ABSTRACT
The administration of analgesic drugs in elderly patients should take into account age-related physiological changes, loss of efficiency of homeostatic mechanisms, and pharmacological interactions with chronic therapies. Underestimation of pain in patients with impaired cognition is often linked to difficulties in pain assessment. In the preoperative phase, it is essential to assess the physical status, cognitive reserve, and previous chronic pain conditions to plan effective analgesia. Furthermore, an accurate pharmacological history of the patient must be collected to establish any possible interaction with the whole perioperative analgesic plan. The use of analgesic drugs with different mechanisms of action for pain relief in the intraoperative phase is a crucial step to achieve adequate postoperative pain control in older adults. The combined multimodal and opioid-sparing strategy is strongly recommended to reduce side effects. The use of various adjuvants is also preferable. Moreover, the implementation of non-pharmacological approaches may lead to faster recovery. High-quality postoperative analgesia in older patients can be achieved only with a collaborative interdisciplinary team. The aim of this review is to highlight the perioperative pain management strategies in the elderly with a special focus on intraoperative pharmacological interventions.
Keywords: Analgesia, elderly patients, multimodal analgesia, opioid-sparing
Introduction
The progressive increase of elderly patients undergoing surgery is closely associated with the need to ensure specific postoperative pain management since inadequate postoperative pain control is associated with adverse outcomes, that in this population can be fatal.[1] Pain management in the elderly is complicated by specific issues due to physio-chronological changes, pre-existing comorbidities, and poly-pharmacotherapy.[2,3] Older people have limited physiological reserves and less effective compensatory mechanisms to deal with unwanted adverse effects of drugs, which also are influenced by inter- or intra-individual variability and interaction with chronic therapies. Pharmacokinetics and pharmacodynamics of analgesic drugs are altered in the elderly leading to implications in everyday practice. This is the reason why the elderly is often excluded from pharmaceutical trials. Careful monitoring of the efficacy and adverse effects of analgesics is an important aspect of pain management practice in older people. Both greater opioid sensitivity and increased risk of postoperative delirium (POD) should be taken into account when planning perioperative analgesic strategies.[4] Assessment of postoperative pain may be challenging in older patients with some degree of chronic pain, or extremely difficult in those with cognitive and/or functional decline.[5] Although numerical verbal pain scales appear to be superior to nonverbal methods of assessment, cognitively impaired patients may not understand these scales, leading to a hazardous underdiagnosis and undertreatment of pain.[3] To date, a multimodal analgesia protocol remains the strategy of choice incorporated into the best practice guidelines, as part of opioid-sparing analgesia.[4,6,7] The aim of this review is to highlight perioperative pain management strategies in the elderly with a special focus on intraoperative pharmacological interventions.
Literature Search
A global literature search was performed in PubMed/MEDLINE, Cochrane Library, EMBASE, Scopus, and Google Scholar to identify the relevant articles (published up to June 1, 2023) relating to postoperative analgesia in the elderly. Reviews, meta-analyses, and randomized clinical trials were selected. A total of 35 articles were finally included based on relevance.
Postoperative pain assessment in older patients
Effective postoperative pain management in the geriatric population, as in any patient, starts with an appropriate quantification of pain. Pain should be reassessed after every intervention and every time there is a change in its characteristics. Particularly after surgery, it is also important to assess the patient’s ability to move and cough without pain, since good pain control after surgery is incorporated in the enhanced recovery after surgery (ERAS) protocols.[7] Pain can be assessed through self-report, behavioral, or physiological measures. Self-report scales require the patient to be able to understand the task and communicate their level of pain. In general, cognitively intact older patients can manage the most commonly used unidimensional pain scales such as the visual analog scale (VAS), verbal rating scale (VRS), and numeric rating scale (NRS).[8,9,10] Pain assessment in elderly patients can be quite challenging in case of cognitive impairment and other age-related factors that may influence the reporting of pain, including fear, anxiety, depression, cultural and social barriers, implications of the disease, and loss of independence.[3,5] In this context, the expression of pain can be under or overestimated, especially in patients with dementia or POD, who are unable to self-evaluate and must be assessed using behavioral scales. In these cases, multidimensional scales—including Pain Assessment in Advanced Dementia (PAINAD) and non-communicative patient’s pain assessment instrument (NOPPAIN) scales—are preferred.[8] Behavioral pain assessment scales—such as Doloplus, Doloplus-2, and Algoplus scales—are also very useful and reliable in cognitively impaired and elderly patients not able to communicate verbally. These scales take into account somatic, psychomotor, and psychosocial domains.[9,10]
Pain management during the perioperative period
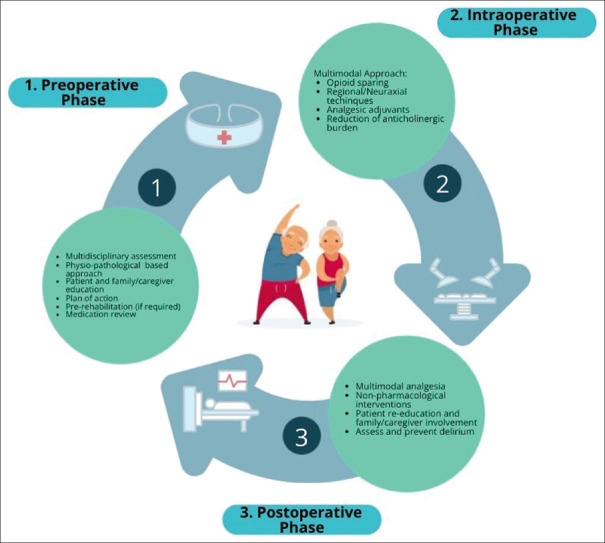
There is an increasing attention to pain control at all stages of the perioperative period to ensure an optimal approach for elderly patients. For this reason, assessment, pharmacological interventions, and environmental strategies throughout all phases of treatment are paramount for patient comfort and care. High-quality postoperative analgesia in older patients can be achieved only with a collaborative interdisciplinary team [Figure 1].
Figure 1.
Perioperative analgesia in the elderly
Preoperative Phase
This phase is hallmarked by a thorough history focusing on prior issues with pain and geriatric-specific issues unique to older adults such as prior falls or delirium. Additionally, there is an emphasis on the education of both the older adult and their family/caregiver regarding postoperative pain expectations and the importance of meeting functional goals such as mobility and nutrition. Several meta-analyses have suggested that preoperative education can decrease postoperative pain and anxiety. This is particularly important in the context of ERAS.[11,12] Misconceptions about pain treatment are common among older adults and can interfere with therapy adherence. Using simple explanations such as infographics or visuals about pain management can be useful in the preoperative setting especially to decrease opioid consumption.[13] An accurate pharmacological history of the patient should be collected to establish the possible interactions with the perioperative complex analgesic plan, mainly underlying cognitive status, medications history, past experiences, and history of opioid abuse, in the context of personalized precision medicine.[6,14] The main objective of high-quality analgesia is to minimize elderly-specific complications while taking into account physiology, pharmacodynamic and pharmacokinetic implications. Paracetamol (N-acetyl-para-aminophenol, acetaminophen) is a centrally acting and nonselective cyclo-oxygenase (COX) inhibitor with few side effects. It exerts mainly analgesic and antipyretic effects, but only weak anti-inflammatory actions at clinically used doses. It is widely used and lacks many of the adverse effects of the nonsteroidal anti-inflammatory drugs (NSAIDs). The most serious adverse effect of an acute overdose of paracetamol is dose-dependent hepatic necrosis. In recommended therapeutic dosages (1 g per 6 h), there are few contraindications, and paracetamol is usually well-tolerated, delivered either orally or intravenously (IV). A reduction in postoperative opioid consumption has been shown when paracetamol is used as preemptive analgesic therapy.[15] The rationale for its use in preemptive analgesia is based on the idea to prevent central sensitization phenomena, or the activation of central neurons by afferent signals that occur during surgery, leading to hypersensitivity postoperatively. NSAIDs reduce pain and inflammation by diminishing peripheral and/or central prostaglandin production attributed to the inhibition of either both COX isoenzymes (non-selective NSAIDs) or only to the inducible form of COX, i.e., the enzyme COX2 (selective NSAIDs). Non-selective NSAIDs, particularly ketorolac, diclofenac, or ketoprofen, enhance pain relief with a potent analgesic efficacy, induce an opioid-sparing effect, thus decreasing opioid-induced adverse effects (such as urinary retention, nausea, and vomiting). However, there are concerns mainly related to the cardiovascular system (CVS) and renal and gastrointestinal (GI) complications. Non-selective NSAIDs can also interfere with the actions of some drugs commonly used in older patients, for example, warfarin, diuretics, and angiotensin-converting enzyme (ACE) inhibitors. The concurrent use of ibuprofen may interfere with the cardioprotective effect of low-dose aspirin.[5] Thoughtful use of short-term non-selective NSAIDS may be appropriate in older adults, accompanied by a 25–50% dose, to minimize opioid consumption. Gabapentin and pregabalin are gamma-aminobutyric acid (GABA) analogs, particularly useful for neuropathic pain control. However, GABA analogs are associated with side effects that may disproportionately impact older adults (e.g., respiratory depression, sedation, dizziness), especially when associated with opioids.[16]
Another goal to be achieved in the preoperative phase is to reduce the inappropriate use of medications with clinically significant anticholinergic or sedative effects in older people.
Intraoperative phase
This phase focuses on maintaining and optimizing the patient’s pain control before awakening from anesthesia as well as minimizing narcotics. Several drugs, including dexamethasone and magnesium, and ketamine delivered either before induction of anesthesia, or intraoperatively have shown to be important adjuncts to prevent sensitization and hyperalgesia. On the other hand, opioids such as fentanyl, alfentanil, sufentanil, and remifentanil remain a mainstay for the induction and maintenance of analgesia intraoperatively. Dexamethasone is frequently given to decrease postoperative nausea and vomiting (PONV) and has been shown to have analgesic and opioid-sparing effects, given at a weight-based dosage.[17] Magnesium sulfate acts as an antagonist to N-methyl-d-aspartate glutamate (NMDA) receptors and can therefore modulate pain response. It has been used for many decades to reduce postoperative pain and opioid-induced hyperalgesia (OIH) in older adults.[2] Ketamine, another NMDA receptor antagonist, at sub-anesthetic doses, can be considered intraoperatively to limit opioid consumption, although there is contrasting evidence on this effect.[18,19] Furthermore, ketamine must be used with caution, particularly in the elderly, due to possible adverse effects such as hallucinations, out-of-body experiences, and nightmares.
In general, it is important to devise strategies that allow for a dose reduction of intraoperative opioids considering the increased risk of serious opioid-related adverse events in the elderly, including bradycardia, sedation, and respiratory depression. Remifentanil (RF), a potent mu-opioid receptor agonist, frequently used during the intraoperative phase, has a discrete hemodynamic stability and a short duration of action.[20,21] The fast offset of action allows for the predictable emergence and recovery from anesthesia, significantly shortening the extubation time and possibly the post-anesthesia care unit (PACU) stay.[22,23,24] In addition, the risk of opioid cumulative adverse effects, such as delayed post-operatory recovery and respiratory depression, is reduced with the use of RF.[24] Notably, the drug is rapidly and completely metabolized by non-specific plasma and tissue esterases,[20] and thus its pharmacokinetics is not affected by reduction in liver or renal function. These characteristics make it an ideal opioid to be used in a variety of surgeries and special populations, including elderly people.[25,26,27]
However, it is important to note that despite the favorable pharmacological properties highlighted above, RF seems to be associated with a small but clinically significant increase in post-surgical pain perception.[28] This effect is certainly related to the rapid offset of clinical actions,[29] although acute pain tolerance and RF-induced hyperalgesia (RIH) may play a role.[30] For this reason, anesthesiologists routinely use a bridge analgesic therapy, often based on long-acting opioids, such as morphine to prevent the occurrence of considerable pain in the immediate postoperative period.[31] On the other hand, there are no currently standardized procedures for the diagnosis of RIH nor any clinical consensus on whether perform such evaluations, thus the incidence of this complication remains unknown.[32] In addition, POD and postoperative cognitive dysfunctions (POCD) can also occur in association with RF use during surgery.[22] The incidence of POCD seems to vary depending on the selected intraoperative opioid therapy; it is lower in RF versus fentanyl,[33] but not versus sufentanil-treated patients.[34] Notably, the risk of developing adverse effects seems to increase with the dose of RF and the length of the surgical procedure,[24,35] although not all studies have confirmed these observations.[29,36,37]
Regarding hyperalgesia a recent study clearly demonstrated an increased incidence of hyperalgesia in patients older than 60 years receiving intraoperative infusions of RF and in patients receiving doses higher than 30 mcg/kg during surgery.[38] An increased consumption of morphine during the immediate postoperative period was first observed in patients treated with continuous infusions of RF at 0.1 mcg/ml/min in addition to desflurane-based anesthesia.[26] The study included 50 patients, 49 of which were analyzed. Patients were randomized 1:1 in two groups to receive desflurane-based anesthesia with or without remifentanil infusions. The enrolled subjects had a mean age (SD) of 60 (13) years in the RF group versus 62 (9) in the desflurane-only group, and thus the study included a certain percentage of subjects older than 65 years. Patients underwent open colon-rectal surgery lasting approximately 2 h. They were trained to use a patient-controlled analgesia (PCA) device for the self-administration of morphine in the postoperative period. In particular, the mean time to first PCA use tended to be 1 h shorter in the RF group compared to the desflurane-only treated group, whereas the cumulative 24-h postoperative morphine consumption was significantly increased in the RF group (mean dose of 59 mg, range 43–71 mg) versus the desflurane only group (mean dose of 32 mg, range 19–59 mg). The authors did not observe significant differences in the rate of PONV or droperidol consumption, nor did they detect episodes of respiratory depression. The study did not report other adverse events, such as dysphoria, dizziness, or drowsiness.[25] Similarly, it has been shown that the awakening and extubation times did not differ between patients assigned to a higher intraoperative dose of RF, i.e., 0.4 mc/kg/min, versus patients treated with a lower dose, i.e., 0.05 mcg/kg/min. The study enrolled slightly younger patients than the previous one, i.e., mean age (SD) of 58 (13) years in the 0.05 mcg/kg/min RF-treated group and 56 (12) years in the 0.4 mcg/kg/min RF-treated group. Interestingly, the authors reported a significant increase in the cumulative morphine consumption in the first 48 h post-surgery in the group treated with the high RF dose compared to the lower dose group.[39] These data suggest that the occurrence of hyperalgesia and its severity may depend on the dose of RF used during surgery, whereas the emergence from anesthesia is largely unaffected by the intraoperative dose as expected considering its PK characteristics.
Notably, it has been shown that the total dose of RF administered during surgery can be significantly reduced using target-controlled infusion (TCI) compared to conventional infusion (CI) at a fixed infusion rate. Moreover, TCI increases hemodynamic stability during and post-surgery in the elderly,[40] thus reducing the extent of peri-incisional hyperalgesia.[27] The study by Rechebé and collaborators enrolled patients scheduled for cardiac elective surgery, with a mean age (SD) of 63.9 (9.9) years in the CI group and 65.9 (7.7) years in the TCI group. It showed a significant reduction of the area of hyperalgesia around the surgical wound in the TCI group at days 1, 2, 4, and 7 following surgery, whereas the pain threshold was significantly increased in the TCI group compared to CI-treated patients. In line with previous findings, the time to extubation tended to be reduced in the TCI group, whereas the time to first postoperative morphine requirement seemed to be shorter in the TCI group versus the CI group. However, the differences between the two groups were not statistically significant. Interestingly, the cumulative consumption of morphine in the first 44 h post-surgery was similar in both groups. Moreover, despite the increased extent of peri-incisional hyperalgesia detected in the TCI group, pain rating, assessed via both a VRS and the horizontal 100-mm VAS at rest, after cough and after painful movements did not differ between the two groups during the first 44 h post-surgery.[27] Different studies have shown that the co-administration of anesthetic drugs, such as propofol, nitrous oxide, magnesium, and dexmedetomidine, seems to be helpful to limit the development of RIH.[41,42,43,44] However, all these studies were carried out in younger patients and their modulatory effect needs to be specifically investigated in the elderly. Numerous studies tested the efficacy of ketamine to prevent RIH, with three meta-analyses carried out over time showing conflicting results.[45,46,47]
In addition, it has been recently shown that the transversus abdominis plane block (TAPB) particularly if associated with transcutaneous electrical acupoint stimulation (TAES) significantly reduced the amount of intraoperative RF, the time to extubation, postoperative time to first ambulation, and flatus as well as the incidence of PONV. Despite the lower intraoperative dose of RF, VAS both at rest and at movement was significantly lower in the TABP/TAES group versus the control group.[48] However, the study lacks an important comparator group, consisting of patients treated only with TEAS during general anesthesia.[49] Notably, it has been also shown that the proportion of patients reporting chronic thoracic pain at 3 months post-surgery is significantly higher in patients treated intraoperatively with RF compared to patients treated with fentanyl.[50] Patients referred pain mostly around the site of incision in both groups. However, there were no differences in pain perception between the two groups at 6- and 12-months following surgery, being the last the primary endpoint of the study. The median consumption of opioids in the immediate postoperative period (24–48 h) was higher in the RF group than in the fentanyl group. Interestingly, the increase in chronic thoracic pain found at 3 months in RF-treated patients seemed to be affected by age and the total RF dose used during surgery. Briefly, in patients younger than 65 years the adjusted odd ratio (OR) for pain was 4.0, with a 95% confidence interval (CI) ranging from 1.3 to 12.2, and P = 0.016. In contrast, no effect was found in patients older than 65 years, with OR of 0.9; 95% CI 0.3 to 2.5. At 3 months, a dose-related effect of RF on pain was observed, with OR of 1.3 (95% 0.5 to 3.1) and 3.3 (95% CI 1.4 to 8.1) for a cumulative dose of <1.875 mcg and ≥1.875 mcg, respectively.[50]
Regarding POCD in the elderly, a large study enrolling 622 patients older than 60 years, scheduled for major abdominal surgery under general anesthesia, showed no significant differences in the rate of POCD at day 1 and 7 post-surgery, in patients treated intraoperatively with RF or fentanyl.[40] Notably, there seemed to be a slight protective effect of RF over the occurrence of POCD, but the trend toward reduced POCD events in RF-treated patients did not reach a statistical significance. In line with previous observations, administration of morphine rescue therapy in the recovery room was higher in the RF group, whereas no significant differences in the cumulative consumption of tramadol via PCA were reported. The authors observed lower VAS in the fentanyl group compared to RF-treated patients only in the immediate postoperative period, 1–2 h post-surgery.[40] Interestingly, they detected lower level of peripheral interleukin 6 (IL-6) at post-surgery day 7 in patients that received RF during surgery versus fentanyl-treated patients, thus suggesting reduced inflammatory processes by RF and/or possibly anti-inflammatory effects. In this regard, both peripheral and central inflammation can contribute to increased pain perception, thus hyperalgesia as well as mediate or aggravate the neurological dysfunctions occurring post-surgery. However, we have recently shown that RF is not able to directly modulate the immune activation of human microglia at clinically relevant concentrations, although it may exert direct pro-nociceptive effects, through activation of the brain-derived neurotrophic factor signaling pathway.[51,52,53] The rate POCD did not vary when RF was used combined with different modalities of general anesthesia, i.e., sevoflurane-based (volatile) anesthesia versus propofol-based anesthesia (total intravenous anesthesia).[54] The study enrolled patients with coronary artery disease (CAD) undergoing non-cardiac interventions. In addition, there were no differences in the occurrence of intra-hospital cardiovascular events (primary endpoint of the study), as well as other anesthesia-related complications, such as PONV. The increased incidence of POCD observed in the elderly compared to younger patients seems to be related to the increased rate of cerebral autoregulation dysfunctions that occur during surgery, particularly in the Trendelenburg position.[55,56] Similarly, no difference in the rate of POD was observed between multimodal general anesthesia performed using a combination of RF, ketamine, magnesium, dexmedetomidine, and bupivacaine for antinociception compared to historical controls.[57] The study enrolled 20 patients undergoing coronary artery bypass graft surgery with or without aortic and/or mitral valve replacement requiring cardiopulmonary bypass. It must be considered that the historical cohort used for comparison in this study derived from the DEXACET clinical trial (non-acetaminophen groups, ClinicalTrials.gov Identifier: NCT02546765), and these patients did not receive RF during general anesthesia.[58]
Over time, different preventive measures have been experimented to reduce the rate of POCD in the elderly. For example, bispectral index monitoring during surgery was shown to be effective in the elderly undergoing colon carcinoma resection.[59] In addition, RF administration according to the surgical pleth index value in patients aged 60–90 years was associated with an increased dose of RF during surgery, but a reduced rate of hypertension and tachycardia compared to RF-treated patients based on hemodynamic parameters. Notably, the SPI-guided analgesia was associated with a lower incidence of delirium in the PACU compared to conventional analgesia.[60] Likewise, the administration of pre-emptive analgesia by continuous femoral nerve block significantly decreased the incidence of POCD in the elderly undergoing total knee arthroplasty.[61] Similar results were obtained in a subsequent study in which quadratus lumborum block (Q-Block) was used to better control postoperative pain perception in elderly patients undergoing laparoscopic radical gastrectomy.[62] At day 7 post-surgery, POCD was diagnosed in two out of 30 patients that received Q-Block during surgery. In the control group (no-block), POCD was observed in eight out of 29 patients, thus at a significantly higher rate of 27.6% versus 6.7% observed in the Q-Block group. Interestingly, the intraoperative doses of RF as well as the cumulative consumption of sufentanil during the first 24 h post-surgery were significantly diminished by the Q-Block. Moreover, increased peripheral pro-inflammatory activation was observed at the end of surgery, although the levels of pro-inflammatory cytokines, including IL-6, tumor necrosis factor α (TNFα), and the high mobility group box protein 1 (HMGB1), were significantly lower in the Q-Block group versus Control.[63] On the other hand, the addition of remimazolam at 0.3–0.5 mg/kg/h during the maintenance phase of anesthesia allowed for the reduction of both propofol and RF intraoperative doses. The reduction of the dose of these drugs during surgery did not affect the recovery time from anesthesia and the PACU stay duration. However, it significantly improved the cognitive performance of patients at 6–7 days after surgery while reducing the incidence of POCD at 5–6 days after surgery. Interestingly, plasma inflammatory cytokines, including TNFα and S100β, were significantly reduced by remimazolam.[64] Similar beneficial effects on postoperative neurocognitive dysfunction were recently observed by using low-dose esketamine during sevoflurane/RF anesthesia in elderly patients with gastrointestinal cancers.[65]
Morphine, often administered intraoperatively 30 minutes to 1 hour after remifentanil infusion is used to manage possible postoperative pain. Subcutaneous and intramuscular routes of administration are discouraged with morphine due to irregular absorption and metabolism leading to early adverse events.[66] Tramadol is an atypical centrally acting analgesic with opioid-like effects that acts by binding to the mu-opioid receptor and inhibiting noradrenaline (norepinephrine) and serotonin (5-hydroxytryptamine) re-uptake. Dosage reduction is preferable in case of hepatic and renal failure.[67] Furthermore, the use of tramadol seems to be associated with an increased risk of POD in patients older than 75, and serotonin syndrome, when concurrently used with monoamine oxidase inhibitors.[67,68]
Postoperative phase
Postoperative management is challenging as there is a delicate balance between adequate pain control using a multimodality approach and avoidance of side effects seen with common opioid pain medications. Uncontrolled pain is more common in older adults and is also a precipitating factor for cognitive impairment, prolonged immobility, increase in length of stay in the hospital, and increased morbidity.[69,70,71] Mitigation of opioid use is crucial and can be achieved using nonpharmacologic methods for pain control, and nonopioid medications in the context of multimodal analgesia during pre- and intraoperative phase.[4] The rationale for this strategy is the achievement of sufficient analgesia using lower doses of analgesics. Particular attention must be paid to drug–drug interactions and potential medication side effects when designing a multimodal pain plan for older adults. Nonpharmacologic pain management therapies (e.g., guided imagery, mindful breathing, superficial massage, acupressure, repositioning, superficial heat or cold, vibration, distraction techniques, and careful scheduling of medical therapy) should be implemented as early as possible in the postoperative setting in the older adult population. Psychological interventions should be considered to relieve anxiety associated with postoperative pain.[72] These therapies seem safe and can relieve postoperative pain, anxiety, and decrease analgesic use and increase adherence to medical therapy.[6] If the use of opioids is necessary, it is suggested to “start low and go slow,” with a reduction of the starting dose of 25–50% and the preference for oral rather than intravenous administration.[4] If intravenous administration is necessary, PCA is suggested, but only if the patient can understand its correct functioning. Long-acting opioids should be avoided in the elderly population, as they require titration and have not shown superiority compared to short-acting drugs; for example, meperidine for the risk of POD and neurotoxicity and tramadol for metabolic variability (Cytochrome P450 2D6) have unpredictable side effects. Continuous pain assessment is necessary to avoid misdiagnosis, and the concomitant use of benzodiazepines should be avoided due to the increased risk of delirium and falling. Regional anesthesia techniques might have an important role at the bedside, even if their role in the postoperative setting need to be further investigated.
Adequate postoperative analgesia is also a key strategy for preventing the risk of postoperative pulmonary complications in elderly patients undergoing major abdominal surgery, as it improves pulmonary compliance and functional residual capacity facilitating deep breathing exercises in the postoperative period.[73,74]
In conclusion, the overarching goal of improving pain management in older adults is to optimize analgesia while minimizing the side effects, in the context of multimodal approach aimed to decrease opioid consumption. High-quality postoperative analgesia in older patients can be achieved only with a collaborative interdisciplinary team. The main objective is to minimize elderly-specific complications while ensuring pain control and patient care. The geriatric patient must be considered a unique category of patient; its frailty and vulnerability must prompt a type of management based on physiology and physiopathology to give the best possible care while increasing patient satisfaction.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Brinson Z, Tang VL, Finlayson E. Postoperative functional outcomes in older adults. Curr Surg Rep. 2016;4:21. doi: 10.1007/s40137-016-0140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shellito AD, Dworsky JQ, Kirkland PJ, Rosenthal RA, Sarkisian CA, Ko CY, et al. Perioperative pain management issues unique to older adults undergoing surgery: A narrative review. Ann Surg Open. 2021;2:e072. doi: 10.1097/AS9.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falzone E, Hoffmann C, Keita H. Postoperative analgesia in elderly patients. Drugs Aging. 2013;30:81–90. doi: 10.1007/s40266-012-0047-7. [DOI] [PubMed] [Google Scholar]
- 4.Aceto P, Antonelli Incalzi R, Bettelli G, Carron M, Chiumiento F, Corcione A, et al. Perioperative management of elderly patients (PriME): Recommendations from an Italian intersociety consensus. Aging Clin Exp Res. 2020;32:1647–73. doi: 10.1007/s40520-020-01624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coldrey JC, Upton RN, Macintyre PE. Advances in analgesia in the older patient. Best Pract Res Clin Anaesthesiol. 2011;25:367–78. doi: 10.1016/j.bpa.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of postoperative pain: A clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists'Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17:131–57. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Ljungqvist O, Hubner M. Enhanced recovery after surgery-ERAS-principles, practice and feasibility in the elderly. Aging Clin Exp Res. 2018;30:249–52. doi: 10.1007/s40520-018-0905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zwakhalen SM, Hamers JP, Abu-Saad HH, Berger MP. Pain in elderly people with severe dementia: A systematic review of behavioral pain assessment tools. BMC Geriatr. 2006;6:3. doi: 10.1186/1471-2318-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torvik K, Kaasa S, Kirkevold Ø, Saltvedt I, Hølen JC, Fayers P, et al. Validation of Doloplus-2 among nonverbal nursing home patients—an evaluation of Doloplus-2 in a clinical setting. BMC Geriatr. 2010;10:9. doi: 10.1186/1471-2318-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rat P, Jouve E, Pickering G, Donnarel L, Nguyen L, Michel M, et al. Validation of an acute pain-behavior scale for older persons with inability to communicate verbally: Algoplus. Eur J Pain. 2011;15:198.e1–10. doi: 10.1016/j.ejpain.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Szeverenyi C, Kekecs Z, Johnson A, Elkins G, Csernatony Z, Varga K. The use of adjunct psychosocial interventions can decrease postoperative pain and improve the quality of clinical care in orthopedic surgery: A systematic review and meta-analysis of randomized controlled trials. J Pain. 2018;19:1231–52. doi: 10.1016/j.jpain.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Ramesh C, Nayak BS, Pai VB, Patil NT, George A, George LS, et al. Effect of preoperative education on postoperative outcomes among patients undergoing cardiac surgery: A systematic review and meta-analysis. J Perianesth Nurs. 2017;32:518–29.e2. doi: 10.1016/j.jopan.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Angelo JL, Wu J, Sirody J, DeUgarte DA. Reduction in prescribed opioids after general surgery procedures at a public hospital. Am Surg. 2019;1:1198–203. [PubMed] [Google Scholar]
- 14.American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: An updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–73. doi: 10.1097/ALN.0b013e31823c1030. [DOI] [PubMed] [Google Scholar]
- 15.Woolf CJ, Chong MS. Preemptive analgesia—treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–79. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 16.By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67:674–94. doi: 10.1111/jgs.15767. [DOI] [PubMed] [Google Scholar]
- 17.De Oliveira GS, Jr, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: A meta-analysis of randomized controlled trials. Anesthesiology. 2011;115:575–88. doi: 10.1097/ALN.0b013e31822a24c2. [DOI] [PubMed] [Google Scholar]
- 18.Hovaguimian F, Tschopp C, Beck-Schimmer B, Puhan M. Intraoperative ketamine administration to prevent delirium or postoperative cognitive dysfunction: A systematic review and meta-analysis. Acta Anaesthesiol Scand. 2018;62:1182–93. doi: 10.1111/aas.13168. [DOI] [PubMed] [Google Scholar]
- 19.Avidan MS, Maybrier HR, Abdallah AB, Jacobsohn E, Vlisides PE, Pryor KO, et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: An international, multicentre, double-blind, randomized clinical trial. Lancet. 2017;15:267–75. doi: 10.1016/S0140-6736(17)31467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glass PSA, Gan TJ, Howell S. A review of the pharmacokinetics and pharmacodynamics of remifentanil. Anesth Analg. 1999;89((4 Suppl)):7. doi: 10.1097/00000539-199910001-00003. [DOI] [PubMed] [Google Scholar]
- 21.Aceto P, Dello Russo C, Lai C, Perilli V, Fucci N, De Giovanni N, et al. Relationship between blood remifentanil concentration and stress hormone levels during pneumoperitoneum in patients undergoing laparoscopic cholecystectomy. Eur Rev Med Pharmacol Sci. 2017;21:4419–22. [PubMed] [Google Scholar]
- 22.De Gaudio A, Ciritella P, Perrotta F, Puopolo M, Lauta E, Mastronardi P, et al. Remifentanil vs fentanyl with a target controlled propofol infusion in patients undergoing craniotomy for supratentorial lesions. Minerva Anestesiol. 2006;72:309–19. [PubMed] [Google Scholar]
- 23.Niedermayer S, Heyn J, Guenther F, Küchenhoff H, Luchting B. Remifentanil for abdominal surgery is associated with unexpectedly unfavorable outcomes. Pain. 2020;161:266–73. doi: 10.1097/j.pain.0000000000001713. [DOI] [PubMed] [Google Scholar]
- 24.De Cosmo G, Congedo E, Clemente A, Aceto P. Sedation in PACU: The role of propofol. Curr Drug Targets. 2005;6:741–4. doi: 10.2174/138945005774574425. [DOI] [PubMed] [Google Scholar]
- 25.Aceto P, Dello Russo C, Punzo G, Luca E, Crea MA, Modesti C, et al. Success of fast-track anesthesia. In: Bernhardt LV, editor. Advances in Medicine and Biology. Nova Science Publishers; 2019. pp. 88–122. [Google Scholar]
- 26.Guignard B, Bossard AE, Coste C, Sessler DI, Lebrault C, Alfonsi P, et al. Acute opioid tolerance: Intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000;93:409–17. doi: 10.1097/00000542-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 27.De Castro V, Godet G, Mencia G, Raux M, Coriat P. Target-controlled infusion for remifentanil in vascular patients improves hemodynamics and decreases remifentanil requirement. Anesth Analg. 2003;96:33–8. doi: 10.1097/00000539-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Richebé P, Pouquet O, Jelacic S, Mehta S, Calderon J, Picard W, et al. Target-controlled dosing of remifentanil during cardiac surgery reduces postoperative hyperalgesia. J Cardiothorac Vasc Anesth. 2011;25:917–25. doi: 10.1053/j.jvca.2011.03.185. [DOI] [PubMed] [Google Scholar]
- 29.Fletcher D, Martinez V. Opioid-induced hyperalgesia in patients after surgery: A systematic review and a meta-analysis. Br J Anaesth. 2014;112:991–1004. doi: 10.1093/bja/aeu137. [DOI] [PubMed] [Google Scholar]
- 30.Angst MS. Intraoperative use of remifentanil for TIVA: Postoperative pain, acute tolerance, and opioid-induced hyperalgesia. J Cardiothorac Vasc Anesth. 2015;29((Suppl 1)):S16–22. doi: 10.1053/j.jvca.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 31.Santonocito C, Noto A, Crimi C, Sanfilippo F. Remifentanil-induced postoperative hyperalgesia: Current perspectives on mechanisms and therapeutic strategies. Local Reg Anesth. 2018;11:15–23. doi: 10.2147/LRA.S143618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muñoz HR, Guerrero ME, Brandes V, Cortínez LI. Effect of timing of morphine administration during remifentanil-based anaesthesia on early recovery from anaesthesia and postoperative pain. Br J Anaesth. 2002;88:814–8. doi: 10.1093/bja/88.6.814. [DOI] [PubMed] [Google Scholar]
- 33.Angst MS Meeting of the Anesthetic and Analgesic Drug Products Advisory Committee. 2023 Apr 19. Available from: https://www.fda.gov/media/167256/download .
- 34.Radtke FM, Franck M, Lorenz M, Luetz A, Heymann A, Wernecke KD, et al. Remifentanil reduces the incidence of post-operative delirium. J Int Med Res. 2010;38:1225–32. doi: 10.1177/147323001003800403. [DOI] [PubMed] [Google Scholar]
- 35.Rasmussen LA, Ryhammer PK, Greisen J, Bhavsar RR, Lorentzen AG, Jakobsen CJ. Ultrashort-acting remifentanil is not superior to long-acting sufentanil in preserving cognitive function-a randomized study. J Clin Anesth. 2016;33:127–34. doi: 10.1016/j.jclinane.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 36.Kim D, Lim HS, Kim MJ, Jeong W, Ko S. High-dose intraoperative remifentanil infusion increases early postoperative analgesic consumption: A prospective, randomized, double-blind controlled study. J Anesth. 2018;32:886–92. doi: 10.1007/s00540-018-2569-6. [DOI] [PubMed] [Google Scholar]
- 37.Treskatsch S, Klambeck M, Mousa SA, Kopf A, Schäfer M. Influence of high-dose intraoperative remifentanil with or without amantadine on postoperative pain intensity and morphine consumption in major abdominal surgery patients: A randomized trial. Eur J Anaesthesiol. 2014;31:41–9. doi: 10.1097/01.EJA.0000434967.03790.0e. [DOI] [PubMed] [Google Scholar]
- 38.Koo CH, Cho YJ, Hong DM, Jeon Y, Kim TK. Influence of high-dose intraoperative remifentanil with intravenous ibuprofen on postoperative morphine consumption in patients undergoing pancreaticoduodenectomy: A randomized trial. J Clin Anesth. 2016;35:47–53. doi: 10.1016/j.jclinane.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 39.Zhang T, Ma F. Changes of entropy index and cerebral oxygen metabolism in the maintenance of remifentanil anesthesia and their predictive value for postoperative hyperalgesia. Comput Math Methods Med. 2022;2022:1080858. doi: 10.1155/2022/1080858. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Joly V, Richebe P, Guignard B, Fletcher D, Maurette P, Sessler DI, et al. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology. 2005;103:147–55. doi: 10.1097/00000542-200507000-00022. [DOI] [PubMed] [Google Scholar]
- 41.De Cosmo G, Sessa F, Fiorini F, Congedo E. Effect of remifentanil and fentanyl on postoperative cognitive function and cytokines level in elderly patients undergoing major abdominal surgery. J Clin Anesth. 2016;35:40–6. doi: 10.1016/j.jclinane.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 42.Su X, Zhu W, Tian Y, Tan L, Wu H, Wu L. Regulatory effects of propofol on high-dose remifentanil-induced hyperalgesia. Physiol Res. 2020;69:157–64. doi: 10.33549/physiolres.934133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wehrfritz A, Bauer M, Noel N, Ramirez-Gil JF, Ihmsen H, Prottengeier J, et al. Evaluation of antihyperalgesic and analgesic effects of 35% nitrous oxide when combined with remifentanil: A randomized phase 1 trial in volunteers. Eur J Anaesthesiol. 2021;38:1230–41. doi: 10.1097/EJA.0000000000001468. [DOI] [PubMed] [Google Scholar]
- 44.Silva Filho SE, Sandes CS, Vieira JE, Cavalcanti IL. Analgesic effect of magnesium sulfate during total intravenous anesthesia: Randomized clinical study. Braz J Anesthesiol. 2021;71:550–7. doi: 10.1016/j.bjane.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Z, Yu J, Lin Q, Li H, Zhang T, Tan H, et al. Effects of an intraoperative intravenous bolus dose of dexmedetomidine on remifentanil-induced postinfusion hyperalgesia in patients undergoing thyroidectomy: A double-blind randomized controlled trial. Anesth Analg. 2021;132:320–8. doi: 10.1213/ANE.0000000000005003. [DOI] [PubMed] [Google Scholar]
- 46.García-Henares JF, Moral-Munoz JA, Salazar A, Del Pozo E. Effects of ketamine on postoperative pain after remifentanil-based anesthesia for major and minor surgery in adults: A systematic review and meta-analysis. Front Pharmacol. 2018;9:921. doi: 10.3389/fphar.2018.00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y, Zheng Y, Gu X, Ma Z. The efficacy of NMDA receptor antagonists for preventing remifentanil-induced increase in postoperative pain and analgesic requirement: A meta-analysis. Minerva Anestesiol. 2012;78:653–67. [PubMed] [Google Scholar]
- 48.Wu L, Huang X, Sun L. The efficacy of N-methyl-D-aspartate receptor antagonists on improving the postoperative pain intensity and satisfaction after remifentanil-based anesthesia in adults: A meta-analysis. J Clin Anesth. 2015;27:311–24. doi: 10.1016/j.jclinane.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 49.Xing R, Yang Y, Zhang M, Wang H, Tan M, Gao C, et al. Effect of transcutaneous electrical acupoint stimulation combined with transversus abdominis plane block on postoperative recovery in elderly patients undergoing laparoscopic gastric cancer surgery: A randomized controlled trial. Pain Ther. 2022;11:1327–39. doi: 10.1007/s40122-022-00429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan YJ, Xue FS, Cheng Y. Letter to the editor regarding “Effect of transcutaneous electrical acupoint stimulation combined with transversus abdominis plane block on postoperative recovery in elderly patients undergoing laparoscopic gastric cancer surgery: A randomized controlled trial”. Pain Ther. 2023;12:885–8. doi: 10.1007/s40122-023-00509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Hoogd S, Ahlers SJGM, van Dongen EPA, van de Garde EMW, Daeter EJ, Dahan A, et al. Randomized controlled trial on the influence of intraoperative remifentanil versus fentanyl on acute and chronic pain after cardiac surgery. Pain Pract. 2018;18:443–51. doi: 10.1111/papr.12615. [DOI] [PubMed] [Google Scholar]
- 52.Dello Russo C, Cappoli N, Tabolacci E, Sollazzi L, Navarra P, Aceto P. Remifentanil does not affect human microglial immune activation in response to pro-inflammatory cytokines. EXCLI J. 2023;22:295–309. doi: 10.17179/excli2022-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cappoli N, Aceto P, Tabolacci E, Mezzogori D, Sollazzi L, Navarra P, et al. Effects of remifentanil on human C20 microglial pro-inflammatory activation. Eur Rev Med Pharmacol Sci. 2021;25:5268–74. doi: 10.26355/eurrev_202108_26547. [DOI] [PubMed] [Google Scholar]
- 54.Cappoli N, Tabolacci E, Aceto P, Dello Russo C. The emerging role of the BDNF-TrkB signaling pathway in the modulation of pain perception. J Neuroimmunol. 2020;349:577406. doi: 10.1016/j.jneuroim.2020.577406. [DOI] [PubMed] [Google Scholar]
- 55.Dai Z, Lin M, Li Y, Gao W, Wang P, Lin J, et al. Sevoflurane-remifentanil versus propofol-remifentanil anesthesia during noncardiac surgery for patients with coronary artery disease –A prospective study between 2016 and 2017 at a single center. Med Sci Monit. 2021;27:e929835. doi: 10.12659/MSM.929835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aceto P, Beretta L, Cariello C, Claroni C, Esposito C, Forastiere EM, et al. Joint consensus on anesthesia in urologic and gynecologic robotic surgery: Specific issues in management from a task force of the SIAARTI, SIGO, and SIU. Minerva Anestesiol. 2019;85:871–85. doi: 10.23736/S0375-9393.19.13360-3. [DOI] [PubMed] [Google Scholar]
- 57.Aceto P, Galletta C, Cambise C, Punzo G, Luca E, Schipa C, et al. Challenges for anaesthesia for robotic-assisted surgery in the elderly: A narrative review. Eur J Anaesthesiol Intensive Care. 2023;2:e0019. [Google Scholar]
- 58.Shanker A, Abel JH, Narayanan S, Mathur P, Work E, Schamberg G, et al. Perioperative multimodal general anesthesia focusing on specific cns targets in patients undergoing cardiac surgeries: The Pathfinder feasibility trial. Front Med (Lausanne) 2021;8:719512. doi: 10.3389/fmed.2021.719512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Subramaniam B, Shankar P, Shaefi S, Mueller A, O'Gara B, Banner-Goodspeed V, et al. Effect of intravenous acetaminophen vs placebo combined with propofol or dexmedetomidine on postoperative delirium among older patients following cardiac surgery: The DEXACET randomized clinical trial. JAMA. 2019;321:686–96. doi: 10.1001/jama.2019.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou Y, Li Y, Wang K. Bispectral index monitoring during anesthesia promotes early postoperative recovery of cognitive function and reduces acute delirium in elderly patients with colon carcinoma: A prospective controlled study using the attention network test. Med Sci Monit. 2018;24:7785–93. doi: 10.12659/MSM.910124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Won YJ, Oh SK, Lim BG, Kim YS, Lee DY, Lee JH. Effect of surgical pleth index-guided remifentanil administration on perioperative outcomes in elderly patients: A prospective randomized controlled trial. BMC Anesthesiol. 2023;23:57. doi: 10.1186/s12871-023-02011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deng LQ, Hou LN, Song FX, Zhu HY, Zhao HY, Chen G, et al. Effect of pre-emptive analgesia by continuous femoral nerve block on early postoperative cognitive function following total knee arthroplasty in elderly patients. Exp Ther Med. 2017;13:1592–7. doi: 10.3892/etm.2017.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu M, Qi Y, He H, Zhang S, Mei Y. Effect of quadratus lumborum block on postoperative cognitive function in elderly patients undergoing laparoscopic radical gastrectomy: A randomized controlled trial. BMC Geriatr. 2021;21:238. doi: 10.1186/s12877-021-02179-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liao YQ, Min J, Wu ZX, Hu Z. Comparison of the effects of remimazolam and dexmedetomidine on early postoperative cognitive function in elderly patients with gastric cancer. Front Aging Neurosci. 2023;15:1123089. doi: 10.3389/fnagi.2023.1123089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma J, Wang F, Wang J, Wang P, Dou X, Yao S, et al. The effect of low-dose esketamine on postoperative neurocognitive dysfunction in elderly patients undergoing general anesthesia for gastrointestinal tumors: A randomized controlled trial. Drug Des Devel Ther. 2023;17:1945–57. doi: 10.2147/DDDT.S406568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keïta H, Geachan N, Dahmani S, Couderc E, Armand C, Quazza M, et al. Comparison between patient-controlled analgesia and subcutaneous morphine in elderly patients after total hip replacement. Br J Anaesth. 2003;90:53–7. doi: 10.1093/bja/aeg019. [DOI] [PubMed] [Google Scholar]
- 67.Brouquet A, Cudennec T, Benoist S, Moulias S, Beauchet A, Penna C, et al. Impaired mobility, ASA status and administration of tramadol are risk factors for postoperative delirium in patients aged 75 years or more after major abdominal surgery. Ann Surg. 2010;251:759–65. doi: 10.1097/SLA.0b013e3181c1cfc9. [DOI] [PubMed] [Google Scholar]
- 68.Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;17:1112–20. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- 69.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63:142–50. doi: 10.1111/jgs.13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aceto P, Lai C, De Crescenzo F, Crea MA, Di Franco V, Pellicano GR, et al. Cognitive decline after carotid endarterectomy: Systematic review and meta-analysis. Eur J Anaesthesiol. 2020;37:1066–74. doi: 10.1097/EJA.0000000000001130. [DOI] [PubMed] [Google Scholar]
- 71.Aceto P, Perilli V, Lai C, Ciocchetti P, Vitale F, Sollazzi L. Postoperative cognitive dysfunction after liver transplantation. Gen Hosp Psychiatry. 2015;37:109–15. doi: 10.1016/j.genhosppsych.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 72.Aceto P, Lai C, Perilli V, Sacco T, Modesti C, Raffaelli M, et al. Factors affecting acute pain perception and analgesics consumption in patients undergoing bariatric surgery. Physiol Behav. 2016;163:1–6. doi: 10.1016/j.physbeh.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 73.Aceto P, Perilli V, Luca E, Schipa C, Calabrese C, Fortunato G, et al. Predictive power of modified frailty index score for pulmonary complications after major abdominal surgery in the elderly: A single centre prospective cohort study. Eur Rev Med Pharmacol Sci. 2021;25:3798–802. doi: 10.26355/eurrev_202105_25947. [DOI] [PubMed] [Google Scholar]
- 74.Perilli V, Aceto P, Sacco T, Modesti C, Ciocchetti P, Vitale F, et al. Anaesthesiological strategies to improve outcome in liver transplantation recipients. Eur Rev Med Pharmacol Sci. 2016;20:3172–7. [PubMed] [Google Scholar]