar melanocytes are activated by paracrine factors (such as SCF) released by neighboring melanoma cells 283, 287. Immunostaining studies have confirmed the presence of TGF-β1-expressing follicular epithelium adjacent to highly TGF-β1-positive melanoma cells 287, supporting this second cascade.
It has been reported that paraneoplastic syndrome caused by lung cancer could induce the darkening of hair and skin in several patients. The potential mechanism may be that a high level of ACTH in the body promotes melanogenesis through the MC1R signaling pathway 1, 288 (Table 1).
Table 1.
Cases of gray hair repigmentation and possible mechanisms
| Cases | possible mechanisms | |
|---|---|---|
| Monoclonal antibody drug | Anti-PD-1/PD-L1 therapy | An inflammatory state caused by PD-1/PD-L1 immunotherapy and cytotoxic tumor destruction results in the collapse of the immune privilege of the hair follicle and promotes hair repigmentation. |
| Dupilumab | It removes IL-4/JAK2/STAT6 signaling pathway's inhibition of melanogenesis. | |
| Adalimumab | It blocks TNF-α and IL-17 signaling and then gets rid of inhibition on melanogenesis. | |
| Scukinumab | It blocks TNF-α and IL-17 signaling and gets rid of inhibition on melanogenesis. | |
| Ustekinumab | It decreases TNF-α and IL-17 produced by TH17 and TH22 cells. | |
| Brentuximab | It blocks CD30 signaling in TH1 and TH17 and then decreased INF-γ, IL-17A, TNF-α and IL-6. | |
| tyrosine kinase inhibitors | Nilotinib | It increases melanogenesis through activation of cAMP/PKA/CREB/MITF signaling pathway and decreases AKT signaling. It also elevates ROS and induces JNK activation to upregulate TYR, TRP-1, TRP-2. |
| Dasatinib | It promotes melanogenesis through MAPK or JNK/ERK/CREB/MITF signaling. | |
| Imatinib | The reason may be similar to Nilotinib and Dasatinib's. | |
| Sorafenib | It represses AKT and ERK pathway and increases β-catenin. | |
| Erlotinib | Post-inflammatory hyperpigmentation after erlotinib-induced follicle inflammation. | |
| Immunomodulatory drugs | lenalidomide or thalidomide | They decrease inhibitors of melanogenesis and increase promotors. |
| Immunosuppressant | Cyclosporine A | CsA downregulates SFRP1, an inhibitor of Wnt ligand, which in turn activates Wnt/β-catenin pathway and then induces hair repigmentation. |
| Other drugs | L-thyroxine | TH inhibits TGF-β2 and promotes melanogenesis. BMAL1 and PER1 are downregulated to prolong anagen. |
| Latanoprost | PGE2 and PGF2α stimulate melanogenesis by cAMP/PLC signaling and promoting melanosome delivery in melanocyte. Promotes hair follicle growth. |
|
| Acetylcholinesterase inhibitor | Not clear but cholinergic signaling is necessary for melanogenesis. Ach also seems to increase α-MSH secretion and regulate stem cells. | |
| Tamoxifen | It induces ROS in melanocyte to stimulate melanogenesis. | |
| L-DOPA | It upregulates TYP and MC1R to enhance melanogenesis. | |
| Cerebrolysin | As a kind of neuropeptide, it causes melanogenesis linked to Melan-A as a kind of neuropeptide | |
| Retinoids | The impact of etretinate and acitretin on RA metabolism, which upregulates C-KIT receptor and leads to ectopic McSCs differentiation, may be an alternate mechanism for hair color change | |
| Mechanical stimulation | Micro-injury | It activates MC1R signaling, Wnt signaling and EDNRB signaling and then promotes melanogenesis. |
| Tumor | Scalp melanoma | Melanoma cells directly deliver melanin to keratinocytes or secrete some factors to activate surrounding normal melanocyte in a paracrine manner. |
| Lung cancer | Paraneoplastic syndrome in lung cancer leads to high level of ACTH, which can stimulate MC1R signaling. |
Conclusion
While widespread repigmentation of gray hair is uncommon, the underlying mechanism remains an important area of study. Understanding the regulatory mechanisms involved in hair follicle melanogenesis and melanocyte stem cells is essential for developing potential clinical therapies to reverse gray hair. However, achieving this goal necessitates extensive research efforts. Given that the skin is an accessible part of the body and the position of the HFs is relatively superficial, topical treatment will have great advantages in safety and effectiveness.
Just as topical minoxidil is commonly used to treat hair loss, topical agents that stimulate melanin synthesis to reverse gray hair will have promising prospects if they can be developed in the future. Physical therapies such as photodynamic therapy and microneedling are also viable options. However, all these treatments rely on the presence of a sufficient population of active McSCs. Therefore, maintaining a healthy population of McSCs is also an urgent problem that needs to be addressed.
Acknowledgments
This work was supported by Affiliated Hospital of Xuzhou Medical University.
Author Contributions
ZRF prepared the related literature, and was a major contributor in writing the manuscript. YQ drew the pictures and wrote part of the manuscript. GJ provided direction throughout the preparation of this manuscript, and made significant revisions to the manuscript. All authors have read and approved the final manuscript.
Abbreviations
- TKIs
tyrosine kinase inhibitors
- HF
hair follicle
- DOPA
dihydroxyphenylalanine
- HFPU
hair follicle pigment unit
- McSC
Melanocyte stem cell
- ORS
outer root sheath
- GSK3β
Glycogen synthase kinase 3β
- MITF
Melanocyte Inducing Transcription Factor
- EDNRB
Endothelin Receptor Type B
- EpSCs
epithelial stem cells
- TACs
transit-amplifying cells
- MC1R
Melanocortin 1 Receptor
- UVB
Ultraviolet B
- POMC
pro-opiomelanocortin
- α-MSH
α-melanocyte-stimulating hormone
- ACTH
adrenocorticotropic hormone
- cAMP
cyclic adenosine monophosphate
- PKA
protein kinase A
- CREB
cAMP responsive element-binding protein
- SOCE
Store‐operated Ca2+ entry
- ER
endoplasmic reticulum
- STIM1
stromal interaction molecule 1
- SOC
store-operated Ca2+
- PLC
phospholipase C
- IP3
inositol triphosphate 3
- ADCY6
adenylyl cyclase 6
- UVR
Ultraviolet radiation
- SCF
stem cell factor
- MAPK
mitogen-activated protein kinase
- ERK
Extracellular signal-regulated kinase
- RSK
ribosomal S6 kinase
- MSK1
Mitogen- and stress-activated kinase 1
- EDN1
Endothelin 1
- PI3K
phosphoinositide 3-kinase
- AKT
serine/threonine-specific protein kinase
- TGF-β
Transforming growth factor-β
- PAX3
paired-box homeotic gene 3
- SOX10
SRY-Box Transcription Factor 10
- CRE
cAMP-response element
- TRP2
tyrosinase-related protein 2
- TYR
tyrosinase
- TYRP-1
tyrosine-related protein-1
- DCT
dopachrome tautomerase, also known as tyrosine-related protein-2, TYRP-2
- GPR143
Protein-Coupled Receptor 143
- MART-1
Melanoma-associated antigen recognized by T cells
- MYO5a
Myosin5a
- CDK2
cyclin dependent kinase 2
- TBX2
T-box transcription factor 2
- CCNB1
cyclin B1
- CCND1
cyclin D1
- PLK1
polo-like kinase 1
- BCL2
B-cell-lymphoma 2
- BIRC7
baculoviral IAP repeat containing 7
- LIG1
DNA ligase I
- TERT
telomerase reverse transcriptase
- EME1
essential meiotic endonuclease 1 homolog 1
- BRCA1
Breast Cancer 1 protein
- FANCA
Fanconi anemia protein A
- GTF2H1
General transcription factor IIH subunit 1
- TFIIH
transcription factor IIH
- ROS
reactive oxygen species
- APE1
apurinic-apyrimidinic endonuclease1
- HIF1α
hypoxia-inducible factor 1
- PGC1α
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- SHH
Sonic hedgehog
- CGRP
calcitonin gene-related peptide
- SP
substance P
- VIP
vasoactive intestinal peptide
- IP
immune privilege
- IGF-I
insulin-like growth factor-I
- NK1R
neurokinin-1 receptors
- NGF
nerve growth factor
- p75NTR
apoptosis- and catagen-inducing receptor
- TrkA
tyrosine kinase receptor type 1
- 5-HT
5-hydroxytryptamine
- S6K1
S6 kinase 1
- HPA
hypothalamic pituitary adrenocortical
- DKK1
Dickkopf-1
- NAS
N-Acetylserotonin
- CRS
chronic restraint stress
- CUMS
chronic unpredicted mild stress
- GABA
Gamma-aminobutyric acid
- DPC
dermal papilla cell
- dWAT
Dermal white adipose tissue
- BMP2
bone morphogenetic protein 2
- PDGFA
platelet‐derived growth factor alpha
- HGF
Hepatocyte growth factor
- SFRP1
frizzled related protein 1
- DDK
dickkopf related protein
- FGF
fibroblast growth factor
- AdipoR1
adiponectin receptor 1
- AMPK
AMP-activated protein kinase
- VEGF
vascular endothelial growth factor
- gAd
globular domain of adiponectin
- CRTCs
CREB-regulated transcription co-activators
- ADSC-Exo
Adipose-derived stem cell exosome
- SKPs
skin-derived precursors
- SVF
Stromal vascular fraction
- IL-6
interleukin-6
- AT-Ex
adipose tissue extracellular fraction
- mAbs
monoclonal antibodies
- PD-1
programmed death-1
- PD-L1
ligand programmed death ligand 1
- ICIs
immune checkpoint inhibitors
- IL-4
interleukin 4
- Th2
T helper 2
- EoE
eosinophilic esophagitis
- JAK2
Janus Kinase 2
- STAT6
Signal Transducer And Activator Of Transcription 6
- HNMs
human normal melanocytes
- TNF
tumor necrosis factor
- MSH-R
melanocyte-stimulating hormones receptor
- FGF2
Fibroblast growth factor 2
- ICAM-1
intracellular adhesion molecule-1
- EDNs
endothelins
- NF-κB
nuclear factor-κB
- NLRP3
NLR Family Pyrin Domain Containing 3
- JNKs
c-Jun N-terminal kinases
- cGVHD
chronic graft-versus-host disease
- CML
chronic myeloid leukemia
- PDGFR
platelet-derived growth factor receptor
- GIST
gastrointestinal stromal tumor
- DDR
discoidin domain receptor
- VEGF
vascular endothelial growth factor
- PDGF
platelet-derived growth factor
- EGFR
epidermal growth factor receptor
- IMiDs
immunomodulatory drugs
- CBRN
lon protease Cereblon
- PBMSs
peripheral blood mononuclear cells
- CRBN
Cereblon
- IκB
inhibitor of NF-κB
- TNFSF14
tumor necrosis factor superfamily member 14
- MM
multiple myeloma
- bFGF
basic fibroblast growth factor
- GM-CSF
granulocyte macrophage-colony stimulating factor
- CsA
Cyclosporine A
- INF-α
interferon-α
- HFs
hair follicles
- TH
thyroid hormone
- PGF2α
Prostaglandin F2α
- PGE2
prostaglandin E2
- MCs
melanocytes
- AChE
Acetylcholinesterase
- Ach
Acetylcholine
- AP1
activator protein 1
- L-DOPA
Levodopa
- p75NTR
p75 neurotrophin receptor
- RA
retinoic acid
- HF-McSCs
hair follicle melanocyte stem cells
- EDN3/EDNRB
endothelin 3/endothelin type-B receptor
- sHG
secondary hair germ
- NBUVB
Narrowband UVB
- c-KIT
tyrosine kinase receptor
References
- 1.O'Sullivan JDB, Nicu C, Picard M, Cheret J, Bedogni B, Tobin DJ. et al. The biology of human hair greying. Biol Rev Camb Philos Soc. 2021;96:107–28. doi: 10.1111/brv.12648. [DOI] [PubMed] [Google Scholar]
- 2.Ji J, Ho BS, Qian G, Xie XM, Bigliardi PL, Bigliardi-Qi M. Aging in hair follicle stem cells and niche microenvironment. J Dermatol. 2017;44:1097–104. doi: 10.1111/1346-8138.13897. [DOI] [PubMed] [Google Scholar]
- 3.Park AM, Khan S, Rawnsley J. Hair Biology: Growth and Pigmentation. Facial Plast Surg Clin North Am. 2018;26:415–24. doi: 10.1016/j.fsc.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 4.He L, Michailidou F, Gahlon HL, Zeng W. Hair Dye Ingredients and Potential Health Risks from Exposure to Hair Dyeing. Chem Res Toxicol. 2022;35:901–15. doi: 10.1021/acs.chemrestox.1c00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg A, Rausser S, Ren J, Mosharov E, Sturm G, Ogden R, Quantitative mapping of human hair greying and reversal in relation to life stress. eLife. 2021. 10. [DOI] [PMC free article] [PubMed]
- 6.Fernandez-Flores A, Saeb-Lima M, Cassarino DS. Histopathology of aging of the hair follicle. J Cutan Pathol. 2019;46:508–19. doi: 10.1111/cup.13467. [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Hammer JA. Melanosome transfer: it is best to give and receive. Curr Opin Cell Biol. 2014;29:1–7. doi: 10.1016/j.ceb.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Q, Lee W, Hu H, Ogawa T, De Leon S, Katehis I, Dedifferentiation maintains melanocyte stem cells in a dynamic niche. Nature. 2023. [DOI] [PMC free article] [PubMed]
- 9.Tobin D, Hagen E, Botchkarev V, Paus R. Do hair bulb melanocytes undergo apoptosis during hair follicle regression (catagen)? The Journal of investigative dermatology. 1998;111:941–7. doi: 10.1046/j.1523-1747.1998.00417.x. [DOI] [PubMed] [Google Scholar]
- 10.Slominski A, Wortsman J, Plonka P, Schallreuter K, Paus R, Tobin D. Hair follicle pigmentation. The Journal of investigative dermatology. 2005;124:13–21. doi: 10.1111/j.0022-202X.2004.23528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh JW, Kloepper J, Langan EA, Kim Y, Yeo J, Kim MJ. et al. A Guide to Studying Human Hair Follicle Cycling In vivo. J Invest Dermatol. 2016;136:34–44. doi: 10.1038/JID.2015.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobin D. A possible role for Langerhans cells in the removal of melanin from early catagen hair follicles. The British journal of dermatology. 1998;138:795–8. doi: 10.1046/j.1365-2133.1998.02215.x. [DOI] [PubMed] [Google Scholar]
- 13.Tobin D, Slominski A, Botchkarev V, Paus R. The fate of hair follicle melanocytes during the hair growth cycle. The journal of investigative dermatology Symposium proceedings. 1999;4:323–32. doi: 10.1038/sj.jidsp.5640239. [DOI] [PubMed] [Google Scholar]
- 14.Commo S, Bernard B. Melanocyte subpopulation turnover during the human hair cycle: an immunohistochemical study. Pigment cell research. 2000;13:253–9. doi: 10.1034/j.1600-0749.2000.130407.x. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura E, Jordan S, Oshima H, Yoshida H, Osawa M, Moriyama M. et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–60. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 16.Commo S, Gaillard O, Bernard B. Human hair greying is linked to a specific depletion of hair follicle melanocytes affecting both the bulb and the outer root sheath. The British journal of dermatology. 2004;150:435–43. doi: 10.1046/j.1365-2133.2004.05787.x. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307:720–4. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- 18.Tanimura S, Tadokoro Y, Inomata K, Binh NT, Nishie W, Yamazaki S. et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011;8:177–87. doi: 10.1016/j.stem.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 19.Lu Z, Xie Y, Huang H, Jiang K, Zhou B, Wang F, Hair follicle stem cells regulate retinoid metabolism to maintain the self-renewal niche for melanocyte stem cells. Elife. 2020. 9. [DOI] [PMC free article] [PubMed]
- 20.Wu S, Yu Y, Liu C, Zhang X, Zhu P, Peng Y. et al. Single-cell transcriptomics reveals lineage trajectory of human scalp hair follicle and informs mechanisms of hair graying. Cell Discov. 2022;8:49. doi: 10.1038/s41421-022-00394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arck PC, Overall R, Spatz K, Liezman C, Handjiski B, Klapp BF. et al. Towards a "free radical theory of graying": melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 2006;20:1567–9. doi: 10.1096/fj.05-4039fje. [DOI] [PubMed] [Google Scholar]
- 22.Takeo M, Lee W, Rabbani P, Sun Q, Hu H, Lim CH. et al. EdnrB Governs Regenerative Response of Melanocyte Stem Cells by Crosstalk with Wnt Signaling. Cell Rep. 2016;15:1291–302. doi: 10.1016/j.celrep.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabbani P, Takeo M, Chou W, Myung P, Bosenberg M, Chin L. et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145:941–55. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi BY. Targeting Wnt/beta-Catenin Pathway for Developing Therapies for Hair Loss. Int J Mol Sci. 2020. 21. [DOI] [PMC free article] [PubMed]
- 25.Yardman-Frank JM, Fisher DE. Skin pigmentation and its control: From ultraviolet radiation to stem cells. Experimental Dermatology. 2020;30:560–71. doi: 10.1111/exd.14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C, Kuai L, Cui R, Miao X. Melanogenesis and the Targeted Therapy of Melanoma. Biomolecules. 2022. 12. [DOI] [PMC free article] [PubMed]
- 27.Manning D, Dart C, Evans RL. Store-operated calcium channels in skin. Front Physiol. 2022;13:1033528. doi: 10.3389/fphys.2022.1033528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motiani RK, Tanwar J, Raja DA, Vashisht A, Khanna S, Sharma S, STIM1 activation of adenylyl cyclase 6 connects Ca(2+) and cAMP signaling during melanogenesis. EMBO J. 2018. 37. [DOI] [PMC free article] [PubMed]
- 29.Shah P, He YY. Molecular regulation of UV-induced DNA repair. Photochem Photobiol. 2015;91:254–64. doi: 10.1111/php.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chou WC, Takeo M, Rabbani P, Hu H, Lee W, Chung YR. et al. Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling. Nat Med. 2013;19:924–9. doi: 10.1038/nm.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahn JH, Jin SH, Kang HY. LPS induces melanogenesis through p38 MAPK activation in human melanocytes. Arch Dermatol Res. 2008;300:325–9. doi: 10.1007/s00403-008-0863-0. [DOI] [PubMed] [Google Scholar]
- 32.Qian W, Liu W, Zhu D, Cao Y, Tang A, Gong G. et al. Natural skin-whitening compounds for the treatment of melanogenesis (Review) Exp Ther Med. 2020;20:173–85. doi: 10.3892/etm.2020.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou L, Panthier J, Arnheiter H. Signaling and transcriptional regulation in the neural crest-derived melanocyte lineage: interactions between KIT and MITF. Development (Cambridge, England) 2000;127:5379–89. doi: 10.1242/dev.127.24.5379. [DOI] [PubMed] [Google Scholar]
- 34.Sato-Jin K, Nishimura EK, Akasaka E, Huber W, Nakano H, Miller A. et al. Epistatic connections between microphthalmia-associated transcription factor and endothelin signaling in Waardenburg syndrome and other pigmentary disorders. FASEB J. 2008;22:1155–68. doi: 10.1096/fj.07-9080com. [DOI] [PubMed] [Google Scholar]
- 35.Terazawa S, Imokawa G. Signaling Cascades Activated by UVB in Human Melanocytes Lead to the Increased Expression of Melanocyte Receptors, Endothelin B Receptor and c-KIT. Photochem Photobiol. 2018;94:421–31. doi: 10.1111/php.12848. [DOI] [PubMed] [Google Scholar]
- 36.Nakajima H, Wakabayashi Y, Wakamatsu K, Imokawa G. An extract of Withania somnifera attenuates endothelin-1-stimulated pigmentation in human epidermal equivalents through the interruption of PKC activity within melanocytes. Phytother Res. 2011;25:1398–411. doi: 10.1002/ptr.3552. [DOI] [PubMed] [Google Scholar]
- 37.Hwang E, Lee TH, Lee W-J, Shim W-S, Yeo E-J, Kim S. et al. A novel syntheticPiperamide derivative NED-180 inhibits hyperpigmentation by activating the PI3K and ERK pathways and by regulating Ca2+influx via TRPM1 channels. Pigment Cell & Melanoma Research. 2016;29:81–91. doi: 10.1111/pcmr.12430. [DOI] [PubMed] [Google Scholar]
- 38.Mosca S, Cardinali G, Flori E, Briganti S, Bottillo I, Mileo AM. et al. The PI3K pathway induced by alphaMSH exerts a negative feedback on melanogenesis and contributes to the release of pigment. Pigment Cell Melanoma Res. 2021;34:72–88. doi: 10.1111/pcmr.12910. [DOI] [PubMed] [Google Scholar]
- 39.Jeon S, Kim NH, Kim JY, Lee AY. Stem cell factor induces ERM proteins phosphorylation through PI3K activation to mediate melanocyte proliferation and migration. Pigment Cell Melanoma Res. 2009;22:77–85. doi: 10.1111/j.1755-148X.2008.00519.x. [DOI] [PubMed] [Google Scholar]
- 40.Todd JR, Scurr LL, Becker TM, Kefford RF, Rizos H. The MAPK pathway functions as a redundant survival signal that reinforces the PI3K cascade in c-Kit mutant melanoma. Oncogene. 2014;33:236–45. doi: 10.1038/onc.2012.562. [DOI] [PubMed] [Google Scholar]
- 41.Nishimura EK, Suzuki M, Igras V, Du J, Lonning S, Miyachi Y. et al. Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell. 2010;6:130–40. doi: 10.1016/j.stem.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murakami M, Matsuzaki F, Funaba M. Regulation of melanin synthesis by the TGF-beta family in B16 melanoma cells. Mol Biol Rep. 2009;36:1247–50. doi: 10.1007/s11033-008-9304-6. [DOI] [PubMed] [Google Scholar]
- 43.Klar AS, Biedermann T, Michalak K, Michalczyk T, Meuli-Simmen C, Scherberich A. et al. Human Adipose Mesenchymal Cells Inhibit Melanocyte Differentiation and the Pigmentation of Human Skin via Increased Expression of TGF-beta1. J Invest Dermatol. 2017;137:2560–9. doi: 10.1016/j.jid.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 44.Joompang A, Anwised P, Klaynongsruang S, Roytrakul S, Taemaitree L, Jangpromma N. Evaluation of TILI-2 as an Anti-Tyrosinase, Anti-Oxidative Agent and Its Role in Preventing Melanogenesis Using a Proteomics Approach. Molecules. 2022. 27. [DOI] [PMC free article] [PubMed]
- 45.Martinez-Esparza M, Jimenez-Cervantes C, Beermann F, Aparicio P, Lozano JA, Garcia-Borron JC. Transforming growth factor-beta1 inhibits basal melanogenesis in B16/F10 mouse melanoma cells by increasing the rate of degradation of tyrosinase and tyrosinase-related protein-1. J Biol Chem. 1997;272:3967–72. doi: 10.1074/jbc.272.7.3967. [DOI] [PubMed] [Google Scholar]
- 46.Kubic JD, Young KP, Plummer RS, Ludvik AE, Lang D. Pigmentation PAX-ways: the role of Pax3 in melanogenesis, melanocyte stem cell maintenance, and disease. Pigment Cell Melanoma Res. 2008;21:627–45. doi: 10.1111/j.1755-148X.2008.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang G, Li Y, Nishimura EK, Xin H, Zhou A, Guo Y. et al. Inhibition of PAX3 by TGF-beta modulates melanocyte viability. Mol Cell. 2008;32:554–63. doi: 10.1016/j.molcel.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Kim DS, Park SH, Park KC. Transforming growth factor-beta1 decreases melanin synthesis via delayed extracellular signal-regulated kinase activation. Int J Biochem Cell Biol. 2004;36:1482–91. doi: 10.1016/j.biocel.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 49.Moon HR, Jung JM, Kim SY, Song Y, Chang SE. TGF-beta3 suppresses melanogenesis in human melanocytes cocultured with UV-irradiated neighboring cells and human skin. J Dermatol Sci. 2020;99:100–8. doi: 10.1016/j.jdermsci.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Hibino T, Nishiyama T. Role of TGF-beta2 in the human hair cycle. J Dermatol Sci. 2004;35:9–18. doi: 10.1016/j.jdermsci.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Li S, Chen J, Chen F, Wang C, Guo X, Wang C. et al. Liposomal honokiol promotes hair growth via activating Wnt3a/beta-catenin signaling pathway and down regulating TGF-beta1 in C57BL/6N mice. Biomed Pharmacother. 2021;141:111793. doi: 10.1016/j.biopha.2021.111793. [DOI] [PubMed] [Google Scholar]
- 52.Pierrat MJ, Marsaud V, Mauviel A, Javelaud D. Expression of microphthalmia-associated transcription factor (MITF), which is critical for melanoma progression, is inhibited by both transcription factor GLI2 and transforming growth factor-beta. J Biol Chem. 2012;287:17996–8004. doi: 10.1074/jbc.M112.358341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pierrat MJ, Marsaud V, Mauviel A, Javelaud D. Transcriptional repression of the tyrosinase-related protein 2 gene by transforming growth factor-beta and the Kruppel-like transcription factor GLI2. J Dermatol Sci. 2019;94:321–9. doi: 10.1016/j.jdermsci.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 54.Steingrimsson E, Copeland NG, Jenkins NA. Melanocyte stem cell maintenance and hair graying. Cell. 2005;121:9–12. doi: 10.1016/j.cell.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 55.Gelmi MC, Houtzagers LE, Strub T, Krossa I, Jager MJ. MITF in Normal Melanocytes, Cutaneous and Uveal Melanoma: A Delicate Balance. Int J Mol Sci. 2022. 23. [DOI] [PMC free article] [PubMed]
- 56.Goding CR, Arnheiter H. MITF-the first 25 years. Genes Dev. 2019;33:983–1007. doi: 10.1101/gad.324657.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du J, Widlund HR, Horstmann MA, Ramaswamy S, Ross K, Huber WE. et al. Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell. 2004;6:565–76. doi: 10.1016/j.ccr.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 58.Carreira S, Liu B, Goding CR. The gene encoding the T-box factor Tbx2 is a target for the microphthalmia-associated transcription factor in melanocytes. J Biol Chem. 2000;275:21920–7. doi: 10.1074/jbc.M000035200. [DOI] [PubMed] [Google Scholar]
- 59.Jacobs J, Keblusek P, Robanus-Maandag E, Kristel P, Lingbeek M, Nederlof P. et al. Senescence bypass screen identifies TBX2, which represses Cdkn2a (p19(ARF)) and is amplified in a subset of human breast cancers. Nature genetics. 2000;26:291–9. doi: 10.1038/81583. [DOI] [PubMed] [Google Scholar]
- 60.Prince S, Carreira S, Vance K, Abrahams A, Goding C. Tbx2 directly represses the expression of the p21(WAF1) cyclin-dependent kinase inhibitor. Cancer research. 2004;64:1669–74. doi: 10.1158/0008-5472.can-03-3286. [DOI] [PubMed] [Google Scholar]
- 61.Strub T, Giuliano S, Ye T, Bonet C, Keime C, Kobi D. et al. Essential role of microphthalmia transcription factor for DNA replication, mitosis and genomic stability in melanoma. Oncogene. 2011;30:2319–32. doi: 10.1038/onc.2010.612. [DOI] [PubMed] [Google Scholar]
- 62.McGill G, Horstmann M, Widlund H, Du J, Motyckova G, Nishimura E. et al. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell. 2002;109:707–18. doi: 10.1016/s0092-8674(02)00762-6. [DOI] [PubMed] [Google Scholar]
- 63.Dynek JN, Chan SM, Liu J, Zha J, Fairbrother WJ, Vucic D. Microphthalmia-associated transcription factor is a critical transcriptional regulator of melanoma inhibitor of apoptosis in melanomas. Cancer Res. 2008;68:3124–32. doi: 10.1158/0008-5472.CAN-07-6622. [DOI] [PubMed] [Google Scholar]
- 64.Beuret L, Flori E, Denoyelle C, Bille K, Busca R, Picardo M. et al. Up-regulation of MET expression by alpha-melanocyte-stimulating hormone and MITF allows hepatocyte growth factor to protect melanocytes and melanoma cells from apoptosis. J Biol Chem. 2007;282:14140–7. doi: 10.1074/jbc.M611563200. [DOI] [PubMed] [Google Scholar]
- 65.Levy C, Khaled M, Robinson KC, Veguilla RA, Chen PH, Yokoyama S. et al. Lineage-specific transcriptional regulation of DICER by MITF in melanocytes. Cell. 2010;141:994–1005. doi: 10.1016/j.cell.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang J, Mou Y, Gong H, Chen H, Xiao H. Microphthalmia-Associated Transcription Factor in Senescence and Age-Related Diseases. Gerontology. 2021;67:708–17. doi: 10.1159/000515525. [DOI] [PubMed] [Google Scholar]
- 67.Seoane M, Buhs S, Iglesias P, Strauss J, Puller AC, Muller J. et al. Lineage-specific control of TFIIH by MITF determines transcriptional homeostasis and DNA repair. Oncogene. 2019;38:3616–35. doi: 10.1038/s41388-018-0661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu F, Fu Y, Meyskens FL Jr. MiTF regulates cellular response to reactive oxygen species through transcriptional regulation of APE-1/Ref-1. J Invest Dermatol. 2009;129:422–31. doi: 10.1038/jid.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buscà R, Berra E, Gaggioli C, Khaled M, Bille K, Marchetti B. et al. Hypoxia-inducible factor 1{alpha} is a new target of microphthalmia-associated transcription factor (MITF) in melanoma cells. The Journal of cell biology. 2005;170:49–59. doi: 10.1083/jcb.200501067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vazquez F, Lim JH, Chim H, Bhalla K, Girnun G, Pierce K. et al. PGC1alpha expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell. 2013;23:287–301. doi: 10.1016/j.ccr.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanwar J, Sharma A, Saurav S, Shyamveer, Jatana N, Motiani R. MITF is a novel transcriptional regulator of the calcium sensor STIM1: Significance in physiological melanogenesis. The Journal of biological chemistry. 2022;298:102681. doi: 10.1016/j.jbc.2022.102681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen J, Zheng Y, Hu C, Jin X, Chen X, Xiao Y. et al. Hair Graying Regulators Beyond Hair Follicle. Front Physiol. 2022;13:839859. doi: 10.3389/fphys.2022.839859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8:552–65. doi: 10.1016/j.stem.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V. et al. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5895–900. doi: 10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang B, Ma S, Rachmin I, He M, Baral P, Choi S. et al. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature. 2020;577:676–81. doi: 10.1038/s41586-020-1935-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan XH, Jin ZH. Paracrine regulation of melanogenesis. Br J Dermatol. 2018;178:632–9. doi: 10.1111/bjd.15651. [DOI] [PubMed] [Google Scholar]
- 77.Moattari CR, Granstein RD. Neuropeptides and neurohormones in immune, inflammatory and cellular responses to ultraviolet radiation. Acta Physiol (Oxf) 2021;232:e13644. doi: 10.1111/apha.13644. [DOI] [PubMed] [Google Scholar]
- 78.Samuelov L, Kinori M, Bertolini M, Paus R. Neural controls of human hair growth: calcitonin gene-related peptide (CGRP) induces catagen. J Dermatol Sci. 2012;67:153–5. doi: 10.1016/j.jdermsci.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 79.Pi LQ, Jin XH, Hwang ST, Lee WS. Effects of calcitonin gene-related peptide on the immune privilege of human hair follicles. Neuropeptides. 2013;47:51–7. doi: 10.1016/j.npep.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 80.Toyoda M, Luo Y, Makino T, Matsui C, Morohashi M. Calcitonin gene-related peptide upregulates melanogenesis and enhances melanocyte dendricity via induction of keratinocyte-derived melanotrophic factors. J Investig Dermatol Symp Proc. 1999;4:116–25. doi: 10.1038/sj.jidsp.5640194. [DOI] [PubMed] [Google Scholar]
- 81.Zhao J, Harada N, Kurihara H, Nakagata N, Okajima K. Dietary isoflavone increases insulin-like growth factor-I production, thereby promoting hair growth in mice. J Nutr Biochem. 2011;22:227–33. doi: 10.1016/j.jnutbio.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 82.Zhou J, Feng JY, Wang Q, Shang J. Calcitonin gene-related peptide cooperates with substance P to inhibit melanogenesis and induces apoptosis of B16F10 cells. Cytokine. 2015;74:137–44. doi: 10.1016/j.cyto.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 83.Liu N, Wang LH, Guo LL, Wang GQ, Zhou XP, Jiang Y. et al. Chronic restraint stress inhibits hair growth via substance P mediated by reactive oxygen species in mice. PLoS One. 2013;8:e61574. doi: 10.1371/journal.pone.0061574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peters EM, Liotiri S, Bodo E, Hagen E, Biro T, Arck PC. et al. Probing the effects of stress mediators on the human hair follicle: substance P holds central position. Am J Pathol. 2007;171:1872–86. doi: 10.2353/ajpath.2007.061206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peters EM, Arck PC, Paus R. Hair growth inhibition by psychoemotional stress: a mouse model for neural mechanisms in hair growth control. Exp Dermatol. 2006;15:1–13. doi: 10.1111/j.0906-6705.2005.00372.x. [DOI] [PubMed] [Google Scholar]
- 86.Paus R, Arck P, Tiede S. (Neuro-)endocrinology of epithelial hair follicle stem cells. Mol Cell Endocrinol. 2008;288:38–51. doi: 10.1016/j.mce.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 87.Ping F, Shang J, Zhou J, Song J, Zhang L. Activation of neurokinin-1 receptor by substance P inhibits melanogenesis in B16-F10 melanoma cells. Int J Biochem Cell Biol. 2012;44:2342–8. doi: 10.1016/j.biocel.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 88.Zhou J, Geng KK, Ping FF, Gao YY, Liu L, Feng BN. Cross-talk between 5-hydroxytryptamine and substance P in the melanogensis and apoptosis of B16F10 melanoma cells. Eur J Pharmacol. 2016;775:106–12. doi: 10.1016/j.ejphar.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 89.Wu H, Zhao Y, Huang Q, Cai M, Pan Q, Fu M. et al. NK1R/5-HT1AR interaction is related to the regulation of melanogenesis. FASEB J. 2018;32:3193–214. doi: 10.1096/fj.201700564RR. [DOI] [PubMed] [Google Scholar]
- 90.Chen M, Cai J, Zhang X, Liao Z, Zhong M, Shang J. et al. Keratinocytes take part in the regulation of substance P in melanogenesis through the HPA axis. J Dermatol Sci. 2022;106:141–9. doi: 10.1016/j.jdermsci.2022.04.011. [DOI] [PubMed] [Google Scholar]
- 91.Park PJ, Lee TR, Cho EG. Substance P stimulates endothelin 1 secretion via endothelin-converting enzyme 1 and promotes melanogenesis in human melanocytes. J Invest Dermatol. 2015;135:551–9. doi: 10.1038/jid.2014.423. [DOI] [PubMed] [Google Scholar]
- 92.Zhou J, Ling J, Song H, Lv B, Wang L, Shang J. et al. Neurokinin-1 receptor is a novel positive regulator of Wnt/ β-catenin signaling in melanogenesis. Oncotarget. 2016;7:81268–80. doi: 10.18632/oncotarget.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bertolini M, Pretzlaff M, Sulk M, Bahr M, Gherardini J, Uchida Y. et al. Vasoactive intestinal peptide, whose receptor-mediated signalling may be defective in alopecia areata, provides protection from hair follicle immune privilege collapse. Br J Dermatol. 2016;175:531–41. doi: 10.1111/bjd.14645. [DOI] [PubMed] [Google Scholar]
- 94.Yuan XH, Yao C, Oh JH, Park CH, Tian YD, Han M. et al. Vasoactive intestinal peptide stimulates melanogenesis in B16F10 mouse melanoma cells via CREB/MITF/tyrosinase signaling. Biochem Biophys Res Commun. 2016;477:336–42. doi: 10.1016/j.bbrc.2016.06.105. [DOI] [PubMed] [Google Scholar]
- 95.Guerrero-Juarez CF, Plikus MV. Emerging nonmetabolic functions of skin fat. Nat Rev Endocrinol. 2018;14:163–73. doi: 10.1038/nrendo.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kruglikov IL, Zhang Z, Scherer PE. The Role of Immature and Mature Adipocytes in Hair Cycling. Trends Endocrinol Metab. 2019;30:93–105. doi: 10.1016/j.tem.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zwick RK, Guerrero-Juarez CF, Horsley V, Plikus MV. Anatomical, Physiological, and Functional Diversity of Adipose Tissue. Cell Metab. 2018;27:68–83. doi: 10.1016/j.cmet.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nicu C, O'Sullivan JDB, Ramos R, Timperi L, Lai T, Farjo N. et al. Dermal Adipose Tissue Secretes HGF to Promote Human Hair Growth and Pigmentation. J Invest Dermatol. 2021;141:1633–45. doi: 10.1016/j.jid.2020.12.019. e13. [DOI] [PubMed] [Google Scholar]
- 99.Chen J, Fan ZX, Zhu DC, Guo YL, Ye K, Dai D. et al. Emerging Role of Dermal White Adipose Tissue in Modulating Hair Follicle Development During Aging. Front Cell Dev Biol. 2021;9:728188. doi: 10.3389/fcell.2021.728188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Won CH, Yoo HG, Park KY, Shin SH, Park WS, Park PJ. et al. Hair growth-promoting effects of adiponectin in vitro. J Invest Dermatol. 2012;132:2849–51. doi: 10.1038/jid.2012.217. [DOI] [PubMed] [Google Scholar]
- 101.Park PJ, Cho EG. Kojyl Cinnamate Ester Derivatives Increase Adiponectin Expression and Stimulate Adiponectin-Induced Hair Growth Factors in Human Dermal Papilla Cells. Int J Mol Sci. 2019. 20. [DOI] [PMC free article] [PubMed]
- 102.Ohn J, Been KW, Kim JY, Kim EJ, Park T, Yoon HJ. et al. Discovery of a transdermally deliverable pentapeptide for activating AdipoR1 to promote hair growth. EMBO Mol Med. 2021;13:e13790. doi: 10.15252/emmm.202013790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nicu C, Jackson J, Shahmalak A, Pople J, Ansell D, Paus R. Adiponectin negatively regulates pigmentation, Wnt/β-catenin and HGF/c-Met signalling within human scalp hair follicles ex vivo. Arch Dermatol Res. 2023;315:603–12. doi: 10.1007/s00403-021-02291-2. [DOI] [PubMed] [Google Scholar]
- 104.Bang S, Won KH, Moon HR, Yoo H, Hong A, Song Y. et al. Novel regulation of melanogenesis by adiponectin via the AMPK/CRTC pathway. Pigment Cell Melanoma Res. 2017;30:553–7. doi: 10.1111/pcmr.12596. [DOI] [PubMed] [Google Scholar]
- 105.Kim Y, Cho JY, Oh SW, Kang M, Lee SE, Jung E. et al. Globular adiponectin acts as a melanogenic signal in human epidermal melanocytes. Br J Dermatol. 2018;179:689–701. doi: 10.1111/bjd.16488. [DOI] [PubMed] [Google Scholar]
- 106.Xiong M, Zhang Q, Hu W, Zhao C, Lv W, Yi Y. et al. Exosomes From Adipose-Derived Stem Cells: The Emerging Roles and Applications in Tissue Regeneration of Plastic and Cosmetic Surgery. Front Cell Dev Biol. 2020;8:574223. doi: 10.3389/fcell.2020.574223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mazini L, Rochette L, Admou B, Amal S, Malka G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int J Mol Sci. 2020. 21. [DOI] [PMC free article] [PubMed]
- 108.Tak YJ, Lee SY, Cho AR, Kim YS. A randomized, double-blind, vehicle-controlled clinical study of hair regeneration using adipose-derived stem cell constituent extract in androgenetic alopecia. Stem Cells Transl Med. 2020;9:839–49. doi: 10.1002/sctm.19-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu J, Yang Q, Wu S, Yuan R, Zhao X, Li Y. et al. Adipose-Derived Stem Cell Exosomes Promoted Hair Regeneration. Tissue Eng Regen Med. 2021;18:685–91. doi: 10.1007/s13770-021-00347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nilforoushzadeh MA, Aghdami N, Taghiabadi E. Effects of Adipose-Derived Stem Cells and Platelet-Rich Plasma Exosomes on The Inductivity of Hair Dermal Papilla Cells. Cell J. 2021;23:576–83. doi: 10.22074/cellj.2021.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liang Y, Tang X, Zhang X, Cao C, Yu M, Wan M. Adipose Mesenchymal Stromal Cell-Derived Exosomes Carrying MiR-122-5p Antagonize the Inhibitory Effect of Dihydrotestosterone on Hair Follicles by Targeting the TGF-beta1/SMAD3 Signaling Pathway. Int J Mol Sci. 2023. 24. [DOI] [PMC free article] [PubMed]
- 112.Lu Q, Gao Y, Fan Z, Xiao X, Chen Y, Si Y. et al. Amphiregulin promotes hair regeneration of skin-derived precursors via the PI3K and MAPK pathways. Cell Prolif. 2021;54:e13106. doi: 10.1111/cpr.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Anderi R, Makdissy N, Azar A, Rizk F, Hamade A. Cellular therapy with human autologous adipose-derived adult cells of stromal vascular fraction for alopecia areata. Stem Cell Res Ther. 2018;9:141. doi: 10.1186/s13287-018-0889-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim DW, Jeon BJ, Hwang NH, Kim MS, Park SH, Dhong ES. et al. Adipose-derived stem cells inhibit epidermal melanocytes through an interleukin-6-mediated mechanism. Plast Reconstr Surg. 2014;134:470–80. doi: 10.1097/PRS.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 115.Chang H, Park JH, Min KH, Lee RS, Kim EK. Whitening effects of adipose-derived stem cells: a preliminary in vivo study. Aesthetic Plast Surg. 2014;38:230–3. doi: 10.1007/s00266-013-0116-2. [DOI] [PubMed] [Google Scholar]
- 116.Jeon BJ, Kim DW, Kim MS, Park SH, Dhong ES, Yoon ES. et al. Protective effects of adipose-derived stem cells against UVB-induced skin pigmentation. J Plast Surg Hand Surg. 2016;50:336–42. doi: 10.1080/2000656X.2016.1175358. [DOI] [PubMed] [Google Scholar]
- 117.Dou S, Yang Y, Zhang J, He Z, Wu Z, Zhao Y. et al. Exploring the Role and Mechanism of Adipose Derived Mesenchymal Stem Cells on Reversal of Pigmentation Model Effects. Aesthetic Plast Surg. 2022;46:1983–96. doi: 10.1007/s00266-022-02872-0. [DOI] [PubMed] [Google Scholar]
- 118.Shen JP, Wu YX, Tang SJ, Peng LH. Experimental study on stromal vascular fraction mediated inhibition of skin pigmentation in guinea pigs. Ann Transl Med. 2022;10:1268. doi: 10.21037/atm-22-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim JY, Park CD, Lee JH, Lee CH, Do BR, Lee AY. Co-culture of melanocytes with adipose-derived stem cells as a potential substitute for co-culture with keratinocytes. Acta Derm Venereol. 2012;92:16–23. doi: 10.2340/00015555-1174. [DOI] [PubMed] [Google Scholar]
- 120.Lim WS, Kim CH, Kim JY, Do BR, Kim EJ, Lee AY. Adipose-derived stem cells improve efficacy of melanocyte transplantation in animal skin. Biomol Ther (Seoul) 2014;22:328–33. doi: 10.4062/biomolther.2014.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim H, Yi N, Do BR, Lee AY. Adipose-Derived Stem Cell Coculturing Stimulates Integrin-Mediated Extracellular Matrix Adhesion of Melanocytes by Upregulating Growth Factors. Biomol Ther (Seoul) 2019;27:185–92. doi: 10.4062/biomolther.2018.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bellei B, Papaccio F, Filoni A, Caputo S, Lopez G, Migliano E. et al. Extracellular fraction of adipose tissue as an innovative regenerative approach for vitiligo treatment. Exp Dermatol. 2019;28:695–703. doi: 10.1111/exd.13954. [DOI] [PubMed] [Google Scholar]
- 123.Bian Y, Yu H, Jin M, Gao X. Repigmentation by combined narrow-band ultraviolet B/adipose-derived stem cell transplantation in the mouse model: Role of Nrf2/HO-1-mediated Ca(2+) homeostasis. Mol Med Rep. 2022. 25. [DOI] [PMC free article] [PubMed]
- 124.Castelli MS, McGonigle P, Hornby PJ. The pharmacology and therapeutic applications of monoclonal antibodies. Pharmacol Res Perspect. 2019;7:e00535. doi: 10.1002/prp2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jiang Y, Chen M, Nie H, Yuan Y. PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations. Hum Vaccin Immunother. 2019;15:1111–22. doi: 10.1080/21645515.2019.1571892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;21:28. doi: 10.1186/s12943-021-01489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rivera N, Boada A, Bielsa MI, Fernandez-Figueras MT, Carcereny E, Moran MT. et al. Hair Repigmentation During Immunotherapy Treatment With an Anti-Programmed Cell Death 1 and Anti-Programmed Cell Death Ligand 1 Agent for Lung Cancer. JAMA Dermatol. 2017;153:1162–5. doi: 10.1001/jamadermatol.2017.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Manson G, Marabelle A, Houot R. Hair Repigmentation With Anti-PD-1 and Anti-PD-L1 Immunotherapy: A Novel Hypothesis. JAMA Dermatol. 2018;154:113. doi: 10.1001/jamadermatol.2017.4421. [DOI] [PubMed] [Google Scholar]
- 129.Sebaratnam DF, Rodriguez Bandera AI, Lowe PM. Hair Repigmentation With Anti-PD-1 and Anti-PD-L1 Immunotherapy: A Novel Hypothesis. JAMA Dermatol. 2018;154:112–3. doi: 10.1001/jamadermatol.2017.4420. [DOI] [PubMed] [Google Scholar]
- 130.Park M, Woo SY, Cho KA, Cho MS, Lee KH. PD-L1 produced by HaCaT cells under polyinosinic-polycytidylic acid stimulation inhibits melanin production by B16F10 cells. PLoS One. 2020;15:e0233448. doi: 10.1371/journal.pone.0233448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Quach HT, Johnson DB, LeBoeuf NR, Zwerner JP, Dewan AK. Cutaneous adverse events caused by immune checkpoint inhibitors. J Am Acad Dermatol. 2021;85:956–66. doi: 10.1016/j.jaad.2020.09.054. [DOI] [PubMed] [Google Scholar]
- 132.Correa-Selm LM, Grichnik JM. PD1 inhibitors and hair repigmentation: A desirable new side effect. Dermatol Ther. 2018. 31. [DOI] [PubMed]
- 133.Navarro-Trivino FJ, Ruiz-Villaverde R, Manuel Ramos-Pleguezuelos F, Vano-Galvan S. Canities Subita after Extreme Trauma Showing Positive Staining for Anti-PD-L1 Antibodies: A New Clue into Etiopathogenesis? Skin Appendage Disord. 2022;8:65–9. doi: 10.1159/000517805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Harb H, Chatila TA. Mechanisms of Dupilumab. Clin Exp Allergy. 2020;50:5–14. doi: 10.1111/cea.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sumitomo C, Akita H, Sugiura K. Unexpected side-effect of dupilumab: Reversal of hair graying. J Dermatol. 2020;47:e316–e7. doi: 10.1111/1346-8138.15472. [DOI] [PubMed] [Google Scholar]
- 136.Choi H, Choi H, Han J, Jin SH, Park JY, Shin DW. et al. IL-4 Inhibits the Melanogenesis of Normal Human Melanocytes through the JAK2-STAT6 Signaling Pathway. J Invest Dermatol. 2013;133:528–36. doi: 10.1038/jid.2012.331. [DOI] [PubMed] [Google Scholar]
- 137.Tintle S, Dabade T, Kalish R, Rosmarin D. Repigmentation of hair following adalimumab therapy. Dermatology online journal. 2015. 21. [PubMed]
- 138.Singh M, Mansuri MS, Kadam A, Palit SP, Dwivedi M, Laddha NC. et al. Tumor Necrosis Factor-alpha affects melanocyte survival and melanin synthesis via multiple pathways in vitiligo. Cytokine. 2021;140:155432. doi: 10.1016/j.cyto.2021.155432. [DOI] [PubMed] [Google Scholar]
- 139.Wang CQF, Akalu YT, Suarez-Farinas M, Gonzalez J, Mitsui H, Lowes MA. et al. IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis. J Invest Dermatol. 2013;133:2741–52. doi: 10.1038/jid.2013.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Grine L, Dejager L, Libert C, Vandenbroucke RE. An inflammatory triangle in psoriasis: TNF, type I IFNs and IL-17. Cytokine Growth Factor Rev. 2015;26:25–33. doi: 10.1016/j.cytogfr.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 141.Martínez-Esparza M, Jiménez-Cervantes C, Solano F, Lozano J, García-Borrón J. Regulation of the murine silver locus product (gp87) by the hypopigmenting cytokines TGF-beta1 and TNF-alpha. Pigment cell research. 2000;13:120–6. doi: 10.1034/j.1600-0749.2000.130211.x. [DOI] [PubMed] [Google Scholar]
- 142.Funasaka Y, Chakraborty A, Hayashi Y, Komoto M, Ohashi A, Nagahama M. et al. Modulation of melanocyte-stimulating hormone receptor expression on normal human melanocytes: evidence for a regulatory role of ultraviolet B, interleukin-1alpha, interleukin-1beta, endothelin-1 and tumour necrosis factor-alpha. The British journal of dermatology. 1998;139:216–24. doi: 10.1046/j.1365-2133.1998.02357.x. [DOI] [PubMed] [Google Scholar]
- 143.Choi H, Ahn S, Lee BG, Chang I, Hwang JS. Inhibition of skin pigmentation by an extract of Lepidium apetalum and its possible implication in IL-6 mediated signaling. Pigment Cell Res. 2005;18:439–46. doi: 10.1111/j.1600-0749.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 144.Choi H, Kim K, Han J, Choi H, Jin SH, Lee EK. et al. Kojic acid-induced IL-6 production in human keratinocytes plays a role in its anti-melanogenic activity in skin. J Dermatol Sci. 2012;66:207–15. doi: 10.1016/j.jdermsci.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 145.Jiang L, Huang J, Lu J, Hu S, Pei S, Ouyang Y. et al. Ganoderma lucidum polysaccharide reduces melanogenesis by inhibiting the paracrine effects of keratinocytes and fibroblasts via IL-6/STAT3/FGF2 pathway. J Cell Physiol. 2019;234:22799–808. doi: 10.1002/jcp.28844. [DOI] [PubMed] [Google Scholar]
- 146.Camara-Lemarroy CR, Salas-Alanis JC. The role of tumor necrosis factor-alpha in the pathogenesis of vitiligo. Am J Clin Dermatol. 2013;14:343–50. doi: 10.1007/s40257-013-0039-3. [DOI] [PubMed] [Google Scholar]
- 147.Manaka L, Kadono S, Kawashima M, Kobayashi T, Imokawa G. The mechanism of hyperpigmentation in seborrhoeic keratosis involves the high expression of endothelin-converting enzyme-1alpha and TNF-alpha, which stimulate secretion of endothelin 1. The British journal of dermatology. 2001;145:895–903. doi: 10.1046/j.1365-2133.2001.04521.x. [DOI] [PubMed] [Google Scholar]
- 148.Imokawa G. Melanocyte Activation Mechanisms and Rational Therapeutic Treatments of Solar Lentigos. Int J Mol Sci. 2019. 20. [DOI] [PMC free article] [PubMed]
- 149.Takenaka Y, Hoshino Y, Nakajima H, Hayashi N, Kawashima M, Imokawa G. Paracrine cytokine mechanisms underlying the hyperpigmentation of seborrheic keratosis in covered skin areas. J Dermatol. 2013;40:533–42. doi: 10.1111/1346-8138.12178. [DOI] [PubMed] [Google Scholar]
- 150.Lu Y, Tonissen KF, Di Trapani G. Modulating skin colour: role of the thioredoxin and glutathione systems in regulating melanogenesis. Biosci Rep. 2021. 41. [DOI] [PMC free article] [PubMed]
- 151.Rongioletti F, Mugheddu C, Murgia S. Repigmentation and new growth of hairs after anti-interleukin-17 therapy with secukinumab for psoriasis. JAAD Case Rep. 2018;4:486–8. doi: 10.1016/j.jdcr.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhou J, An X, Dong J, Wang Y, Zhong H, Duan L. et al. IL-17 induces cellular stress microenvironment of melanocytes to promote autophagic cell apoptosis in vitiligo. FASEB J. 2018;32:4899–916. doi: 10.1096/fj.201701242RR. [DOI] [PubMed] [Google Scholar]
- 153.Kotobuki Y, Tanemura A, Yang L, Itoi S, Wataya-Kaneda M, Murota H. et al. Dysregulation of melanocyte function by Th17-related cytokines: significance of Th17 cell infiltration in autoimmune vitiligo vulgaris. Pigment Cell Melanoma Res. 2012;25:219–30. doi: 10.1111/j.1755-148X.2011.00945.x. [DOI] [PubMed] [Google Scholar]
- 154.Kholmanskikh O, van Baren N, Brasseur F, Ottaviani S, Vanacker J, Arts N. et al. Interleukins 1alpha and 1beta secreted by some melanoma cell lines strongly reduce expression of MITF-M and melanocyte differentiation antigens. Int J Cancer. 2010;127:1625–36. doi: 10.1002/ijc.25182. [DOI] [PubMed] [Google Scholar]
- 155.Arts N, Cane S, Hennequart M, Lamy J, Bommer G, Van den Eynde B. et al. microRNA-155, induced by interleukin-1ss, represses the expression of microphthalmia-associated transcription factor (MITF-M) in melanoma cells. PLoS One. 2015;10:e0122517. doi: 10.1371/journal.pone.0122517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhuang T, Li S, Yi X, Guo S, Wang Y, Chen J. et al. Tranilast Directly Targets NLRP3 to Protect Melanocytes From Keratinocyte-Derived IL-1beta Under Oxidative Stress. Front Cell Dev Biol. 2020;8:588. doi: 10.3389/fcell.2020.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang CY, Guo Y, Wu WJ, Man MQ, Tu Y, He L. UVB-Induced Secretion of IL-1beta Promotes Melanogenesis by Upregulating TYR/TRP-1 Expression In vitro. Biomed Res Int. 2022;2022:8230646. doi: 10.1155/2022/8230646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Singh RK, Lee KM, Vujkovic-Cvijin I, Ucmak D, Farahnik B, Abrouk M. et al. The role of IL-17 in vitiligo: A review. Autoimmun Rev. 2016;15:397–404. doi: 10.1016/j.autrev.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bernardini N, Skroza N, Tolino E, Mambrin A, Anzalone A, Balduzzi V. et al. IL-17 and its role in inflammatory, autoimmune, and oncological skin diseases: state of art. Int J Dermatol. 2020;59:406–11. doi: 10.1111/ijd.14695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bhardwaj S, Rani S, Srivastava N, Kumar R, Parsad D. Increased systemic and epidermal levels of IL-17A and IL-1beta promotes progression of non-segmental vitiligo. Cytokine. 2017;91:153–61. doi: 10.1016/j.cyto.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 161.Tomaszewska K, Kozlowska M, Kaszuba A, Lesiak A, Narbutt J, Zalewska-Janowska A. Increased Serum Levels of IFN-gamma, IL-1beta, and IL-6 in Patients with Alopecia Areata and Nonsegmental Vitiligo. Oxid Med Cell Longev. 2020;2020:5693572. doi: 10.1155/2020/5693572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Di Cesare A, Fargnoli MC, Marinucci A, Peris K. Rationale for the development of speckled hyperpigmentation in the areas of psoriatic plaques after treatment with biologic agents. J Invest Dermatol. 2015;135:318–20. doi: 10.1038/jid.2014.297. [DOI] [PubMed] [Google Scholar]
- 163.Zhang S, Liang J, Tian X, Zhou X, Liu W, Chen X. et al. Secukinumab-induced multiple lentigines in areas of resolved psoriatic plaques: A case report and literature review. Dermatol Ther. 2021;34:e15048. doi: 10.1111/dth.15048. [DOI] [PubMed] [Google Scholar]
- 164.Park S, Ahn G, Park J, Seo S. The First Case of Ustekinumab-Associated Hair Repigmentation and a Proposed Mechanism of Action. Annals of dermatology. 2021;33:300–1. doi: 10.5021/ad.2021.33.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Armstrong AW, Read C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA. 2020;323:1945–60. doi: 10.1001/jama.2020.4006. [DOI] [PubMed] [Google Scholar]
- 166.Penzi LR, Manatis-Lornell A, Saavedra A, Fisher D, Senna MM. Hair repigmentation associated with the use of brentuximab. JAAD Case Rep. 2017;3:563–5. doi: 10.1016/j.jdcr.2017.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Deutsch YE, Tadmor T, Podack ER, Rosenblatt JD. CD30: an important new target in hematologic malignancies. Leuk Lymphoma. 2011;52:1641–54. doi: 10.3109/10428194.2011.574761. [DOI] [PubMed] [Google Scholar]
- 168.So T, Ishii N. The TNF-TNFR Family of Co-signal Molecules. Advances in experimental medicine and biology. 2019;1189:53–84. doi: 10.1007/978-981-32-9717-3_3. [DOI] [PubMed] [Google Scholar]
- 169.Robert C, Spatz A, Faivre S, Armand JP, Raymond E. Tyrosine kinase inhibition and grey hair. Lancet. 2003;361:1056. doi: 10.1016/S0140-6736(03)12805-X. [DOI] [PubMed] [Google Scholar]
- 170.Kockerols C, Westerweel P. Hair Repigmentation Induced by Nilotinib. The New England journal of medicine. 2022;387:e12. doi: 10.1056/NEJMicm2119953. [DOI] [PubMed] [Google Scholar]
- 171.Cheng Y, Chen H, Chiu H. Erlotinib-induced hair repigmentation. International journal of dermatology. 2014;53:e55–7. doi: 10.1111/j.1365-4632.2011.05422.x. [DOI] [PubMed] [Google Scholar]
- 172.Robert C, Mateus C, Spatz A, Wechsler J, Escudier B. Dermatologic symptoms associated with the multikinase inhibitor sorafenib. J Am Acad Dermatol. 2009;60:299–305. doi: 10.1016/j.jaad.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 173.Cohen P, Cross D, Janne PA. Kinase drug discovery 20 years after imatinib: progress and future directions. Nat Rev Drug Discov. 2021;20:551–69. doi: 10.1038/s41573-021-00195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Quintás-Cardama A, Cortes J. Nilotinib: a phenylamino-pyrimidine derivative with activity against BCR-ABL, KIT and PDGFR kinases. Future oncology (London, England) 2008;4:611–21. doi: 10.2217/14796694.4.5.611. [DOI] [PubMed] [Google Scholar]
- 175.Contreras O, Villarreal M, Brandan E. Nilotinib impairs skeletal myogenesis by increasing myoblast proliferation. Skelet Muscle. 2018;8:5. doi: 10.1186/s13395-018-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kim J, Lee HJ, Park JH, Cha BY, Hoe HS. Nilotinib modulates LPS-induced cognitive impairment and neuroinflammatory responses by regulating P38/STAT3 signaling. J Neuroinflammation. 2022;19:187. doi: 10.1186/s12974-022-02549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jeitany M, Leroy C, Tosti P, Lafitte M, Le Guet J, Simon V, Inhibition of DDR1-BCR signalling by nilotinib as a new therapeutic strategy for metastatic colorectal cancer. EMBO Mol Med. 2018. 10. [DOI] [PMC free article] [PubMed]
- 178.Wu J, Xu X, Zheng L, Mo J, Jin X, Bao Y. Nilotinib inhibits microglia-mediated neuroinflammation to protect against dopaminergic neuronal death in Parkinson's disease models. Int Immunopharmacol. 2021;99:108025. doi: 10.1016/j.intimp.2021.108025. [DOI] [PubMed] [Google Scholar]
- 179.Kim KI, Jo JW, Lee JH, Kim CD, Yoon TJ. Induction of pigmentation by a small molecule tyrosine kinase inhibitor nilotinib. Biochem Biophys Res Commun. 2018;503:2271–6. doi: 10.1016/j.bbrc.2018.06.148. [DOI] [PubMed] [Google Scholar]
- 180.Chang SP, Huang HM, Shen SC, Lee WR, Chen YC. Nilotinib induction of melanogenesis via reactive oxygen species-dependent JNK activation in B16F0 mouse melanoma cells. Exp Dermatol. 2018;27:1388–94. doi: 10.1111/exd.13797. [DOI] [PubMed] [Google Scholar]
- 181.Kang B, Kim Y, Park TJ, Kang HY. Dasatinib, a second-generation tyrosine kinase inhibitor, induces melanogenesis via ERK-CREB-MITF-tyrosinase signaling in normal human melanocytes. Biochem Biophys Res Commun. 2020;523:1034–9. doi: 10.1016/j.bbrc.2020.01.051. [DOI] [PubMed] [Google Scholar]
- 182.Jeong SM, Yoon TJ. Development of Pigmentation-Regulating Agents by Drug Repositioning. Int J Mol Sci. 2021. 22. [DOI] [PMC free article] [PubMed]
- 183.Kim KI, Jung KE, Shin YB, Kim CD, Yoon TJ. Sorafenib induces pigmentation via the regulation of beta-catenin signalling pathway in melanoma cells. Exp Dermatol. 2022;31:57–63. doi: 10.1111/exd.14112. [DOI] [PubMed] [Google Scholar]
- 184.Steins M, Thomas M, Geissler M. Erlotinib. Recent Results Cancer Res. 2018;211:1–17. doi: 10.1007/978-3-319-91442-8_1. [DOI] [PubMed] [Google Scholar]
- 185.Lin KY, Chen CM, Lu CY, Cheng CY, Wu YH. Regulation of miR-21 expression in human melanoma via UV-ray-induced melanin pigmentation. Environ Toxicol. 2017;32:2064–9. doi: 10.1002/tox.22421. [DOI] [PubMed] [Google Scholar]
- 186.Yun WJ, Bang SH, Min KH, Kim SW, Lee MW, Chang SE. Epidermal growth factor and epidermal growth factor signaling attenuate laser-induced melanogenesis. Dermatol Surg. 2013;39:1903–11. doi: 10.1111/dsu.12348. [DOI] [PubMed] [Google Scholar]
- 187.AlGhamdi KM, Kumar A. Depigmentation therapies for normal skin in vitiligo universalis. J Eur Acad Dermatol Venereol. 2011;25:749–57. doi: 10.1111/j.1468-3083.2010.03876.x. [DOI] [PubMed] [Google Scholar]
- 188.Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203–18. doi: 10.1016/j.jaad.2014.07.032. quiz 19-20. [DOI] [PubMed] [Google Scholar]
- 189.Zuo RC, Apolo AB, DiGiovanna JJ, Parnes HL, Keen CM, Nanda S. et al. Cutaneous adverse effects associated with the tyrosine-kinase inhibitor cabozantinib. JAMA Dermatol. 2015;151:170–7. doi: 10.1001/jamadermatol.2014.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lovering S, Miao W, Bailie T, Amato D. Hair repigmentation associated with thalidomide use for the treatment of multiple myeloma. BMJ Case Rep. 2016. 2016. [DOI] [PMC free article] [PubMed]
- 191.Dasanu CA, Mitsis D, Alexandrescu DT. Hair repigmentation associated with the use of lenalidomide: graying may not be an irreversible process! J Oncol Pharm Pract. 2013;19:165–9. doi: 10.1177/1078155212442561. [DOI] [PubMed] [Google Scholar]
- 192.Zhu YX, Kortuem KM, Stewart AK. Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma. Leuk Lymphoma. 2013;54:683–7. doi: 10.3109/10428194.2012.728597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chang X, Zhu Y, Shi C, Stewart AK. Mechanism of immunomodulatory drugs' action in the treatment of multiple myeloma. Acta Biochim Biophys Sin (Shanghai) 2014;46:240–53. doi: 10.1093/abbs/gmt142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xu Y, Sun J, Sheard MA, Tran HC, Wan Z, Liu WY. et al. Lenalidomide overcomes suppression of human natural killer cell anti-tumor functions by neuroblastoma microenvironment-associated IL-6 and TGFbeta1. Cancer Immunol Immunother. 2013;62:1637–48. doi: 10.1007/s00262-013-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liang CJ, Yen YH, Hung LY, Wang SH, Pu CM, Chien HF. et al. Thalidomide inhibits fibronectin production in TGF-beta1-treated normal and keloid fibroblasts via inhibition of the p38/Smad3 pathway. Biochem Pharmacol. 2013;85:1594–602. doi: 10.1016/j.bcp.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 196.Bian C, Qin WJ, Zhang CY, Zou GL, Zhu YZ, Chen J. et al. Thalidomide (THD) alleviates radiation induced lung fibrosis (RILF) via down-regulation of TGF-beta/Smad3 signaling pathway in an Nrf2-dependent manner. Free Radic Biol Med. 2018;129:446–53. doi: 10.1016/j.freeradbiomed.2018.10.423. [DOI] [PubMed] [Google Scholar]
- 197.Lu Y, Zhao C, Lei L, Tao Z, Zheng L, Wen J. et al. Effects of thalidomide on Th17, Treg cells and TGF-beta1/Smad3 pathway in a mouse model of systemic sclerosis. Int J Rheum Dis. 2020;23:406–19. doi: 10.1111/1756-185X.13769. [DOI] [PubMed] [Google Scholar]
- 198.Amirshahrokhi K, Khalili AR. Thalidomide ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in an experimental model. Inflammation. 2015;38:476–84. doi: 10.1007/s10753-014-9953-7. [DOI] [PubMed] [Google Scholar]
- 199.Paravar T, Lee DJ. Thalidomide: mechanisms of action. Int Rev Immunol. 2008;27:111–35. doi: 10.1080/08830180801911339. [DOI] [PubMed] [Google Scholar]
- 200.Keifer JA, Guttridge DC, Ashburner BP, Baldwin AS Jr. Inhibition of NF-kappa B activity by thalidomide through suppression of IkappaB kinase activity. J Biol Chem. 2001;276:22382–7. doi: 10.1074/jbc.M100938200. [DOI] [PubMed] [Google Scholar]
- 201.Fu C, Chen J, Lu J, Yi L, Tong X, Kang L. et al. Roles of inflammation factors in melanogenesis (Review) Mol Med Rep. 2020;21:1421–30. doi: 10.3892/mmr.2020.10950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhou J, Ling J, Song J, Wang Y, Feng B, Ping F. Interleukin 10 protects primary melanocyte by activation of Stat-3 and PI3K/Akt/NF-kappaB signaling pathways. Cytokine. 2016;83:275–81. doi: 10.1016/j.cyto.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 203.Semeraro M, Vacchelli E, Eggermont A, Galon J, Zitvogel L, Kroemer G. et al. Trial Watch: Lenalidomide-based immunochemotherapy. Oncoimmunology. 2013;2:e26494. doi: 10.4161/onci.26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Englaro W, Bahadoran P, Bertolotto C, Buscà R, Dérijard B, Livolsi A. et al. Tumor necrosis factor alpha-mediated inhibition of melanogenesis is dependent on nuclear factor kappa B activation. Oncogene. 1999;18:1553–9. doi: 10.1038/sj.onc.1202446. [DOI] [PubMed] [Google Scholar]
- 205.Kim K, Choi H, Kim H, Lee T. TNFSF14 inhibits melanogenesis via NF-kB signaling in melanocytes. Cytokine. 2018;110:126–30. doi: 10.1016/j.cyto.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 206.Zhou J, Shang J, Song J, Ping F. Interleukin-18 augments growth ability of primary human melanocytes by PTEN inactivation through the AKT/NF-kappaB pathway. Int J Biochem Cell Biol. 2013;45:308–16. doi: 10.1016/j.biocel.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 207.Sun L, Pan S, Yang Y, Sun J, Liang D, Wang X. et al. Toll-like receptor 9 regulates melanogenesis through NF-kappaB activation. Exp Biol Med (Maywood) 2016;241:1497–504. doi: 10.1177/1535370216642529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chaiprasongsuk A, Panich U. Role of Phytochemicals in Skin Photoprotection via Regulation of Nrf2. Front Pharmacol. 2022;13:823881. doi: 10.3389/fphar.2022.823881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bodera P, Stankiewicz W. Immunomodulatory properties of thalidomide analogs: pomalidomide and lenalidomide, experimental and therapeutic applications. Recent patents on endocrine, metabolic & immune drug discovery. 2011;5:192–6. doi: 10.2174/187221411797265890. [DOI] [PubMed] [Google Scholar]
- 210.Wang Y, Viennet C, Robin S, Berthon JY, He L, Humbert P. Precise role of dermal fibroblasts on melanocyte pigmentation. J Dermatol Sci. 2017;88:159–66. doi: 10.1016/j.jdermsci.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 211.Kim NH, Lee AY. Growth Factors Upregulated by Uric Acid Affect Guanine Deaminase-Induced Melanogenesis. Biomol Ther (Seoul) 2023;31:89–96. doi: 10.4062/biomolther.2022.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhu JW, Ni YJ, Tong XY, Guo X, Wu XP. Activation of VEGF receptors in response to UVB promotes cell proliferation and melanogenesis of normal human melanocytes. Exp Cell Res. 2020;387:111798. doi: 10.1016/j.yexcr.2019.111798. [DOI] [PubMed] [Google Scholar]
- 213.Zhu JW, Ni YJ, Tong XY, Guo X, Wu XP, Lu ZF. Tranexamic Acid Inhibits Angiogenesis and Melanogenesis in vitro by Targeting VEGF Receptors. Int J Med Sci. 2020;17:903–11. doi: 10.7150/ijms.44188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Amor KT, Ryan C, Menter A. The use of cyclosporine in dermatology: part I. J Am Acad Dermatol. 2010;63:925–46. doi: 10.1016/j.jaad.2010.02.063. quiz 47-8. [DOI] [PubMed] [Google Scholar]
- 215.Rebora A, Delmonte S, Parodi A. Cyclosporin A-induced hair darkening. International journal of dermatology. 1999;38:229–30. [PubMed] [Google Scholar]
- 216.Sadighha A, Zahed GM. Hair darkening after treatment with cyclosporin in a patient with psoriasis. J Eur Acad Dermatol Venereol. 2008;22:1239–41. doi: 10.1111/j.1468-3083.2008.02598.x. [DOI] [PubMed] [Google Scholar]
- 217.Gohar A. Comment on the letter by Sadighha and Zahed on Hair darkening after treatment with cyclosporin in a patient with psoriasis. Journal of the European Academy of Dermatology and Venereology: JEADV. 2009;23:862. doi: 10.1111/j.1468-3083.2009.03275.x. [DOI] [PubMed] [Google Scholar]
- 218.Hawkshaw NJ, Paus R. Beyond the NFAT Horizon: From Cyclosporine A-Induced Adverse Skin Effects to Novel Therapeutics. Trends Pharmacol Sci. 2021;42:316–28. doi: 10.1016/j.tips.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 219.Hawkshaw NJ, Hardman JA, Haslam IS, Shahmalak A, Gilhar A, Lim X. et al. Identifying novel strategies for treating human hair loss disorders: Cyclosporine A suppresses the Wnt inhibitor, SFRP1, in the dermal papilla of human scalp hair follicles. PLoS Biol. 2018;16:e2003705. doi: 10.1371/journal.pbio.2003705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Redondo P, Guzmán M, Marquina M, Pretel M, Aguado L, Lloret P. et al. [Repigmentation of gray hair after thyroid hormone treatment] Actas dermo-sifiliograficas. 2007;98:603–10. [PubMed] [Google Scholar]
- 221.van Beek N, Bodo E, Kromminga A, Gaspar E, Meyer K, Zmijewski MA. et al. Thyroid hormones directly alter human hair follicle functions: anagen prolongation and stimulation of both hair matrix keratinocyte proliferation and hair pigmentation. J Clin Endocrinol Metab. 2008;93:4381–8. doi: 10.1210/jc.2008-0283. [DOI] [PubMed] [Google Scholar]
- 222.Hardman JA, Haslam IS, Farjo N, Farjo B, Paus R. Thyroxine differentially modulates the peripheral clock: lessons from the human hair follicle. PLoS One. 2015;10:e0121878. doi: 10.1371/journal.pone.0121878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mancino G, Miro C, Di Cicco E, Dentice M. Thyroid hormone action in epidermal development and homeostasis and its implications in the pathophysiology of the skin. J Endocrinol Invest. 2021;44:1571–9. doi: 10.1007/s40618-020-01492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Di Cicco E, Moran C, Visser WE, Nappi A, Schoenmakers E, Todd P. et al. Germ Line Mutations in the Thyroid Hormone Receptor Alpha Gene Predispose to Cutaneous Tags and Melanocytic Nevi. Thyroid. 2021;31:1114–26. doi: 10.1089/thy.2020.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bellandi S, Amato L, Cipollini E, Antiga E, Brandini L, Fabbri P. Repigmentation of hair after latanoprost therapy. Journal of the European Academy of Dermatology and Venereology: JEADV. 2011;25:1485–7. doi: 10.1111/j.1468-3083.2010.03949.x. [DOI] [PubMed] [Google Scholar]
- 226.Digiuni M, Fogagnolo P, Rossetti L. A review of the use of latanoprost for glaucoma since its launch. Expert opinion on pharmacotherapy. 2012;13:723–45. doi: 10.1517/14656566.2012.662219. [DOI] [PubMed] [Google Scholar]
- 227.Scott G, Leopardi S, Printup S, Malhi N, Seiberg M, Lapoint R. Proteinase-activated receptor-2 stimulates prostaglandin production in keratinocytes: analysis of prostaglandin receptors on human melanocytes and effects of PGE2 and PGF2alpha on melanocyte dendricity. The Journal of investigative dermatology. 2004;122:1214–24. doi: 10.1111/j.0022-202X.2004.22516.x. [DOI] [PubMed] [Google Scholar]
- 228.Scott G, Jacobs S, Leopardi S, Anthony FA, Learn D, Malaviya R. et al. Effects of PGF2alpha on human melanocytes and regulation of the FP receptor by ultraviolet radiation. Exp Cell Res. 2005;304:407–16. doi: 10.1016/j.yexcr.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 229.Gledhill K, Rhodes LE, Brownrigg M, Haylett AK, Masoodi M, Thody AJ. et al. Prostaglandin-E2 is produced by adult human epidermal melanocytes in response to UVB in a melanogenesis-independent manner. Pigment Cell Melanoma Res. 2010;23:394–403. doi: 10.1111/j.1755-148X.2010.00696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Starner RJ, McClelland L, Abdel-Malek Z, Fricke A, Scott G. PGE(2) is a UVR-inducible autocrine factor for human melanocytes that stimulates tyrosinase activation. Exp Dermatol. 2010;19:682–4. doi: 10.1111/j.1600-0625.2010.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ma HJ, Ma HY, Yang Y, Li PC, Zi SX, Jia CY. et al. alpha-Melanocyte stimulating hormone (MSH) and prostaglandin E2 (PGE2) drive melanosome transfer by promoting filopodia delivery and shedding spheroid granules: Evidences from atomic force microscopy observation. J Dermatol Sci. 2014;76:222–30. doi: 10.1016/j.jdermsci.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 232.Shin DW. The physiological and pharmacological roles of prostaglandins in hair growth. Korean J Physiol Pharmacol. 2022;26:405–13. doi: 10.4196/kjpp.2022.26.6.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yazdanian N, Mozafarpoor S, Goodarzi A. Phosphodiesterase inhibitors and prostaglandin analogues in dermatology: A comprehensive review. Dermatol Ther. 2021;34:e14669. doi: 10.1111/dth.14669. [DOI] [PubMed] [Google Scholar]
- 234.Sasaki S, Hozumi Y, Kondo S. Influence of prostaglandin F2alpha and its analogues on hair regrowth and follicular melanogenesis in a murine model. Experimental dermatology. 2005;14:323–8. doi: 10.1111/j.0906-6705.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- 235.Chan LKM, Braidy N, Ng W, Xu YH, Chen J, McDonald R. et al. Re-pigmentation of hair after prolonged cholinesterase inhibitor therapy in a Chinese population. Australas J Dermatol. 2020;61:e417–e20. doi: 10.1111/ajd.13356. [DOI] [PubMed] [Google Scholar]
- 236.Wu Q, Xia Y, Dai K, Bai P, Kwan KKL, Guo MSS. et al. Solar light induces the release of acetylcholine from skin keratinocytes affecting melanogenesis. FASEB J. 2020;34:8941–58. doi: 10.1096/fj.202000708R. [DOI] [PubMed] [Google Scholar]
- 237.Wu Q, Fung AHY, Xu ML, Poon K, Liu EYL, Kong XP. et al. Microphthalmia-associated transcription factor up-regulates acetylcholinesterase expression during melanogenesis of murine melanoma cells. J Biol Chem. 2018;293:14417–28. doi: 10.1074/jbc.RA118.003729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wu Q, Bai P, Xia Y, Lai QWS, Guo MSS, Dai K. et al. Solar light induces expression of acetylcholinesterase in skin keratinocytes: Signalling mediated by activator protein 1 transcription factor. Neurochem Int. 2020;141:104861. doi: 10.1016/j.neuint.2020.104861. [DOI] [PubMed] [Google Scholar]
- 239.Guo M, Wu Q, Dong T, Tsim K. The UV-induced uptake of melanosome by skin keratinocyte is triggered by α7 nicotinic acetylcholine receptor-mediated phagocytosis. The FEBS journal. 2022. [DOI] [PubMed]
- 240.Hasse S, Chernyavsky AI, Grando SA, Paus R. The M4 muscarinic acetylcholine receptor plays a key role in the control of murine hair follicle cycling and pigmentation. Life Sci. 2007;80:2248–52. doi: 10.1016/j.lfs.2007.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Enkhtaivan E, Lee CH. Role of Amine Neurotransmitters and Their Receptors in Skin Pigmentation: Therapeutic Implication. Int J Mol Sci. 2021. 22. [DOI] [PMC free article] [PubMed]
- 242.Takahashi T. Multiple Roles for Cholinergic Signaling from the Perspective of Stem Cell Function. Int J Mol Sci. 2021. 22. [DOI] [PMC free article] [PubMed]
- 243.Hampson J, Donnelly A, Lewis-Jones M, Pye J. Tamoxifen-induced hair colour change. The British journal of dermatology. 1995;132:483–4. doi: 10.1111/j.1365-2133.1995.tb08690.x. [DOI] [PubMed] [Google Scholar]
- 244.Cario M. How hormones may modulate human skin pigmentation in melasma: An in vitro perspective. Exp Dermatol. 2019;28:709–18. doi: 10.1111/exd.13915. [DOI] [PubMed] [Google Scholar]
- 245.Jian D, Jiang D, Su J, Chen W, Hu X, Kuang Y. et al. Diethylstilbestrol enhances melanogenesis via cAMP-PKA-mediating up-regulation of tyrosinase and MITF in mouse B16 melanoma cells. Steroids. 2011;76:1297–304. doi: 10.1016/j.steroids.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 246.Sun M, Xie HF, Tang Y, Lin SQ, Li JM, Sun SN. et al. G protein-coupled estrogen receptor enhances melanogenesis via cAMP-protein kinase (PKA) by upregulating microphthalmia-related transcription factor-tyrosinase in melanoma. J Steroid Biochem Mol Biol. 2017;165:236–46. doi: 10.1016/j.jsbmb.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 247.Filoni A, Mariano M, Cameli N. Melasma: How hormones can modulate skin pigmentation. J Cosmet Dermatol. 2019;18:458–63. doi: 10.1111/jocd.12877. [DOI] [PubMed] [Google Scholar]
- 248.Matama T, Araujo R, Preto A, Cavaco-Paulo A, Gomes AC. In vitro induction of melanin synthesis and extrusion by tamoxifen. Int J Cosmet Sci. 2013;35:368–74. doi: 10.1111/ics.12052. [DOI] [PubMed] [Google Scholar]
- 249.Yang G, Nowsheen S, Aziz K, Georgakilas AG. Toxicity and adverse effects of Tamoxifen and other anti-estrogen drugs. Pharmacol Ther. 2013;139:392–404. doi: 10.1016/j.pharmthera.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 250.Komagamine T, Suzuki K, Hirata K. Darkening of white hair following levodopa therapy in a patient with Parkinson's disease. Mov Disord. 2013;28:1643. doi: 10.1002/mds.25696. [DOI] [PubMed] [Google Scholar]
- 251.Slominski A, Zmijewski MA, Pawelek J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012;25:14–27. doi: 10.1111/j.1755-148X.2011.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Langan EA, Lisztes E, Biro T, Funk W, Kloepper JE, Griffiths CE. et al. Dopamine is a novel, direct inducer of catagen in human scalp hair follicles in vitro. Br J Dermatol. 2013;168:520–5. doi: 10.1111/bjd.12113. [DOI] [PubMed] [Google Scholar]
- 253.Villarreal-Reyna G, Garza-Morales R, Soto-Dominguez A, Montanez-Guerrero L, Saucedo-Cardenas O, Gomez-Flores M. et al. Cerebrolysin induces hair repigmentation associated to MART-1/Melan-A reactivation. Eur J Med Res. 2022;27:257. doi: 10.1186/s40001-022-00889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Botchkarev V, Botchkareva N, Albers K, Chen L, Welker P, Paus R. A role for p75 neurotrophin receptor in the control of apoptosis-driven hair follicle regression. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2000;14:1931–42. doi: 10.1096/fj.99-0930com. [DOI] [PubMed] [Google Scholar]
- 255.Nagase K, Inoue T, Narisawa Y. Manifest hair repigmentation associated with etretinate therapy. J Dermatol. 2017;44:e34–e5. doi: 10.1111/1346-8138.13514. [DOI] [PubMed] [Google Scholar]
- 256.Seckin D, Yildiz A. Repigmentation and curling of hair after acitretin therapy. Australas J Dermatol. 2009;50:214–6. doi: 10.1111/j.1440-0960.2009.00542.x. [DOI] [PubMed] [Google Scholar]
- 257.Ward PD, Miller HL, Shipman AR. A case of repigmentation and curling of hair on acitretin therapy. Clin Exp Dermatol. 2014;39:91–2. doi: 10.1111/ced.12212. [DOI] [PubMed] [Google Scholar]
- 258.Vesper J, Fenske N. Hair darkening and new growth associated with etretinate therapy. Journal of the American Academy of Dermatology. 1996;34:860. doi: 10.1016/s0190-9622(96)90048-1. [DOI] [PubMed] [Google Scholar]
- 259.VanBuren CA, Everts HB. Vitamin A in Skin and Hair: An Update. Nutrients. 2022. 14. [DOI] [PMC free article] [PubMed]
- 260.Wang Z, Coleman DJ, Bajaj G, Liang X, Ganguli-Indra G, Indra AK. RXRalpha ablation in epidermal keratinocytes enhances UVR-induced DNA damage, apoptosis, and proliferation of keratinocytes and melanocytes. J Invest Dermatol. 2011;131:177–87. doi: 10.1038/jid.2010.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Taguchi N, Hata T, Kamiya E, Homma T, Kobayashi A, Aoki H. et al. Eriodictyon angustifolium extract, but not Eriodictyon californicum extract, reduces human hair greying. Int J Cosmet Sci. 2020;42:336–45. doi: 10.1111/ics.12620. [DOI] [PubMed] [Google Scholar]
- 262.Taguchi N, Homma T, Aoki H, Kunisada T. Dietary Eriodictyon angustifolium Tea Supports Prevention of Hair Graying by Reducing DNA Damage in CD34+ Hair Follicular Keratinocyte Stem Cells. Biological & pharmaceutical bulletin. 2020;43:1451–4. doi: 10.1248/bpb.b20-00455. [DOI] [PubMed] [Google Scholar]
- 263.Taguchi N, Hata T, Kamiya E, Kobayashi A, Aoki H, Kunisada T. Reduction in human hair graying by sterubin, an active flavonoid of Eriodictyon angustifolium. J Dermatol Sci. 2018;92:286–9. doi: 10.1016/j.jdermsci.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 264.Taguchi N, Kitai R, Ando T, Nishimura T, Aoki H, Kunisada T. Protective Effect of Hydroxygenkwanin against Hair Graying Induced by X-Ray Irradiation and Repetitive Plucking. JID Innov. 2022;2:100121. doi: 10.1016/j.xjidi.2022.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Han MN, Lu JM, Zhang GY, Yu J, Zhao RH. Mechanistic Studies on the Use of Polygonum multiflorum for the Treatment of Hair Graying. Biomed Res Int. 2015;2015:651048. doi: 10.1155/2015/651048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sextius P, Betts R, Benkhalifa I, Commo S, Eilstein J, Massironi M. et al. Polygonum multiflorum Radix extract protects human foreskin melanocytes from oxidative stress in vitro and potentiates hair follicle pigmentation ex vivo. International journal of cosmetic science. 2017;39:419–25. doi: 10.1111/ics.12391. [DOI] [PubMed] [Google Scholar]
- 267.Thang ND, Diep PN, Lien PT, Lien LT. Polygonum multiflorum root extract as a potential candidate for treatment of early graying hair. J Adv Pharm Technol Res. 2017;8:8–13. doi: 10.4103/2231-4040.197332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Jo SJ, Shin H, Paik SH, Na SJ, Jin Y, Park WS. et al. Efficacy and Safety of Pueraria lobata Extract in Gray Hair Prevention: A Randomized, Double-Blind, Placebo-Controlled Study. Ann Dermatol. 2013;25:218–22. doi: 10.5021/ad.2013.25.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Park WS, Kwon O, Yoon TJ, Chung JH. Anti-graying effect of the extract of Pueraria thunbergiana via upregulation of cAMP/MITF-M signaling pathway. J Dermatol Sci. 2014;75:153–5. doi: 10.1016/j.jdermsci.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 270.Chavez A, Tiger J. Hair Repigmentation After Mohs Micrographic Surgery and Secondary Intention Wound Healing on the Scalp of an 84-Year-Old Woman. Dermatologic surgery: official publication for American Society for Dermatologic Surgery [et al] 2021;47:1281–3. doi: 10.1097/DSS.0000000000003094. [DOI] [PubMed] [Google Scholar]
- 271.Kubelis-Lopez DE, Zapata-Salazar NA, Said-Fernandez SL, Sanchez-Dominguez CN, Salinas-Santander MA, Martinez-Rodriguez HG. et al. Updates and new medical treatments for vitiligo (Review) Exp Ther Med. 2021;22:797. doi: 10.3892/etm.2021.10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ziaeifar E, Ziaeifar F, Mozafarpoor S, Goodarzi A. Applications of microneedling for various dermatologic indications with a special focus on pigmentary disorders: A comprehensive review study. Dermatologic therapy. 2021;34:e15159. doi: 10.1111/dth.15159. [DOI] [PubMed] [Google Scholar]
- 273.York K, Meah N, Bhoyrul B, Sinclair R. A review of the treatment of male pattern hair loss. Expert Opin Pharmacother. 2020;21:603–12. doi: 10.1080/14656566.2020.1721463. [DOI] [PubMed] [Google Scholar]
- 274.Yuriguchi M, Aoki H, Taguchi N, Kunisada T. Pigmentation of regenerated hairs after wounding. Journal of Dermatological Science. 2016;84:80–7. doi: 10.1016/j.jdermsci.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 275.Goldstein NB, Koster MI, Jones KL, Gao B, Hoaglin LG, Robinson SE. et al. Repigmentation of Human Vitiligo Skin by NBUVB Is Controlled by Transcription of GLI1 and Activation of the beta-Catenin Pathway in the Hair Follicle Bulge Stem Cells. J Invest Dermatol. 2018;138:657–68. doi: 10.1016/j.jid.2017.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Han X, Chang L, Qiu Z, Lin M, Wang Y, Liu D. et al. Micro-Injury Induces Hair Regeneration and Vitiligo Repigmentation Through Wnt/beta-Catenin Pathway. Stem Cells Dev. 2022;31:111–8. doi: 10.1089/scd.2021.0276. [DOI] [PubMed] [Google Scholar]
- 277.Li H, Fan L, Zhu S, Shin MK, Lu F, Qu J. et al. Epilation induces hair and skin pigmentation through an EDN3/EDNRB-dependent regenerative response of melanocyte stem cells. Sci Rep. 2017;7:7272. doi: 10.1038/s41598-017-07683-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Dummer R. Clinical picture: hair repigmentation in lentigo maligna. Lancet. 2001;357:598. doi: 10.1016/s0140-6736(00)04059-9. [DOI] [PubMed] [Google Scholar]
- 279.Rahim RR, Husain A, Tobin DJ, Lawrence CM. Desmoplastic melanoma presenting with localized hair repigmentation. Br J Dermatol. 2013;169:1371–3. doi: 10.1111/bjd.12521. [DOI] [PubMed] [Google Scholar]
- 280.Inzinger M, Massone C, Arzberger E, Hofmann-Wellenhof R. Hair repigmentation in melanoma. Lancet. 2013;382:1224. doi: 10.1016/S0140-6736(13)61604-9. [DOI] [PubMed] [Google Scholar]
- 281.Tiger JB, Habeshian KA, Barton DT, Brennick JB. Repigmentation of hair associated with melanoma in situ of scalp. J Am Acad Dermatol. 2014;71:e144–5. doi: 10.1016/j.jaad.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 282.Amann VC, Dummer R. Localized Hair Repigmentation in a 91-Year-Old Woman. JAMA Dermatol. 2016;152:81–2. doi: 10.1001/jamadermatol.2015.3812. [DOI] [PubMed] [Google Scholar]
- 283.Chan C, Magro C, Pham A, LeBlanc R, Yan S, Barton D. et al. Spontaneous Hair Repigmentation in an 80-Year-Old Man: A Case of Melanoma-Associated Hair Repigmentation and Review of the Literature. The American Journal of dermatopathology. 2019;41:671–4. doi: 10.1097/DAD.0000000000001353. [DOI] [PubMed] [Google Scholar]
- 284.Lackey AE, Glassman G, Grichnik J, McDonald J, Correa-Selm L. Repigmentation of gray hairs with lentigo maligna and response to topical imiquimod. JAAD Case Rep. 2019;5:1015–7. doi: 10.1016/j.jdcr.2019.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Lopez-Sanchez C, Collgros H. Hair repigmentation as a clue for scalp melanoma. Australas J Dermatol. 2020;61:179–80. doi: 10.1111/ajd.13213. [DOI] [PubMed] [Google Scholar]
- 286.Chew T, Pannell M, Jeeves A. Focal hair re-pigmentation associated with melanoma of the scalp. ANZ J Surg. 2020;90:1175–6. doi: 10.1111/ans.15453. [DOI] [PubMed] [Google Scholar]
- 287.Hasegawa T, Iino S, Kitakaze K, Kato T, Kabata D, Oyama N. et al. Repigmentation of aging gray hair associated with unrecognized development and progression of amelanotic melanoma of the scalp: A physiological alert underlying hair rejuvenation. J Dermatol. 2021;48:e281–e3. doi: 10.1111/1346-8138.15881. [DOI] [PubMed] [Google Scholar]
- 288.Paus R. A neuroendocrinological perspective on human hair follicle pigmentation. Pigment Cell Melanoma Res. 2011;24:89–106. doi: 10.1111/j.1755-148X.2010.00808.x. [DOI] [PubMed] [Google Scholar]