Abstract
Introduction
Interleukin-4 (IL-4) and interleukin-13 (IL-13) are two essential cytokines involved in the T helper 2 (Th2)-mediated inflammatory response to diseases, such as atopic dermatitis (AD). AK120 is a humanized immunoglobulin G subclass 4 (IgG4) monoclonal antibody (mAb) directed against the IL-4 receptor alpha (IL-4Rα) subunit shared by the IL-4 and IL-13 receptor complexes. This mAb inhibits the signaling of the IL-4 and IL-13 cytokines.
Methods
The study consisted of two parts. Part 1 was a single ascending dose (SAD) study with five cohorts (receiving 15, 50, 150, 300 or 600 mg of AK120, respectively) of healthy subjects; part 2 was a multiple ascending dose (MAD) study with four cohorts (receiving AK120 at doses of 300 mg once every 2 weeks [Q2W], 300 mg once weekly [QW], 150 mg QW or 75 mg QW) of subjects with AD. A total of 81 subjects (40 in part 1, 41 in part 2) were enrolled in the study.
Results
The compound was safe and well tolerated in both a SAD up to 600 mg in healthy subjects and in a MAD from 75 to 600 mg in subjects with AD. The exposure of AK120 increased in an approximately dose-dependent manner upon subcutaneous dosing. The levels of the biomarkers serum thymus and activation-regulated chemokine ligand 17 (TARC/CCL17) and immunoglobulin E decreased from baseline after AK120 administration, indicating the inhibition of the IL-4/IL-13 signaling pathways. AK120 showed improved Eczema Area and Severity Index (EASI) scores, and the proportion of subjects with Investigator Global Assessment (IGA) score 0/1 increased after AK120 treatment.
Conclusions
AK120 exhibited an acceptable safety profile in healthy and AD subjects, and showed preliminary efficacy. These findings support the continued investigation of AK120 for treating AD.
Clinical Trial Registration
ClinicalTrials.gov identification number: NCT04256174.
Keywords: Interleukin-4, Interleukin-13, Atopic dermatitis, Monoclonal antibody, Clinical study, First-in-human
Key Summary Points
| Why carry out the study? |
| AK120 is a novel monoclonal antibody targeting interleukin 4 receptor alpha (IL-4Rα). The aim of this study was to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics and clinical preliminary efficacy of AK120 in both healthy subjects and subjects with moderate-to-severe atopic dermatitis (AD). |
| What was learned from the study? |
| AK120 was found to be safe and well tolerated in both healthy and AD subjects. There was no significant difference between the AK120 and placebo arms in terms of the incidence of treatment-emergent adverse events (TEAEs) and treatment-related TEAEs. There were no deaths nor treatment-related serious adverse events among the study cohorts. |
| The study provides preliminary efficacy results in the treatment of patients with AK120 by achieving improved Eczema Area and Severity Index (EASI) and Investigator Global Assessment (IGA) scores, which could be a reference for future studies of AK120. |
| This study provides the first evidence from a study involving humans that AK120 is safe and well tolerated in both healthy and AD subjects |
Introduction
Atopic dermatitis (AD) is a chronic type 2 inflammatory skin disease characterized by pruritus, erythematous and eczematous lesions [1, 2]. Factors such as epidermal gene mutation, skin barrier dysfunction and immune dysregulation play critical roles in the development of AD [3]. Epidemiology studies show that AD is most common in children and adolescents, with a reported prevalence ranging up to 26% in the age group 6–11 years [4] and up to 24.6% in the age group (13–14 years [5]. It was reported that around 10% of adults have been diagnosed with AD [6], which negatively affects the quality-of-life (QoL) of these patients and their families. A previous study carried out in the USA found that AD was always associated with worse results of QoL compared to other common chronic diseases, such as heart disease, diabetes and high blood pressure [7]. Children with AD often suffer from intense pruritus, skin lesions involving a large body surface area, sleep deprivation, depression and poor school performance [8]. In addition, AD also poses a significant economic burden to patients due to its relapsing feature. In the Global Burden of Disease Study 2019, AD was ranked 28th in 369 diseases [9]. Mild AD can be adequately controlled by topical corticosteroid therapies, but moderate-to-severe AD, which accounts for nearly one third of all AD cases in children [10], often requires systemic treatment. Current systemic therapy for moderate-to-severe AD mainly includes nonspecific anti-inflammatory agents such as corticosteroids to prevent exacerbation and improve epidermal function. However, systemic corticosteroid therapy is usually not recommended for children due to the risk of rebound after short-term treatment and long-term toxic effects [11]. Targeted biological treatment is a strategy for the local treatment of refractory AD patients with fewer side effects [12, 13].
The pathogenesis of AD involves dysregulation of systemic T helper 2 (Th2) cells, with increased immunoglobulin E (IgE) levels and eosinophilia. It is widely accepted that infiltration of group 2 innate lymphoid cells (ILC2s) in AD lesions promotes the response of Th2 cells and increases the Th2-associated cytokine release of interleukin (IL)-4 and IL-13 [14]. IL-4 and IL-13 play an important role in the Th2-mediated inflammatory response. IL-4 drives the differentiation of Th2 cells and IgE production in B cells [15], and IL-13 participates in the maturation and differentiation of B-cells and eosinophil chemotaxis [15]. These two cytokines also downregulate the expression of essential genes involved in skin barrier function and integrity [14]. In addition, increased levels of IL-4 and IL-13 hamper the production of antimicrobial peptides in response to bacterial and viral stimuli, thus increasing the risk of Staphylococcus aureus infection [16]. Therefore, inhibiting the IL-4/IL-13 pathway may prevent or reverse the development of AD. The critical roles of IL-4/IL-13 make them ideal targets for the development of novel therapies with higher specificity and less toxicity compared with systemic corticosteroid therapy.
AK120 is a novel humanized immunoglobulin G subclass 4 (IgG4) monoclonal antibody (mAb) targeting the IL-4 receptor alpha subunit (IL-4Rα), a component shared by both the IL-4 and IL-13 receptors, thereby blocking the pathways mediated by both cytokines. AK120 is expected to selectively inhibit the Th2 pathway, consequently preventing or reversing the development of AD. Currently, AK120 is also being developed to treat asthma, eosinophilic esophagitis and chronic rhinosinusitis, all of which share the same underlying pathology of type 2 inflammatory skin disease. Here we report the results of a phase I, first-in-human (FIH) study that we carried out to assess the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of AK120 in both healthy and AD subjects.
Methods
Study Design
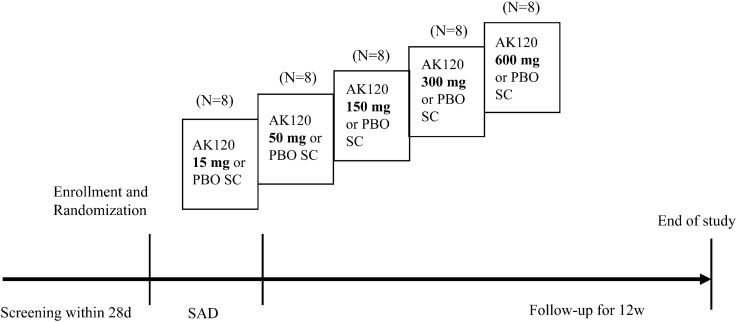
The study consisted of two parts. Part 1 was a phase I, randomized, double-blind, placebo-controlled study with the aim to evaluate the safety, tolerability, PK and PD of AK120 following the administration of a single subcutaneous (SC) dose to healthy subjects. This part of the study consisted of five sequential dose escalation cohort (15, 50, 150, 300 or 600 mg of AK120). A total of 40 subjects were planned to be enrolled in this study, with eight subjects in each cohort (N = 8). A step-wise single-dose escalation plan was adopted. The first cohort of subjects received the lowest single dose of 15 mg of AK120 or placebo subcutaneously; escalated dose levels were 50, 150, 300 and 600 mg of AK120 or placebo for cohorts 2, 3, 4 and 5 respectively. Eligible subjects were randomized and assigned to receive a single dose of AK120 or matching placebo on day 1. After dosing, the subjects were required to remain in the study center for 48 h for safety monitoring, continuous cardiac monitoring and PK assessments. The subjects were discharged from the study center on day 3. All subjects were followed-up to week 12 for safety, tolerability and PK/PD assessment (Fig. 1).
Fig. 1.
Overall study design (part 1). Healthy subjects were screened for 28 days, and those assessed to be eligible were enrolled and randomized (AK120:placebo = 3:1). AK120 humanized immunoglobulin G subclass 4 monoclonal antibody, d day, PBO placebo, SAD single ascending dose, SC subcutaneous, w week
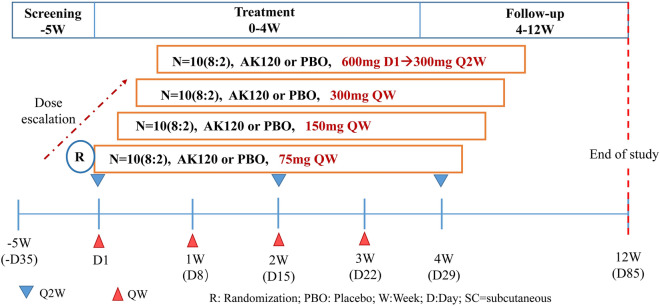
Part 2 of the study was a 12-week, sequential ascending, repeated-dose study with the aim to evaluate the safety, tolerability, PK, PD and preliminary efficacy of AK120 in subjects with moderate-to-severe AD. The treatment period was 4 weeks in duration; subjects were followed for 8 weeks after the end of the treatment period. There were four sequential dose escalation cohorts, with approximately ten subjects randomized in a 4:1 ratio to receive multiple SC doses (total of 4 doses weekly) of either the active drug AK120 (N = 8) or matching placebo (N = 2) in each cohort (Fig. 2). Dose escalation to subsequent cohorts only continued if (1) the Safety Review Committee (SRC) reviewed the available safety data at least up to day 29 and available PK data at least through to day 25 and (2) the previous dose level was considered to be safe and well tolerated. Cohorts 1, 2 and 3 received a SC injection of 75, 150 and 300 mg AK120 or matching placebo; cohort 4 received a SC loading dose of 600 mg of AK120 or a matching placebo on day 1, followed by a dose of 300 mg of AK120 or a matching placebo every 2 weeks (Q2W) thereafter.
Fig. 2.
Overall study design (part 2). Subjects with AD were screened for 35 days and those deemed eligible were enrolled and randomized (AK120:placebo = 4:1). AD Atopic dermatitis, Q2W every 2 weeks, QW once weekly
The study was designed and conducted according to guidelines of Good Clinical Practice (GCP), the current Declaration of Helsinki and National Medical Product Administration (NMPA). The protocol of this study was approved by the ethics committee. An informed consent document approved by independent ethics committee was signed by the subjects or their legally authorized representative before the participant entered in the study. This trial is registered on ClinicalTrials.gov (NCT04256174).
Subjects
Eligible subjects for the study were men and women aged ≥ 18–55 years who were willing and able to comply with the requirements for clinic visits and study-related procedures. In part 1, all subjects were healthy, as determined by medical history, physical examination, 12-lead electrocardiogram (ECG) and clinical laboratory tests. Patients with moderate to severe AD were enrolled in part 2.
Objective and Endpoints
The primary objective of the study was to evaluate the safety and tolerability of single/multiple SC dose of AK120 in both healthy subjects and subjects with moderate-to-severe AD. The secondary objectives were to assess the PK and PD of AK120 in healthy and AD subjects and to evaluate the clinical preliminary efficacy of AK120 following the administration of multiple SC doses to subjects with AD.
The primary endpoints of this study were (1) the incidence of treatment-emergent adverse events (TEAEs) and serious adverse events (SAEs) and (2) changes in laboratory results, ECGs and vital signs from baseline. The secondary endpoints were (1) serum AK120 concentrations at different time points after administration, (2) PK and PD parameters and (3) detectable anti-drug antibody (ADA), (4) the proportion of subjects achieving ≥ 50%/75% improvement in the Eczema Area and Severity Index (EASI 50/EASI 75) from baseline and (5) the proportion of subjects with Investigator Global Assessment (IGA) ranging from 0 to 1 and IGA reduction from baseline of ≥ 2 points.
Safety Analysis
Safety of AK120 was monitored through the recording of TEAEs/SAEs, physical examinations, clinical laboratory tests (hematology, biochemistry and urinalysis), vital sign assessments, standard 12-lead ECG recordings and local injection-site reactions. All TEAEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 23.0. The number and percentage of TEAEs/SAEs were tabulated by preferred term (PT) with a breakdown by treatment group. Deaths and other significant AEs/SAEs, including those leading to discontinuation of the study, were summarized and listed separately.
PK Analysis
Blood samples were collected at baseline (pre-dose), at 2 h, 8 h post administration and on days 1, 2, 3, 6, 8, 11, 15, 22, 29, 43, 57, 71 and 85 post administration (15 time points in total) to measure the concentration of functional AK120 in serum. PK parameters were determined based on non-compartmental analysis, including maximum observed concentration (Cmax), time to Cmax (Tmax), area under the curve from 0 to the time of the last quantifiable concentration (AUC0-t) and terminal elimination half-life (T1/2). Phoenix® WinNonlin® software (version 8.2; Certara, Princeton, NJ, USA) was used to estimate the PK parameters.
PD Analysis
Blood samples were collected at baseline (day -1) and days 2, 8, 15, 29, 57 and 85 post administration (a total of 7 time points) to measure the concentration of thymus and activation-regulated chemokine (TARC/CCL17) and immunoglobulin E (IgE). Serum TARC/CCL17 was measured using and a validated electrochemiluminescent immunoassay, and IgE was measured in serum samples using a validated enzyme-linked immunosorbent assay (ELISA) with a human IgE ELISA kit (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA; catalog no.: BMS2097TEN). Comparisons between post-baseline evaluations and baseline within each dosage cohort and comparisons between the dosage groups and placebo group were performed.
Efficacy Analysis
The efficacy analyses were performed using the safety analysis set. All continuous efficacy variables were summarized by summary statistics by treatment group. The categorical efficacy variables were summarized by number and percentage of subjects for each category. Data from subjects receiving placebo were pooled across cohorts for analysis.
Results
Subject Characteristics
In part 1 of this study, 40 healthy subjects were enrolled and allocated to five treatment groups (N = 30) and the placebo group (N = 10). All cohorts were well balanced with respect to baseline characteristics. Most of subjects in the study were white: up to 83.3% (25/30) in the AK120 treatment group and 90.0% in the placebo group (9/10). Mean age was 27.9 (± standard deviation [SD] 9.2) years for the AK120 group and 33.3 (± 7.8) years for the placebo group; mean body mass index (BMI) was 23.7 (± 2.8) kg/m2 for the AK120 group and 25.4 (± 3.6) kg/m2 for the placebo group. In the second part of the study, 41 subjects with AD were enrolled and allotted to four treatment groups (N = 32) and the placebo group (N = 9). The mean EASI score of subjects in the AK120 treatment group and placebo group was 33.0 (± 14.5) and 32.7 (± 11.9), respectively; 65.6% (21/32) of the subjects in the AK120 group and 55.6% (5/9) in the placebo group had IGA score 3; 28.1% (9/32) of the subjects in the AK120 group and 44.4%(4/9) in the placebo group had IGA score 4; and 6.3% (2/32) of the subjects in the AK120 group had IGA score 5. The demographics and characteristics of the subjects are shown in Table 1.
Table 1.
Baseline demographic and disease characteristics of subjects
| Category | Part 1 (healthy subjects) | Part 2 (subjects with moderate-to-severe AD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AK120 15 mg (N = 6) |
AK120 50 mg (N = 6) |
AK120 150 mg (N = 6) |
AK120 300 mg (N = 6) |
AK120 600 mg (N = 6) |
AK120 Total (N = 30) |
Placebo (N = 10) |
AK120 75 mg QW (N = 8) |
AK120 150 mg QW (N = 8) |
AK120 300 mg QW (N = 8) |
AK120 300 mg Q2W (N = 8) |
AK120 Total (N = 32) |
Placebo (N = 9) |
|
| Age (years), mean (SD) | 30.8 (13.5) | 24.8 (8.7) | 26.2 (6.2) | 32.2 (11.2) | 25.5 (3.3) | 27.9 (9.2) | 33.3 (7.8) | 28.6 (9.26) | 39.4 (13.94) | 33.8 (15.04) | 33.8 (11.88) | 33.9 (12.69) | 24.3 (6.48) |
| Male sex, n (%) | 1 (16.7) | 2 (33.3) | 1 (16.7) | 1 (16.7) | 1 (16.7) | 6 (20.0) | 2 (20.0) | 5 (62.5) | 5 (62.5) | 2 (25.0) | 5 (62.5) | 17 (53.1) | 4 (44.4) |
| Race, n (%) | |||||||||||||
| White | 5 (83.3) | 6 (100.0) | 4 (66.7) | 5 (83.3) | 5 (83.3) | 25 (83.3) | 9 (90.0) | 5 (62.5) | 7 (87.5) | 5 (62.5) | 5 (62.5) | 22 (68.8) | 3 (33.3) |
| Asian | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 1 (3.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (25.0) |
2 (6.3) |
4 (44.4) |
| Native Hawaiian or other Pacific Islander | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 1 (12.5) | 0 (0.0) |
2 (6.3) |
0 (0.0) |
| Other | 1 (16.7) | 0 (0.0) | 2 (33.3) | 0 (0.0) | 1 (16.7) | 4 (13.3) | 1 (10.0) | 3 (37.5) | 0 (0.0) | 2 (25.0) | 1 (12.5) |
6 (18.8) |
2 (22.2) |
| BMI (kg/m2), mean (SD) | 21.9 (1.8) | 23.6 (1.8) | 24.0 (4.2) | 23.1 (2.5) | 25.7 (2.5) | 23.7 (2.8) | 25.4 (3.6) | 24.8 (5.9) | 29.7 (5.6) | 29.5 (5.7) | 27.7 (4.1) |
27.9 (5.5) |
22.2 (3.5) |
| EASI score, mean (SD) | NA | 38.7 (14.6) | 21.9 (9.4) | 31.1 (8.4) | 40.4 (17.7) |
33.0 (14.5) |
32.7 (11.9) | ||||||
| IGA score, n (%) | |||||||||||||
| 3 | NA | 5 (62.5) | 7 (87.5) | 5 (62.5) | 4 (50.0) |
21 (65.6) |
5 (55.6) | ||||||
| 4 | NA | 3 (37.5) | 1 (12.5) | 2 (25.0) | 3 (37.5) |
9 (28.1) |
4 (44.4) | ||||||
| 5 | NA | 0 (0.0) | 0 (0.0) | 1 (12.5) | 1 (12.5) |
2 (6.3) |
0 (0.0) | ||||||
| BSA affected, mean (SD) | NA | 78.5 (19.8) | 51.1 (24.8) | 56.9 (18.1) | 75.0 (16.0) |
65.4 (22.3) |
55.2 (24.9) | ||||||
| Average P-NRS score, mean (SD) | NA | 5.8 (1.8) | 6.3 (1.9) | 6.4 (1.3) | 7.3 (1.7) |
6.5 (1.7) |
5.8 (1.6) | ||||||
AD Atopic dermatitis, BMI body mass index, BSA body surface area, EASI Eczema Area and Severity Index, IGA Investigator Global Assessment, NA not applicable, P-NRS Pruritus-Numeric Rating Scale, QW once weekly, Q2W every 2 weeks, SD standard deviation
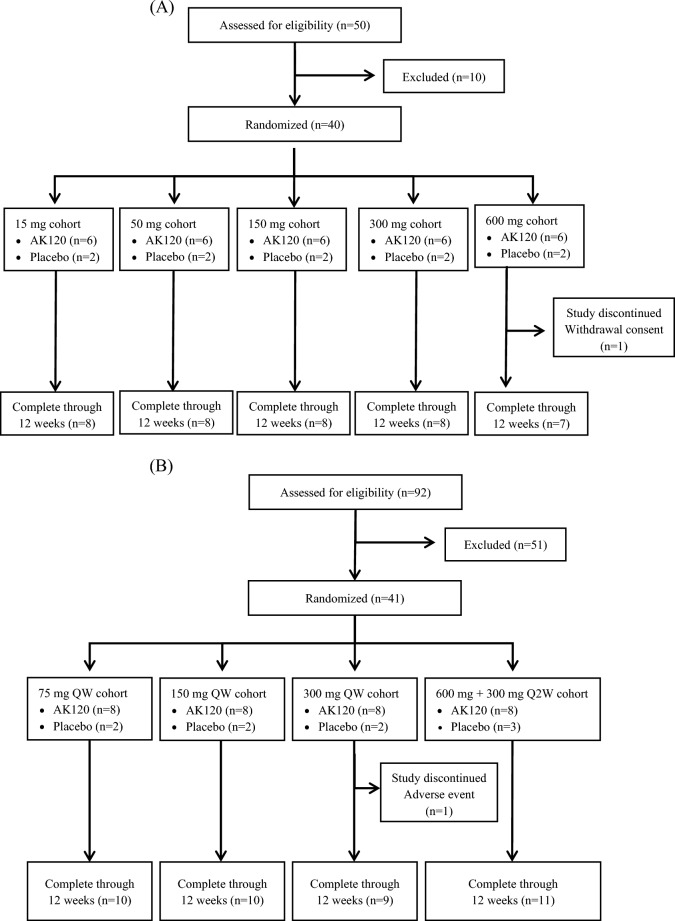
In Part 1 of the study (Fig. 3a), 50 subjects were screened and 40 subjects were ultimately enrolled in the five cohorts and randomized to receive either AK120 or placebo at the ratio of 6:2. A total of 30 subjects received AK120 and ten subjects received placebo; 39 subjects completed all the study visits; there was one study discontinuation due to withdrawal of consent with personal reasons given instead of any AE. A total of 92 subjects were screened in part 2 of the study (Fig. 3b), of whom 41 were randomized in the study in total; 51 subjects were discontinued prior to randomization, with the majority of these (50 subjects) not meeting the eligibility criteria; one subject discontinued before randomization due to being informed by the sponsor that the study was fully recruited.
Fig. 3.
CONSORT flow diagram of patient disposition through week 12 showing patient enrollment, randomization and discontinuation. The primary reasons for discontinuation are listed. a Diagram of part 1 (healthy subjects), b diagram of part 2 (subjects with moderate-to-severe AD)
Safety and Tolerability of AK120
In the first part of the study, the overall incidence of TEAEs in the AK120 treatment groups and placebo group was 86.7% (26/30) and 90.0% (9/10), respectively; the difference was not significant. The incidence of TEAEs was similar across the five cohorts of the AK120 group, indicating that AK120 at a dose up to 600 mg was well tolerated by healthy subjects. The majority of the TEAEs which did occur were mild (50% [20/40]) in severity; moderate TEAEs accounted for 35% (14/40) of all TEAEs. The only SAE that was reported occurred in one subject receiving 50 mg of AK120; this was a case of appendicitis and was not considered to be treatment-related by the investigator. The most common TEAEs (incidence ≥ 5%) in the AK120 treatment groups were upper respiratory tract infection (33.3%), headache (30.0%), abdominal pain (13.3%), migraine (10.0%), mouth ulceration (6.7%), catheter site phlebitis (6.7%) and limb injury (6.7%). The incidence of injection-related reaction was 6.7% (2/30) and 10% (1/10) in the AK120 treatment groups and placebo group, respectively; the difference was not significant. No death or TEAE leading to discontinuation of the study was reported. Analysis of vital sign findings did not reveal any clinically relevant effect of AK120 treatment. Although some abnormal laboratory values and ECG assessments were reported, there were no clinically significant changes in the subjects of all cohorts (Table 2).
Table 2.
Summary of overall adverse events
| Category | Part 1 (healthy subjects) | Part 2 (subjects with moderate-to-severe AD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AK120 15 mg (N = 6) |
AK120 50 mg (N = 6) |
AK120 150 mg (N = 6) |
AK120 300 mg (N = 6) |
AK120 600 mg (N = 6) |
AK120 Total (N = 30) |
Placebo (N = 10) |
AK120 75 mg, QW (N = 8) |
AK120 150 mg, QW (N = 8) |
AK120 300 mg, QW (N = 8) |
AK120 300 mg, Q2W (N = 8) |
AK120 Total (N = 32) |
Placebo (N = 9) |
|
| Subjects with any TEAE | 5 (83.3) | 4 (66.7) | 6 (100.0) | 5 (83.3) | 6 (100.0) | 26 (86.7) | 9 (90.0) | 8 (100.0) | 6 (75.0) | 8 (100.0) | 6 (75.0) | 28 (87.5) | 7 (77.8) |
| Mild | 4 (66.7) | 2 (33.3) | 5 (83.3) | 1 (16.7) | 3 (50.0) | 15 (50.0) | 5 (50.0) | 6 (75.0) | 4 (50.0) | 2 (25.0) | 1 (12.5) | 13 (40.6) | 1 (11.1) |
| Moderate | 1 (16.7) | 1 (16.7) | 1 (16.7) | 4 (66.7) | 3 (50.0) | 10 (33.3) | 4 (40.0) | 2 (25.0) | 2 (25.0) | 6 (75.0) | 5 (62.5) | 15 (46.9) | 6 (66.7) |
| Severe | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Subjects with any treatment-related TEAEs | 1 (16.7) | 2 (33.3) | 1 (16.7) | 4 (66.7) | 5 (83.3) | 13 (43.3) | 2 (20.0) | 2 (25.0) | 4 (50.0) | 3 (37.5) | 3 (37.5) | 12 (37.5) | 4 (44.4) |
| Mild | 1 (16.7) | 2 (33.3) | 1 (16.7) | 3 (50.0) | 4 (66.7) | 11 (36.7) | 2 (20.0) | 1 (12.5) | 4 (50.0) | 0 (0.0) | 2 (25.0) | 7 (21.9) | 2 (22.2) |
| Moderate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (16.7) | 2 (6.7) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 3 (37.5) | 1 (12.5) | 5 (15.6) | 2 (22.2) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Subjects with any SAEs | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| TEAEs leading to study drug injection interruption | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 1 (3.1) | 1 (11.1) |
| TEAEs leading to study permanent discontinuation | NA | NA | NA | NA | NA | NA | NA | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (11.1) |
| TEAEs reported with an incidence ≥ 10% | |||||||||||||
| Upper respiratory tract infection | 2 (33.3) | 1 (16.7) | 2 (33.3) | 3 (50.0) | 2 (33.3) | 10 (33.3) | 4 (40.0) | 0 (0.0) | 1 (12.5) | 1 (12.5) | 1 (12.5) | 3 (9.4) | 3 (33.3) |
| Headache | 4 (66.7) | 0 (0.0) | 2 (33.3) | 1 (16.7) | 2 (33.3) | 9 (30.0) | 6 (60.0) | 0 (0.0) | 1 (12.5) | 1 (12.5) | 1 (12.5) | 3 (9.4) | 1 (11.1) |
| Abdominal pain | 1 (16.7) | 0 (0.0) | 1 (16.7) | 1 (16.7) | 1 (16.7) | 4 (13.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 1 (3.1) | 1 (11.1) |
| Migraine | 0 (0.0) | 1 (16.7) | 1 (16.7) | 1 (16.7) | 0 (0.0) | 3 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Oral herpes | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (3.3) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Injection site bruising | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dermatitis contact | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Muscle strain | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (10.0) | ||||||
| Epistaxis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (11.1) |
| Back pain | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Muscle spasms | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Lymphadenopathy | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.1) | 1 (11.1) |
| Dermatitis contact | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Erythema | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Rash | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Tooth extraction | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Skin infection | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 1 (12.5) | 2 (25.0) | 4 (12.5) | 0 (0.0) |
| Cellulitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (11.1) |
| Pruritus | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 1 (12.5) | 1 (12.5) | 0 (0.0) | 3 (9.4) | 1 (11.1) |
| Acne | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (11.1) |
| Rash erythematous | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (11.1) |
| Toothache | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 1 (3.1) | 1 (11.1) |
| Rhinitis allergic | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 2 (6.3) | 1 (11.1) |
| Injection site pain | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 1 (12.5) | 1 (12.5) | 1 (12.5) | 4 (12.5) | 1 (11.1) |
| Insomnia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 1 (12.5) | 1 (12.5) | 3 (9.4) | 1 (11.1) |
| Blood triglycerides increased | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 1 (3.1) | 1 (11.1) |
| Blood lactate dehydrogenase increased | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (11.1) |
| Liver function test increased | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (11.1) |
| Dry eye | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (11.1) |
| Hypersensitivity | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (11.1) |
Values in table are presented as the number with the percentage in parentheses
NA Not applicable, SAE serious adverse event, TEAE treatment-emergent adverse event
In the second part of the study, 28 (87.5%) and seven (77.8%) subjects experienced at least one TEAE in the AK120 treatment groups and placebo group, respectively. The number of TEAEs reported in the AK120 dosage groups of 75 mg QW, 150 mg QW, 300 mg QW and 300 mg Q2W were eight (100.0%), six (75.0%), eight (100.0%) and six (75.0%), respectively. There were no dose-related increases in TEAEs reported in the subjects in the active treatment groups. All TEAEs were mild and moderate in severity. The most common TEAEs occurring in the AK120 treatment groups (total) were skin infection (12.5%, 4/32) and injection site pain (12.5%, 4/32). In the placebo group, the most common TEAEs were upper respiratory tract infection (33.3%, 3/9). There were 12 (37.5%) subjects who experienced at least one treatment-related TEAE in the AK120 treatment group (total), and four (44.4%) subjects in the placebo group. One (11.1%) subject had hypersensitivity that ended in permanent study discontinuation in the placebo group; this TEAE was assessed as moderate by the investigator. No SAE, treatment-related SAE or adverse events of special interest (AESIs) were reported (Table 2).
PK and PD Results of AK120
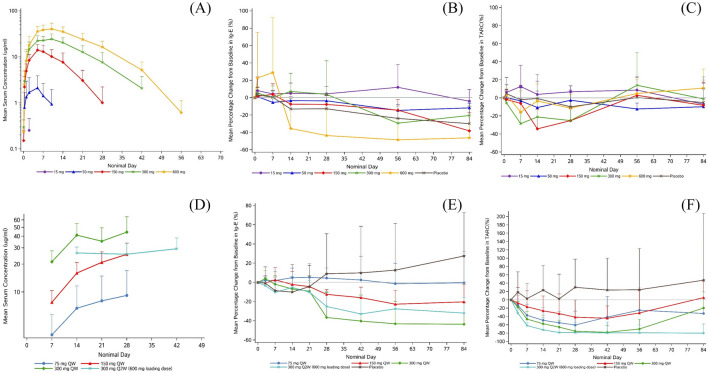
In the first part of the study, following a single SC dose of AK120 in healthy subjects, the median time to Cmax (Tmax) ranged from 120 to 239 h in the 50 mg-600 mg groups. The mean AUC0-t observed in the 15 mg, 50 mg, 150 mg, 300 mg and 600 mg dosage groups was 10.773, 390.583, 4446.3, 12,260.7, and 21,744.2 h µg/ml, respectively; and the mean Cmax was 0.251, 2.126, 14.042, 25.811 and 41.896 ug/ml, respectively. AUC0-t and Cmax increased in an approximately dose proportional manner in the dose range of 150–600 mg. Mean T½ ranged from 101 to 153 h in the 150–600 mg groups (Fig. 4a). After a single SC injection of AK120 in healthy subjects, serum thymus and activation regulated chemokine ligand 17 (TARC/CCL17) level significantly decreased in the AK120 150–600 mg groups compared with baseline, whereas there were no significant changes in AK120 15–50 mg groups and placebo group (Fig. 4c). The total serum IgE level in AK120 600 mg group decreased significantly with a minimum average value of 73.03 IU/ml in 12 weeks. A similar trend was not observed in other dosage groups (Fig. 4B).
Fig. 4.
Pharmacokinetics and pharmacodynamics. a–c Part 1 PK. a Arithmetic mean (± SD) serum concentration—time profile in healthy subjects following a single subcutaneous doses of AK120, b IgE, c TARC/CCL17. d–e Part 2 PK. d Arithmetic mean (± SD) serum trough concentration-time profiles in patients with AD following multiple doses of AK120, e IgE, f SD standard deviation, TARC/CCL17. TARC/CCL17 thymus and activation-regulated chemokine ligand 17
In the second part of the study, the AD patients received SC 75 mg QW, 150 mg QW, 300 mg QW or 300 Q2W (with 600 mg loading dose on day 1) AK120. The average serum concentration (Ctrough,sd) at 168 h after the first dose of AK120 in the QW dosage groups (75 mg QW, 150 mg QW, 300 mg QW) was 3.446, 7.744 and 21.196 ug/ml, respectively. In the 300 mg Q2W dosage group, the average serum concentration was 26.396 ug/ml at 336 h after first administration. After multiple doses. the average trough-concentration (Ctrough,md) in the 75 mg QW, 150 mg QW, 300 mg QW and 300 Q2W reached 9.183, 25.486, 44.284 and 29.302 ug/ml, respectively, and the average drug accumulation ratios (Rac [Ctrough,md/Ctrough,sd]) for Ctrough were 2.364, 3.465, 2.141 and 1.122 fold, respectively. Mild to moderate drug accumulation was observed in 75 mg QW, 150 mg QW and 300 mg QW dosage groups but no apparent drug accumulation was observed in 300 mg Q2W dosage group (Fig. 4d). A decrease in TARC/CCL17 level from baseline is shown in Fig. 4f. Serum TARC decreased by an average of 66% and 79% at day 22 and day 29 (300 mg QW group). In the AK120 300 mg Q2W treatment group (with 600 mg loading dose on day 1), SC serum TARC decreased by an average of 79% at bot day 22 and day 29. The decrease in serum total IgE level from baseline was dose dependent after day 22 among the AK120 treatment groups (Fig. 4e).
Preliminary Efficacy of AK120
The proportion of subjects who achieved EASI 50 and EASI 75 was higher in the AK120 treatment groups (total) than in the placebo group on days 8, 15, 22 and 29. On day 29, the proportion of subjects who reached EASI 50 and EASI 75 in the AK120 treatment groups (total) was 64.5% (20/31) and 35.5% (11/31), respectively; the proportion in the placebo group was 12.5% (1/8) and 0%, respectively (Table 3).
Table 3.
Summary of reduction in Eczema Area and Severity Index and Investigator Global Assessment score from baseline
| Visit category | AK120 treatment groups | Placebo (N = 9) |
||||
|---|---|---|---|---|---|---|
| 75 mg QW (N = 8) |
150 mg QW (N = 8) |
300 mg QW (N = 8) |
300 mg Q2W (N = 8) |
Total (N = 32) |
||
| Day 8 | 8 | 8 | 8 | 7 | 31 | 9 |
| EASI 50, n (%) | 2 (25.0) | 1 (12.5) | 2 (25.0) | 1 (14.3) | 6 (19.4) | 0 (0.0) |
| EASI 75, n (%) | 1 (12.5) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 2 (6.5) | 0 (0.0) |
| IGA score 0 or 1, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| IGA reduction from baseline of ≥ 2 points, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Day 15 | 8 | 8 | 7 | 8 | 31 | 9 |
| EASI 50, n (%) | 1 (12.5) | 5 (62.5) | 3 (42.9) | 2 (25.0) | 11 (35.5) | 2 (22.2) |
| EASI 75, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| IGA score 0 or 1, n (%) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 0 (0.0) | 1 (3.2) | 0 (0.0) |
| IGA reduction from baseline of ≥ 2 points, n (%) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 1 (12.5) | 2 (6.5) | 1 (11.1) |
| Day 22 | 8 | 8 | 7 | 8 | 31 | 8 |
| EASI 50, n (%) | 2 (25.0) | 6 (75.0) | 4 (57.1) | 4 (50.0) | 16 (51.6) | 1 (12.5) |
| EASI 75, n (%) | 1 (12.5) | 2 (25.0) | 1 (14.3) | 1 (12.5) | 5 (16.1) | 0 (0.0) |
| IGA score 0 or 1, n (%) | 1 (12.5) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 2 (6.5) | 0 (0.0) |
| IGA reduction from baseline of ≥ 2 points, n (%) | 1 (12.5) | 0 (0.0) | 2 (28.6) | 1 (12.5) | 4 (12.9) | 0 (0.0) |
| Day 29 | 8 | 8 | 7 | 8 | 31 | 8 |
| EASI 50, n (%) | 3 (37.5) | 7 (87.5) | 4 (57.1) | 6 (75.0) | 20 (64.5) | 1 (12.5) |
| EASI 75, n (%) | 1 (12.5) | 4 (50.0) | 4 (57.1) | 2 (25.0) | 11 (35.5) | 0 (0.0) |
| IGA score 0 or 1, n (%) | 1 (12.5) | 4 (50.0) | 1 (14.3) | 2 (25.0) | 8 (25.8) | 0 (0.0) |
| IGA reduction from baseline of ≥ 2 points, n (%) | 2 (25.0) | 4 (50.0) | 2 (28.6) | 2 (25.0) | 10 (32.3) | 0 (0.0) |
| Day 43 | 7 | 8 | 7 | 8 | 30 | 8 |
| EASI 50, n (%) | 2 (28.6) | 6 (75.0) | 4 (57.1) | 4 (50.0) | 16 (53.3) | 0 (0.0) |
| EASI 75, n (%) | 1 (14.3) | 5 (62.5) | 2 (28.6) | 3 (37.5) | 11 (36.7) | 0 (0.0) |
| IGA score 0 or 1, n (%) | 1 (14.3) | 3 (37.5) | 2 (28.6) | 1 (12.5) | 7 (23.3) | 0 (0.0) |
| IGA reduction from baseline of ≥ 2 points, n (%) | 2 (28.6) | 3 (37.5) | 3 (42.9) | 1 (12.5) | 9 (30.0) | 0 (0.0) |
| Day 57 | 8 | 8 | 7 | 7 | 30 | 8 |
| EASI 50, n (%) | 4 (50.0) | 7 (87.5) | 5 (71.4) | 4 (57.1) | 20 (66.7) | 0 (0.0) |
| EASI 75, n (%) | 1 (12.5) | 3 (37.5) | 4 (57.1) | 2 (28.6) | 10 (33.3) | 0 (0.0) |
| IGA score 0 or 1, n (%) | 1 (12.5) | 1 (12.5) | 1 (14.3) | 0 (0.0) | 3 (10.0) | 0 (0.0) |
| IGA reduction from baseline of ≥ 2 points, n (%) | 2 (25.0) | 2 (25.0) | 2 (28.6) | 0 (0.0) | 6 (20.0) | 0 (0.0) |
| Day 85 | 8 | 8 | 7 | 8 | 31 | 7 |
| EASI 50, n (%) | 3 (37.5) | 5 (62.5) | 3 (42.9) | 4 (50.0) | 15 (48.4) | 1 (14.3) |
| EASI 75, n (%) | 1 (12.5) | 2 (25.0) | 2 (28.6) | 1 (12.5) | 6 (19.4) | 0 (0.0) |
| IGA score 0 or 1, n (%) | 1 (12.5) | 1 (12.5) | 1 (14.3) | 0 (0.0) | 3 (9.7) | 0 (0.0) |
| IGA reduction from baseline of ≥ 2 points, n (%) | 2 (25.0) | 2 (25.0) | 1 (14.3) | 0 (0.0) | 5 (16.1) | 0 (0.0) |
On day 29, the proportion of subjects who reached EASI 50 in the AK120 75 mg QW, 150 mg QW, 300 mg QW and 300 mg Q2W dosage groups was 37.5% (3/8), 87.5% (7/8), 57.1% (4/7) and 75.0% (6/8), respectively. The proportion of subjects who reached EASI 75 in the AK120 75 mg QW, 150 mg QW, 300 mg QW and 300 mg Q2W treatment groups was 12.5% (1/8), 50.0% (4/8), 57.1% (4/7) and 25.0% (2/8), respectively. More subjects (87.5%) treated with AK120 150 mg QW achieved EASI 50 compared to the other dosage groups, possibly due to mild disease severity at baseline compared with the other AK120 dosage groups.
The percentage of subjects with IGA improvement was observed on day 15 post AK120 administration and reached a peak on day 29 with 25.8% (8/31) and 32.3% (10/31) IGA score 0 or 1 and reduction of ≥ 2-point, respectively (Table 3). The percentage of treatment responders with IGA score 0 or 1 decreased gradually when the AK120 injection was stopped.
Discussion
In part 1 of this study, a total of 86.7% subjects in the AK120 treatment groups experienced at least one TEAE, which was comparable with the rate in the placebo group (90.0%). No dose-limiting TEAEs were identified. The majority of TEAEs were mild or moderate in severity. The most common TEAEs occurring in the AK120 treatment groups were upper respiratory tract infection (33.3%) and headache (30.0%). As a reference, a review which compiled six phase I studies on dupilumab reported that injection site reactions (0–66.7%), headache (5.3–33.3%), gastrointestinal disorders (16.7–25.0%) and upper respiratory tract infection (5.3–15.8%) were the most common TEAEs associated with dupilumab in healthy subjects [17]. In part 2 of this study, a total of 28 (87.5%) and seven (77.8%) subjects experienced at least one TEAE in the AK120 treatment groups and placebo group, respectively. The majority of TEAEs were mild or moderate in severity. No SAE or TEAEs leading to permanent discontinuation from the study occurred in the AK120 treatment groups. The most common TEAEs occurring in the AK120 treatment groups were skin infection (12.5%, 4/32) and injection site pain (12.5%, 4/32). Upper respiratory tract infection (33.3%, 3/9 subjects) was the most common TEAE in the placebo group. In a 12-week monotherapy study of dupilumab as reference, 76% (42/55) subjects in the dupilumab group experienced at least one TEAE, injection-site reactions were the AEs that occurred at a higher frequency in the dupilumab group, one (2%) subject had a SAE and one (2%) subject discontinued the study due to an AE [18]. These results show that the safety profile of AK120 compared with that of dupilumab is better. However, given the present study’s small sample size and short exposure duration, the ability of this study to assess the safety profile of AK120 is limited. Additional clinical trials are required to thoroughly identify the safety features of AK120.
The first part of the study assessed serum TARC/CCL17 [19] and IgE [20]; these are widely accepted biomarkers induced by the IL-4/IL-4 receptor pathway and related to type 2 inflammation. A decrease in serum TARC/CCL17 level was observed after the administration of 150 mg and 300 mg of AK120; a decrease in serum IgE was observed after the administration of 600 mg of AK120; these results suggest a successful inhibition of IL-4 and IL-13 activity mediated by the IL-4Rα pathway. No significant change from baseline in cytokines IL-1β, IL-6 and tumor necrosis factor alpha (TNF-α) was observed in all groups, indicating that AK120 was safe and did not induce a cytokine storm. In part 2 of the study, serum TARC decreased by an average of 66% and 79% at day 22 and day 29, respectively, in the AK120 300 mg QW group; this result indicates that the TARC decrease was dose dependent. In the AK120 600 mg group (loading on day 1 then 300 mg Q2W SC), serum TARC level decreased by an average of 79% on both day 22 and day 29. Serum total IgE level decreased by an average of 44% to the maximum at day 57 following AK120 300 mg QW SC.
The proportion of subjects who achieved EASI 50 and EASI 75 was higher in the AK120 treatment groups (64.5% [20/31] and 35.5% [11/31], respectively), than than in the placebo group (12.5% [1/8] and 0, respectively). The percentage of subjects with IGA improvement was observed on day 15 post AK120 administration and reached a peak on day 29, when 25.8% (8/31) and 32.3% (10/31) of subjects had an IGA score 0 or 1 and a reduction of ≥ 2 points, respectively. As a reference, a study of 4-week monotherapy of dupilumab reported that the percentage of subjects who reached EASI 50 and EASI 75 on day 29 was 59% (30/51) and 29% (15/51), respectively [18]. When the results of the M4A and M4B studies are compared with our results from AK120 groups (75 mg, 150 mg and 300 mg), we note that the EASI 50 on day 29 is almost same (58% [14/24] vs. 59%[30/51]) and the EASI 75 on day 29 is much higher (38% [9/24] vs. 29%[15/51]). In addition, the results of IGA on day 29 also showed better improvement (25% [6/24] vs. 12% [6/51]). Considering the insufficient sample size, this advantage needs to be confirmed by further research.
Conclusion
Our FIH study provides evidence that AK120 was safe and well tolerated in both healthy and AD subjects up to 600 mg for single and multiple dose administration. There was no correlation between the occurrence of AEs and AK120 dosage. AK120 demonstrated an ability to decrease serum TARC/CCL17 and IgE levels. The exposure of AK120 increased in an approximately dose-dependent proportional manner in the dose range of 150–600 mg. Mild to moderate accumulation was observed after multiple doses of AK120 in QW dosage groups. Subjects treated with AK120 showed improved EASI scores, and the proportion of subjects reaching IGA score 0 or 1 and reduction of ≥ 2-point was increased after AK120 treatment. The efficacy of AK120 presented in a dose-dependent manner. In summary, current data showed that AK120 has potential advantages compared to dupilumab. The limitation of the study was small sample size. Further exploration of AK120 for safety and efficacy in larger-scale clinical studies is needed.
Acknowledgements
We thank all the subjects who participated in this study.
Medical Writing/Editorial Assistance
Medical writing and editorial assistance were provided by Yueqiao Liu, Liwen Liang and Min Zhang of Akeso Biopharma Inc.
Authors’ contributions
Guoqin Wang, Yu Zhang and Benchao Chen developed the study design. Christopher John Wynne, Alexandra Cole, Charlotte Lemech and Rodney Sinclair contributed to the final version of the manuscript. Max Wang, Baiyong Li and Michelle Xia supervised the project.
Funding
This clinical study and the journal’s Rapid Service Fee were sponsored by Akeso Biopharma Inc.
Data availability
The data support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of Interest
The authors declare no conflict of interest.
Ethical Approval
The study was designed and conducted according to guidelines of Good Clinical Practice(GCP), the current Declaration of Helsinki and National Medical Product Administration (NMPA). The protocol of this study was approved by the ethics committee. An informed consent document approved by independent ethics committee was signed by the subjects or their legally authorized representative before the participant entered in the study.
Authorship
All named authors made substantial contributions to the conception or design of the work, or to the acquisition, analysis or interpretation of data. All named authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy of integrity of any part of the work are appropriately investigated and resolved and have given their approval for this version to be published. All named authors meet the international Committee of Medical Journal(ICMJE) criteria for authorship for this manuscript.
References
- 1.David Boothe W, Tarbox JA, Tarbox MB. Atopic Dermatitis: Pathophysiology. Adv Exp Med Biol. 2017;1027:21–37. doi: 10.1007/978-3-319-64804-0_3. [DOI] [PubMed] [Google Scholar]
- 2.Douladiris N, Vakirlis E, Vassilopoulou E. Atopic dermatitis and water: is there an optimum water intake level for improving atopic skin? Children (Basel) 2023;10(2):273. doi: 10.3390/children10020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J, Kim BE, Leung DYM. Pathophysiology of atopic dermatitis: clinical implications. Allergy Asthma Proc. 2019;40(2):84–92. doi: 10.2500/aap.2019.40.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverberg JI, Barbarot S, Gadkari A, et al. Epidemiology of atopic dermatitis (AD) in children aged 6–11 years: a cross-sectional study in the United States (US), Canada, Europe, and Japan. Skin. 2020;1:2. [Google Scholar]
- 5.Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124(6):1251–8.e23. doi: 10.1016/j.jaci.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22(2):125–137. doi: 10.5021/ad.2010.22.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: A population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–347. doi: 10.1016/j.anai.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: A randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83(5):1282–1293. doi: 10.1016/j.jaad.2020.06.054. [DOI] [PubMed] [Google Scholar]
- 9.Dong WL, An J, Yu M, et al. The prevalence and year lived with disability of atopic dermatitis in China: Findings from the global burden of disease study 2019. World Allergy Organ J. 2021;14(11):100604. doi: 10.1016/j.waojou.2021.100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(1):44–56. doi: 10.1001/jamadermatol.2019.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 12.Ratchataswan T, Banzon TM, Thyssen JP, Weidinger S, Guttman-Yassky E, Phipatanakul W. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract. 2021;9(3):1053–1065. doi: 10.1016/j.jaip.2020.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saini S, Pansare M. New insights and treatments in atopic dermatitis. Pediatr Clin North Am. 2019;66(5):1021–1033. doi: 10.1016/j.pcl.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139(4S):S65–S76. doi: 10.1016/j.jaci.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhi NA, Pirozzi G, Graham N. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425–437. doi: 10.1080/1744666X.2017.1298443. [DOI] [PubMed] [Google Scholar]
- 16.Kisich KO, Carspecken CW, Fiéve S, Boguniewicz M, Leung DY. Defective killing of Staphylococcus aureus in atopic dermatitis is associated with reduced mobilization of human beta-defensin-3. J Allergy Clin Immunol. 2008;122(1):62–68. doi: 10.1016/j.jaci.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Radin A, Li M, et al. Pharmacokinetics, Pharmacodynamics, Safety, and Tolerability of Dupilumab in Healthy Adult Subjects. Clin Pharmacol Drug Dev. 2020;9(6):742–755. doi: 10.1002/cpdd.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. New Engl J Med. 2014;371(2):130–139. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 19.Wirnsberger G, Hebenstreit D, Posselt G, Horejs-Hoeck J, Duschl A, et al. IL-4 induces expression of TARC/CCL17 via two STAT6 binding sites. Eur J Immunol. 2006;36(7):1882–1891. doi: 10.1002/eji.200635972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izuhara K, Yanagihara Y, Hamasaki N, Shirakawa T, Hopkin JM, et al. Atopy and the human IL-4 receptor α chain. J Allergy Clin Immunol. 2000;106(1):S65–S71. doi: 10.1067/mai.2000.106776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data support the findings of this study are available from the corresponding author upon reasonable request.