Abstract
Consumption of St. John’s wort plant is high worldwide due to its various medicinal properties. However, herbal products containing St. John’s wort may be contaminated with toxic metals. This is often related to contamination of both water and the atmosphere, lack of proper cultivation methods, and inadequate plant storage conditions, as well as a lack of stricter sanitary supervision. A safety assessment of copper (Cu), lead (Pb), cadmium (Cd) and arsenic (As) content in 23 products containing St. John’s wort (pharmaceutical herbal products, food supplements and traditional herbal remedies) sold in the metropolitan area of Mexico City was conducted. The analysis of metals was determined using a graphite-furnace atomic absorption spectrometer. All herbal products were contaminated with Cu, Pb, Cd and As. The pharmaceutical herbal items showed less contamination by metals. The daily human intake (DHI) values for Pb exceeded the permissible limits in the group of traditional herbal remedies. The DHI calculation for As exceeded the permitted intake values for all items in the group of traditional herbal remedies, five food supplements and one pharmaceutical herbal product. The hazard indicator calculation of the non-carcinogenic cumulative risk values for traditional herbal remedies was greater than 1, suggesting a risk to human health.
Keywords: St. John’s wort, Hypericum sp., heavy metal content, pharmaceutical herbal products, food supplements, arsenic, cadmium, lead
1. Introduction
Human activities have had an enormous impact on environmental pollution. In particular, metals are a relevant source of this pollution, as they remain in the environment for long periods of time [1,2]. The main factors contributing to this contamination are both natural (climate, soil mineral composition) and anthropogenic activities (transport, industry and agriculture). Therefore, toxic metal contamination in agricultural soil and crops for human consumption has major implications for human health [1,3]. But the main causes of metal contamination in plants growing in developing countries are related to pollution conditions of both the atmosphere and water, as well as soil sterilization methods and storage conditions [1,4].
It is important to mention that various metals have biological activity in plants as essential elements or toxic agents that adversely affect the physiological growth of plants and are capable of generating chronic diseases when they enter the food chain [3,5,6]. Toxic metals cause serious health damage such as renal failure, pulmonary emphysema, injury of kidney, liver damage, and symptoms of chronic toxicity [7]. These effects depend on the concentrations of the metals and the co-exposure to other metals [8,9]. However, even low concentrations of some metals can damage various organs, leading to functional damage [10]. It is important to note that essential metals known as trace elements are required for metabolic and physiological functions of both plants and the human body, such as is the case with copper (Cu) [11,12]. But contamination by heavy metals, such as lead (Pb) and cadmium (Cd), in herbal medicine is well known [13,14]. In addition, both metals have carcinogenic potential [7]. In particular, Pb is associated to cognitive impairment, visual dysfunction, renal tumors, and raised blood pressure [7,15,16]. Cd induces pathologies in the cardiovascular system [7,17], anemia, osteoporosis, emphysema, anosmia, and impairment of the kidney and immune systems [7,15,16,18]. Another metal that is toxic to humans is arsenic (As), as chronic exposure is related to cancer in skin, lungs, bladder, kidney and liver [7,15,16].
The main molecular mechanism of toxicity promoted by heavy metals in biological systems is the formation of reactive oxygen species that generate oxidative stress in: (i) DNA—damages it and alters its repair mechanism; (ii) proteins—misfolding, denaturation and inactivation of enzymes, as well as their aggregation; (iii) lipids—damage to the cell membrane and damage to the cell [7,19]. In addition, there is impairment of the antioxidant defense system [20].
Currently 70–80% of the world’s population primarily uses medicinal plants for primary health care [21], and in some developing countries it is the only option, and the plants may be contaminated with toxic metals. In addition, it is widely known that plants are an important source of pharmaceutical drugs for the treatment of various diseases of worldwide relevance, such as depression [22].
In particular, the consumption of St. John’s wort (Hypericum spp.) is very high worldwide [23]. This herb belongs to a genus of plants that includes almost 500 species [24]. Especially Hypericum perforatum has been considered of great medicinal value since the time of the ancient Greeks to treat internal and external diseases such as burns, eczema, skin wounds, inflammatory conditions, gastritis, bronchitis, hypothyroidism, cancer, insomnia, hemorrhoids and anxiety [25,26,27]. Currently, its main use is as an antidepressant [26,27,28]. These medicinal properties have also been described for several species of St. John’s wort [29].
However, not all herbal products containing St John’s wort are safe for human consumption, but due to their demand, their production worldwide has increased, and in many cases, without proper sanitary regulation. Thus, products such as food supplements and traditional herbal remedies may lack the strict regulation required for pharmaceutical herbal products and may be contaminated with toxic metals. In Mexico, there is little information on the metal content in herbal products to assess their quality and food safety. There are also different plants that receive the common name of St John’s wort in Mexico, such as the species Artemisia vulgaris and Tagetes lucida.
The purpose of this study is to analyze the concentrations of metals such as Pb, Cd, As and Cu in pharmaceutical herbal medicines, food supplements, and traditional herbal remedies containing St. John’s wort that are sold in the metropolitan area of Mexico City and to determine if their content is consistent with acceptable standards.
2. Materials and Methods
2.1. Chemicals and Reagents
Standard commercial solutions for atomic absorption spectroscopy for each element (Cu, Pb, Cd, and As; 1000 µg/mL) were used in this study as certified calibration standards (Perkin Elmer, Norwalk, CT, USA). Nitric acid 65%, suprapur® quality (Merck, Darmstadt, Germany) was used for digestion and preparation of dilutions. Dibasic ammonium phosphate (Sigma-Aldrich, St. Louis MO, USA) and Triton X-100 (J.T. Baker, Saint Paul, MN, USA) were used for this study. All solutions were carried out using deionized water obtained from an Elix®Essential 3V water purification system (Millipore, Bedford, MA, USA).
2.2. Collection of Plant-Based Products
Twenty-three commercial products, containing St. John’s wort as the main ingredient, were purchased in pharmacies, supermarkets, health food stores, and local markets located in the Mexico City metropolitan area between January 2021 and June 2021. The presentation of the products was in both solid (tablets, capsules and dry leaves) and liquid (tincture) form.
2.3. Processing of Herbal Products
Both the plastic and glass materials were cleaned with soap and tap water and soaked in a nitric acid solution (3%) overnight followed by rinsing with deionized water [30] to remove metal residues.
To carry out the metal analysis, the products purchased were classified considering their main uses as pharmaceutical herbal products, food supplements, and traditional herbal remedies, as well as their main characteristics (Table 1).
Table 1.
Characteristics of the investigated products containing St. John’s wort.
| Pharmaceutical Herbal Products | ||||
|---|---|---|---|---|
| Product Code | Origin * | Label Statement (Main Compounds) | Formulation Presentation | Therapeutic Uses |
| P1 | Mexico | St. John’s wort (Hypericum perforatum) | Herbal drug (drops) | Treatment of depressive state |
| P2 | Mexico | St. John´s wort (Hypericum perforatum) 150 mg (dry extract of the aerial parts), equivalent to not less than 0.40 mg and not more than 0.54 mg of Hypericin | Herbal drug (tablets) | Auxiliary in the treatment of mild and moderate depressive states |
| P3 | Germany | St. John´s wort (Hypericum perforatum) (Hypericum) 300 mg (flower dry extract) | Herbal drug (tablets) | Auxiliary in the treatment of mild and moderate depressive states |
| P4 | Spain | St. John´s wort (Hypericum perforatum) (Hypericum) 275 mg (flower powder) | Herbal drug (capsules) | Auxiliary in the treatment of nervousness, anxiety, mild depression and sleep disorders |
| P5 | USA | St. John´s wort (Hypericum perforatum) (Hypericum) 300 mg (dry extract) |
Herbal drug (tablets) | Treatment of depressive states (listlessness, apathy, loss of self-esteem) and anxiety |
| P6 | Switzerland | St. John´s wort (Hypericum perforatum) 250 mg (dry extract) equivalent to 0.5 mg hypericin |
Herbal drug (tablets) | Antidepressant for the treatment of mild and moderate depression, in the treatment of transitory alterations of mood, sadness, melancholy, discouragement, lack of personal interest |
| P7 | Mexico | St. John´s wort—Artemisia vulgaris | Herbal drug (drops) | Depression |
| Food Supplements | ||||
| Product Code | Origin * | Label Statement (Main Compounds) | Formulation Presentation | Therapeutic Uses |
| S1 | Mexico | St. John´s wort (Perforatum SD) Tribulus terrestres Stachys officinalis Euphorbi lathyris |
Dietary supplement (drops) | Depression treatment assistant |
| S2 | Mexico | St. John’s wort (Tagetes lucida) Tribulus terrestris Tumera aphrodisiaca L. Curcuma longa Apple vinegar |
Dietary supplement (drops) | Anxiety |
| S3 | Mexico | St. John’s wort (Hypericum perforatum) standardized to 0.3% Hypericin | Dietary supplement (drops) | Natural antidepressant, it promotes a general sense of well-being. It is a supplement to strengthen the nervous system |
| S4 | Mexico | St. John’s wort (Hypericum perforatum L.) | Dietary supplement (capsules) | Helps in the control of anxiety, anguish, depression, and irritability |
| S5 | Mexico | St. John’s wort (Hiperycum perforatum), water, cane alcohol | Dietary supplement (drops) | Help in stress management Help in falling asleep |
| S6 | Mexico | St. John’s wort, White Sapote, Valerian Root, Maca, Royal Jelly, California Damiana | Dietary supplement (drops) | Helps in the control of pressure, anxiety, lack of sleep, and anguish |
| S7 | Mexico | St. John’s wort (Tagetes lucida) | Dietary supplement (tablets) | Auxiliary in the treatment of stress, depression, nervousness, and anxiety |
| S8 | Mexico | St. John’s wort (Hypericum perforatum) cbp vehicle 50 mL |
Dietary supplement (drops) | Natural antidepressant, auxiliary in the treatment of anxiety, melancholy and nervousness |
| S9 | Mexico | St. John´s wort (Hyperucum perforatum), White Sapote, Passion flower |
Dietary supplement (capsules) | Helps in the control of pressure, anxiety, lack of sleep, anguish and headache |
| S10 | Mexico | St. John’s wort, California Damiana, White hawthorn, guarana, zarzapilla, royal jelly Vitamins: A, B1, B2, B3, B6; glutamic acid, calcium |
Dietary supplement (tablets) | Removes anguish and anxiety due to depression (antidepressant) |
| S11 | Mexico | St. John´s wort (Hypericum perforatum), Turnera difusa, Crataegus oxyacantha l, flower pollen, honey bee, Lepidium meyenii, Paullinia cupana, Smilax aspera, royal jelly, vitamin A (Palmitate), vitamin B1 (Thiamine), vitamin B2 (Riboflavin), vitamin B3 (Nicotinamide), vitamin B6 (Pyridoxine), vitamin E (D alpha tocopherol), glutamic acid, calcium pantothenate | Dietary supplement (tablets) | Calms and soothes anxiety, relieves headaches, acts on the nervous system, energizing, helps in prostate problems, helps in the immune system, gives energy |
| S12 | Mexico | St. John’s wort (Hypericum perforatum) leaf and stem, gelatin, magnesium stearate | Dietary supplement (capsules) | Treatment for depression and anxiety |
| Traditional Herbal Remedies | ||||
| Product Code | Origin * | Label Statement (Main Compounds) | Formulation Presentation | Therapeutic Uses |
| T1 | Mexico | St. John´s wort (Tagetes lucida) | Traditional herbal remedy (leaves) | Auxiliary in the treatment of nervous depression, anguish and stress |
| T2 | Mexico | St. John’s wort | Traditional herbal remedy (leaves and stems) | Nerves |
| T3 | Mexico | St. John’s wort | Traditional herbal remedy (leaves and stems) | Nerves and anxiety |
| T4 | México | St. John’s wort | Traditional herbal remedy (leaves and stems) | Depression |
* The nationality of the products for sale is in accordance with the manufacturer’s declaration, but the origin of the natural compounds for their manufacture is unknown.
The solid products were micronized with a porcelain mortar/pestle (Thomas Scientific, Swedesboro, NJ, USA). For metal analysis, 300 mg of powder or 300 µL for liquids were taken. The material acquired as leaves was processed as an infusion according to the instructions for use, and subsequently, 1 mL was used for further processing. All products were digested with HNO3 suprapur (Merck, Darmstadt, Germany) using 1.5 mL for solids, 1.2 mL for liquids and 1 mL for infusion samples. If organic matter remained in the samples, it was processed in nitric acid at room temperature overnight for digestion. Then, the samples were heated in a 60 °C water bath until the orange smoke disappeared. After digestion and cooling, the samples were diluted for analysis. The results are reported as the average of two repeated measurements.
2.4. Metal Contamination Status of Herbal Products Containing St. John’s Wort
The determination of Cu, Pb, As and Cd concentrations was performed before the expiry date of the products. The quantification of each metal was carried out using an atomic absorption spectrophotometer (Perkin Elmer 3110) with a graphite furnace (Perkin Elmer AA600) coupled to an AS800 autosampler (Perkin Elmer, Norwalk, CT, USA) [30]. A specific hollow cathode lamp at wavelengths of 324.8 nm, 228.8 nm, 283.30 nm, and 193.7 nm for Cu, Cd, Pb and As, respectively, was used in the experimental tests for each metal. Metal concentrations were calculated using a calibration curve of six points at ranges from 2 to 45 µg/L for Cu and 2.5 to 45 µg/L for Pb. For Cd and As, the calibration curve was of five points at ranges from 2 to 6 µg/L and 10 to 100 µg/L, respectively. The solutions for the calibration curves were prepared fresh each time the samples were analyzed, using a dilution of a stock solution with 0.2% HNO3, and the coefficient of determination was at least 0.99. For Pb determination, a solution containing 0.2 mL of dibasic ammonium phosphate, 0.5 mL of Triton X-100, and 0.2 mL ultrapure HNO3 per 100 mL of deionized water was used as matrix modifier. Each sample was tested in duplicate.
2.5. Daily Human Intake Dose Calculation Equation for Cu, Pb, As, and Cd
The calculations of daily human intake (DHI) were performed with the dose recommended by a health professional or as written on herbal pharmaceutical products and herbal food supplements. In the specific case of traditional herbal remedies, this calculation was carried out with the dose recommended, not written, by the seller of the herbs.
| DHI = recommended daily intake dose × metal concentration (µg) | (1) |
The results obtained were compared with the daily intake limits issued for each metal by the following associations or government agencies: US California Proposition 65 [31], the American Herbal Products Association [32] and Canada Natural and Non-Prescription Health Products [33].
2.6. Non-Carcinogenic Health Risk Assessment of Herbal Products
2.6.1. Human Health Risk Equation
The estimate of non-carcinogenic risk from ingestion of metals present in products containing St. John’s wort was calculated using the hazard quotient (HQ) for As and Cd following the guidelines of the United States Environmental Protection Agency (US EPA, 2011) [34].
The formula for calculating HQ was as follows:
| HQ = EHDI/RfD | (2) |
The calculation for the estimated human daily intake (EHDI) of each metal was carried out according to the recommended daily dose for humans for an adult person weighing 65 kg [35].
| EHDI (mg/kg/day) = DHI/body weight (kg) | (3) |
The reference dose (RfD) is an estimated value for human daily oral exposure to a metal that does not represent a significant lifetime health risk [35].
The RfD values available from the US EPA for the metals analyzed in the current study were those reported for inorganic As (3 × 10 −4 mg/kg/day) and Cd (5 × 10 −4 mg/kg/day) [35]. An HQ value equal to or greater than 1 indicates a potential health risk.
2.6.2. Hazardousness Indicator Calculation
The hazard indicator calculation (HIC) value was performed using the sum of the HQs of As and Cd [34] to obtain the cumulative risk for each product.
| HIC= HQ (Cd) + HQ (As) | (4) |
According to the US EPA, if an HI value equal to or less than 1 is obtained, it indicates that exposure to these metals will not cause non-carcinogenic adverse effects. Nevertheless, an HI value greater than 1 does not constitute a statistical probability of adverse health effects.
2.7. Statistical Analyses
The software program used for statistical analysis was IBM SPSS 20 for Windows (Armonk, NY, USA). Since most of the data did not have a normal distribution (p < 0.05, Shapiro–Wilk test), the non-parametric Kruskal–Wallis test and the post hoc test for pairwise comparisons were used to examine the differences between groups for each metal. Significant values were adjusted by the Bonferroni correction for multiple testing.
3. Results
We analyzed samples obtained from 23 products containing St. John’s wort as the main ingredient (Table 1). In particular, the food supplements studied had other ingredients besides St. John’s wort. Products sold in Mexico under the common name of St. John’s wort, such as items containing Tagetes lucida and Artemisia vulgaris as the main ingredient, were also included in this study.
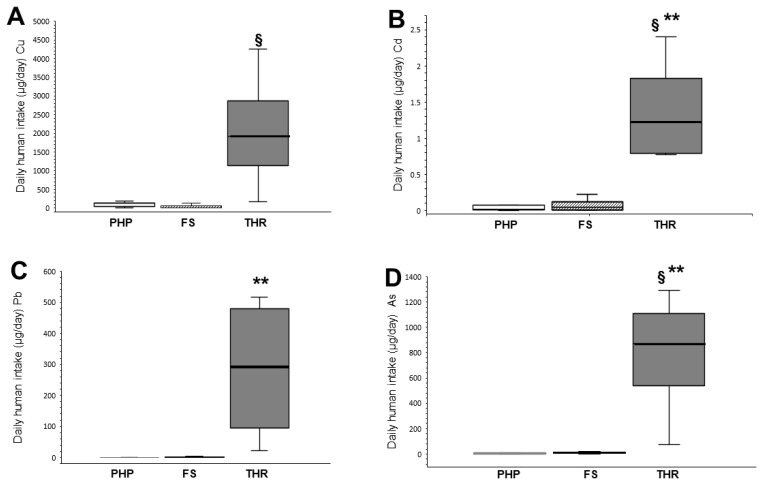
The items were manufactured in Mexico (82.60%), Germany (4.35%), Spain (4.35%), Switzerland (4.37%) and the USA (4.37%), but the origin of the raw material is unknown. The goods purchased in the metropolitan area of Mexico City were classified as pharmaceutical herbal products (P, 30.44%), food supplements (S, 52.17%) and traditional herbal remedies (T, 17.39%). The metals analyzed (Cu, Cd, and Pb) were detected in 100% of the products, and As was present in 95.65% of them, all except in one pharmaceutical herbal product (Figure 1).
Figure 1.
The THR group showed the highest levels of DHI for the different metals that were analyzed with respect to the other groups in the study. Box plots represent the 25 and 75 percentile (box), median (heavy line) and extreme values (whiskers) for the DHI of three groups for the four metals: Cu (A), Cd (B), Pb (C) and As (D). Differences were analyzed using a Kruskal–Wallis test with post hoc test and Bonferroni correction. ** p ≤ 0.01 compared to PHP group, § p ≤ 0.05 compared to the FS group. Cu: copper; Cd: cadmium; Pb: lead; As: arsenic; DHI: daily human intake; PHP: pharmaceutical herbal products; FS: food supplement; THR: traditional herbal remedies.
3.1. Daily Human Intake (DHI)
The daily human intake (DHI; µg/day) of Cu, Cd, Pb and As in the various categories of St. John’s wort-containing products is shown in Figure 1.
Cu is an essential element for the organism and was identified in all items, finding that the DHI ranged from 1.22 µg/day for a pharmaceutical herbal product (item code P7) to 4256 µg/day for a traditional herbal remedy (item code T2). For this metal, the daily limits for the Mexican population are 750 µg/day for women and 730 µg/day for men [36,37]. However, three products (75%; item codes T2, T3 and T4) belonging to the group of traditional herbal remedies had more than twice the suggested limit. The lowest amount of DHI for this metal was obtained in the food supplement group when compared to the traditional herbal remedies group (Table 2; p = 0.013). The highest DHI levels were also shown for the traditional herbal remedies group. No statistically significant differences were found between the groups of herbal pharmaceuticals and traditional herbal remedies.
Table 2.
Differences between the studied groups containing St. John’s wort for each analyzed metal.
| Daily Human Intake (µg/Day) | Pharmaceutical Herbal Products n = 7 |
Food Supplements n = 12 |
Traditional Herbal Remedies n = 4 |
||||
|---|---|---|---|---|---|---|---|
| Metals | Mean ± SD | Median (25–75 Percentile) |
Mean ± SD | Median (25–75 Percentile) |
Mean ± SD | Median (25–75 Percentile) |
p |
| Cu | 99.07 ± 71.56 | 127.04 (50.64–138.72) |
61.91 ± 96.77 | 12.7 (7.26–86.77) |
2067 ± 1721.6 | 1923.2 (809–3325) |
0.016 * 1 a 0.071 b 0.013 c |
| Cd | 0.08 ± 0.13 | 0.02 (0.01–0.08) |
0.10 ± 0.15 | 0.05 (0.01–0.13) |
1.40 ± 0.78 | 1.22 (0.79–2.02) |
0.006 * 1 a 0.006 b 0.021 c |
| Pb | 0.84 ± 0.72 | 0.76 (0.28–1.11) |
2.19 ± 1.16 | 2.3 (1.09–3) |
281.5 ± 247.11 | 294 (71–492) |
0.001 * 0.126 a 0.001 b 0.061 c |
| As | 4.29 ± 4.17 | 3 (1.10–6.88) |
9.42 ± 5.26 | 8.81 (5.01–13.23) |
776.6 ± 530.04 | 871.2 (382–1171.2) |
0.002 * 0.277 a 0.001 b 0.046 c |
SD, Standard deviation. Results of * Kruskal–Wallis test with post hoc test and Bonferroni correction for each metal. a Comparison between pharmaceutical herbal products and food supplements; b comparison between pharmaceutical herbal products and traditional herbal remedies; c comparison between food supplements and traditional herbal remedies.
For the toxic metals Cd, Pb and As, we found differences in the products analyzed. The DHI results obtained for Cd were in the range of 0.003–2.40 µg/day, with the smallest amount in a pharmaceutical herbal product (item code P1), and the largest quantity in a traditional herbal remedy (item T4). All products analyzed for Cd were within the recommended range of 4.1–6.0 µg/day reported by US CA P65, AHPA and the CNNHP [31,32,33], respectively. The group of pharmaceutical herbal products showed the lowest DHI levels for Cd with respect to the group of traditional herbal remedies (Table 2; p = 0.006). The highest DHI levels for Cd were found for the traditional herbal remedies group, with a statistical difference when compared to the food supplements group (Table 2; p = 0.021).
Regarding Pb, the lowest DHI value was 0.168 µg/day in a pharmaceutical herbal product (item 7), and the highest value was 516 µg/day for product T3, a traditional herbal remedy. All traditional herbal remedies (100%; T1 to T4) exceeded the limits reported by US CA P65, AHPA and the CNNHP [31,32,33], where the values reached 516 µg/day for product T3, which represents from 34 to 85 times the allowed limits of 6 to 15 µg/day. For Pb, the lowest DHI levels were obtained in the pharmaceutical herbal products group, with a statistically significant difference from the traditional herbal remedies group (Table 2; p = 0.001). Also, the highest DHI levels for this metal were for the traditional herbal remedies group.
The lowest DHI value for As was 0.611 µg/day for the pharmaceutical herbal product P7, and the highest level was 1291.2 µg/day for a traditional herbal remedy (item T2). Also, several products exceeded the permitted limit of As for human intake; for example, in the group of pharmaceutical herbal products, the item P3 (14.3%) exceeded the values reported by US CA P65, AHPA and the CNNHP [31,32,33]. Food supplements S4, S7, S10, S11 and S12 (41.7%) exceeded the limits issued by the same agencies. However, all traditional herbal remedies (100%) exceeded the permissible limit of 10 µg/day (Figure 1), with a maximum value of 1291.2 µg/day for product T2, representing 129 times more than recommended for humans on a daily basis. But, in the pharmaceutical herbal product P5, it was not possible to detect As contamination. The lowest As values were reported in the group of pharmaceutical herbal products, and the highest values were found for the traditional herbal remedies group, finding a statistically significant difference between these groups (Table 2; p = 0.001). Likewise, these differences were also obtained between the groups of food supplements and traditional herbal remedies (Table 2; p = 0.046).
Table 2 shows that the DHI values for the analyzed metals from pharmaceutical herbal products, food supplements and traditional herbal remedies presented the following pattern: Cu >>> As >> Pb > Cd. The average DHI values obtained for the toxic metals Cd, Pb and As showed lower levels for pharmaceutical herbal products, followed by food supplements and finally traditional herbal remedies (Figure 2). All products were contaminated with Cd and Pb, and As was present in 96% of the products analyzed.
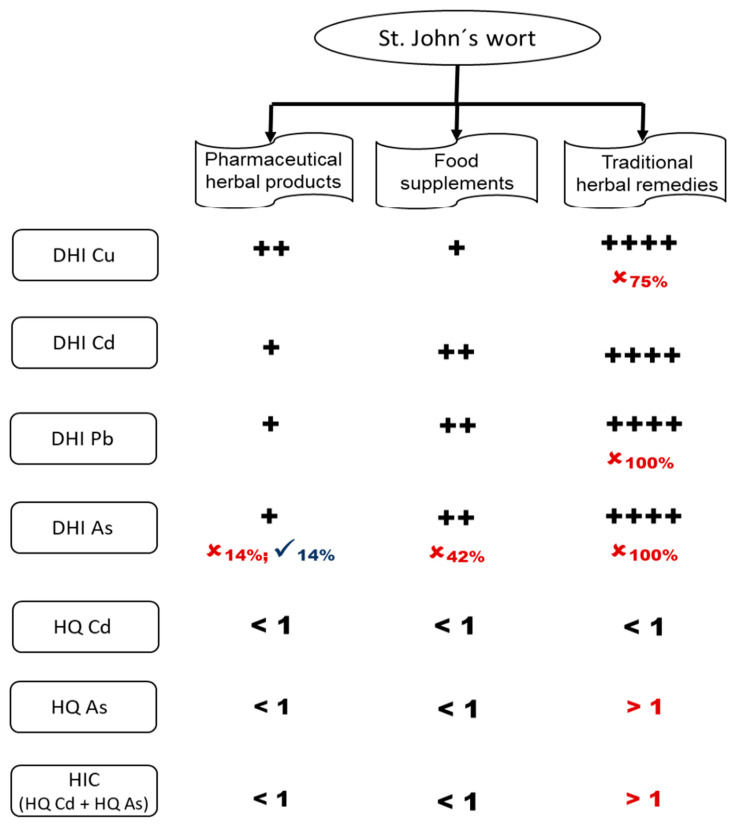
Figure 2.
Representation of DHI, HQ and HIC values obtained for Cu, Cd, Pb and As metals analyzed in pharmaceutical herbal products, food supplements and traditional herbal remedies, where (+) represents the lowest value and (++++) the highest value obtained. Cu, copper; Cd, cadmium; Pb, lead; As, arsenic; DHI, daily human intake; HQ, hazard quotient (potential risks to human health); HIC, hazard indicator calculation (cumulative non-carcinogenic risk). ✕ red letters, percentage of products with a value greater than the recommended limit; ✓ and blue letters, percentage of products without metal detection.
All herbal pharmaceutical products showed values within the permitted limits for Cu, Cd and Pb, and As exceeded the allowed values only in product P3 (14.3%) of German origin. The DHI values for Cu, Cd and Pb of the food supplements were in the permitted range for human consumption, but in this group, the As levels for products S4, S7, S10, S11 and S12 (41.7%) were above the accepted values. Finally, for traditional herbal remedies, only Cd values were in the accepted range; for Cu, 25% of the products (item T1) were within the established values; for metals Pb and As, all products (100%) exceeded the acceptable limits.
3.2. Non-Carcinogenic Health Risk Assessment of Herbal Products
3.2.1. Human Health Risk Estimation
Table 3 shows the estimated human daily intake (EHDI) values (mg/kg/day) calculated for Cu, Cd, Pb and As in the different types of herbal products analyzed (pharmaceutical herbal products, food supplements and traditional herbal remedies). The EHDI values obtained for Cu, analyzing all product categories, were in the range from 3.134 × 10−5 mg/kg/day (item S1) to 6.548 mg/kg/day × 10−2 mg/kg/day (item T2) for a food supplement and a traditional herbal remedy, respectively.
Table 3.
Estimated human daily intake (mg/kg per day) of metals analyzed in pharmaceutical herbal products, food supplements and traditional herbal remedies.
| Pharmaceutical Herbal Products | ||||
|---|---|---|---|---|
| Product Code | EHDI (mg/kg/day) Cu |
EHDI (mg/kg/day) Cd |
EHDI (mg/kg/day) Pb |
EHDI (mg/kg/day) As |
| P1 | 3.135 × 10−5 | 4.615 × 10−8 | 3.877 × 10−6 | 2.448 × 10−5 |
| P2 | 1.996 × 10−3 | 2.749 × 10−7 | 2.092 × 10−5 | 1.272 × 10−4 |
| P3 | 2.869 × 10−3 | 1.231 × 10−6 | 3.352 × 10−5 | 1.704 × 10−4 |
| P4 | 1.527 × 10−3 | 5.785 × 10−6 | 1.332 × 10−5 | 4.609 × 10−5 |
| P5 | 2.272 × 10−3 | 1.107 × 10−6 | 1.177 × 10−5 | N.D. |
| P6 | 1.954 × 10−3 | 9.231 × 10−8 | 4.677 × 10−6 | 8.446 × 10−5 |
| P7 | 1.883 × 10−5 | 1.231 × 10−7 | 2.585 × 10−6 | 9.4 × 10−6 |
| Food Supplements | ||||
| Product Code | EHDI (mg/kg/day) Cu |
EHDI (mg/kg/day) Cd |
EHDI (mg/kg/day) Pb |
EHDI (mg/kg/day) As |
| S1 | 3.134 × 10−5 | 9.231 × 10−8 | 1.408 × 10−5 | 7.449 × 10−5 |
| S2 | 7.786 × 10−5 | 1.077 × 10−7 | 1.943 × 10−5 | 3.708 × 10−5 |
| S3 | 1.404 × 10−4 | 1.231 × 10−7 | 3.808 × 10−5 | 5.463 × 10−5 |
| S4 | 7.107 × 10−4 | 1.815 × 10−6 | 4.078 × 10−5 | 1.958 × 10−4 |
| S5 | 1.862 × 10−4 | 2.923 × 10−7 | 4.292 × 10−5 | 7.975 × 10−5 |
| S6 | 2.044 × 10−4 | 1.692 × 10−7 | 1.192 × 10−5 | 1.511 × 10−4 |
| S7 | 2.306 × 10−4 | 2.323 × 10−6 | 4.928 × 10−5 | 2.901 × 10−4 |
| S8 | 8.595 × 10−5 | 1.231 × 10−6 | 7.846 × 10−6 | 9.757 × 10−5 |
| S9 | 1.959 × 10−3 | 7.969 × 10−6 | 3.257 × 10−5 | 1.202 × 10−4 |
| S10 | 1.373 × 10−4 | 3.231 × 10−7 | 2.901 × 10−5 | 1.752 × 10−4 |
| S11 | 3.008 × 10−3 | 1.385 × 10−6 | 5.283 × 10−5 | 2.513 × 10−4 |
| S12 | 4.657 × 10−3 | 3.462 × 10−6 | 6.566 × 10−5 | 2.112 × 10−4 |
| Traditional Herbal Remedies | ||||
| Product Code | EHDI (mg/kg/day) Cu |
EHDI (mg/kg/day) Cd |
EHDI (mg/kg/day) Pb |
EHDI (mg/kg/day) As |
| T1 | 2.548 × 10−3 | 1.192 × 10−5 | 3.385 × 10−4 | 1.12 × 10−3 |
| T2 | 6.548 × 10−2 | 2.52 × 10−5 | 7.2 × 10−3 | 1.986 × 10−2 |
| T3 | 3.683 × 10−2 | 1.231 × 10−5 | 7.938 × 10−3 | 1.063 × 10−2 |
| T4 | 2.234 × 10−2 | 3.692 × 10−5 | 1.846 × 10−3 | 1.617 × 10−2 |
EHDI: estimated human daily intake; Cu: copper; Cd: cadmium; Pb: lead; As: arsenic; N.D.: Non-detectable.
The EHDIs for Cd ranged from 4.615 × 10−8 mg/kg/day (item P1) to 3.692 × 10−5 mg/kg/day (product T4), the former being a pharmaceutical herbal product and the latter a traditional herbal remedy.
In particular, the EHDI results for Pb ranged between 3.877 × 10−6 mg/kg/day (pharmaceutical herbal product P1) and 7.938 × 10−3 mg/kg/day (traditional herbal remedy T3). Finally, the lowest As value was 9.4 × 10−6 for pharmaceutical herbal remedy P7, and the highest value was 1.986 × 10−2 for the traditional herbal remedy T2.
3.2.2. Hazard Quotient
The hazard quotient (HQ) indicates the potential risks to human health of the contaminants and was calculated for Cd and As in pharmaceutical herbal products, food supplements and traditional herbal remedies (Table 4).
Table 4.
Hazard quotient and hazard indicator calculation for cadmium and arsenic metals analyzed in pharmaceutical herbal products, food supplements and traditional herbal remedies.
| Pharmaceutical Herbal Products | |||
|---|---|---|---|
| Product Code | HQ Cd | HQ As | HIC |
| P1 | 0.00009 | 0.08159 | 0.08168 |
| P2 | 0.00055 | 0.42385 | 0.42440 |
| P3 | 0.00246 | 0.56815 | 0.57062 |
| P4 | 0.01157 | 0.15364 | 0.16521 |
| P5 | 0.00222 | N.A. | 0.00222 |
| P6 | 0.00018 | 0.28154 | 0.28172 |
| P7 | 0.00025 | 0.03133 | 0.03158 |
| Food Supplements | |||
| S1 | 0.00018 | 0.24831 | 0.24849 |
| S2 | 0.00022 | 0.12359 | 0.12381 |
| S3 | 0.00025 | 0.18210 | 0.18235 |
| S4 | 0.00363 | 0.65262 | 0.65625 |
| S5 | 0.00058 | 0.26585 | 0.26643 |
| S6 | 0.00034 | 0.50359 | 0.50393 |
| S7 | 0.00465 | 0.96708 | 0.97172 |
| S8 | 0.00246 | 0.32523 | 0.32769 |
| S9 | 0.01594 | 0.40056 | 0.41650 |
| S10 | 0.00065 | 0.58410 | 0.58475 |
| S11 | 0.00277 | 0.83774 | 0.84051 |
| S12 | 0.00692 | 0.70410 | 0.71103 |
| Traditional Herbal Remedies | |||
| T1 | 0.02385 | 3.73333 | 3.75718 |
| T2 | 0.05040 | 66.21538 | 66.26578 |
| T3 | 0.02462 | 35.44615 | 35.47077 |
| T4 | 0.07383 | 53.90769 | 53.98154 |
HQ: Hazard quotient; HIC: Hazard indicator calculation; N.A: not applicable; Cd: cadmium; As: arsenic.
The HQ for Cd was less than 1 (HQ < 1) for all of the herbal products analyzed. However, the highest values of this ratio were for traditional herbal remedies. The calculated HQ for As was greater than 1 (HQ > 1), also for traditional herbal remedies.
3.2.3. Hazard Indicator Calculation
The hazard indicator calculation (HIC) of cumulative non-carcinogenic risk was performed with the sum of HQs of Cd and As for each herbal product (Figure 2). The HIC value for all of the traditional herbal remedies was greater than 1 (Table 4) but not for pharmaceutical herbal products and food supplements.
4. Discussion
It is well known that medicinal plants play a relevant role in both the pharmaceutical industry and health foods [38]. However, it is important that these products have close health surveillance because medicines made from plants, as well as health foods, have been reported to contain highly toxic heavy metals [5].
In addition, it is well known that plants are capable of uptake of heavy metals that enter the food chain, causing a threat to animal and human health [13,39]. This is because heavy metals accumulate in tissues and are not easily metabolized in the body [16].
In this sense, it is important to know if the herbal products containing St John’s wort on sale in Mexico, and given their high consumption, are safe for human health, since there is scarce information on contamination with toxic metals in these products. In addition, it should be noted that in our study, we analyzed products known and sold in Mexico under the name of St. John’s wort, such as items containing Tagetes lucida and Artemisia vulgaris as the main ingredient.
We found that the herbal products containing St. John’s wort that were analyzed (pharmaceutical herbal products, food supplements and traditional herbal remedies) and marketed in the Mexico City metropolitan area all contained Cu, Cd and Pb, while As was only detected in 95% of the herbal items. In particular, the group of pharmaceutical herbal products showed, on average, the lowest levels of Cd, Pb and As, except for Cu, where the group of food supplements had the lowest levels (Table 2). Our results confirm that herbal products containing St. John’s wort for sale in Mexico are contaminated with toxic metals, such as Pb, As and Cd, as has been reported for other products used as food supplements in Mexico [40], as well as for other herbal products in different countries [41,42,43,44,45,46,47,48], including those with rigorous sanitary surveillance such as Singapore [47].
Toxic heavy metal contamination of herbal products obtained in our study, as well as those reported worldwide, may be related to different human activities such as agriculture, construction, mining, overuse of pesticides, improper waste disposal, and industrial processes such as obtaining raw material (plants) to packing and distribution of these products [16,49].
A relevant aspect that should be considered is that during the cultivation and development of medicinal plants, they could have been contaminated with heavy metals because they may have been irrigated for long periods of time with untreated water or wastewater or growing in areas of intense traffic or near areas of industrial waste. For example, several studies have been conducted in Asia that clearly showed soil contamination by heavy metals on roadsides with different volumes of traffic [50,51]. These soils may subsequently be used for agricultural practices and enter the food chain. It has also been reported that the main source of soil contamination with toxic metals such as Cd, Pb, As and Cu is related to the prolonged use of phosphate fertilizers and/or inadequate irrigation practices [52,53].
Heavy metals may be present in a soluble form in soil and thus available for uptake and transport from contaminated soils to plants [4,52]. The uptake of heavy metals by plants is mainly through root and/or foliar uptake [53]. Subsequent to the uptake of heavy metals into plant leaves, translocation of these metals occurs by chemical, physical and biological mechanisms [52]. Additionally, translocation of toxic metals from roots to shoots causes redistribution of nutrients and toxic metals in plant tissues [4]. This occurs with As and Cd, which are stored in the cellular components of roots [13,54,55] and subsequently can be redistributed, leading to high content of these heavy metals in different parts of the plant.
Exposure of plants to toxic concentrations of heavy metals generates reactive oxygen species, such as superoxide and hydroxyl radicals, as well as hydrogen peroxide and singlet oxygen, which can induce oxidative stress, damaging the growth, development and cellular metabolism of plants [56]. For example, As can alter the activities of proteins/enzymes involved in the regulation of cell division and in the substitution of the phosphate group of ATP, affecting plant growth. Pb toxicity affects seed morphology, germination, physiology and early crop growth of several plant species. Cd induces nitric oxide accumulation, which is involved in the inhibition of auxin transport to the root, causing a reduction in meristem size [57,58].
It has also been reported that although essential metals are required for plant development, growth and maintenance, the uptake of heavy metals [59,60] can interfere with phosphorous, potassium and nitrogen uptake and cause deficiencies of these macronutrients in plants, thus affecting the biochemical and physical processes of plants (photosynthesis, chlorophyll biosynthesis, protein modification, DNA synthesis) [52]. This generates changes in medicinal plants that are mediated by heavy metals, causing a decrease in their medicinal properties.
In the current study, the traditional herbal remedies had the highest levels of Cd, Pb and As contamination, compared to pharmaceutical herbal medicines and food supplements. This may be due to the fact that the highest accumulation of heavy metals in plants is mainly in the leaves [61], which in this study were analyzed together with the stems (Table 1 and Table 3) and are generally used to prepare infusions. In addition, previous studies have reported the presence of heavy metals in medicinal plants used for the preparation of herbal teas [62,63,64,65,66,67]. In this regard, toxic metals such as Cd, Pb and As affect the size, number, pigmentation and thickness of leaves [68]. Thus, it should be noted that these toxic metals can ultimately affect the quality, efficacy and safety of products for human consumption.
Studies have been conducted in Turkey to analyze the concentration of metals (Cu, Cd, Pb, Ni and Cr) in St. John’s wort that was cultivated in various geographical locations, reporting very low or absent content of Cd and Pb analyzed in its stems, leaves, flowers and fruits [69,70]. That report is very similar to the findings of our study, because we detected Cd and Pb levels that did not exceed the allowable limits, except for all traditional herbal remedy products, which exceeded the permitted Pb levels.
The content of metals found may be related to the different geographical locations where the plant grew, where each place has a particular characteristic of soil and atmosphere that may be contaminated by heavy metals due, in part, to anthropogenic activities as mentioned previously [71,72]. This is supported by a study conducted in the mountains of Poland, two regions of Romania and eastern Serbia where the concentrations of Cd and Pb in St. John’s wort exceeded the permissible limits [73,74,75]. Thus, we can note that there are no constant levels of metals in the analyzed products containing St. John’s wort. Nevertheless, plant-based products should conform to permitted intake margins for toxic metals such as Pb, Cd, and As to be acceptable for human health.
These aspects are relevant for obtaining quality St. John’s wort products that must comply with good practices and ensure a plant-based product without risks to human health [76]. In addition, studies have been conducted in controlled environments where St. John’s wort can grow efficiently, finding that these environments favor an increase in the biomass and medicinal content of this plant [77]. Our results suggest that the products analyzed could have been contaminated with toxic metals (Cd, Pb and As) at some stage of the product production, such as during cultivation, harvesting, storage and processing, until the product was obtained for marketing. In the particular case of the traditional herbal remedies, these were purchased at popular markets in bulk, where the seller packaged them on the spot, which might have increased the risk of contamination.
On the other hand, the calculation of DHI for Cu indicated that all pharmaceutical herbal products and food supplements were within the maximum limits for the Mexican population [36,37]. However, 75% of the traditional herbal remedies (T2, T3 and T4) exceeded twice those limits. Although Cu is necessary for the body, its excess in the diet is related to irritation of the upper respiratory tract, abdominal pain, dermatitis, vomiting, diarrhea, nausea and liver damage [78], so it is important that intake is within permissible limits for humans.
Additionally, although all of the herbal products analyzed (pharmaceutical herbal products, food supplements and traditional herbal remedies) were contaminated with Cd, the calculated values for DHI of this toxic metal were within the recommended range of 0.003 (P1, pharmaceutical herbal product) to 2.40 µg/day (T4, traditional herbal remedy), complying with the recommendations of US CA P65, AHPA and the CNNHP [31,32,33], respectively. It is important to note that Cd is naturally present in the environment and is a contaminant that comes from industry and agricultural activities, and is present in water, fertilizers and cigarettes [4]. It is also relevant to note that the main source of Cd exposure in non-smokers is food (approximately 90%) [79]. Therefore, it is recommended that herbal products should be monitored more closely to avoid health risks due to this toxic metal. This is because it can cause damage to reproductive tissues and contribute to infertility [80]. It also damages the kidney and lungs, which can result in pulmonary edema and death, and damages bones, leading to osteoporosis and spontaneous fractures, as well as increased blood pressure [78]. Severe exposure also causes alterations in behavior and learning ability [80]. A very relevant fact to mention is that St. John’s wort is a Cd accumulator plant [81]. However, the DHI value for all of the herbal products analyzed was within the permitted limits, although it may contribute, together with other toxic metals, to increased health risk.
The DHI calculation for Pb showed that 100% of the traditional herbal remedies exceeded the permitted limits by up to 85 times, according to the values of US CA P65 (15 µg/day), AHPA (6 µg/day) and the CNNHP (10 µg/day) [31,32,33] (Figure 1), while pharmaceutical herbal products and food supplements remained within the margins allowed for Pb.
Thus, we found that the DHI estimates obtained for traditional herbal remedies exceeded these limits, and although one of the main routes of Pb exposure is by inhalation or ingestion of dust, food is also a source of contamination [79,82], as we report in this study. In humans, the main organs that absorb lead are the kidney, liver, brain, and heart. But in the long term, 90% of lead is located in the bones. This metal causes kidney disease, cognitive impairment, headache, memory loss, inattention and an increased risk of cancer [75].
Finally, the DHI for As was shown to be present in a range from 0.611 µg/day for a pharmaceutical herbal (P7) to 1291.2 µg/day for a traditional herbal remedy (item T2). However, the three types of herbal product groups showed contamination with this heavy metal. Product P3, which belonged to the group of pharmaceutical herbal products, had a value of 11.08 µg/day, exceeding the maximum allowed value of As for human intake [31,32,33]. Regarding the food supplements group, several products exceeded the permitted DHI limit of 10 µg/day reported by various agencies [31,32,33], as was the case for items S4 (12.72 µg/day), S7 (18.86 µg/day), S10 (11.39 µg/day), S11 (16.33 µg/day) and S12 (13.73 µg/day). Additionally, all of the products in the traditional herbal remedies group exceeded the permitted limit of 10 µg/day, reaching values of 1291.2 µg/day (product T2) which represents 129 times the daily values allowed for humans [31,32,33].
Our results show that one herbal pharmaceutical product, five food supplements and all traditional herbal remedies presented DHI values higher than allowed limits. This As contamination may be due to the fact that the main routes of exposure to the metal are food, water, soil and air [83], showing that greater scrutiny of herbal products containing St. John’s wort on sale in Mexico is required. It is worth mentioning that As causes damage to the brain, lungs, kidney, abdominal damage, skin rash, and intestinal ulcer, among others, as well as various types of cancer [83]. In addition, sensory, psychological and cognitive dysfunctions also occur [84].
The calculation of the estimate of non-carcinogenic risk from ingestion of metals present in the herbal products containing St. John’s wort analyzed gave an HQ value for Cd of less than 1 for all of the groups of products analyzed, showing that this metal does not have a non-carcinogenic risk. However, the HQ for As showed that only the traditional herbal remedies (T1 to T4) had an HQ greater than 1, suggesting that the ingestion of these products may pose a risk to human health, due to the high As content as reported in the guidelines of the United States Environmental Protection Agency (US EPA, 2011) [34].
Additionally, the HIC values, to obtain the non-carcinogenic cumulative hazard estimation, were calculated for Cd and As, and only the traditional herbal remedies showed values higher than 1, obtaining HIC values from 3.75 to 66.26, which shows that this group of herbal products presents a risk to human health. It should be considered that it was not possible to obtain the human health risk estimation for Cu and Pb because there is no RfD available for these two metals. In particular Pb does not have a threshold value and, therefore, a RfD value cannot be recommended [35]. Thus, our results are not conclusive with regard to the possible health risk posed by some products because there is no information available to perform the calculations for some metals.
Our study has some limitations; it would be an additional contribution to analyze other heavy metals to obtain the non-carcinogenic cumulative index and evaluate if the health risk index increases. In addition, other toxic metals may accumulate in the human body due to the continuous use of plant-derived products that could cause health damage. It is also important to extend this study to include more herbal products containing St. John’s wort on sale in the Mexican territory. The lack of information on the origin of the raw material used to obtain the herbal products in this study, as well as the form of commercialization of the traditional herbal remedies, did not allow us to identify with certainty the source(s) of metal contamination.
5. Conclusions
Our study showed that all herbal products were contaminated with Cu, Pb, Cd and As. The pharmaceutical herbal products showed less contamination with these metals. The DHI values for Pb exceeded the permitted limits exclusively in the traditional herbal remedies group. The DHI calculation for As exceeded the permitted intake values for all items in the traditional herbal remedies group, as well as for five food supplements and one pharmaceutical herbal product. This indicates that even though pharmaceutical herbal products have strict surveillance, this should be monitored and assessed continuously. In addition, the hazard indicator calculation of the non-carcinogenic cumulative risk values for the traditional herbal remedies was greater than 1, suggesting a risk to human health. Thus, although this cumulative indicator was obtained with the sum of As and Cd, contamination by these metals is important because they could reach plant-based products through different sources. Therefore, food safety should be improved with better agricultural methods as well as plant processing procedures to reduce the availability of heavy metals.
Acknowledgments
We thank Patricia Rojas for the donation of the herbal products analyzed, which were purchased with personal resources.
Author Contributions
Conceptualization, P.R. and C.R. (Carolina Rojas); Formal analysis, P.R., E.R.-S., A.M.L.-R., C.R. (Carolina Rojas) and B.A.G.-M.; Investigation, P.R., E.R.-S., L.O.-R. and C.R. (Carolina Rojas); Methodology, P.R., E.R.-S., A.M.L.-R., B.A.G.-M., C.R. (Camilo Ríos) and A.A.R.-A.; Resources, P.R. and C.R. (Camilo Ríos); Writing—original draft, P.R.; Writing—review and editing, P.R., L.O.-R., E.R.-S., C.R. (Carolina Rojas), A.M.L.-R., B.A.G.-M., C.R. (Camilo Ríos) and A.A.R.-A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the current study are available under reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Saxena G., Purchase D., Mulla S.I., Dattatraya-Saratale G., Naresh-Bharagava R. Phyroremediation of heavy metals-contaminated sites: Eco-environmental concerns, field studies, sustainability issues, and future prospects. Rev. Environ. Contam. Toxicol. 2020;249:71–131. doi: 10.1007/398_3019_24. [DOI] [PubMed] [Google Scholar]
- 2.Valdés Durán A., Aliaga G., Deckart K., Karas C., Cáceres D., Nario A. The environmental geochemical baseline, background and sources of metal and metalloids present in urban, peri-urban and rural soils in the O’Higgins region, Chile. Environ. Geochem. Health. 2022;44:3173–3189. doi: 10.1007/s10653-021-01098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okereafor U., Marhatha M., Mekuto L., Uche-Okereafor N., Sebola T., Mavumengwana V. Toxic metals implications on agricultural soils, plants, animals, aquatic life and human health. Int. J. Environ. Res. Public Health. 2020;17:2204. doi: 10.3390/ijerph17072204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao F.J., Tang Z., Song J.J., Huang X.Y., Wang P. Toxic metals and metalloids: Uptake, transport, detoxification, phyroremediation, and crop improvement for safer food. Mol. Plant. 2022;15:27–44. doi: 10.1016/j.molp.2021.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Hlihor R.M. Medicinal plant growth in heavy metals contaminated soils: Responses to metal stress and induced risks to human health. Toxics. 2022;10:499. doi: 10.3390/toxics10090499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mihaljev Z., Zivkov-Balos M., Cupić Z., Jaksić S. Levels of some microelements and essential heavy metals in herbal teas in Siberia. Acta Pol. Pharm. 2014;71:385–391. [PubMed] [Google Scholar]
- 7.Balali-Mood M., Naseri K., Tahergorabi Z., Khazdair M.R., Sadeghi M. Toxic mechanisms of five metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021;12:643972. doi: 10.3389/fphar.2021.643972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crichton R.R. Metal Toxicity—An introduction. In: Crichton R.R., Ward R.J., Hider R.C., editors. Metal Chelation in Medicine. 1st ed. Royal Society of Chemistry; Cambridge, UK: 2016. pp. 1–23. [Google Scholar]
- 9.Aaseth J.O. Toxic and essential metals in human health and disease 2021. Biomolecules. 2022;12:1375. doi: 10.3390/biom12101375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crisponi G. Essential and toxic metal ions in human health and disease; from chemical features to clinical roles. Curr. Med. Chem. 2021;18:7187–7189. doi: 10.2174/092986732835211116120553. [DOI] [PubMed] [Google Scholar]
- 11.Mir A.R., Pichtel J., Hayat S. Copper: Uptake, toxicity and tolerance in plants and management of Cu-contaminated soil. Biometal. 2021;34:737–759. doi: 10.1007/s10534-021-00306-z. [DOI] [PubMed] [Google Scholar]
- 12.Jomova K., Makova M., Alomar S.Y., Alwase S.H., Nepovimova E., Kuca K., Rhodes C.J., Valko M. Essential metals in health and disease. Chem. Biol. Interact. 2022;367:110173. doi: 10.1016/j.cbi.2022.110173. [DOI] [PubMed] [Google Scholar]
- 13.Luo L., Wang B., Jiang J., Fitzgerald M., Huang Q., Yu Z., Li H., Zhang J., Wei J., Yang C., et al. Heavy metal contaminations in herbal medicines: Determination, comprehensive risk assessments, and solutions. Front. Pharmacol. 2021;11:595335. doi: 10.3389/fphar.2020.595335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Locatelli C., Melucci D., Locatelli M. Toxic metals in herbal medicines. A review. Curr. Bioact. Compd. 2014;10:181–188. doi: 10.2174/1573407210666140716164321. [DOI] [Google Scholar]
- 15.Rehman K., Fatima F., Waheed I., Akash M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell Biochem. 2018;119:157–184. doi: 10.1002/jcb.26234. [DOI] [PubMed] [Google Scholar]
- 16.Jaishankar M., Tseten T., Anbalagan N., Mathew B.B., Beeregowda K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014;7:60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obeng-Gyasi E. Chronic cadmium exposure and cardiovascular disease in adults. J. Environ. Sci. Health. A Tox. Hazard. Subst. Environ. Eng. 2020;55:726–729. doi: 10.1080/10934529.2020.1737459. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z., Sun Y., Ba Q., Wan H. Effects of cadmium exposure on the immune system and immunoregulation. Front Immunol. 2021;12:695484. doi: 10.3389/fimmu.2021.695484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abd Elnabi M.K., Elkaliny N.E., Elyazied M.M., Azab S.H., Elkhalifa S.A., Elmasry S., Mouhamed M.S., Shalamesh E.M., Alhorieny N.A., Abd Elaty A.E., et al. Toxicity of heavy metals and recent advances in their removal: A review. Toxics. 2023;11:580. doi: 10.3390/toxics11070580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jan A.T., Azam M., Siddiqui K., Ali A., Choi I., Haq Q.M. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015;16:29592–29630. doi: 10.3390/ijms161226183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO Global Report on Traditional and Complementary Medicine 2019. [(accessed on 27 January 2023)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/312342/9789241515436-eng.pdf?sequence=1&isAllowed=y.
- 22.Fathinezhad Z., Sewell R.D.E., Lorigooini Z., Rafieian-Kopaei M. Depression and Treatment with Effective Herbs. Curr. Pharm. Des. 2019;25:738–745. doi: 10.2174/1381612825666190402105803. [DOI] [PubMed] [Google Scholar]
- 23.Hypericum perforatum (St John’s Wort), CABI Compendium. CABI International. [(accessed on 1 February 2023)]. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.28268.
- 24.The Editors of Encyclopaedia Britannica. “Saint-John’s-Wort”. Encyclopedia Britannica, 31 May 2020. [(accessed on 27 January 2023)]. Available online: https://www.britannica.com/plant/Hypericum.
- 25.WHO Monographs on Selected Medicinal Plants, Volume 2. 2002. [(accessed on 27 January 2023)]. Available online: http://apps.who.int/iris/bitstream/handle/10665/42052/9241545372.pdf?sequence=2.
- 26.Klemow K.M., Bartlow A., Crawford J., Kocher N., Shah J., Ritsick M. Medical Attributes of St. John’s Wort (Hypericum perforatum) In: Benzie I.F.F., Wachtel-Galor S., editors. Herbal Medicine: Biomolecular and Clinical Aspects. 2nd ed. CRC Press; Boca Raton, FL, USA: Taylor & Francis; Oxfordshire, UK: 2011. [PubMed] [Google Scholar]
- 27.St. John’s Wort. [Updated 2022 May 19]. In StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; January 2023. [(accessed on 26 January 2023)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK557465/
- 28.Nobakht S.Z., Akaberi M., Mohammadpour A.H., Tafazoli M.A., Emami S.A. Hypericum perforatum: Traditional uses, clinical trials, and drug interactions. Iran. J. Basic Med. Sci. 2022;25:1045–1058. doi: 10.22038/IJBMS.2022.65112.14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kladar N., Srđenović B., Grujić N., Rat M., Gavarić N., Anačkov G., Božin B. St. John’s Wort (Hypericum Spp.)–Relation between the biological source and medical properties. In: Davis H.R., editor. Hypericum: Botanical Sources, Medical Properties and Health Effects. 1st ed. Nova Science Publishers, Inc.; Hauppauge, NY, USA: 2015. pp. 53–80. [Google Scholar]
- 30.Rojas P., Ruiz-Sánchez E., Ríos C., Ruiz-Chow A., Reséndiz-Albor A.A. A health risk assessment of lead and other metals in pharmaceutical herbal products and dietary supplements containing Ginkgo biloba in the Mexico City Metropolitan Area. Int. J. Environ. Res. Public Health. 2021;18:8285. doi: 10.3390/ijerph18168285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.California Office of Environmental Health Hazard Assessment Proposition 65 No Significant Risk Levels (NSRLs) and Maximum Allowable Dose Levels (MADLs). (Revised 2021) [(accessed on 14 February 2023)]; Available online: https://oehha.ca.gov/proposition-65/general-info/current-proposition-65-no-significant-risk-levels-nsrls-maximum.
- 32.American Herbal Product Association AHPA Guidance Policy. Heavy Metals (Revised 2012) [(accessed on 1 February 2023)]. Available online: www.ahpa.org/Files/Document%20Library/AHPAGuidancePolicies/AHPA_Guidance_Heavy_Metals.pdf.
- 33.Natural and Non-Prescription Health Products (Canada). Quality of Natural Health Products Guide. 2015. [(accessed on 14 February 2023)]. Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/dhp-mps/alt_formats/pdf/prodnatur/legislation/docs/eq-paq-eng.pdf.
- 34.US EPA . Exposure Factors Handbook 2011 Edition (Final Report) 1st ed. United States Environmental Protection Agency; Washington, DC, USA: 2011. pp. 1–56. [Google Scholar]
- 35.US EPA . Integrated Risk Information System. United States Environmental Protection Agency; Washington, DC, USA: 2008. [(accessed on 5 February 2023)]. Available online: https://iris.epa.gov/AtoZ/?list_type=alpha. [Google Scholar]
- 36.Morales Guerrero J.C., Camacho Parra M.E., García Morales C., Juárez Ramos P., Flores Sánchez J.J. ¿Hay riesgo de efectos adversos por el consumo de nutrimentos a partir de productos alimenticios adicionados en México? Nutr. Hosp. 2018;35:1356–1365. doi: 10.20960/nh.1832. [DOI] [PubMed] [Google Scholar]
- 37.Bourges H.E., Casanueva E., Rosado J.L. Recomendaciones de ingestión de nutrimentos para la población mexicana. In: Casanueva E., Rosado J.L., editors. Bases Fisiológicas, I. Apéndice 2. 1st ed. Editorial Médica Panamericana; Mexico City, Mexico: 2005. pp. 1–372. [Google Scholar]
- 38.Thomford N.E., Senthebane D.S., Rowe A., Munro D., Seele P., Maroyi A., Dzobo K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018;19:1578. doi: 10.3390/ijms19061578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaiswal A., Verma A., Jaiswal P. Detrimental effects of heavy metals in soil, plants, and aquatic ecosystems and in humans. J. Environ. Pathol. Toxicol. Oncol. 2018;37:183–197. doi: 10.1615/JEnvironPatholToxicolOncol.2018025348. [DOI] [PubMed] [Google Scholar]
- 40.García-Rico I., Leyva-Pérez J., Jara-Marini M.E. Content and daily intake of copper, zinc, lead, cadmium, and mercury from dietary supplements in Mexico. Food Chem. Toxicol. 2007;45:1599–1605. doi: 10.1016/j.fct.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 41.Tschinkel P.F.S., Melo E.S.P., Pereira H.S., Silva K.R.N., Arakaki D.G., Lima N.V., Fernandes M.R., Leite L.C.S., Melo E.S.P., Melnikov P., et al. The hazardous level of heavy metals in different medicinal plants and their decoctions in water: A public health problem in Brazil. Biomed. Res. Int. 2020;2020:1465051. doi: 10.1155/2020/1465051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mikulski M.A., Wichman M.D., Simmons D.L., Pham A.N., Clottey V., Fuortes L.J. Toxic metals in ayurvedic preparations from a public health lead poisoning cluster investigation. Int. J. Occup. Environ. Health. 2017;23:187–192. doi: 10.1080/10773525.2018.1447880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris E.S., Cao S., Littlefield B.A. Heavy metal and pesticide content in commonly prescribed individual raw Chinese Herbal Medicines. Sci. Total Environ. 2011;409:4297–4305. doi: 10.1016/j.scitotenv.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X., Chi W., Tian H. Probabilistic ecological risk assessment of heavy metals in western Laizhou Bay, Shandong Province, China. PLoS ONE. 2018;14:e0213011. doi: 10.1371/journal.pone.0213011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh N.K., Raghubanshi A.S., Upadhyay A.K. Arsenic and other heavy metal accumulation in plants and algae growing naturally in contaminated area of West Bengal, India. Ecotox. Environ. Saf. 2016;130:224–233. doi: 10.1016/j.ecoenv.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 46.Mulaudzi R.B., Tshikalange T.E., Olowoyo J.O. Antimicrobial activity, cytotoxicity evaluation and heavy metal content of five commonly used South African herbal mixtures. South Afr. J. Bot. 2017;112:314–318. doi: 10.1016/j.sajb.2017.06.024. [DOI] [Google Scholar]
- 47.Kin K.A., Dickson R., Amponsah I. The heavy metal contents of some selected medicinal plants sampled from different geographical locations. Pharmacogn. Res. 2013;5:103. doi: 10.1080/15569543.2016.1225768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leal A.S., Prado G., Gomes T.C.B. Determination of metals in medicinal plants highly consumed in Brazil. Braz. J. Pharm. Sci. 2013;499:599–607. doi: 10.1590/S1984-82502013000300022. [DOI] [Google Scholar]
- 49.Yee S.K., Chu S.S., Xu Y.M., Choo P.L. Regulatory control of Chinese proprietary medicines in Singapore. Health Policy. 2005;71:133–149. doi: 10.1016/j.healthpol.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 50.Alinia-Ahandani E., Sheydaei M., Akram M., Selamoglu Z., Alizadeh-Terepoei Z., Alinia-Ahandani M. Heavy metals concentrations in some roadsides with different traffic volumes in Rasht City-Iran. Op. Acc. J. Bio. Sci. Res. 2021;7:1–4. doi: 10.46718/JBGSR.2021.07.000169. [DOI] [Google Scholar]
- 51.Alinia-Ahandani E., Alizadeh-Terepoei Z., Sheydaei M., Peysepar-Balalami F. Assessment of soil on some heavy metals and its pollution in Roodsar-Iran. Biomed. J. Sci. Tech. Res. 2020;28:21977–21979. doi: 10.26717/BJSTR.2020.28.004714. [DOI] [Google Scholar]
- 52.Asiminicesei D.-M., Vasilachi I.C., Gavrilescu M. Heavy metal contamination of medicinal plants and potential implications on human health. Rev. Chim. 2020;71:16–36. doi: 10.37358/RC.20.7.8222. [DOI] [Google Scholar]
- 53.Chen G., Li J., Han H., Du R., Wang X. Physiological and molecular mechanisms of plant responses to copper stress. Int. J. Mol. Sci. 2022;23:12950. doi: 10.3390/ijms232112950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y., Moore K.L., Miller A.J., McGrath S.P., Ma J.F., Zhao F.-J. The role of nodes in arsenic storage and distribution in rice. J. Exp. Bot. 2015;66:3717–3724. doi: 10.1093/jxb/erv164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore K.L., Chen Y., van de Meene A.M.L., Hughes L., Liu W.J., Geraki T., Mosselmans F., McGrath S.P., Grovenor C., Zhao F.J. Combined NanoSIMS and synchrotron X-ray fluorescence reveals distinct cellular and subcellular distribution patterns of trace elements in rice tissues. New Phytol. 2014;201:104–115. doi: 10.1111/nph.12497. [DOI] [PubMed] [Google Scholar]
- 56.Berni R., Luyckx M., Xu X., Legay S., Sergeant K., Hausman J.-F., Lutts S., Cai G., Guerriero G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019;161:98–106. doi: 10.1016/j.envexpbot.2018.10.017. [DOI] [Google Scholar]
- 57.Skuza L., Szucko-Kaciuba I., Filip E., Bozek I. Natural molecular mechanisms of plant hyperaccumulation and hypertolerance heavy metals. Int. J. Mol. Sci. 2022;23:9335. doi: 10.3390/ijms23169335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou J., Zhang Z., Zhang Y., Wei Y., Jiang Z. Effects of lead stress on the growth, physiology, and cellular structure of privet seedings. PLoS ONE. 2018;13:e0191139. doi: 10.1371/journal.pone.0191139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ibrahim M.H., Kong Y.C., Zain N.A.M. Effect of cadmium and copper exposure on growth, secondary metabolites and antioxidant activity in the medicinal plant Sambung nyawa (Gynura procumbens (Lour.) Merr) Molecules. 2017;22:1623. doi: 10.3390/molecules22101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sobariu D.L., Fertu D.I.T., Diaconu M., Pavel L.V., Hlihor R.M., Dragoi E.N., Curteanu S., Lenz M., Corvini P.F.X., Gavrilescu M. Rhizobacteria and plant symbiosis in heavy metal uptake and its implications for soil bioremediation. N. Biotechnol. 2017;39 Pt A:125–134. doi: 10.1016/j.nbt.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 61.Shahid M., Dumat C., Khalid S., Schreck E., Xiong T., Niazi N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard Mater. 2017;325:36–58. doi: 10.1016/j.jhazmat.2016.11.063. [DOI] [PubMed] [Google Scholar]
- 62.Nica Badea D. Determination of potentially toxic heavy metals (Pb, Hg, Cd) in popular medicinal herbs in the coal power plant tea. Rev. Chim. 2015;66:1132–1136. [Google Scholar]
- 63.Diaconu D., Diaconu R., Navrotescu T. Estimation of heavy metals in medicinal plants and their infusions. Ovidius Univ. Ann Chem. 2012;23:115–120. doi: 10.2478/v10310-012-0019-0. [DOI] [Google Scholar]
- 64.Muntean N., Muntean E., Creta C., Duda M. Heavy metals in some commercial herbal teas. ProEnvironment. 2013;6:591–594. [Google Scholar]
- 65.Ghazala Y., Fizza I., Muniba I., Vania M. Monitoring and risk assessment due to presence of heavy metals and pesticides in tea samples. Food Sci. Technol. 2018;38:625–628. doi: 10.1590/fst.07417. [DOI] [Google Scholar]
- 66.Miroslawski J., Paukszto A. Determination of the cadmium, chromium, nickel and lead ions relays in selected polish medicinal plants and their infusion. Biol. Trace Elem. Res. 2018;182:147–151. doi: 10.1007/s12011-017-1072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Samuel Nakamura K., Hodge F.S. Occurrence and risk of metal(loid)s in Thelesperma megapotamicum tea plant. Plants. 2019;9:21. doi: 10.3390/plants9010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riyazuddin R., Nisha N., Ejaz B., Khan M.J., Kumar M., Ramteke P.W., Gupta R. A comprehensive review on the heavy metal toxicity and sequestration in plants. Biomolecules. 2021;28:43. doi: 10.3390/biom12010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leblebici S., Bahtiyar S.D., Özyurt M.S. Determination of the amount of heavy metal in some medicinal plants sold in herbalist in Kütahya. DPU J. Grad. Sch. Nat. Appl. Sci. Mehmet Akif Ersoy Univ. 2012;29:1–6. [Google Scholar]
- 70.Ergün N., Yolcu H., Karanlik S., Dikkaya E. Heavy metal accumulation and mineral contents of some plants on Amanos Mountains (Hatay), Turkey. BIBAD Biyol. Bilim. Arast. Derg. 2010;3:121–127. [Google Scholar]
- 71.Bin C., Xiaoru W., Lee F.S.C. Pyrolisis coupled with atomic absorption spectrometry for determination of mercury in Chinese medicinal materials. Anal. Chim. Acta. 2001;447:161–169. doi: 10.1016/S0003-2670(01)01218-1. [DOI] [Google Scholar]
- 72.Geneva M. Metal uptake by Saint John’s wort (Hypericum perforatum L.) grown on industrially polluted soil; Proceedings of the 6th Conference on Aromatic and Medicinal Plants of Southeast European Countries; Antalaya, Turkey. 18–22 April 2010. [Google Scholar]
- 73.Đurović D., Bulat Z., Buha A., Matovic V. Cadmium, mercury and lead in Hypericum perforatum L. collected in Western Serbia; Proceedings of the 16th International Conference on Heavy Metals in the Environment; Rome, Italy. 23–27 September 2012. [Google Scholar]
- 74.Fischer A., Brodziak-Dopierała B., Loska K., Stojko J. The assessment of toxic metals in plants used in cosmetics and cosmetology. Int. J. Environ. Res. Public Health. 2017;14:1280. doi: 10.3390/ijerph14101280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barbulescu A., Berbes L., Dumitriu C.S. Impact of soil pollution on Melliferous plants. Toxics. 2022;10:239. doi: 10.3390/toxics10050239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scotti F., Löbel K., Booker A., Heinrich M. St. John’s wort (Hypericum perforatum) products- How variable is the primary material? Front. Plant Sci. 2019;9:1973. doi: 10.3389/fpls.2018.01973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zobayed S., Saxena P.K. Production of St. John’s wort plants under controlled environment for maximizing biomass and secondary metabolites. In Vitro Cell. Dev. Biol. Plant. 2004;40:108–114. doi: 10.1079/IVP2003498. [DOI] [Google Scholar]
- 78.Shelar M., Gawade V., Bhujbal S. A review on heavy metal contamination in herbals. J. Pharm. Res. Int. 2021;33:7–16. doi: 10.9734/jpri/2021/v33i29A31561. [DOI] [Google Scholar]
- 79.Clemens S. Safer food through plant science: Reducing toxic element accumulation in crops. J. Exp. Bot. 2019;70:5537–5557. doi: 10.1093/jxb/erz366. [DOI] [PubMed] [Google Scholar]
- 80.Perrelli M., Wu R., Liu D., Lucchini R.G., Del Bosque-Plata L., Vergare M.J., Akhter M.P., Ott J., Cragnoli C. Heavy metals as risk factors for human diseases-a Bayesian network approach. Eur. Rev. Med. Pharmacol. Sci. 2022;26:9275–9310. doi: 10.26355/eurrev_202212_30681. [DOI] [PubMed] [Google Scholar]
- 81.Masarovičová E., KráĬov K., Kummerová M. Principles of classification of medicinal plants as hyperaccumulators or excluders. Acta Physiol. Plant. 2010;32:823–829. doi: 10.1007/s11738-010-0474-1. [DOI] [Google Scholar]
- 82.Norton G.J., Williams P.N., Adomako E.E., Price A.H., Zhu Y., Zhao F.-J., McGrath S., Deacon C.M., Villada A., Sommella A., et al. Lead rice: Analysis of baseline lead levels in market and field collected rice grains. Sci. Total Environ. 2014;485:428–434. doi: 10.1016/j.scitotenv.2014.03.090. [DOI] [PubMed] [Google Scholar]
- 83.Palma-Lara I., Martínez-Castillo M., Quintana-Pérez J.C., Arellano-Mendoza M.G., Tamay-Cach F., Valenzuela-Limón O.L.O., García-Montalvo E.A., Hernández-Zavala A. Arsenic exposure: A public health problema leading to several cancers. Regul. Toxicol. Pharmacol. 2020;110:104539. doi: 10.1016/j.yrtph.2019.104539. [DOI] [PubMed] [Google Scholar]
- 84.Karri V., Schuhmacher M., Kumar V. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ. Toxicol. Pharmacol. 2016;48:203–213. doi: 10.1016/j.etap.2016.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the current study are available under reasonable request from the corresponding author.