Abstract
The integrity of 3′-ends of tRNAs is essential for aminoacylation and consequently for protein synthesis. The CCA-termini are generated and, if truncated by exonucleolytic activity, restored by tRNA nucleotidyltransferase. However, further truncations at the 3′-end can occur by exonuclease activity or during processing of overlapping tRNA primary transcripts in metazoan mitochondria. In the latter case, the upstream tRNA is released in a 3′-truncated form (lacking up to six bases) and subsequently completed. In human mitochondria, tRNATyr (missing the discriminator nucleotide A73) is completed by a discriminator adding activity followed by CCA addition. Since in vivo a high percentage of further 3′-terminally degraded human tRNATyr transcripts could be observed, it was tested in an in vitro system whether this repair mechanism for tRNA 3′-ends acts also on these further degraded tRNA versions. Additionally, 3′-truncated versions of two non-overlapping mitochondrial tRNAs (tRNAThr and tRNAPhe) were examined. The results show that these transcripts can be repaired during incubation. A similar base incorporating activity was observed in mouse mitochondria, indicating that a repair mechanism for the 3′-end of several tRNAs exists in mitochondria of humans and possibly other metazoans which goes beyond the CCA addition.
INTRODUCTION
In the mitochondrial genome of many metazoans, several tRNA genes lying on one strand and sharing 1–6 nt are described (1–3). The sequences of these genes are therefore overlapping. In the processing of the corresponding primary transcripts, the downstream located tRNA is liberated as a complete molecule carrying the overlapping region at the 5′-end, while the upstream tRNA is released in a 3′-truncated form. In the subsequent completion of this tRNA polyadenylation may be involved in some cases (1,2), and for the human mitochondrial tRNATyr a discriminator adding activity has been described (4). To test whether the described discriminator addition represents a specialized RNA editing reaction for the completion of processing products of overlapping tRNA precursors, or a more general repair mechanism which acts also on tRNA molecules carrying additional deletions at the 3′-ends, further truncated versions of tRNATyr similar to those found in vivo and truncated run-off transcripts of two non-overlapping mitochondrial tRNA genes (tRNAPhe and tRNAThr) were examined in an in vitro system.
MATERIALS AND METHODS
PCR and preparation of substrate RNA
Templates for in vitro transcription were prepared by PCR amplification (standard conditions) of the corresponding mitochondrial genes using the following primers synthesized on an ABI DNA/RNA synthesizer:
RT/PCR1 (circularized tRNATyr): 5′-ATGCTTCACTCAGCCATT-3′
RT/PCR2 (circularized tRNATyr): 5′-AATCTAAAGACAGGGGTT-3′
T7 (Tyr): 5′-CTAATACGACTCACTATAGGTAAAATGGCTGAG-3′
Downstream (Tyr): 5′-TGGTAAAAAGAGGCT*TAA-3′ (*diagnostic mutation)
Downstream (Tyr-1): 5′-GGTAAAAAGAGGCT*TAA-3′ (*diagnostic mutation)
Downstream (Tyr-2): 5′-GTAAAAAGAGGCT*TAA-3′ (*diagnostic mutation)
Downstream (Tyr-4): 5′-AAAAAGAGGCT*TAA-3′ (*diagnostic mutation)
T7 (chimpPhe): 5′-CTAATACGACTCACTATAGTTTATGTAGCTTACC-3′
Downstream (chimpPhe): 5′-TGTTTATGGGGTGATGTAAACC-3′
Downstream (chimpPhe-1): 5′-GTTTATGGGGTGATGTAAACC-3′
T7 (chimpThr): 5′-CTAATACGACTCACTATAGCCCTTGTAGTATAAAC-3′
Downstream (chimpThr): 5′-TGTCCTTGGAAGAAAGTTTTCG-3′
Downstream (chimpThr-1): 5′-GTCCTTGGAAGAAAGTTTTCG-3′
Downstream (chimpThr-2): 5′-TCCTTGGAAGAAAGTTTTCG-3′
Transcription was carried out according to the manufacturer (New England Biolabs, Schwalbach, Germany). Radioactively labeled transcripts or reaction products were separated by denaturing polyacrylamide gel electrophoresis and purified as described by Peattie (5). Dephosphorylation using calf intestinal alkaline phosphatase and 5′-end-labeling using polynucleotide kinase and [γ-33P]ATP (Amersham Pharmacia Biotech, Freiburg, Germany) were carried out according to the manufacturer (New England Biolabs).
Preparation of mitochondrial S100 extract
Mitochondria were prepared from frozen HeLa or FM3A cells (Computer Cell Culture Center, Mons, Belgium) by differential centrifugation and sucrose step gradient ultracentrifugation (6,7). The S100 protein extract was prepared and stored at –80°C as described by Reichert et al. (4) and had a concentration of 3.5–5 mg/ml.
The purity of the mitochondrial preparation was confirmed by northern blot analyses for nuclear (U6) and cytoplasmic (tRNALeu) marker RNA molecules and showed no detectable hybridization signals in the mitochondrial preparations, but strong signals in nuclear and cytoplasmic fractions, respectively. Furthermore, the activity of lactate dehydrogenase as a marker enzyme for cytoplasm was analyzed. The mitochondrial fraction contained 5.6% specific activity in comparison to the cytoplasmic fraction, a result that indicates (in addition to the northern blot experiments) a high purity of the mitochondrial preparations.
In vitro assay for tRNA repair
1–10 pmol 5′-33P-labeled tRNA substrate were incubated with 3.5–5 µg of mitochondrial protein extract (HeLa or FM3A cells) in the presence of 1 mM NTPs (Amersham Pharmacia Biotech), 30 mM HEPES–KOH (pH 7.6), 6 mM MgCl2, 30 mM KCl, 2 mM DTT and 30 U RNase inhibitor (Roche Molecular Biochemicals, Mannheim, Germany) in a total volume of 20 µl for 6 h at 30°C. The products were ethanol precipitated and separated by electrophoresis on a 10% polyacrylamide gel containing 8 M urea and visualized by autoradiography. The correct 3′-ends of the transcripts were confirmed for each experiment by 3′-sequence analysis of the isolated RNA after incubation without protein extract (data not shown).
3′-sequence analysis
Ligation of isolated tRNAs to an agarose gel purified DNA EcoRI-restriction fragment with T4 RNA ligase and subsequent cDNA synthesis with M-MuLV Reverse Transcriptase (New England Biolabs) was performed as described by Hetzer and Mueller (8). Alternatively, the tRNAs were circularized by 5′- and 3′-end ligation (1) and converted into cDNA. cDNA solution (5 µl) was used for 100 µl standard PCR reactions. PCR products were cloned using a TA cloning kit according to the manufacturer (Invitrogen, Groningen, The Netherlands). Chain termination sequencing of individual clones was carried out on an ALFexpress sequencer (Amersham Pharmacia Biotech).
RESULTS
Repair of further truncated versions of tRNATyr
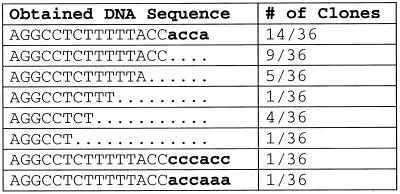
In order to determine the nature of the 3′-ends of tRNATyr in vivo as a product of an overlapping human mitochondrial gene, total mitochondrial tRNA was isolated from HeLa cells, circularized, and the tRNATyr fraction was amplified by RT–PCR and cloned. Of 36 sequenced clones, 39% (14 of 36) represented the complete tRNA carrying the discriminator base and the CCA terminus (A73C74C75A76), while 25% (9 of 36) were identical to the processing product and lacked the discriminator position and the CCA end (Table 1) (4). Interestingly, 31% (11 of 36) were further truncated at the 3′-end, while 6% (2 of 36) showed misincorporations and addition of extra nucleotides. The possibility of PCR artefacts being responsible for this misincorporation is very unlikely, since none of the other clones showed any variation at this or other positions of the amplified tRNA sequence. Furthermore, PCR errors lead predominantly to base exchanges, but do not explain the observed extra bases at the tRNA 3′-terminus. In addition, the results do not represent a degradation of the tRNA preparation, since another tRNA (tRNACys) which was analyzed as a control, carried complete 5′- and 3′-ends and showed no truncations (17 of 17 clones, data not shown).
Table 1. Sequence analysis of acceptor stem 3′ part of in vivo mitochondrial tRNATyr.
Bold, lower case characters, incorporated nucleotides; dots, missing nucleotides in cleavage products and further truncated molecules.
Overlapping regions in tRNA genes in other metazoans lead to similar 3′-deletions in the released upstream tRNAs, which are subjected to RNA editing (1–3). To investigate whether the found truncated transcripts can be restored to full-length tRNA, a protein extract (S100) prepared from isolated mitochondria from HeLa cells was incubated with 33P-5′-end-labeled run-off transcripts of mitochondrial tRNATyr-2 (missing C72A73, Fig. 1B) and tRNATyr-4 (missing A70C71C72A73, Fig. 1B) in the presence of all four NTPs. In order to get more information about the type and extent of base incorporation, the elongation of tRNATyr-1 (described in 4) was studied in more detail. All transcripts carried diagnostic mutations (based on chimpanzee mitochondrial tRNA genes) for discrimination between substrate and endogenous tRNA of the S100 extract. The reaction products were separated on a denaturing 10% polyacrylamide gel and visualized by autoradiography (Fig. 1A).
Figure 1.
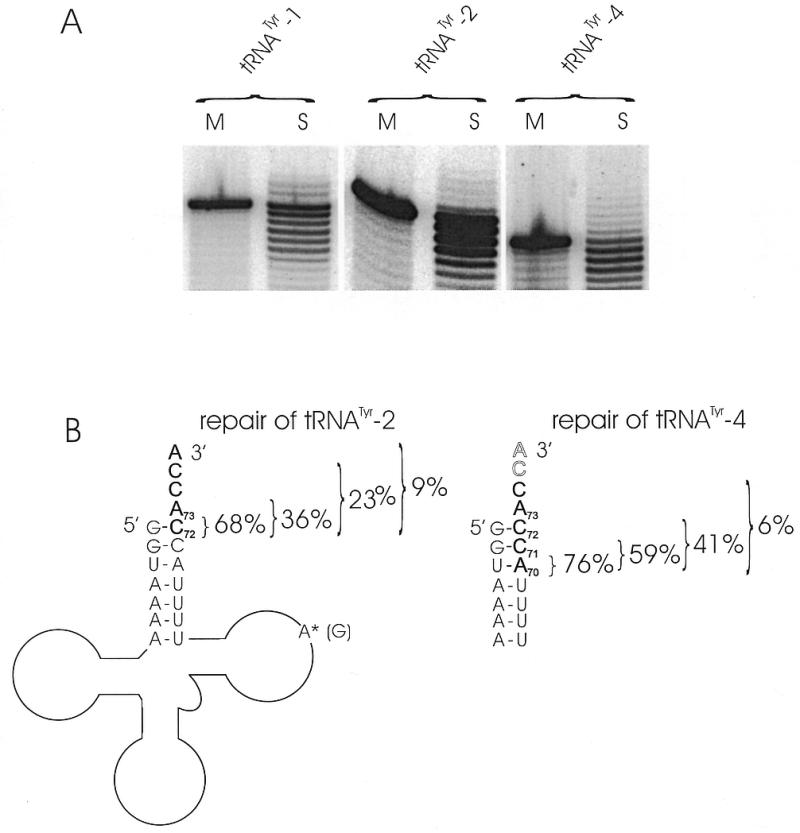
In vitro base incorporation in 3′-truncated versions of tRNATyr. (A) Incubation of the transcripts with nucleotides in the presence (S) or absence (M) of S100 protein extract. All offered transcripts are accepted as substrates and elongated for several bases, leading to a reduced electrophoretic mobility of the products. (B) Secondary structures of tRNATyr-2 and tRNATyr-4. The asterisk indicates the diagnostic base of the substrate tRNAs, while the human residue at that position is indicated in brackets. The correctly incorporated bases are numbered and drawn in bold characters, the percentage values indicate the fidelity. In the case of tRNATyr-4, only partial addition of the CCA terminus was observed.
All tRNA versions (tRNATyr-1, tRNATyr-2 and tRNATyr-4) were accepted as substrates and showed incorporation of several nucleotides, while a considerable amount of RNA was further degraded (Fig. 1A). An explanation for this background degradation might be the presence of nucleases in the crude mitochondrial S100 extract and is not unusual for such an in vitro system. In the cases of tRNATyr-2 and tRNATyr-4 the intensity of the bands decreases with the number of incorporated nucleotides, indicating a slow elongation process that is not completed after 6 h. This was confirmed by time course experiments which showed an increasing intensity of shifted bands with increasing incubation time (data not shown).
In order to resolve the nature of the incorporated nucleotides, the 3′-terminal sequences of the reaction products were determined. For the tRNATyr-1 transcript the previously published results (4) were verified: 9 of 58 analyzed clones (16%) contained the correct discriminator A73, whereas incorporation of CMP at this position was observed in 32 of 58 clones (55%), of UMP in 4 of 58 clones (7%) and of GMP in 1 of 58 clones (2%). This is consistent with assays of tRNATyr-1 in the presence of only one type of NTP, which are incorporated at different efficiencies (CMP > AMP > UMP > GMP, data not shown). In addition, in 12 of 58 clones tRNATyr-1 were further truncated during incubation by 1–2 nt and subsequently elongated—a reaction that could be detected due to the misincorporation of bases (Table 2).
Table 2. Sequence analysis of tRNATyr, tRNAPhe, tRNAThr and after base incorporation.
Incorporated nucleotides are shown in bold, lower case characters.
In the case of tRNATyr-2 (Fig. 1B, left), 68% (15 of 22 clones) had incorporated the correct nucleotide C at position 72, while 27% (6 of 22 clones) carried a U residue at that position. Eight of the clones (36%) carried additionally the correct discriminator base A73, whereas in four clones (18%) incorporation of C at this position was observed. Furthermore, two clones were repaired to a tRNA molecule containing the correct nucleotides C72A73 and a partial (23%) or complete CCA terminus (9%), while one clone showed further 3′-truncation with subsequent polyadenylation.
In the analysis of the tRNATyr-4 reaction products (Fig. 1B, right) incorporation of the correct bases at position 70 (A70) were detected in 76% (13 of 17 clones), at positions 70 and 71 (A70C71) in 59% (10 of 17 clones) and at position 70–72 (A70C71C72) in 41% (7 of 17 clones). One of them (6%) contained additionally the correct discriminator A73 and the first base of the CCA terminus. Similar to one of the tRNATyr-2 clones, two tRNATyr-4 sequences showed polyadenylation. Taken together, these results indicate that tRNATyr transcripts lacking several nucleotides at the 3′-end besides the CCA terminus can be repaired in human mitochondria.
3′-truncated transcripts of tRNAPhe and tRNAThr can be repaired in vitro
To investigate whether the repair activity is restricted to tRNATyr (as the processing product of an overlapping tRNA precursor molecule) or whether it also acts on other truncated, non-overlapping tRNAs, transcripts of mitochondrial tRNAPhe and tRNAPhe-1 (missing the discriminator nucleotide A73, Fig. 2) were tested. In order to differentiate between endogenous tRNAs of the human mitochondrial extract and substrate tRNAs, transcripts based on chimpanzee mitochondrial tRNA genes were used. tRNAPhe was elongated for 3 nt indicating a highly active tRNA nucleotidyltransferase in the extract, while tRNAPhe-1 was extended by 4 nt as expected for incorporation of the discriminator base in addition to the CCA-terminus (Fig. 2A). This indicates that the discriminator adding activity (4) can also use a tRNA other than tRNATyr as a substrate. The 3′-end analysis of the reaction products of tRNAPhe-1 revealed that 78% carried the correct discriminator base A (seven of nine clones), while 11% (one of nine clones) incorporated CMP or UMP (one of nine clones) at that position. Additionally, all clones showed complete or partial addition of the CCA-terminus (Fig. 2B and Table 2), demonstrating that a truncated tRNAPhe-1 transcript can be restored to a full-length tRNA in vitro.
Figure 2.

Repair of tRNAPhe-1. (A) 33P-5′-end-labeled in vitro transcripts representing tRNAPhe and tRNAPhe-1 were incubated in the presence of human S100 mitochondrial protein extract and nucleotides, leading to base incorporation and shifted product bands migrating at identical positions (S). In parallel, mock incubation in the absence of protein was performed (M). For determination of the nature of the incorporated nucleotides, the shifted bands were isolated and their 3′-terminal sequence determined (Table 2). (B) Schematic drawing of tRNAPhe-1 carrying incorporated bases (bold characters). Seventy-eight percent of the analyzed clones showed the correct base A73 added at the discriminator position. Additionally, all sequences carried either the complete or partial CCA terminus. Diagnostic bases (chimpanzee Pan troglodytes sequence) are indicated by asterisks, the nucleotides in the human transcript are shown in brackets.
Furthermore, two 3′-truncated versions of another tRNA (tRNAThr-1, lacking A73, and tRNAThr-2, lacking C72A73) as well as the complete tRNAThr (with A73) were tested (Fig. 3A). All transcripts were extended to the same length. While the main reaction product of tRNAThr was elongated by 3 nt as expected for CCA addition, an additional signal indicating the incorporation of 4 nt could be observed. In the case of tRNAThr-1 and tRNAThr-2, up to 5 or 6 nt, respectively, were incorporated, showing that these tRNAs were accepted as substrates as well.
Figure 3.
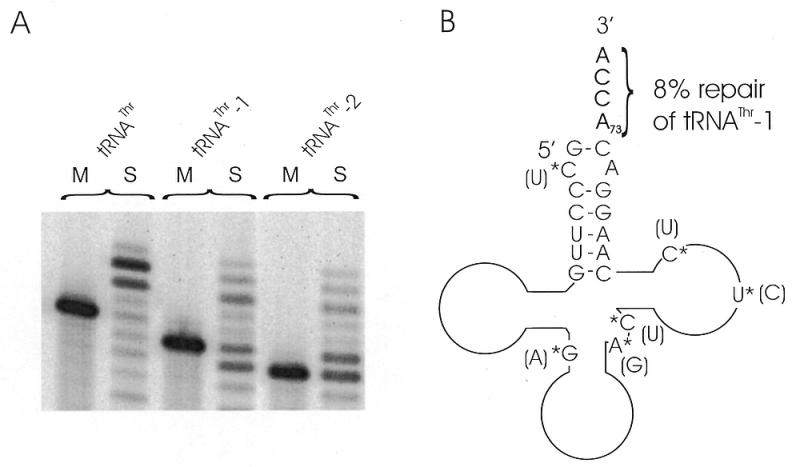
In vitro base incorporation in tRNAThr-1 and tRNAThr-2. (A) In the presence of S100 extract and NTPs (S) the tRNAThr as well as the truncated versions were extended to the same length, as indicated by identical migration properties. M, mock incubation without S100 extract. (B) Inferred secondary structure of tRNAThr-1 showing a low discriminator (A73) repair fidelity of 8%. Diagnostic bases are indicated by asterisks, the original bases in the human tRNAThr are shown in brackets.
To identify the incorporated bases, the product bands of tRNAThr-1 were analyzed. In 1 of 13 clones (8%) the tRNA was completed to a full-length molecule containing the correct discriminator A73 and the CCA-terminus (Fig. 3B). In contrast, all other clones showed misincorporation of C at the discriminator position and of C and A in varying sequence after that nucleotide (Table 2). Furthermore, three clones carried in addition to the CCA-end an extra C residue at the 3′-end. These clones represent probably the observed extra band in Figure 3A. The predominant misincorporation of C is similar to that observed for the elongation of tRNATyr-1 (50–55%) (4, and this article) but might here be promoted by the unusual structure in the acceptor stem (mismatch C2< >A71) of the chimpanzee tRNAThr, which might also explain the additionally incorporated nucleotide mentioned above. Further experiments are necessary to address this. Taken together, one can conclude that besides tRNATyr and tRNAPhe another tRNA (tRNAThr) can be repaired, although to a rather low extent.
tRNA repair in other species?
To test whether the repair activity is found in other metazoan mitochondria (to our knowledge all analyzed so far carry overlapping tRNA genes), 5′-end-labeled in vitro transcripts of tRNAPhe and tRNAPhe-1 were incubated in the presence of a mitochondrial protein extract from mouse FM3A cells. The incorporation of 3 nt in tRNAPhe was observed as the main reaction product (as expected for CCA addition). For the truncated transcript tRNAPhe-1 a product of the same size as the complete tRNAPhe was visible corresponding to the addition of the discriminator nucleotide and the CCA-terminus (data not shown). These data imply that in mouse mitochondria a 3′-truncated tRNA can also be completed.
DISCUSSION
The observation that in human mitochondria, 31% of the analyzed tRNATyr sequences show deletions at the 3′-terminus led to the question whether these transcripts can be restored by a base incorporation activity that is believed to complete tRNAs of overlapping genes in mitochondria. The results presented indicate that this seems to be the case: several tRNAs, lacking the discriminator nucleotide A73 and other nucleotides in the acceptor stem, are completed at varying fidelity (up to 78% for tRNAPhe) in an in vitro system. This is probably dependent on the structure of the substrate tRNAs. However, one important discrepancy to the in vivo situation (only 6% of tRNATyr show anomalous base additions) is the high rate of CMP misincorporation. A possible explanation is that the tRNA nucleotidyltransferase supplies the nucleotide adding activity as CMP and AMP were the predominantly incorporated nucleotides. Several arguments favor this idea although only tRNAs containing a discriminator base are thought to be substrates for this enzyme and heterogeneity in the CCA sequence has not been observed in vivo (9–11). It was reported that the tRNA nucleotidyltransferase has poly(C)-polymerase activity (12,13) and that in the presence of solely CTP tRNA-N-C-C is converted to tRNA-N-C-C-C (9). Furthermore, the Km values for the Escherichia coli enzyme (0.2 mM for CTP, 0.6 mM for ATP and 8.8 mM for UTP) (9) support the incorporation efficiency of CMP > AMP > UMP at the discriminator position of tRNATyr-1 (Table 2 and data not shown). Additionally, a yeast mitochondrial protein extract extends tRNATyr-1 by 3 nt (presumably CCA) rather than 4 (as observed with the human extract), indicating that tRNA nucleotidyltransferase accepts a truncated tRNA as a substrate at least in vitro (4).
Therefore, it seems possible that the mitochondrial tRNA nucleotidyltransferase is responsible for the addition of nucleotides at the discriminator position (and other bases of the acceptor stem) of a truncated tRNA. However, it is also conceivable that discriminator nucleotide and CCA terminus are incorporated by separate activities. Some of the sequencing results presented in Table 2 indicate that polyadenylation might be involved, something that has been suggested also on the basis of sequences of tRNAs in land snail and chicken mitochondria (1,14). Nevertheless, incorporation of A residues could only account for the addition of the discriminator nucleotide in the tested tRNAs but not for the observed repair of further truncated transcripts since in this case CMP as well as AMP was incorporated. This resembles the tRNA editing event observed in platypus mitochondria, where the 3′-part of the acceptor stem of tRNASer is edited towards 5′-C70C71C72A73-3′ prior to the addition of the CCA-end (2). Since in the experiments described above UMP and GMP were also incorporated (although at low levels), it is possible that these nucleotides are used as well in repair. Furthermore, since A and C residues are frequently found at position 73 in tRNAs (60 and 15%, respectively) (15), the repair activity is likely to have a rather broad substrate range. It is not possible so far to decide whether one or several simultaneously acting activities are responsible for the repair of the three investigated tRNAs. However, the fact that a nucleotide adding activity is essential in vivo to complete released 3′-truncated tRNAs of overlapping precursor molecules indicates that the base incorporating activity is not an in vitro artifact, but has biological significance. This is further supported by the observation that a minihelix transcript consisting of the acceptor and the T stem of tRNATyr including the discriminator A73 is elongated by 3 nt (presumably CCA), while a version lacking the discriminator base (minihelix-1) is not extended in the in vitro system (data not shown). Therefore, the repair activity does not elongate all RNA primers but rather seems to be specific for tRNA molecules.
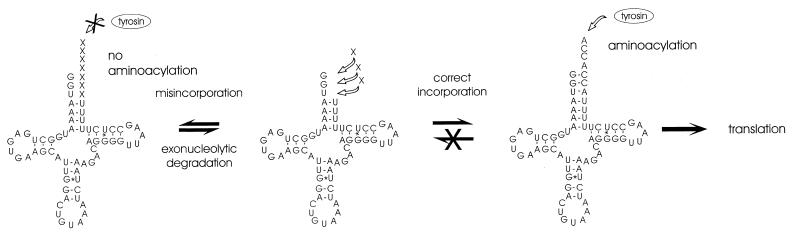
The dynamics of tRNA 3′-ends in vivo involve a steady turnover by degradation and elongation, while biologically active 3′-ends are protected against exonucleolytic activity by aminoacylation (10,16,17). Therefore, it is tempting to speculate that the aminoacylation of the completed tRNAs has an influence on the repair reaction (Fig. 4). If nucleotide misincorporation occurs, the tRNA is probably not recognized and charged by the appropriate aminoacyl–tRNA synthetase and remains therefore unprotected. However, after removal of the misincorporated base(s), the tRNA has a second chance to be completed accurately. The correct incorporation of nucleotides has two consequences. First, aminoacylation becomes possible and the charged tRNA can participate in translation and is therefore selectively removed from equilibrium. Second, such tRNAs are protected from exonucleolytic degradation. Such a dynamic polymerization/degradation model requires neither high fidelity nor template dependence of nucleotide incorporation. Nevertheless, the in vitro base incorporation shows percentage values of accurate repair that indicate an error-prone but non-random mechanism. A process with this error rate and the selective force for stabilizing correctly completed (repaired) tRNAs by aminoacylation might lead to the very low percentage of misincorporation observed in vivo. Since the in vitro system does not include amino acids and therefore cannot produce charged tRNA molecules, no selection for functional tRNAs (carrying the correct nucleotide sequence) can occur. Hence, repaired tRNAs are not protected from degradation by aminoacylation and might explain (in addition to a possible higher error rate in vitro) the observed variety of 3′-end sequences. However, it has to be tested whether the presence of the corresponding amino acid allows aminoacylation and leads to a change in the accuracy of base incorporation in the in vitro system.
Figure 4.
Dynamic model of tRNA repair. tRNATyr-4 is presented as an example. The 3′-end of the tRNA is subjected to a permanent turnover by elongation and degradation. The incorporation of the correct sequence leading to aminoacylation of the tRNA has two consequences (right). First, the molecule becomes protected against exonucleolytic degradation. Second, translation removes this tRNA from the equilibrium, leading thereby to an increase of repaired tRNA molecules in the pool. In contrast, misincorporation of nucleotides at the truncated 3′-end impedes aminoacylation of the tRNA, which is therefore not protected against exonucleolytic activity (left). After removal of the (incorrect) 3′-terminal nucleotides, the tRNA has a second chance to be elongated correctly.
Addition of the 3′-terminal CCA sequence by tRNA nucleotidyltransferase is essential for all organisms that do not encode the CCA terminus in tRNA genes (all eukaryotes, many eubacteria and some archaea; 9,18). Since deletion or mutation of the nucleotidyltransferase gene leads to growth defects and reduced fitness in E.coli (16,19) and Saccharomyces cerevisiae (20), it is conceivable that the extended repair of further truncated tRNA 3′-ends is a considerable contribution to the fitness of an organism. This is probably very valuable for organisms that do not encode the CCA terminus in their tRNA genes: Before CCA addition occurs, the 3′-terminus of the acceptor stem of a eukaryotic tRNA could be degraded, leading to the inactivation of the molecule, while in certain prokaryotes the 3′-end is ‘protected’ by the encoded CCA-end that can be reconstituted by tRNA nucleotidyl transferase. The repair mechanism described here might protect immature eukaryotic tRNAs lacking the CCA-end in a similar way and therefore compensate for the fact that the CCA terminus is not encoded in the corresponding genes.
During the condensation of the mitochondrial genome (21), an ancestral tRNA repair mechanism may have allowed the evolution of overlapping tRNA genes. Therefore, editing of truncated processing products might represent just one aspect of tRNA repair. Possibly both the increased fitness of an organism (due to tRNA repair) and this editing reaction act as a selective force for the evolutionary preservation of the repair function. Whether it is restricted to mitochondrial systems carrying overlapping tRNA genes or takes place in other mitochondria or compartments as well (with the exception of yeast; 4), remains to be clarified. The observation of tRNA repair in human mitochondria suggests that this reaction is of general functional importance rather than a specialized mechanism for completing truncated tRNAs resulting from overlapping primary transcripts. Furthermore, the existence of a similar activity in mouse mitochondria indicates that tRNA repair might be common to all metazoan mitochondria.
Acknowledgments
ACKNOWLEDGEMENTS
We are especially grateful to S. Pääbo for discussions and would like to thank M. Dörner, A. Greenwood, U. Rothbauer, W. Schaffner and M. Stoneking for valuable suggestions and critical reading of the manuscript. Furthermore, we thank S. El-Gogo and M. Kimm for experimental assistance. This work was financially supported by the Deutsche Forschungsgemeinschaft (Mo 634).
REFERENCES
- 1.Yokobori S.-I. and Pääbo,S. (1995) Proc. Natl Acad. Sci. USA, 92, 10432–10435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yokobori S.-I. and Pääbo,S. (1995) Nature, 377, 490. [DOI] [PubMed] [Google Scholar]
- 3.Tomita K., Ueda,T. and Watanabe,K. (1996) Nucleic Acids Res., 24, 4987–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reichert A., Rothbauer,U. and Mörl,M. (1998) J. Biol. Chem., 273, 31977–31984. [DOI] [PubMed] [Google Scholar]
- 5.Peattie D.A. (1979) Proc. Natl Acad. Sci. USA, 76, 1760–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tapper D.P., Van Etten,R.A. and Clayton,D.A. (1983) Methods Enzymol., 97, 426–434. [DOI] [PubMed] [Google Scholar]
- 7.Rossmanith W., Tullo,A., Potuschak,T., Karwan,R. and Sbisa,E. (1995) J. Biol. Chem., 270, 12885–12891. [DOI] [PubMed] [Google Scholar]
- 8.Hetzer M. and Mueller,M.W. (1993) Nucleic Acids Res., 21, 5526–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sprinzl M. and Cramer,F. (1979) Prog. Nucleic Acids Res. Mol. Biol., 22, 2–69. [DOI] [PubMed] [Google Scholar]
- 10.Deutscher M.P. (1990) Prog. Nucleic Acids Res. Mol. Biol., 39, 209–240. [DOI] [PubMed] [Google Scholar]
- 11.Yue D., Maizels,N. and Weiner,A.M. (1996) RNA, 2, 895–908. [PMC free article] [PubMed] [Google Scholar]
- 12.Best A.N. and Novelli,G.D. (1971) Arch. Biochem. Biophys., 142, 539–547. [DOI] [PubMed] [Google Scholar]
- 13.Deutscher M.P. (1972) J. Biol. Chem., 247, 469–480. [PubMed] [Google Scholar]
- 14.Yokobori S.-I. and Pääbo,S. (1997) J. Mol. Biol., 265, 95–99. [DOI] [PubMed] [Google Scholar]
- 15.Sprinzl M., Horn,C., Brown,M., Ioudovitch,A. and Steinberg,S. (1998) Nucleic Acids Res., 26, 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deutscher M.P., Lin,J.J. and Evans,J.A. (1977) J. Mol. Biol., 117, 1081–1094. [DOI] [PubMed] [Google Scholar]
- 17.Solari A., Gatica,M. and Allende,J.E. (1977) Nucleic Acids Res., 4, 1873–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deutscher M.P. (1982) in Jacob,S.T. (ed.), Enzymes of Nucleic Acid Synthesis and Modification. CRC Press, Boca Raton, FL, pp. 159–183.
- 19.Zhu L. and Deutscher,M.P. (1987) EMBO J., 6, 2473–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aebi M., Kirchner,G., Chen,J.-Y., Vijayraghavan,U., Jacobson,A., Martin,N.C. and Abelson,J. (1990) J. Biol. Chem., 265, 16216–16220. [PubMed] [Google Scholar]
- 21.Gray M.W., Burger,G. and Lang,B.F. (1999) Science, 283, 1476–1481. [DOI] [PubMed] [Google Scholar]