ABSTRACT
All nitrogen-fixing bacteria and archaea (diazotrophs) use molybdenum (Mo) nitrogenase to reduce dinitrogen (N2) to ammonia, with some also containing vanadium (V) and iron-only (Fe) nitrogenases that lack Mo. Among diazotrophs, the regulation and usage of the alternative V-nitrogenase and Fe-nitrogenase in methanogens are largely unknown. Methanosarcina acetivorans contains nif, vnf, and anf gene clusters encoding putative Mo-nitrogenase, V-nitrogenase, and Fe-nitrogenase, respectively. This study investigated nitrogenase expression and growth by M. acetivorans in response to fixed nitrogen, Mo/V availability, and CRISPRi repression of the nif, vnf, and/or anf gene clusters. The availability of Mo and V significantly affected growth of M. acetivorans with N2 but not with NH4Cl. M. acetivorans exhibited the fastest growth rate and highest cell yield during growth with N2 in medium containing Mo, and the slowest growth in medium lacking Mo and V. qPCR analysis revealed the transcription of the nif operon is only moderately affected by depletion of fixed nitrogen and Mo, whereas vnf and anf transcription increased significantly when fixed nitrogen and Mo were depleted, with removal of Mo being key. Immunoblot analysis revealed Mo-nitrogenase is detected when fixed nitrogen is depleted regardless of Mo availability, while V-nitrogenase and Fe-nitrogenase are detected only in the absence of fixed nitrogen and Mo. CRISPRi repression studies revealed that V-nitrogenase and/or Fe-nitrogenase are required for Mo-independent diazotrophy, and unexpectedly that the expression of Mo-nitrogenase is also required. These results reveal that alternative nitrogenase production in M. acetivorans is tightly controlled and dependent on Mo-nitrogenase expression.
IMPORTANCE
Methanogens and closely related methanotrophs are the only archaea known or predicted to possess nitrogenase. Methanogens play critical roles in both the global biological nitrogen and carbon cycles. Moreover, methanogens are an ancient microbial lineage and nitrogenase likely originated in methanogens. An understanding of the usage and properties of nitrogenases in methanogens can provide new insight into the evolution of nitrogen fixation and aid in the development nitrogenase-based biotechnology. This study provides the first evidence that a methanogen can produce all three forms of nitrogenases, including simultaneously. The results reveal components of Mo-nitrogenase regulate or are needed to produce V-nitrogenase and Fe-nitrogenase in methanogens, a result not seen in bacteria. Overall, this study provides a foundation to understand the assembly, regulation, and activity of the alternative nitrogenases in methanogens.
KEYWORDS: methanogens, nitrogen fixation, methanogenesis, metalloregulation, nitrogenase, Methanosarcina, archaea
INTRODUCTION
Microbes are the primary drivers of the global biological nitrogen (N) cycle (1, 2). For example, only select bacteria and archaea are capable of biological nitrogen fixation, whereby dinitrogen gas (N2) is reduced to ammonia (NH3), the preferred “fixed” form of N used directly by most organisms. The biological reduction of the triple bond of N2 is difficult and is catalyzed by nitrogenase, a unique metalloenzyme (3, 4). To date, all known and predicted N2-fixing prokaryotes (diazotrophs) possess molybdenum (Mo) nitrogenase that contains a Mo atom within the unique iron (Fe) Mo-cofactor or M-cluster of the active site (5, 6). Mo-nitrogenase consists of two components; the Fe protein that contains a single iron-sulfur (Fe-S) cluster, and the MoFe protein that contains the active site FeMo-cofactor and the [8Fe-7S] P-cluster. The Fe protein, encoded by nifH, is the dinitrogenase reductase that donates electrons to the MoFe protein, the dinitrogenase composed of a heterotetramer of subunits encoded by nifD and nifK. Together NifH and NifDK catalyzes the energy intensive reduction of N2 as shown: N2 + 16 ATP + 8e− + 8H+ → 2NH3 + H2 + 16 ADP + 16 Pi (7). As such, Mo-nitrogenase production and activity are highly regulated in diazotrophs and are only synthesized when a fixed N source is unavailable. When needed, Mo-nitrogenase is produced in high quantities and can comprise as much as 10% of the total protein of the cell (8).
In addition to having Mo-nitrogenase, some diazotrophs possess alternative nitrogenases that lack Mo (9, 10). The vanadium (V) nitrogenase and the Fe-only (Fe) nitrogenase contain an active site FeV-cofactor and FeFe-cofactor, respectively, instead of FeMo-cofactor (11, 12). The understanding of the genetic, biochemical, and catalytic properties of the alternative nitrogenases has primarily come from a few model bacteria (e.g., Azotobacter vinelandii). V-nitrogenase and Fe-nitrogenase have a similar subunit composition as Mo-nitrogenase, comprised of VnfH/VnfDK and AnfH/AnfDK subunits, respectively. However, a distinguishing feature of V-nitrogenase and Fe-nitrogenase is the presence of an additional subunit (G) that associates with the dinitrogenase component (i.e., VnfDGK and AnfDGK) (9, 11). The precise role of the G subunit is unknown, but it is required for diazotrophy in the absence of Mo (13). V-nitrogenase and Fe-nitrogenase are less efficient at reducing N2 than Mo-nitrogenase. More electron flux is directed to H2 production during reduction of N2 by the alternative nitrogenases leading to substantially more ATP consumption. The V-nitrogenase and Fe-nitrogenase are estimated to produce 3 and 7 H2 molecules and consume 24 ATPs and 40 ATPs, respectively, during the reduction of a single N2 to 2 NH3 (14, 15). As such, alternative nitrogenases in bacteria are only produced when insufficient levels of Mo are present to support usage of Mo-nitrogenase. In studied bacteria that possess all three nitrogenases, the expression and activity of each nitrogenase are highly regulated in response to metal and fixed N availability (9, 16). For example, metal-dependent expression of V-nitrogenase and Fe-nitrogenase in A. vindelandii involves the specific transcription regulators VnfA and AnfA that activate expression of V-nitrogenase and Fe-nitrogenase, respectively, in response to the absence of Mo (17, 18).
In addition to N2, nitrogenases from bacteria can reduce other double and triple-bonded substrates (e.g., CO, CO2, and acetylene). Moreover, in the absence of another substrate, nitrogenase reduces protons to H2, a feature that has been exploited to use nitrogenase to produce H2 as a biofuel (19, 20). The substrate, product, and activity profiles are also different between the three nitrogenases. The reduction of acetylene (C2H2) to ethylene (C2H4) is commonly used to measure nitrogenase activity (21). Mo-nitrogenase reduces acetylene at a higher rate than both V-nitrogenase and Fe-nitrogenase, both of which also further reduce ethylene to produce ethane (C2H6) as a minor product (22). Mo-nitrogenase does not produce ethane. Compared to Mo-nitrogenase, bacterial V-nitrogenase is also more adept at reducing CO to alkanes, and Fe-nitrogenase is better at reducing CO2 to CH4 (11, 23 – 25).
In contrast to bacterial diazotrophs, the regulation, assembly, and activity of nitrogenase, especially the alternative nitrogenases, are largely unknown in archaeal diazotrophs. Among archaea, only methanogens and the closely related anaerobic methanotrophs are known or predicted to fix N2 (5, 26, 27). N2 fixation has been studied in a few species of methanogens. The primary models are the obligate CO2-reducing methanogen Methanococcus maripaludis, and the more versatile species Methanosarcina mazei and Methanosarcina barkeri (28, 29). Methanosarcina species can grow using methylated compounds (e.g., methanol) and acetate, in addition to reducing CO2 with H2 (30). M. maripaludis and M. mazei only contain Mo-nitrogenase, whereas strains of M. barkeri are predicted to contain all three nitrogenases (31, 32). Mo-dependent and V-dependent N2 fixation has been demonstrated in M. barkeri (33 – 35). To our knowledge, diazotrophy under Fe-only conditions using the Fe-nitrogenase has not been documented for any methanogen. Previous research has primarily focused on elucidating the mechanisms that regulate the production and activity of Mo-nitrogenase in methanogens, revealing that the regulatory proteins used to control transcription and activity of Mo-nitrogenase are distinct from those used by most bacteria (36, 37). Recently, small RNAs (sRNAs) have also been demonstrated to play roles in N2 fixation and assimilation in methanogens (38, 39).
Methanosarcina acetivorans serves as an ideal model methanogen to understand the regulation and usage of the alternative nitrogenases in methanogens, since its genome encodes all three nitrogenases and it has a robust genetic system (40 – 43). Recently, it was shown that M. acetivorans can fix N2 using Mo-nitrogenase. Like M. maripaludis, M. mazei, and M. barkeri, Mo-nitrogenase is only produced in M. acetivorans when cells are grown in the absence of a fixed N source (e.g., NH4Cl). Silencing of the nif operon in M. acetivorans using the recently developed CRISPRi-dCas9 system confirmed that Mo-nitrogenase is required for diazotrophy when cells are supplied Mo (43). However, to our knowledge, the ability of M. acetivorans to fix N2 when Mo is not available has not been documented nor have the activities of M. acetivorans V-nitrogenase or Fe-nitrogenase been reported. Presumably, M. acetivorans produces V-nitrogenase and/or Fe-nitrogenase when both fixed N and Mo are limiting. In this study, we show that M. acetivorans can grow by fixing N2 in the absence of Mo with growth dependent on the production of V-nitrogenase and/or Fe-nitrogenase. These results provide a foundation to understand the regulation and properties of the three nitrogenases in methanogens.
RESULTS
Organization of nitrogenase genes in M. acetivorans and prevalence of alternative nitrogenases in methanogens
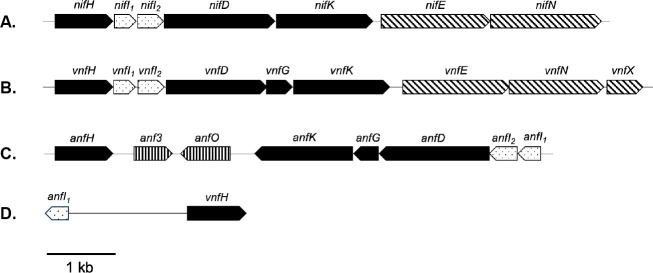
The annotated genome of M. acetivorans contains three separate nitrogenase gene clusters (Fig. 1), designated nif, vnf, and anf, encoding putative Mo-nitrogenase, V-nitrogenase, and Fe-nitrogenase, respectively (40). The gene arrangement of the nif cluster is similar to the characterized nif operons from M. maripaludis, M. barkeri, and M. mazei (32, 44, 45). In addition to encoding the nitrogenase structural components (NifH and NifDK), the operon also encodes the regulatory proteins NifI1 and NifI2 and the FeMo-cofactor scaffold proteins NifEN (12, 46). The M. acetivorans vnf cluster contains the same gene arrangement as nif, including its own regulatory and scaffold genes, but also includes vnfG and a homolog of nifX, designated vnfX. Additional copies of nifX are found in the genome of M. acetivorans. NifX is involved in FeMo-cofactor assembly in bacteria (12). The gene arrangement of the M. acetivorans anf cluster is like the vnf cluster, except anfH encoding the putative Fe-protein is divergent and downstream of anfK. The anf and vnf gene clusters are opposite each other in the chromosome of M. acetivorans (Fig. 1). Interestingly, the amino acid sequences of VnfH and AnfH are identical, indicating the same Fe-protein functions with both V-nitrogenase and Fe-nitrogenase. Also unique to the anf cluster is the presence of homologs of Anf3 and AnfO found in anf operons of bacteria. Anf3 is essential for diazotrophy with the Fe-nitrogenase in Rhodobacter capsulatus (47). An Anf3 homolog characterized in A. vinelandii is a heme-binding and FAD-binding oxidase that may protect the Fe-nitrogenase from oxygen (48). Recently, AnfO was shown to control misincorporation of the FeV-cofactor in AnfDGK (49).
Fig 1.
Arrangement of nitrogenase gene clusters in the genome of M. acetivorans. (A) nif; Mo-nitrogenase, (B) vnf; V-nitrogenase, (C) anf; Fe-nitrogenase. Locus tags: nifHI1I2DKEN (ma3895-3901), vnfHI1I2DGKENX (ma1213-1221), anfI1I2DKEN (ma1212-1205). Black arrows: nitrogenase subunits, diagonal striped arrows: cofactor assembly proteins, dotted arrows: regulatory proteins and vertical striped arrows: unknown function. (D) The vnf and anf gene clusters are opposite each other in the chromosome as shown.
The nif, vnf, and anf gene clusters are widely distributed within genera of bacteria. However, nitrogenase genes are found only in a subset of archaea, restricted to methanogens and closely related anaerobic methanotrophs. The nif operon is distributed across six of the seven orders of methanogens, whereas the vnf and anf genes are restricted to the Methanosarcinales, with few exceptions, namely Methanobacterium lacus, which contains an anf gene cluster (5, 26, 27). Like bacteria, all methanogens that contain putative vnf and anf clusters also contain the nif operon. Out of the 41 complete Methanosarcinales genome sequences analyzed from the NCBI database, ~66% contain the nif genes. Of those containing nif, ~44% contain the vnf and/or anf genes (Table S1). The arrangement of the vnf and anf gene clusters are similar across the Methanosarcinales (Fig. S1). Of note is a hypothetical protein encoded by a gene between vnfDGK and vnfEN in several Methanosarcina species.
Molybdenum and vanadium availability affect diazotrophic growth of M. acetivorans
To ascertain the effect of molybdenum and vanadium availability on nitrogenase utilization by M. acetivorans, the wild-type strain WWM73 (Table 1) that is commonly used for genetic analysis (42) was passed in high-salt (HS) standard medium lacking Mo for >100 generations to deplete molybdate to <1 ppb as determined by ICP-MS. Molybdate is the biological available form of Mo. Vanadium is not present in standard HS medium. Mo-depleted cells were used to inoculate Mo-depleted HS medium devoid of NH4Cl (fixed N source). Methanol was used as the carbon and energy source in all experiments. Molybdate, vanadate, and NH4Cl were added from sterile anaerobic stocks to separate cultures to compare the effect of Mo, V, and fixed N on growth and nitrogenase expression. Neither the depletion of Mo nor the addition of V affects the generation time, or cell yield when NH4Cl is supplied as the fixed N source (Fig. 2; Table 2). However, the depletion of Mo and the addition of V significantly affect the generation time and cell yield in cultures without NH4Cl (diazotrophic). When M. acetivorans is provided Mo in the absence of NH4Cl, the generation time increases approximately threefold, and the cell yield decreases by approximately 37% compared to non-diazotrophic cultures (Table 2). Diazotrophic cultures lacking Mo but provided V have an even longer generation time and further reduction in cell yield (~50% that of non-diazotrophic cultures). Diazotrophic growth is further impacted by the absence of both Mo and V, with an ~10-fold increase in generation time and an ~70% reduction in cell yield compared to non-diazotrophic cultures (Fig. 2; Table 2). Diazotrophic cultures lacking Mo also have an extended lag phase compared to diazotrophic cultures containing Mo (Fig. 2; Table 2). These data reveal that M. acetivorans is capable of diazotrophy in the absence of Mo, and that V availability impacts N2 fixation. These results are consistent with M. acetivorans utilizing Mo-nitrogenase, V-nitrogenase, and Fe-nitrogenase to fix N2 according to Mo and V availability.
TABLE 1.
M. acetivorans strains used in this study
| Strain | Description | gRNA | References |
|---|---|---|---|
| WWM73 | Wild-type strain used for genetic analysis | None | (42) |
| DJL72 | WWM73 with integrated CRISPRi plasmid | None | (43) |
| DJL74 | WWM73 with integrated CRISPRi plasmid | gRNA-nifH | (43) |
| DJL107 | WWM73 with integrated CRISPRi plasmid | gRNA-vnfH | This study |
| DJL120 | WWM73 with integrated CRISPRi plasmid | gRNA-anfI1 | This study |
| DJL119 | WWM73 with integrated CRISPRi plasmid | gRNA-vnfH + gRNA-anfI1 | This study |
Fig 2.
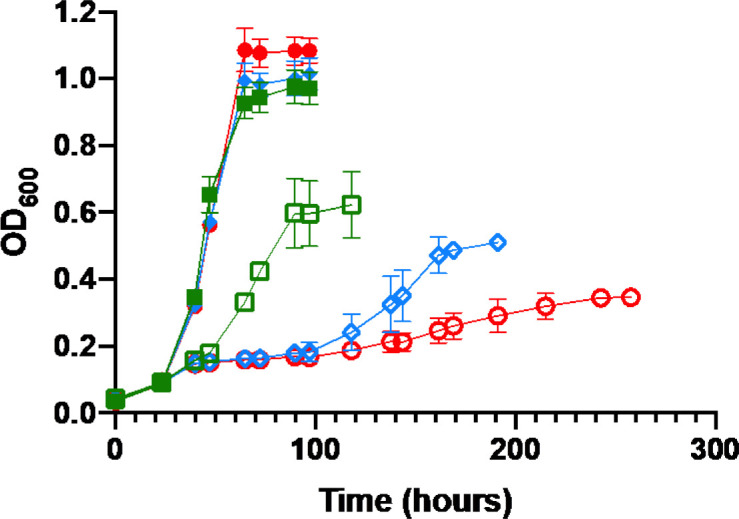
Comparison of the growth of M. acetivorans in the presence (closed) or absence (open) of NH4Cl in HS medium with Mo + Fe (green squares), V + Fe (blue diamonds), or Fe alone (red circles). Inoculum: stationary phase cells grown in Mo-depleted HS medium with NH4Cl. Error bars represent mean ± 1 SD from at least three biological replicates.
TABLE 2.
Effect of metal and NH4Cl availability on growth of M. acetivorans with methanol
| Relevant metals | Nitrogen source | Lag time (h) a | Generation time (h) b | Cell yield (cells/mL) b |
|---|---|---|---|---|
| Mo + Fe | NH4Cl | 30 | 8.2 ± 0.5 | 3.02 ± 0.2 × 108 |
| N2 | 48 | 28.5 ± 4 | 1.92 ± 0.3 × 108 | |
| V + Fe | NH4Cl | 30 | 8.5 ± 0.1 | 3.08 ± 0.2 × 108 |
| N2 | 90 | 44.5 ± 4.1 | 1.53 ± 0.6 × 108 | |
| Fe-only | NH4Cl | 30 | 8.7 ± 0.1 | 3.34 ± 0.2 × 108 |
| N2 | 96 | 82 ± 4.1 | 9.88 ± 0.4 × 107 |
Approximate time when culture entered exponential phase.
Generation time and cell yield represent the mean ± 1 SD from at least three biological replicates. Cell yield was determined by cell counts (initial cells/mL = 6.0 x× 106).
Methylotrophic methanogenesis is not altered by diazotrophy or the availability of molybdenum or vanadium
Growth of M. acetivorans with methanol utilizes the methylotrophic pathway of methanogenesis, where one methyl group of methanol is oxidized to CO2, and the resulting three electron pairs are used to reduce three additional methyl groups to CH4 (50). To determine if diazotrophy and metal availability affect the flow of carbon during methylotrophic methanogenesis, contributing to the slower growth rate and lower cell yields in the absence of Mo, total CH4 was determined after the cessation of growth of nondiazotrophic and diazotrophic cultures. Similar amounts of CH4 were observed across all growth conditions (Table 3), revealing N2 fixation and differences in Mo and V availability does not significantly alter the flow of carbon during methylotrophic methanogenesis. Therefore, the observed hierarchical decrease in cell yields during diazotrophic growth under Mo + Fe, V + Fe, or Fe-only conditions (Table 3) is not due to altered methanogenesis but is likely due to the increased ATP consumption needed to support N2 reduction by Mo-nitrogenase, V-nitrogenase, and Fe-nitrogenase, as seen in bacteria (9).
TABLE 3.
Effect of metal and NH4Cl availability on total CH4 production by M. acetivorans with methanol
| Relevant metals | Nitrogen source | CH4 produced (μmol) a |
|---|---|---|
| Mo + Fe | NH4Cl | 1,004 ± 109 |
| N2 | 1,092 ± 58 | |
| V + Fe | NH4Cl | 926 ± 193 |
| N2 | 823 ± 24 | |
| Fe-only | NH4Cl | 1,031 ± 48 |
| N2 | 1,079 ± 41 |
Data represent the mean ± 1 SD from at least three biological replicates (10 mL cultures).
Molybdenum availability affects the expression of V-nitrogenase and Fe-nitrogenase but not Mo-nitrogenase in M. acetivorans
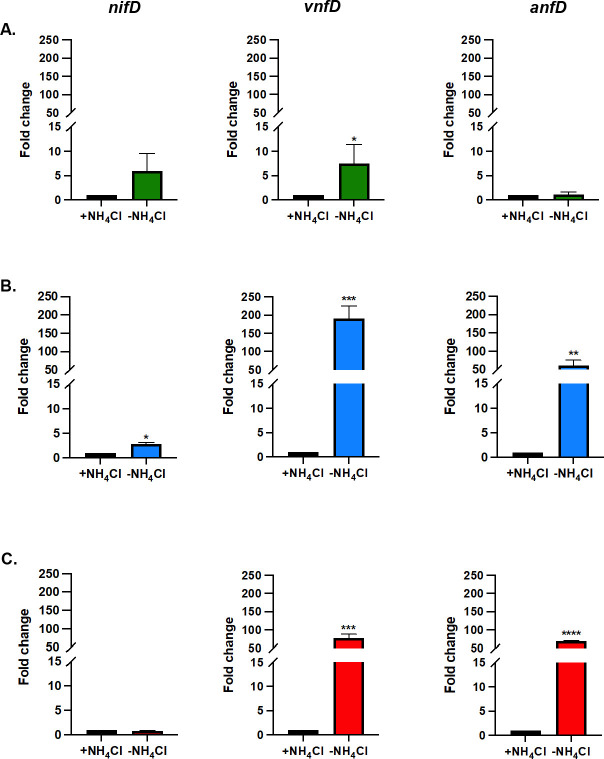
Previous results demonstrated that Mo-nitrogenase is not produced in M. acetivorans cells grown in the presence of NH4Cl. Removal of NH4Cl results in a modest increase in nif transcription and production of Mo-nitrogenase, allowing growth with N2. Moreover, CRISPRi repression of the nif operon abolished the ability to grow with N2 in medium containing Mo (43). To determine the effect of fixed N and Mo depletion on Mo-nitrogenase, V-nitrogenase, and Fe-nitrogenase expression, qPCR was performed using primers specific for nifD, vnfD, and anfD to analyze transcript abundance in cells grown in medium with or without NH4Cl and containing Mo + Fe, V + Fe, or Fe-only (Fig. 3). An increase in transcript abundance for nifD and vnfD was observed in cells grown in Mo + Fe medium without NH4Cl, relative to the transcript abundance in cells grown with NH4Cl (Fig. 3A). However, only the fold change for vnfD was significant. Comparison of nifD, vnfD, and anfD transcript abundance from cells grown with V + Fe showed a significant fold change for vnfD and anfD (Fig. 3B). The transcript abundance of vnfD is ~180-fold higher in cells grown in V + Fe medium without NH4Cl compared to cells grown with NH4Cl. Transcript abundance for anfD is ~60-fold higher in cells grown in V + Fe medium without NH4Cl compared to cells grown with NH4Cl. In contrast, only a slight increase (~threefold) was observed for nifD transcript abundance. Like the transcript abundance of vnfD and anfD in cells grown with V + Fe, cells grown in Fe-only medium lacking NH4Cl had a significant increase in vnfD and anfD transcript abundance compared to cells grown with NH4Cl (Fig. 3C). No change in the expression of nifD was detected in cells grown in Fe-only medium lacking NH4Cl relative to that with NH4Cl (Fig. 3C).
Fig 3.
Effect of fixed N availability on the transcription of the nif, vnf, and anf gene clusters in M. acetivorans as determined by qPCR. The relative abundance of nifD, vnfD, and anfD transcripts in M. acetivorans cells grown with NH4Cl (normalized to one) were compared to cells grown without NH4Cl and 16s rRNA was used as the control. M. acetivorans was grown with methanol in HS medium containing (A) Mo + Fe (B) V + Fe, or (C) Fe-only. Error bars represent mean ± 1SD for two technical replicates and three biological replicates. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
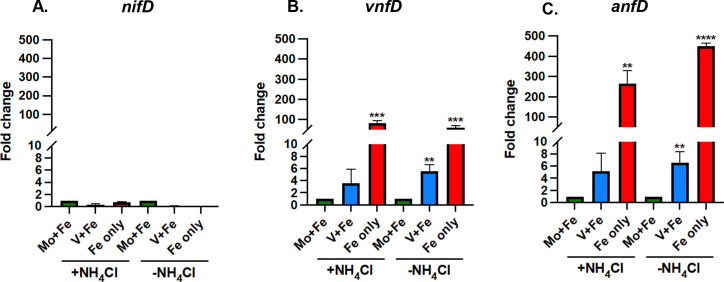
To further determine the effect of Mo depletion on transcription of each nitrogenase gene cluster, the fold change in nifD, vnfD, and anfD transcript abundance was also calculated by comparing the relative abundance in cells grown in V + Fe or Fe-only medium to the transcript abundance in cells grown in Mo + Fe medium (Fig. 4). The expression of nifD did not significantly change in cells grown in medium with or without Mo, regardless of the presence or absence of NH4Cl (Fig. 4A). However, the removal of Mo significantly affected the transcription of both vnfD and anfD in cells grown with or without NH4Cl (Fig. 4B and C). The transcript abundance of vnfD is highest in cells grown in Fe-only medium, with the fold change higher than when V is present. A similar pattern was observed for the expression of anfD. However, the fold change in expression of anfD in cells grown with Fe-only compared to Mo + Fe was much higher (~300- to 600-fold). These results indicate there is significant regulatory control of transcription of the vnf and anf gene clusters, whereas there is only modest transcriptional control of the nif operon. The results also show that the depletion of Mo is the key signal that increases transcription of the vnf and anf gene clusters. Removal of a fixed N source (NH4Cl) when Mo is available has only a slight effect on the transcription of the vnf and anf gene clusters (Fig. 3A).
Fig 4.
Effect of molybdenum availability on the transcription of the nif, vnf, and anf gene clusters in M. acetivorans as determined by qPCR. The relative abundance of (A) nifD, (B) vnfD, and (C) anfD transcripts in cells grown with Mo (normalized to 1) were compared to cells grown without Mo, and 16s rRNA was used as the control. Error bars represent mean ± 1SD for two technical replicates and three biological replicates. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
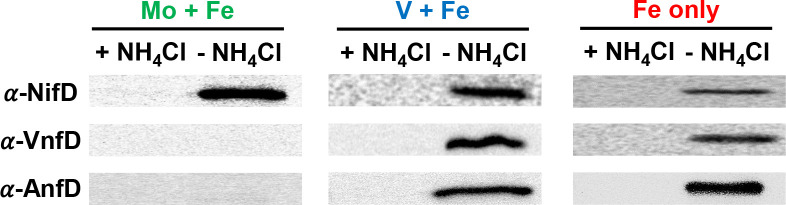
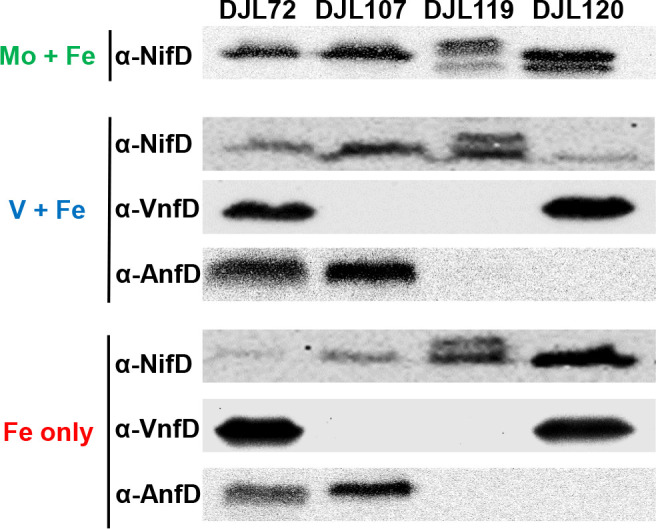
The production of Mo-nitrogenase, V-nitrogenase, and Fe-nitrogenase in M. acetivorans grown under the same conditions for qPCR analysis was determined by Western blot using antibodies specific to NifD, VnfD, and AnfD (Fig. 5), and a loading control SDS-Page gel is shown in Fig. S2. Consistent with previous results (43), NifD was only detected in lysate from M. acetivorans cells grown in Mo + Fe medium lacking NH4Cl. Neither VnfD nor AnfD was detected in lysate from cells grown in Mo + Fe medium regardless of the presence or absence of NH4Cl. However, both VnfD and AnfD were detected in lysate from cells grown in Mo-depleted medium lacking NH4Cl. Interestingly, NifD was also detected in lysate from cells grown in Mo-depleted medium. The availability of V does not appear to affect the production of VnfD or AnfD. These results indicate that both the depletion of fixed N and Mo are required for production of V-nitrogenase and Fe-nitrogenase in M. acetivorans.
Fig 5.
Western blot analysis using NifD-specific, VnfD-specific, and AnfD-specific antibodies on lysate from M. acetivorans cells grown with or without NH4Cl and the indicated metals.
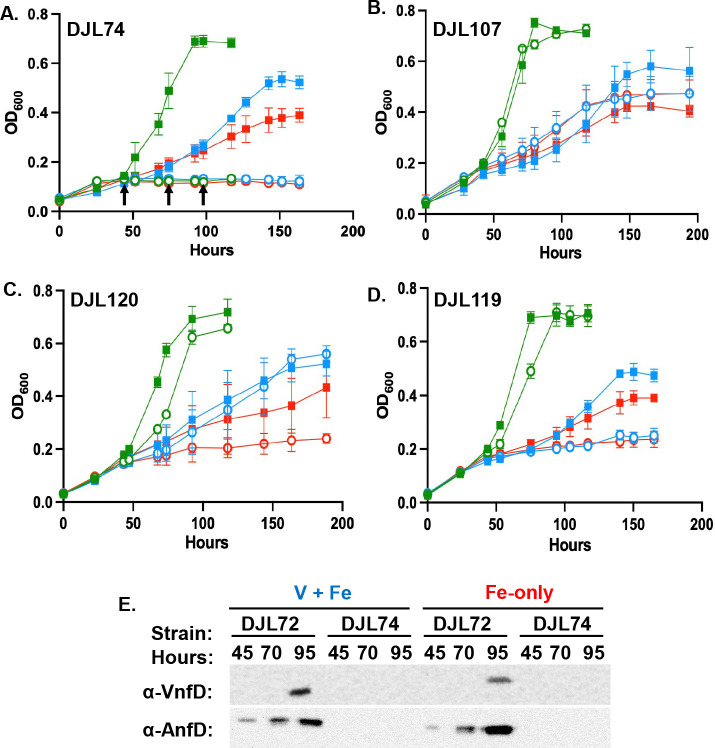
Mo-nitrogenase is required for V-nitrogenase and Fe-nitrogenase expression
To determine the role of each nitrogenase during diazotrophy in Mo-repleted and Mo-depleted medium, growth studies were performed with the previously described nif operon CRISPRi repression strain DJL74 and new vnf and/or anf operon CRISPRi repression strains (Table 1). As described previously, strain DJL74 is unable to grow in the absence of fixed nitrogen and presence of Mo (Fig. 6A). Surprisingly, strain DJL74 is also unable to grow in the absence of Mo when V-nitrogenase and/or Fe-nitrogenase should support N2 fixation (Fig. 6A). This result reveals that Mo-nitrogenase expression is required for diazotrophy under all conditions tested, consistent with NifD detection when fixed nitrogen is depleted regardless of the presence/absence of Mo (Fig. 5). VnfD is not detected by Western blot (loading control gel in Fig. S4) in lysates from strain DJL107 (Fig. 7), consistent with complete repression of the vnf operon. However, strain DJL107 grows comparable to the control strain DJL72 under all conditions (Fig. 6B). Similarly, AnfD is not detected by Western blot (loading control gel in Fig. S4) in lysates from strain DJL120 (Fig. 7). Strain DJL120 also grows similar to strain DJL72, except under Fe-only conditions, where it is unable to grow, indicating that expression of the Fe-nitrogenase is required for diazotrophy in the absence of Mo and V. Taken together, these data indicate the V-nitrogenase likely only supports diazotrophy in presence of V, while the Fe-nitrogenase supports diazotrophy in the presence and absence of V. Importantly, neither VnfD nor AnfD is detected by Western blot (loading control gel in Fig. S4) in cell lysates of the dual vnf/anf repression strain DJL119, with these cells only producing NifD (Fig. 7). Strain DJL119 grows similar to the control strain in the presence of Mo, but is unable to grow in medium lacking Mo, regardless of the presence/absence of V (Fig. 6D), revealing that the alternative nitrogenases are required for diazotrophy in the absence of Mo. This result also demonstrates that Mo-nitrogenase alone in strain DJL119 is unable to support diazotrophy in the absence of Mo.
Fig 6.
Comparison of the growth of M. acetivorans CRISPRi repression strains. (A) DJL74 (gRNA-nifH), (B) DJL107 (gRNA-vnfH), (C) DJL120 (gRNA-anfI1), and (D) DJL119 (gRNA-vnfH/anfI1) to the control strain DJL72 in the absence of NH4Cl in HS medium with Mo + Fe (green), V + Fe (blue), or Fe alone (red). In all panels, strain DJL72 is represented by squares and the CRISPRi repression strain by circles. Error bars represent mean ± 1SD from at least three biological replicates. (E) Western blot of lysates from cells of strains DJL72 and DJL74 taken at the timepoints indicated by the arrows in panel (A).
Fig 7.
Western blot analysis using NifD-specific, VnfD-specific, and AnfD-specific antibodies on lysate from cells of M. acetivorans CRISPRi repression strains DJL107, DJL120, DJL119, and control strain DJL72 grown without NH4Cl and the indicated metals.
To test if nif operon expression is required for alternative nitrogenase production, V-nitrogenase and Fe-nitrogenase expression over time in strains DJL72 and DJL74 grown in Mo-depleted medium was probed by Western blot (loading control gel in Fig. S5) using antibodies specific to VnfD and AnfD (Fig. 6E). Whereas the detection of both VnfD and AnfD occurs in cells of strain DJL72 grown in V + Fe and Fe-only medium, both VnfD and AnfD are not detected in strain DJL74. This result clearly demonstrates that the expression of the nif operon is required for production of both V-nitrogenase and Fe-nitrogenase and explains why strain DJL74 is unable to grow fix N2 in the absence of Mo (Fig. 6A).
DISCUSSION
The regulation, assembly, and activity of the three forms of nitrogenase are well understood in diazotrophic bacteria, especially in the principal model A. vinelandii that contains all three nitrogenases. A. vinelandii is an obligate aerobe; thus, in addition to nitrogenase structural proteins, A. vinelandii requires accessory proteins to prevent oxidative damage to nitrogenase and to integrate nitrogen fixation into central metabolism. At least 82 genes are predicted to be involved in the formation and regulation of Mo-nitrogenase, V-nitrogenase, and Fe-nitrogenase in A. vinelandii (16). Moreover, there is complex regulatory control over hierarchal nitrogenase expression, with only one nitrogenase produced at a time. When fixed N is absent and Mo is available, Mo-nitrogenase is preferentially produced over V-nitrogenase and Fe-nitrogenase, followed by V-nitrogenase if Mo is absent and V is present. If neither Mo nor V is available, then Fe-nitrogenase is produced (26). Among anaerobic methanogens, the alternative nitrogenases are restricted primarily to the Methanosarcinales, the most metabolically diverse methanogens with the largest genomes. Nonetheless, the genomes of sequenced Methanosarcinales contain simpler nitrogenase gene clusters and lack many of the accessory and regulatory proteins found in A. vinelandii and other diazotrophic bacteria (27). The formation and regulation of the alternative nitrogenases are likely simpler in methanogens compared to aerobic diazotrophic bacteria. The results presented here demonstrate that M. acetivorans produces all three nitrogenases and is capable of diazotrophy in the absence of available Mo and V (Fe-only condition). Results from the CRISPRi repression strains reveal that diazotrophy in the absence of Mo requires V-nitrogenase and/or Fe-nitrogenase. To our knowledge, this is the first direct evidence of a methanogen using a Fe-nitrogenase.
Like other diazotrophs, M. acetivorans only produces nitrogenase in the absence of fixed N. The diazotrophic growth profiles of M. acetivorans correlate with reported ATP requirements by Mo-nitrogenase, V-nitrogenase, and Fe-nitrogenase from bacteria (14). M. acetivorans has the fastest growth rate and highest cell yield during diazotrophic growth when utilizing only Mo-nitrogenase. Only a modest increase in transcription of the nif operon was observed in response to fixed N depletion, even though NifD is only detected in cells not provided fixed N. The high basal level of transcription of the nif operon likely allows M. acetivorans to be poised for rapid Mo-nitrogenase production. The relatively short lag time before the onset of diazotrophic growth in Mo + Fe medium (Table 2; Fig. 2) supports the rapid production of Mo-nitrogenase.
The results indicating minimal transcriptional control of the nif operon further support that post-transcriptional regulation is a key factor controlling Mo-nitrogenase production. Previous studies investigated the role of NrpR in regulating the expression of Mo-nitrogenase in M. acetivorans. NrpR is the repressor of the nif operon in methanogens and indirectly senses fixed N availability by directly sensing intracellular 2-oxogluatrate levels (51). A mutant strain of M. acetivorans where nrpR transcription was silenced using the CRISPRi-dCas9 system revealed that the depletion of NrpR results in an increase in the transcription of the nif operon, but the mutant still fails to produce detectable nitrogenase when grown with fixed N (43). In M. mazei, a small RNA (sRNA154) is exclusively expressed when fixed N is limiting and functions to stabilize the polycistronic mRNA produced from the nif operon (38). The genome of M. acetivorans encodes a sRNA154 homolog, indicating similar post-transcriptional regulation of the nif operon. Interestingly, removal of Mo did not significantly alter transcription of the nif operon or the production of nitrogenase (Fig. 4A and 5). Therefore, the critical and likely only signal for Mo-nitrogenase production in M. acetivorans is fixed N limitation. This is distinct from diazotrophic bacteria that contain V-nitrogenase and Fe-nitrogenase. For example, A. vinelandii and the purple nonsulfur phototroph Rhodopseudomonas palustris both stop producing Mo-nitrogenase when Mo is depleted (26, 52).
While Mo-depletion had little effect on Mo-nitrogenase expression, it is critical for the expression of V-nitrogenase and Fe-nitrogenase in M. acetivorans. Both fixed N and Mo depletion are required for production of V-nitrogenase and Fe-nitrogenase (Fig. 5). Importantly, Mo depletion resulted in a significant increase in the relative transcript abundance of vnfD and anfD (Fig. 3 and 4). Thus, unlike production of Mo-nitrogenase, transcriptional regulation is a key mechanism to control production of V-nitrogenase and Fe-nitrogenase in M. acetivorans. The overall transcript abundance profiles for vnfD and anfD are similar across all growth conditions. Mo depletion appears to be a key effector as cells grown with NH4Cl exhibited a significant increase in transcript abundance of vnfD and anfD (Fig. 4). Nonetheless, neither VnfD nor AnfD was detected in cells grown with NH4Cl in Mo-depleted medium (Fig. 5), indicating post-transcriptional regulation of vnf and anf genes is also likely involved. Unexpectedly, in the absence of Mo, the presence of V does not increase the transcript abundance of vnfD and anfD as much as the increase during Fe-only conditions (Fig. 4). The role V plays in nitrogenase regulation is unknown in most diazotrophs. Nevertheless, when comparing the effect of fixed N depletion, a large relative fold change in transcript abundance for vnfD and anfD was observed in cells grown in V + Fe medium (Fig. 3B). Expression of the vnf and anf operons in A. vinelandii in the absence of Mo results in the production of either V-nitrogenase or Fe-nitrogenase depending on V availability, but not both. In contrast, V availability had no effect on V-nitrogenase or Fe-nitrogenase production in M. acetivorans, as each was produced in cells grown in Mo-depleted medium (Fig. 5). Notably, VnfH and AnfH are identical in amino acid sequence, indicating a single dinitrogenase reductase (VnfH/AnfH) can support the in vivo activities of separate dinitrogenases (VnfDGK and AnfDGK).
Production of both V-nitrogenase and Fe-nitrogenase in M. acetivorans clearly requires fixed N depletion since neither VnfD nor AnfD was detected by immunoblot in lysate from cells grown with NH4Cl regardless of Mo availability. Regulation of V-nitrogenase and Fe-nitrogenase expression in response to fixed N availability does not likely involve direct control of vnf and anf transcription since fixed N depletion in the presence of Mo did not alter anfD transcript abundance and only had a modest effect on vnfD transcript abundance (Fig. 3A). These results are consistent with the promotor regions of both the vnf and anf gene clusters lacking the identified NrpR operator sequence (53). The promoter regions also lack identified binding sites for NrpA, an activator of the nif operon in M. mazei, for which M. acetivorans encodes two homologs (MA0545 and MA0546) (54). Thus, post-transcriptional regulation is likely the primary mechanism of control of V-nitrogenase and Fe-nitrogenase production in response to fixed N availability. It is possible sRNA154, or another sRNA, is responsive to fixed N depletion and functions to stabilize vnf and anf mRNAs, which allows for V-nitrogenase and Fe-nitrogenase production only when fixed N is depleted.
Mo availability is the key factor controlling transcription of both the vnf and anf gene clusters in M. acetivorans. In non-diazotrophic (e.g., Escherichia coli) and diazotrophic bacteria, the molybdate-responsive transcriptional regulator ModE controls the expression of the high-affinity molybdate transporter ModABC as well as Mo-dependent enzymes (55). In A. vinelandii, ModE indirectly represses the expression of both V-nitrogenase and Fe-nitrogenase by directly repressing the transcription of the genes encoding the regulators VnfA and AnfA. VnfA activates transcription of the vnf operon and AnfA activates transcription of the anf operon in A. vinelandii (55). The genome of M. acetivorans encodes several homologs of ModABC (MA0325-27, MA1235-37, and MA2280-82), including additional homologs of ModBC (MA3902-03) downstream of the nif operon. M. acetivorans contains a ModE homolog (MA0283) but lacks homologs to VnfA and AnfA. Potential ModE-binding sites are located upstream of vnfH and anfI1, the first genes in the vnf and anf gene clusters (56). Therefore, it is highly plausible that ModE is responsible for repressing transcription of vnf and anf when sufficient Mo is available to support Mo-nitrogenase activity. Depletion of Mo (corepressor) likely results in removal of DNA-bound ModE and de-repression of transcription of the vnf and anf gene clusters, leading to the simultaneous production of V-nitrogenase and Fe-nitrogenase in M. acetivorans. The results are consistent with this regulatory mechanism. Interestingly, the starter inoculum used in all expression studies was maintained in Mo-depleted medium, which should result in an increase in vnf and anf transcription even during growth with NH4Cl (Fig. 4). As such, the starter inoculum should be primed to use the alternative nitrogenases once fixed N is depleted, yet there was a much longer period before the onset of growth in Mo-depleted medium compared to the onset of growth in Mo-depleted medium with added Mo (Table 2; Fig. 2). This result indicates that there are likely other unknown regulatory factors involved in controlling the production of V-nitrogenase and Fe-nitrogenase in response to fixed N and Mo depletion.
To our knowledge, M. acetivorans is the first diazotroph shown to simultaneously produce all three nitrogenases when grown in Mo-depleted conditions, indicating that the function of the alternative nitrogenases is intimately connected to Mo-nitrogenase. Although M. acetivorans may continue to produce Mo-nitrogenase when fixed N is limiting regardless of Mo availability to be poised to use the most efficient nitrogenase. since energy conservation (i.e., ATP generation) during methanogenesis by M. acetivorans is significantly lower compared to studied diazotrophic bacteria (57), the results with strain DJL74 clearly demonstrate that nif operon expression is required for expression of functional V-nitrogenase and Fe-nitrogenase. Of the proteins encoded in the nif operon (Fig. 1), NifH could be the critical factor, since in addition to providing electrons to NifDK during N2 reduction, it serves multiple roles in nitrogenase maturation in bacteria. For example, NifH is involved in the synthesis of the complex metalloclusters within NifDK (e.g., P-cluster) (3, 12, 58). Therefore, NifH could be required for metallocluster synthesis in VnfDGK and AnfDGK. Although VnfEN scaffold proteins are encoded in the vnf gene cluster, it is also possible NifEN is needed for metallocluster synthesis in VnfDGK and/or AnfDGK. Alternatively, inactive NifDK may serve a regulatory role in controlling the production of active V-nitrogenase and Fe-nitrogenase. We are currently investigating which nif operon proteins are required for alternative nitrogenase production.
Overall, the results from this study reveal that the expression and usage of the alternative nitrogenases in M. acetivorans is distinct from studied bacteria, and that Mo-nitrogenase expression is directly linked to the production of functional alternative nitrogenases. Finally, the results highlight the utility of M. acetivorans as a model to understand the regulation, maturation, and activity of the three forms of nitrogenase in methanogens.
MATERIALS AND METHODS
M. acetivorans strains and growth
M. acetivorans strain WWM73, a pseudo-wild type strain used for genetic manipulation (42), was used for all experiments. Anoxic HS medium was prepared as previously described with some modifications (59). To prepare Mo-depleted HS medium, all glassware was washed twice with 1 M HCl, once with 1 M H2SO4, and then rinsed with ultrapure water to remove any residual molybdate prior to use. NH4Cl and molybdate were omitted and the HS medium was reduced with 1.5 mM DTT. The concentration of molybdate in Mo-depleted HS medium was determined <1 ppb by ICP-MS (University of Arkansas Stable Isotope Facility). Methanol, NH4Cl, sodium sulfide (Na2S), sodium molybdate (Na2MoO4), and sodium vanadate (Na3VO4) were added from anoxic sterile stocks using sterile syringes prior to inoculation. M. acetivorans strain WWM73 was grown in Balch tubes containing 10 mL of HS medium with 125 mM methanol and 0.025% Na2S (wt/vol). Molybdate (1 µM), vanadate (1 µM), and NH4Cl (18 mM) were added to cultures as indicated. M. acetivorans strain WWM73 was grown for more than 100 generations in Mo-depleted HS medium containing methanol and NH4Cl prior to the growth experiments. The inoculum used in growth experiments was stationary phase cells grown in Mo-depleted HS medium with NH4Cl. Growth was measured by monitoring optical density at 600 nm (OD600) using a spectrophotometer. Cell density was determined from OD600 using a standard curve generated by direct cell counts with a hemocytometer.
Quantitative PCR analysis of gene expression
M. acetivorans cells were harvested during mid-log phase (0.3–0.4 OD600) by anaerobic centrifugation of 4–8 mL of culture. Cell pellets were resuspended in 1 mL TRIzol and frozen at −80°C. RNA was extracted using the Zymo Direct-zol Miniprep kit (#R2052) and further purified using the Invitrogen DNA-free DNA Removal Kit (#AM1906). cDNA was generated using the Bio-Rad iScript Select cDNA Synthesis Kit (#1708896). qPCR primers were designed using Geneious Prime to analyze nifD (Forward: 5′-CGCCCGCTGTGAAGGATATA-3′, Reverse: 5′-TTATGTCAAAGGGAGTGGGGTC-3′), vnfD (Forward: 5′-GTCGGAAAGAGGTTGCAGCTA-3, Reverse: 5′-GCTTCGTGTGCCAGGTATCA-3′), anfD (Forward: 5′-GTCTCCCTGATGGCCGAATT-3′, Reverse: 5′-AGATCTGTCTCTGGCCTGGT-3′), and 16srRNA (Forward: 5′-GGTACGGGTTGTGAGAGCAA-3′, Reverse: 5′-CTCGGTGTCCCCTTATCACG-3′).
qPCR of three biological replicates and two technical replicates was performed with the SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, #1725271). Relative quantification was determined using the 2−ΔΔCq method with 16srRNA as a standard as described (43).
Western blot analysis
Separate custom polyclonal antibodies specific for M. acetivorans NifD, VnfD, or AnfD were generated using the PolyExpress Silver package (two epitopes) from Genscript. Specificity of the antibodies was confirmed using recombinant NifD, VnfD, and AnfD expressed in E. coli (data not shown). M. acetivorans cells were harvested during mid-log phase (0.3–0.4 OD600) by aerobic centrifugation (8500 × g for 10 min at 4°C) of 6 mL of culture. The cell pellet was resuspended in 50 mM Tris, 150 mM NaCl pH 7.2 with 1 mM PMSF and 1 mM benzamidine, normalized based on OD600, and frozen at −80°C. Whole-cell lysate was generated by five freeze/thaw cycles and a 1-h DNase (5 µg) treatment at 37°C. Protein concentration was determined using the Bradford assay. After blocking for 1 h in TBST (20 mM Tris, 150 mM NaCl, and 0.1% Tween pH 7.6) with 5% milk, membranes were incubated for 18 h with the primary antibodies specific for NifD, VnfD, or AnfD, then washed three times with TBST. Membranes were then incubated with an HRP-conjugated secondary antibody (Promega) for 1 h, followed by three washes with TBST. Finally, membranes were visualized using an enhanced chemiluminescent reagent (Thermo Scientific) and an Alpha Innotech imaging system.
Methane determination by gas chromatography
After the cessation of growth, the total volume of gas produced by each culture was measured using a glass syringe, which also normalized the pressure to 1 atm. The amount of CH4 produced was determined by injection of 50 µL of headspace gas into a Shimadzu Nexis GC-2030 gas chromatograph fitted with an Rt-Q-BOND fused silica PLOT column with a 0.32-mm internal diameter, a 30-m length, and a 10.00-µm film thickness (Restek, VWR #89166-308) and BID detector. The sample split ratio was 42.6, and the carrier gas was helium at 4.44 mL/min. The injection port temperature was 100°C, column temperature was 27°C, and BID temperature was 220°C. Peak integration was performed using Shimadzu LabSolutions software and moles of CH4 were determined using methane standards.
Construction of CRISPRi gene repression strains
CRISPRi repression of the vnf and anf operons in M. acetivorans was designed and strains constructed as previously described for CRISPRi repression of the nif operon in strain DJL74 (43). Briefly, 20 bp guide RNAs (gRNA) targeting dCas9 to the vnf (gRNA-vnfH-5′-CCCGTAAAAAGCAATTTTTC-3′) or anf (gRNA-anfI1-5′-ATAACCTTCGTACTACCTTT-3′) gene cluster were designed using Geneious Prime (Fig. S3). Synthetic DNA oligos (gBlocks) (IDT) were designed for assembly with CRISPRi-dCas9 plasmid pDL734. gBlocks and pDL734 were assembled using the Gibson assembly Ultra Kit (Synthetic Genomics). The assembly mix was used to transform E. coli strain WM4489, transformants were screened for assembled plasmids using standard methods, and final plasmids were sequenced. Each plasmid was used to transform M. acetivorans strain WWM73 as described (60). Integration of each plasmid into the chromosome of strain WWM73 was screened using PCR. Repression of the vnf and/or anf operons in the resultant CRISPRi repression strains DJL107, DJl120, and DJl119 (Table 1) was confirmed by Western blot as described above.
ACKNOWLEDGMENTS
The authors thank Tom Deere for helpful discussions and assistance with gas chromatography. The authors also thank Erik Pollock of the University of Arkansas Stable Isotope Facility for assistance with metals analysis.
This work was supported in part by DOE Biosciences grant number DE-SC0019226 (D.J.L.), NSF grant number MCB1817819 (D.J.L.), NSF Graduate Research Fellowship under grant number 1842401 (M.C.), and the Arkansas Biosciences Institute (D.J.L.), the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000.
Contributor Information
Daniel J. Lessner, Email: dlessner@uark.edu.
Haruyuki Atomi, Kyoto University, Kyoto, Japan .
DATA AVAILABILITY
The raw data from growth studies and qPCR will be available upon request.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aem.01033-23.
Supplemental tables and figures.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Fowler D, Coyle M, Skiba U, Sutton MA, Cape JN, Reis S, Sheppard LJ, Jenkins A, Grizzetti B, Galloway JN, Vitousek P, Leach A, Bouwman AF, Butterbach-Bahl K, Dentener F, Stevenson D, Amann M, Voss M. 2013. The global nitrogen cycle in the twenty-first century. Philos Trans R Soc Lond B Biol Sci 368:20130164. doi: 10.1098/rstb.2013.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stein LY, Klotz MG. 2016. The nitrogen cycle. Curr Biol 26:R94–8. doi: 10.1016/j.cub.2015.12.021 [DOI] [PubMed] [Google Scholar]
- 3. Rubio LM, Ludden PW. 2008. Biosynthesis of the iron-molybdenum cofactor of nitrogenase. Annu Rev Microbiol 62:93–111. doi: 10.1146/annurev.micro.62.081307.162737 [DOI] [PubMed] [Google Scholar]
- 4. Dixon R, Kahn D. 2004. Genetic regulation of biological nitrogen fixation. Nat Rev Microbiol 2:621–631. doi: 10.1038/nrmicro954 [DOI] [PubMed] [Google Scholar]
- 5. Dos Santos PC, Fang Z, Mason SW, Setubal JC, Dixon R. 2012. Distribution of nitrogen fixation and nitrogenase-like sequences amongst microbial genomes. BMC Genomics 13:162. doi: 10.1186/1471-2164-13-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burris RH. 1991. Nitrogenases. J Biol Chem 266:9339–9342. [PubMed] [Google Scholar]
- 7. Peters JW, Fisher K, Dean DR. 1995. Nitrogenase structure and function: a biochemical-genetic perspective. Annu Rev Microbiol 49:335–366. doi: 10.1146/annurev.mi.49.100195.002003 [DOI] [PubMed] [Google Scholar]
- 8. Dingler C, Kuhla J, Wassink H, Oelze J. 1988. Levels and activities of nitrogenase proteins in Azotobacter vinelandii grown at different dissolved oxygen concentrations. J Bacteriol 170:2148–2152. doi: 10.1128/jb.170.5.2148-2152.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eady RR. 1996. Structure-function relationships of alternative nitrogenases. Chem Rev 96:3013–3030. doi: 10.1021/cr950057h [DOI] [PubMed] [Google Scholar]
- 10. Harwood CS. 2020. Iron-only and vanadium nitrogenases: fail-safe enzymes or something more? Annu Rev Microbiol 74:247–266. doi: 10.1146/annurev-micro-022620-014338 [DOI] [PubMed] [Google Scholar]
- 11. Hu Y, Ribbe MW. 2015. Nitrogenase and homologs. J Biol Inorg Chem 20:435–445. doi: 10.1007/s00775-014-1225-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu Y, Ribbe MW. 2016. Biosynthesis of the metalloclusters of nitrogenases. Annu Rev Biochem 85:455–483. doi: 10.1146/annurev-biochem-060614-034108 [DOI] [PubMed] [Google Scholar]
- 13. Waugh SI, Paulsen DM, Mylona PV, Maynard RH, Premakumar R, Bishop PE. 1995. The genes encoding the delta subunits of dinitrogenases 2 and 3 are required for mo-independent diazotrophic growth by Azotobacter vinelandii. J Bacteriol 177:1505–1510. doi: 10.1128/jb.177.6.1505-1510.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris DF, Lukoyanov DA, Kallas H, Trncik C, Yang ZY, Compton P, Kelleher N, Einsle O, Dean DR, Hoffman BM, Seefeldt LC. 2019. Mo-, V-, and Fe-nitrogenases use a universal eight-electron reductive-elimination mechanism to achieve N2 reduction. Biochemistry 58:3293–3301. doi: 10.1021/acs.biochem.9b00468 [DOI] [PubMed] [Google Scholar]
- 15. Eady RR, Robson RL, Richardson TH, Miller RW, Hawkins M. 1987. The vanadium nitrogenase of Azotobacter chroococcum. Purification and properties of the VFe protein. Biochem J 244:197–207. doi: 10.1042/bj2440197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hamilton TL, Ludwig M, Dixon R, Boyd ES, Dos Santos PC, Setubal JC, Bryant DA, Dean DR, Peters JW. 2011. Transcriptional profiling of nitrogen fixation in Azotobacter vinelandii. J Bacteriol 193:4477–4486. doi: 10.1128/JB.05099-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luque F, Pau RN. 1991. Transcriptional regulation by metals of structural genes for Azotobacter vinelandii nitrogenases. Molec Gen Genet 227:481–487. doi: 10.1007/BF00273941 [DOI] [PubMed] [Google Scholar]
- 18. Premakumar R, Pau RN, Mitchenall LA, Easo M, Bishop PE. 1998. Regulation of the transcriptional activators AnfA and VnfA by metals and ammonium in Azotobacter vinelandii . FEMS Microbiol Lett 164:63–68. doi: 10.1111/j.1574-6968.1998.tb13068.x [DOI] [PubMed] [Google Scholar]
- 19. McKinlay J. B., Harwood CS. 2010. Photobiological production of hydrogen gas as a biofuel. Curr Opin Biotechnol 21:244–251. doi: 10.1016/j.copbio.2010.02.012 [DOI] [PubMed] [Google Scholar]
- 20. McKinlay JB, Harwood CS, Newman DK. 2011. Calvin cycle flux, pathway constraints, and substrate oxidation state together determine the H2 biofuel yield in photoheterotrophic bacteria. mBio 2:e00323-10. doi: 10.1128/mBio.00323-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Postgate JR. 1982. Biological nitrogen-fixation - fundamentals. Phil Trans R Soc Lond B 296:375–385. doi: 10.1098/rstb.1982.0013 [DOI] [Google Scholar]
- 22. Dilworth MJ, Eady RR, Eldridge ME. 1988. The vanadium nitrogenase of Azotobacter chroococcum reduction of acetylene and ethylene to ethane. Biochem J 249:745–751. doi: 10.1042/bj2490745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu Y, Lee CC, Ribbe MW. 2011. Extending the carbon chain: hydrocarbon formation catalyzed by vanadium/molybdenum nitrogenases. Science 333:753–755. doi: 10.1126/science.1206883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seefeldt LC, Yang ZY, Duval S, Dean DR. 1827. Nitrogenase reduction of carbon-containing compounds. Biochim Biophys Acta 1827:1102–1111. doi: 10.1016/j.bbabio.2013.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zheng Y, Harris DF, Yu Z, Fu Y, Poudel S, Ledbetter RN, Fixen KR, Yang ZY, Boyd ES, Lidstrom ME, Seefeldt LC, Harwood CS. 2018. A pathway for biological methane production using bacterial iron-only nitrogenase. Nat Microbiol 3:281–286. doi: 10.1038/s41564-017-0091-5 [DOI] [PubMed] [Google Scholar]
- 26. Mus F, Alleman AB, Pence N, Seefeldt LC, Peters JW. 2018. Exploring the alternatives of biological nitrogen fixation. Metallomics 10:523–538. doi: 10.1039/c8mt00038g [DOI] [PubMed] [Google Scholar]
- 27. Mus F, Colman DR, Peters JW, Boyd ES. 2019. Geobiological feedbacks, oxygen, and the evolution of nitrogenase. Free Radic Biol Med 140:250–259. doi: 10.1016/j.freeradbiomed.2019.01.050 [DOI] [PubMed] [Google Scholar]
- 28. Kessler PS, Leigh JA. 1999. Genetics of nitrogen regulation in Methanococcus maripaludis. Genetics 152:1343–1351. doi: 10.1093/genetics/152.4.1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kessler PS, McLarnan J, Leigh JA. 1997. Nitrogenase phylogeny and the molybdenum dependence of nitrogen fixation in Methanococcus maripaludis. J Bacteriol 179:541–543. doi: 10.1128/jb.179.2.541-543.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thauer RK, Kaster AK, Seedorf H, Buckel W, Hedderich R. 2008. Methanogenic archaea: ecologically relevant differences in energy conservation. Nat Rev Microbiol 6:579–591. doi: 10.1038/nrmicro1931 [DOI] [PubMed] [Google Scholar]
- 31. Hendrickson EL, Kaul R, Zhou Y, Bovee D, Chapman P, Chung J, Conway de Macario E, Dodsworth JA, Gillett W, Graham DE, Hackett M, Haydock AK, Kang A, Land ML, Levy R, Lie TJ, Major TA, Moore BC, Porat I, Palmeiri A, Rouse G, Saenphimmachak C, Söll D, Van Dien S, Wang T, Whitman WB, Xia Q, Zhang Y, Larimer FW, Olson MV, Leigh JA. 2004. Complete genome sequence of the genetically tractable hydrogenotrophic methanogen Methanococcus maripaludis. J Bacteriol 186:6956–6969. doi: 10.1128/JB.186.20.6956-6969.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ehlers C, Veit K, Gottschalk G, Schmitz RA. 2002. Functional organization of a single nif cluster in the mesophilic archaeon Methanosarcina mazei strain Go1. Archaea 1:143–150. doi: 10.1155/2002/362813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chien YT, Auerbuch V, Brabban AD, Zinder SH. 2000. Analysis of genes encoding an alternative nitrogenase in the archaeon Methanosarcina barkeri 227. J Bacteriol 182:3247–3253. doi: 10.1128/JB.182.11.3247-3253.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lobo AL, Zinder SH. 1988. Diazotrophy and nitrogenase activity in the archaebacterium Methanosarcina barkeri 227. Appl Environ Microbiol 54:1656–1661. doi: 10.1128/aem.54.7.1656-1661.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lobo A.L. 1990. Nitrogenase in the archaebacterium Methanosarcina barkeri 227. J Bacteriol 172:6789–6796. doi: 10.1128/jb.172.12.6789-6796.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leigh JA. 2000. Nitrogen fixation in methanogens: the archaeal perspective, current issues in molecular biology. Curr Issues Mol Biol 2:125–131. [PubMed] [Google Scholar]
- 37. Leigh JA, Dodsworth JA. 2007. Nitrogen regulation in bacteria and archaea. Annu Rev Microbiol 61:349–377. doi: 10.1146/annurev.micro.61.080706.093409 [DOI] [PubMed] [Google Scholar]
- 38. Prasse D, Förstner KU, Jäger D, Backofen R, Schmitz RA. 2017. sRNA154 a newly identified regulator of nitrogen fixation in Methanosarcina mazei strain Go1. RNA Biol 14:1544–1558. doi: 10.1080/15476286.2017.1306170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Buddeweg A, Sharma K, Urlaub H, Schmitz RA. 2018. sRNA41 affects ribosome binding sites within polycistronic mRNAs in Methanosarcina mazei Go1. Mol Microbiol 107:595–609. doi: 10.1111/mmi.13900 [DOI] [PubMed] [Google Scholar]
- 40. Galagan JE, Nusbaum C, Roy A, Endrizzi MG, Macdonald P, FitzHugh W, Calvo S, Engels R, Smirnov S, Atnoor D, Brown A, Allen N, Naylor J, Stange-Thomann N, DeArellano K, Johnson R, Linton L, McEwan P, McKernan K, Talamas J, Tirrell A, Ye W, Zimmer A, Barber RD, Cann I, Graham DE, Grahame DA, Guss AM, Hedderich R, Ingram-Smith C, Kuettner HC, Krzycki JA, Leigh JA, Li W, Liu J, Mukhopadhyay B, Reeve JN, Smith K, Springer TA, Umayam LA, White O, White RH, Conway de Macario E, Ferry JG, Jarrell KF, Jing H, Macario AJL, Paulsen I, Pritchett M, Sowers KR, Swanson RV, Zinder SH, Lander E, Metcalf WW, Birren B. 2002. The genome of M. acetivorans reveals extensive metabolic and physiological diversity. Genome Res 12:532–542. doi: 10.1101/gr.223902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nayak DD, Metcalf WW. 2017. Cas9-mediated genome editing in the methanogenic archaeon Methanosarcina acetivorans Proc Natl Acad Sci U S A 114:2976–2981. doi: 10.1073/pnas.1618596114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guss AM, Rother M, Zhang JK, Kulkarni G, Metcalf WW. 2008. New methods for tightly regulated gene expression and highly efficient chromosomal integration of cloned genes for Methanosarcina species. Archaea 2:193–203. doi: 10.1155/2008/534081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dhamad AE, Lessner DJ. 2020. A CRISPRi-dCas9 system for archaea and its use to examine gene function during nitrogen fixation by Methanosarcina acetivorans. Appl Environ Microbiol 86:e01402-20. doi: 10.1128/AEM.01402-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kessler PS, Blank C, Leigh JA. 1998. The nif gene operon of the methanogenic Archaeon Methanococcus maripaludis. J Bacteriol 180:1504–1511. doi: 10.1128/JB.180.6.1504-1511.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chien YT, Zinder SH. 1996. Cloning, functional organization, transcript studies, and phylogenetic analysis of the complete nitrogenase structural genes (nifHDK2) and associated genes in the archaeon Methanosarcina Barkeri 227. J Bacteriol 178:143–148. doi: 10.1128/jb.178.1.143-148.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kessler PS, Daniel C, Leigh JA. 2001. Ammonia switch-off of nitrogen fixation in the methanogenic archaeon Methanococcus maripaludis: mechanistic features and requirement for the novel GlnB homologues, NifI(1) and NifI(2). J Bacteriol 183:882–889. doi: 10.1128/JB.183.3.882-889.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sicking C, Brusch M, Lindackers A, Riedel KU, Schubert B, Isakovic N, Krall C, Klipp W, Drepper T, Schneider K, Masepohl B. 2005. Identification of two new genes involved in diazotrophic growth via the alternative fe-only nitrogenase in the phototrophic purple bacterium Rhodobacter capsulatus. J Bacteriol 187:92–98. doi: 10.1128/JB.187.1.92-98.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Varghese F, Kabasakal BV, Cotton CAR, Schumacher J, Rutherford AW, Fantuzzi A, Murray JW. 2019. A low-potential terminal oxidase associated with the iron-only nitrogenase from the nitrogen-fixing bacterium Azotobacter vinelandii. J Biol Chem 294:9367–9376. doi: 10.1074/jbc.RA118.007285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pérez-González A, Jimenez-Vicente E, Salinero-Lanzarote A, Harris DF, Seefeldt LC, Dean DR. 2022. AnfO controls fidelity of nitrogenase FeFe protein maturation by preventing misincorporation of FeV-cofactor. Mol Microbiol 117:1080–1088. doi: 10.1111/mmi.14890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ferry JG. 1999. Enzymology of one-carbon metabolism in methanogenic pathways. FEMS Microbiol Rev 23:13–38. doi: 10.1111/j.1574-6976.1999.tb00390.x [DOI] [PubMed] [Google Scholar]
- 51. Lie TJ, Leigh JA. 2002. Regulatory response of Methanococcus maripaludis to alanine, an intermediate nitrogen source. J Bacteriol 184:5301–5306. doi: 10.1128/JB.184.19.5301-5306.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Oda Y, Samanta SK, Rey FE, Wu L, Liu X, Yan T, Zhou J, Harwood CS. 2005. Functional genomic analysis of three nitrogenase isozymes in the photosynthetic bacterium Rhodopseudomonas palustris. J Bacteriol 187:7784–7794. doi: 10.1128/JB.187.22.7784-7794.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lie TJ, Dodsworth JA, Nickle DC, Leigh JA. 2007. Diverse Homologues of the archaeal repressor NrpR function similarly in nitrogen regulation. FEMS Microbiol Lett 271:281–288. doi: 10.1111/j.1574-6968.2007.00726.x [DOI] [PubMed] [Google Scholar]
- 54. Weidenbach K, Ehlers C, Schmitz RA. 2014. The transcriptional activator NrpA is crucial for inducing nitrogen fixation in Methanosarcina mazei Go1 under nitrogen-limited conditions. FEBS J 281:3507–3522. doi: 10.1111/febs.12876 [DOI] [PubMed] [Google Scholar]
- 55. Demtröder L, Narberhaus F, Masepohl B. 2019. Coordinated regulation of nitrogen fixation and molybdate transport by molybdenum. Mol Microbiol 111:17–30. doi: 10.1111/mmi.14152 [DOI] [PubMed] [Google Scholar]
- 56. Studholme DJ, Pau RN. 2003. A DNA element recognised by the molybdenum-responsive transcription factor mode is conserved in proteobacteria, green sulphur bacteria and archaea. BMC Microbiol 3:24. doi: 10.1186/1471-2180-3-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ferry JG. 2010. How to make a living by exhaling methane. Annu Rev Microbiol 64:453–473. doi: 10.1146/annurev.micro.112408.134051 [DOI] [PubMed] [Google Scholar]
- 58. Rangaraj P, Shah VK, Ludden PW. 1997. Aponifh functions in iron-molybdenum cofactor synthesis and apodinitrogenase maturation. Proc Natl Acad Sci U S A 94:11250–11255. doi: 10.1073/pnas.94.21.11250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sowers KR, Boone JE, Gunsalus RP. 1993. Disaggregation of Methanosarcina spp. and growth as single cells at elevated osmolarity. Appl Environ Microbiol 59:3832–3839. doi: 10.1128/aem.59.11.3832-3839.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Metcalf WW, Zhang JK, Apolinario E, Sowers KR, Wolfe RS. 1997. A genetic system for archaea of the genus Methanosarcina: liposome-mediated transformation and construction of shuttle vectors. Proc Natl Acad Sci U S A 94:2626–2631. doi: 10.1073/pnas.94.6.2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental tables and figures.
Data Availability Statement
The raw data from growth studies and qPCR will be available upon request.