Abstract
Botulinum toxin is a potent neuromodulator commonly used for cosmetic applications in the clinic. In this article, we reviewed the various formulations of botulinum toxin type A commercially available in the United States, as well as clinical pearls for preprocedural planning, common in‐office injections, and management of complications.
Keywords: botulinum toxin, facial plastics, facial wrinkles
BACKGROUND
Botulinum exotoxin is produced by the bacteria Clostridium botulinum. There exist several different serotypes ranging from A to G, with types A and B commonly used in medical applications. Botulinum toxin is a potent neuromodulator with a variety of functional and cosmetic uses. 1 It was first described in 1980 in the ophthalmologic literature for the treatment of strabismus. 2 Later, in 1993, Blitzer et al. 3 reported the use of botulinum toxin for cosmetic purposes. The toxin acts at the neuromuscular junction, where it enters the presynaptic nerve terminal via receptor‐mediated endocytosis. 4 , 5 Once intracellular, the toxin cleaves SNARE proteins (SNAP‐25) required for exocytosis, resulting in blockade of acetylcholine release from the presynaptic terminal and inhibition of muscle contraction. 4 , 5 The effects of botulinum toxin are typically seen between 2 and 5 days following injection, with maximal effect achieved in 1–2 weeks. The duration of the effect has been variably described in the literature, possibly owing to differences in formulation, injection site, dosage, and outcome measures, but effects typically begin to wear off around 3–4 months. 6 There is no definitive consensus on the mechanism of toxin metabolism and the ebbing of its effects, although the regeneration of SNAP‐25 seems to play a role in recovery of muscle function. 1
PRODUCT FORMULATIONS
Currently, there are four formulations of botulinum toxin A that are approved by the Food and Drug Administration (FDA) for clinical use (Table 1). These are OnabotulinumtoxinA (Botox), AbobotulinumtoxinA (Dysport), IncobotulinumtoxinA (Xeomin), and DaxibotulinumtoxinA‐lanm (Daxxify), which differ by the purification method used in production. Safety profiles and rates of adverse effects have been reported to be similar between different formulations. 5 , 10 Prior studies comparing Botox and Dysport have reported conflicting results in the difference in effectiveness between the two products. Lowe et al. 11 , 12 showed in two randomized trials that Botox provides greater and prolonged effects for the treatment of glabellar lines. However, these trials had notable limitations in power and randomization. 13 Conversely, Michaels et al. 7 and Lowe et al. 14 reported superior effects of Dysport compared to Botox for the treatment of upper face lines and forehead wrinkles. However, this finding may have resulted from a disproportionate dose of Dysport used (four units Dysport to one unit Botox and three units Dysport to one unit Botox, respectively). When a ratio of 2:1 and 2.5:1 of Dysport to Botox was used, there was a similar efficacy in the treatment of glabellar lines and upper face lines. 8 , 15 At present, there are no trials that directly compare Xeomin to other formulations.
Table 1.
Summary of botulinum toxin A formulations approved for clinical use.
| Trade name | Generic name | Serotype | Equivalent dose relative to Botox (Units) | Approved indications by Food and Drug Administration |
|---|---|---|---|---|
| Botox | OnabotulinumtoxinA | Exotoxin A | 1.0 | Forehead, glabella, crow's feet |
| Dysport | AbobotulinumtoxinA | Exotoxin A | 2.0–2.5 7 , 8 | Glabella |
| Xeomin | IncobotulinumtoxinA | Exotoxin A | 1.0–1.5 | Glabella |
| Daxxify | DaxibotulinumtoxinA‐Ianm | Exotoxin A | 2.0 9 | Glabella |
In September 2022, Revance Therapeutics Inc, announced FDA approval for Daxxify (DaxibotulinumtoxinA‐Ianm) for use for injection of glabellar lines. Their Phase 3 clinical trial included 2700 patients who underwent nearly 4200 treatment injections. In the study, 40 U of Daxxify was used to treat glabellar rhytids. The median duration of effect was 6 months, with some patients having results lasting up to 9 months. Daxxify was well‐tolerated in these trials and had a safety profile similar to current neuromodulators in the market. 9 , 16 Their study was limited due to a predominantly Caucasian female patient population with each patient only receiving a single treatment. 16 However, the increased duration of the effect for Daxxify could make it a unique and useful addition to the armamentarium of neuromodulators available to providers, although this will remain to be seen once it becomes available for widespread clinical use.
PREPROCEDURE COUNSELING
Before treatment, patients should be thoroughly counseled on expectations, risks, and postprocedure care. The specific areas on the face to be treated should be elicited directly from the patient, rather than assumed by the provider. The senior author has each patient point out using a mirror and cotton‐tip applicator particular areas of concern on their face. Oftentimes, patients may seek results that cannot be fully addressed by neuromodulator injection and are better achieved or augmented through other treatments such as fillers, skin resurfacing, or surgical procedures. Educating patients on the expected effects and limitations of neuromodulators is crucial to achieving patient satisfaction.
Contraindications to botulinum toxin injection include known allergies or prior adverse reactions, history of keloids, and history of neuromuscular disease (e.g. myasthenia gravis, Lambert‐Eaton, amyotrophic lateral sclerosis). Patients taking aminoglycosides, muscle relaxants, or anticholinergics should avoid treatment with botulinum toxin due to the risk of potentiation of muscle weakness. 3 Given the lack of strong evidence regarding safety of neuromodulator treatment in pregnant or breastfeeding women, the senior author also avoids treatment in these patients. Once a treatment plan is established, the patient must also be educated on treatment risks and post‐procedural care. Risks include injection site pain, bruising, bleeding, allergic reaction, and unsatisfactory functional or cosmetic outcomes, the latter of which will be discussed in more detail in the following sections.
Most postprocedure care recommendations following botulinum toxin injection are anecdotally based and have limited supporting evidence in the literature. 13 Common recommendations are typically aimed at reducing pain and bruising following injection, as well as preventing unwanted spread of toxin to muscles near the areas of injection. While some providers warn against massage and manipulation of facial muscles after injection to reduce the risk of this spread, others advocate for massage to increase uptake of toxin. 17 Interestingly, Wei et al. noted that in patients who underwent masseter injections with botulinum toxin, those who were instructed to increase masticatory effort following injection experienced a more prolonged reduction in masseter thickness and volume, suggesting a possible benefit of increased activity to treated muscles following injection. 18 For postprocedure care, the senior author typically recommends avoidance of facial massage or manipulation, lying flat, strenuous activity or exercise, or any activity that creates significant vasodilation (e.g., taking a hot bath or using a sauna) for several hours following treatment. Frozen gel‐packs are offered to patients after injection and can be used if the patient desires.
PROCEDURAL CONSIDERATIONS
Drug reconstitution and preparation
Botulinum toxin is supplied as a vacuum‐dried powder and is reconstituted using sterile injectable saline. Several studies have shown improved patient comfort with injection using saline‐containing preservatives as compared to preservative‐free saline. 19 , 20 , 21 , 22 , 23 There is also mixed evidence regarding whether aggressive versus gentle reconstitution can impact drug potency and outcomes. 24 , 25 The authors recommend against shaking the drug vial during reconstitution, at least to prevent formation of air bubbles that make it difficult to completely draw up the reconstituted solution into a syringe.
Reconstitution can be performed at various concentrations. Two of the largest randomized studies comparing the effects of different concentrations of Botox showed no difference in reduction of rhytids in the lateral orbit 22 or glabella. 26 However, a smaller randomized study reported a larger area of effect for a larger volume injection of equivalent dose. 27 The senior author reconstitutes Botox using sterile saline with preservative at a concentration of 50 units/mL. The same concentration is used for Xeomin, while Dysport is reconstituted at a three‐fold higher concentration (150 units/mL) to account for its relative difference in potency. Injections are performed through a 32‐gauge needle on a 1 mL syringe. In the author's experience, the small gage needle minimizes injection pain but is quicker to blunt with repeated injections. For this reason, a fresh needle is used after every 6–10 injection sites.
After reconstitution. Botox, Dysport, and Xeomin manufacturers recommend that they be administered with 24 h and to be stored in a refrigerator at 2°C–8°C until administered. Yang et al. 28 reviewed the efficacy of Botox that was used immediately after reconstitution and 2 weeks after reconstitution while being stored at 4°C and 20°C in the treatment of horizontal forehead rhytids. They found there was no difference in timing of onset, duration of action, or measurable reduction of forehead movement between the three samples. 28 Similar studies have been performed suggesting the continued efficacy of botulinum toxin A after reconstitution and storage in a refrigerated setting. 29 , 30
Common areas of treatment
A thorough understanding of facial anatomy and musculature is critical to achieving optimal results. Moreover, patients will differ in terms of muscular strength, bulk, positioning, and orientation, as well as other important factors such as skin and aging. Consequently, there is no single injection pattern or map that will work for all patients. The injector must ask the patient to contract the target muscles or muscle groups to appreciate the full extent of the area to be treated and determine the appropriate injection sites and dosage. The following section highlights important considerations in common areas of treatment. For the sake of simplicity, the dosages reported below are in Botox and Xeomin equivalent units. Dysport equivalent dosages can be derived by multiplying by a factor of three.
Frontalis
The frontalis muscle is responsible for brow elevation and the formation of horizontal rhytids on the forehead. Due to the relatively large area covered by the muscle, multiple small‐dose injections of 1.5–2.5 units are typically used to minimize the appearance of these rhytids symmetrically and uniformly. When treating the forehead, the patient's hairline and forehead height must be taken into consideration. Patients with a short forehead and low hairline may only require a single row of injections, while those with a high hairline may require multiple rows. In older patients, frontalis hyperfunction may be a compensatory mechanism for brow ptosis, which can be unmasked and become symptomatic if the brow is overtreated. This is especially true for the lateral brow, which can create visual hooding and unsightly sagging if injections are performed too low or at too high a dose. For this reason, the authors recommend avoiding injections within 2 cm above the lateral 1/3 of the eyebrow, especially in older and female patients. At the same time, the lateral forehead cannot be ignored altogether, as undertreatment in this area relative to the central forehead can create an unnatural “Spock” appearance. In the author's experience, a single injection of 2.5 units 2 cm above the highest point of the lateral brow is sufficient to avoid this deformity. As with any part of the face, treatment of a muscle or muscle group will not only reduce movement in the area affected by the toxin but will also highlight movement in areas that are undertreated. This is especially apparent for the frontalis given its wide area of action.
Glabella
The glabellar muscles (corrugator, depressor supercilia, and procerus), are responsible for brow depression and medialization. Treatment of this area is targeted towards the vertical scowl lines between the brows, as well as horizontal rhytids above the nasal root. Injection sites at the glabella typically take on a v‐shaped orientation in 2.5–5.0 unit aliquots. The angulation and degree of lateral extension of the “v,” however, are entirely dependent on the size, shape, and orientation of the underlying brow depressors, which can vary widely between patients. The lateral extent of corrugator action can be ascertained when the patient is asked to scowl, and injections planned accordingly. In patients with a heavy horizontal rhytid above the nasal root, an additional injection can be placed in the midline below the tip of the “v” to target the inferior aspect of the procerus. Because the brow depressors work antagonistically with the frontalis, treatment of these two areas should be balanced; overtreating the frontalis relative to the depressors will result in lowering the brow, and vice versa. The injector should note that medially, injections should be targeted deeper through the frontalis where the bellies of the depressor muscles originate, while lateral injections should be aimed more superficially at their subdermal attachments. Furthermore, injections should be placed at least 1 cm above the orbital rim to minimize the risk of drug diffusion across the orbital septum, which can lead to levator hypofunction and blepharoptosis. Last, a higher overall dose is often required in male versus female patients in this area, due to a more stark difference in muscle bulk at the brow.
Crow's feet
Contraction of the lateral orbicularis oculi causes radially oriented rhytids of the lateral eyelids known commonly as Crow's feet or smile lines. Given the thin skin and musculature in this area, injections are typically placed more superficially in the subdermal plane. Injection sites are positioned in an arc around the lateral orbital rim in 2.5 unit aliquots, with the extent and number of injections determined by the extent of rhytids visible during contraction. Injections should be placed at least 1 cm away from the orbital rim to avoid lagophthalmos as well as diffusion through the septum which can affect the lid retractors as well as other intraorbital musculature. Care should be taken to not overtreat the inferior aspect of the Crow's feet, as contraction of the orbicularis oculi here contributes to a sincere‐appearing Duchenne smile. Furthermore, inferior injections should meticulously follow the curvature of the orbital rim, as injections placed too low may affect midface musculature and lead to asymmetric lip movement.
Chemical brow lift
By combining the treatment of the brow depressors of the superolateral orbicularis oculi, a subtle browlift can be achieved. The brow depressors of the glabella are treated as noted above, while the orbicularis is treated with 1.5–2.5 unit injections in 2–3 points along the lateral extent of the eyebrow. These lateral injections are placed superficially, following the curvature of the lateral brow (Figure 1). The injector's free hand can be used to elevate the lateral brow such that the needle enters the skin at least 1 cm away from the orbital rim to avoid the complications noted above. 25 Patients should be counseled that the lift provided will be subtle in comparison to surgical correction, and the injector should keep in mind that any concurrent treatment of the frontalis should be done conservatively, as this can easily negate the effects of a chemical browlift.
Figure 1.
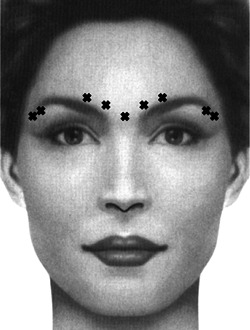
Example injection sites for chemical browlift.
Depressor anguli oris
To address drooping of the corner of the mouth, which contributes to the appearance of aging, the depressor anguli oris can be treated to create a subtle lift of the oral commissures. A single 2.5–5.0 unit injection may be placed at a point traced 1 cm lateral and then 1 cm down from the oral commissure (Figure 2). 31 If a greater effect is desired, a second injection may be performed just inferolateral to the first. Care should be taken to stay at least 1 cm lateral to the oral commissures with these injections to avoid affecting the depressor labii inferioris, leading to asymmetric lower lip depression and asymmetric dental show.
Figure 2.
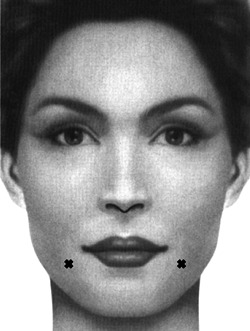
Injection sites for depressor anguli oris.
Upper lip lift
The upper orbicularis oris can be treated to negate its pull against the lip elevators, thus creating a slight lift of the upper lip. The author performs these injections in four locations along the vermilion border, at the philtral peaks as well as about 1/3 of the distance from the philtral peaks to the oral commissures (Figure 3). Patients should be counseled that due to reduced orbicularis oris function, they may have difficulty with activities that require puckering (e.g., sucking through a straw, whistling). Furthermore, injections in this area can be especially sensitive and as such, proper counseling and preapplication of a topical anesthetic may aid in patient comfort and compliance.
Figure 3.

Injection sites for upper lip lift.
Masseter
The masseter muscle contributes significantly to the shape of the lower face and jawline. The use of botulinum toxin in the masseter has been reported for the treatment of refractory bruxism, but it has also been described for cosmetic use in facial contouring. Reduction of masseteric volume can lead to a slimmer‐appearing face and more defined jawline. The belly of the masseter can be easily palpated just above the angle of the mandible when the patient is asked to bite down. The senior author places three injections aimed deep into the muscle where most of the bulk is palpated on contraction. During injection, an imaginary line should be drawn from the inferior aspect of the tragus to the ipsilateral oral commissure. Injections should not be placed near or above this line to avoid diffusion to the midfacial musculature (Figure 4). Furthermore, care should be taken to avoid placing injections too superficial or medial, as both the platysma and risorius can be affected leading to facial asymmetry. Finally, a sufficiently long needle should be used for this injection to reach the deep belly of the muscle, since asymmetric bulging has been reported from injections limited to the superficial masseter. 30 The author typically initiates treatment with a conservative dose of 20–30 units per side. If the patient tolerates this well and desires a more pronounced effect, the dose can be increased up to double that amount. When planning subsequent injections, the injector should keep in mind that repeated treatment of the masseter leads to cumulative volume loss, and so an increased dose is not always necessary to achieve more facial slimming.
Figure 4.
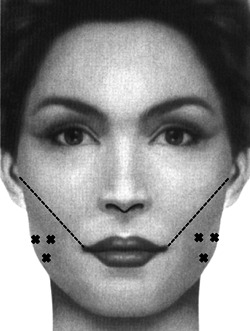
Injection sites for treatment of the masseter. Injections should be kept below a line connecting the inferior border of the tragus and the oral commissure.
PITFALLS AND COMPLICATIONS
The most common complications of Botulinum toxin injection are fortunately mild and consist of bruising and protracted pain at the injection site. 32 , 33 Dissatisfaction with the cosmetic outcome typically involves one of the following: (1) Undertreatment or imbalanced treatment, which manifests as persistent rhytids, asymmetry, or an unnatural appearance (e.g., Spock eyebrows) (2) Overtreatment, which results in excessive hypofunction (e.g., loss of Duchenne smile, brow ptosis), and (3) Diffusion of toxin to an unintended muscle (e.g., upper eyelid ptosis from impaired levator function, asymmetric smile from impaired zygomaticus or risorius). These complications are often technique‐dependent and decline in incidence with injector skill and experience. 34 , 35
In certain cases of persistent rhytids or facial asymmetry, additional injections may be performed. Since the effect of botulinum toxin continues to increase 1–2 weeks following injection, patients who notice unsatisfactory effects or asymmetry in the first few days after treatment should be counseled to wait several days before pursuing additional injections.
In cases of overtreatment or unintended drug diffusion, the best course of action is often to provide reassurance and close follow‐up for the patient. Since the effects of botulinum toxin are temporary, unsatisfactory cosmesis resulting from neuromodulator injection is reversible by default. If blepharoptosis occurs due to diffusion of the drug to the levator, prescription apraclonidine 0.5% may be used to stimulate Müller's muscle and improve lid elevation. One to two drops may be placed three times daily for the duration of lid ptosis. Caution should be exercised in patients with glaucoma and are glaucoma‐suspect, as apraclonidine may increase intraocular pressure. 36 Oxymetazoline HCl 0.1% (Upneeq) has also been FDA approved for the treatment of acquired blepharaoptosis. In two randomized phase III trials, there was a statistically significant improvement in subjects' visual fields and eyelid elevation when treated with one drop to the affected eye daily. 37 , 38
In rare cases, patients may develop neutralizing IgG antibodies against botulinum toxin, resulting in decreased treatment effect and duration. 39 Overall, the incidence of neutralizing antibodies is reported between 0.3%–6.0% for all uses of neuromodulatora. However, it is seen at a lower rate for cosmetic applications and its incidence is not well established. 39 , 40 If neutralizing antibodies are suspected in patients who stop responding to treatment, an alternative formulation/brand can be often used with success.
AUTHOR CONTRIBUTIONS
Both authors contributed meaningfully to the review of literature, review of current techniques, and the authorship of this manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
None.
ACKNOWLEDGMENTS
None.
Johnson AJ, Chen DS. Office‐based facial plastics procedures: neuromodulators. World J Otorhinolaryngol Head Neck Surg. 2023;9:220‐226. 10.1002/wjo2.121
DATA AVAILABILITY STATEMENT
The data and information reviewed in this article can be accessed through the citation listed below. No independent data was collected by the authors in this review paper.
REFERENCES
- 1. Montecucco C, Molgó J. Botulinal neurotoxins: revival of an old killer. Curr Opin Pharmacol. 2005;5:274‐279. [DOI] [PubMed] [Google Scholar]
- 2. Carruthers A, Carruthers J. History of the cosmetic use of Botulinum A exotoxin. Dermatol Surg. 1998;24:1168‐1171. [DOI] [PubMed] [Google Scholar]
- 3. Blitzer A, Brin MF, Keen MS, Aviv JE. Botulinum toxin for the treatment of hyperfunctional lines of the face. Arch Otolaryngol Head Neck Surg. 1993;119:1018‐1022. [DOI] [PubMed] [Google Scholar]
- 4. Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3‐9. [DOI] [PubMed] [Google Scholar]
- 5. Samizadeh S, De Boulle K. Botulinum neurotoxin formulations: overcoming the confusion. Clin Cosmet Investig Dermatol. 2018;11:273‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wright G, Lax A, Mehta SB. A review of the longevity of effect of botulinum toxin in wrinkle treatments. Br Dent J. 2018;224:255‐260. [DOI] [PubMed] [Google Scholar]
- 7. Michaels BM, Csank GA, Ryb GE, Eko FN, Rubin A. “Prospective randomized comparison of onabotulinumtoxinA (Botox) and abobotulinumtoxinA (Dysport) in the treatment of forehead, glabellar, and periorbital wrinkles. Aesthet Surg J. 2012;32:96‐102. [DOI] [PubMed] [Google Scholar]
- 8. Rzany B, Nast A. Head‐to‐head studies of botulinum toxin A in aesthetic medicine: which evidence is good enough. J Am Acad Dermatol. 2007;56:1066‐1067. [DOI] [PubMed] [Google Scholar]
- 9. Carruthers JD, Fagien S, Joseph JH, et al. DaxibotulinumtoxinA for injection for the treatment of glabellar lines: results from each of two multicenter, randomized, double‐blind, placebo‐controlled, phase 3 studies (SAKURA 1 and SAKURA 2). Plast Reconstr Surg. 2020;145:45‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walker TJ, Dayan SH. Comparison and overview of currently available neurotoxins. J Clin Aesthet Dermatol. 2014;7:31‐39. [PMC free article] [PubMed] [Google Scholar]
- 11. Lowe PL, Patnaik R, Lowe NJ, Lowe PL, Patnaik R, Lowe NJ. A comparison of two botulinum type a toxin preparations for the treatment of glabellar lines: double‐blind, randomized, pilot study. Dermatol Surg. 2005;31:1651‐1654. [DOI] [PubMed] [Google Scholar]
- 12. Lowe P, Patnaik R, Lowe N. Comparison of two formulations of botulinum toxin type A for the treatment of glabellar lines: a double‐blind, randomized study. J Am Acad Dermatol. 2006;55:975‐980. [DOI] [PubMed] [Google Scholar]
- 13. Dover JS, Monheit G, Greener M, Pickett A. Botulinum toxin in aesthetic medicine: myths and realities. Dermatol Surg. 2018;44:249‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lowe NJ, Shah A, Lowe PL, Patnaik R. Dosing, efficacy and safety plus the use of computerized photography for botulinum toxins type A for upper facial lines. J Cosmetic Laser Ther. 2010;12:106‐111. [DOI] [PubMed] [Google Scholar]
- 15. Karsai S, Adrian R, Hammes S, Thimm J, Raulin C. A randomized double‐blind study of the effect of Botox and Dysport/Reloxin on forehead wrinkles and electromyographic activity. Arch Dermatol. 2007;143:1447‐1449. [DOI] [PubMed] [Google Scholar]
- 16. Green JB, Mariwalla K, Coleman K, et al. A large, open‐label, phase 3 safety study of DaxibotulinumtoxinA for injection in glabellar lines: a focus on safety from the SAKURA 3 study. Dermatol Surg. 2021;47:42‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rzany B, Fratila AA, Fischer TC, et al. Recommendations for the best possible use of botulinum neurotoxin type a (Speywood units) for aesthetic applications. J Drugs Dermatol. 2013;12:80‐84. [PubMed] [Google Scholar]
- 18. Wei J, Xu H, Dong J, Li Q, Dai C. Prolonging the duration of masseter muscle reduction by adjusting the masticatory movements after the treatment of masseter muscle hypertrophy with botulinum toxin type a injection. Dermatol Surg. 2015;41(suppl 1):S101‐S109. [DOI] [PubMed] [Google Scholar]
- 19. Alam M. Pain associated with injection of botulinum A exotoxin reconstituted using isotonic sodium chloride with and without preservative: a double‐blind, randomized controlled trial. Arch Dermatol. 2002;138:510‐514. [DOI] [PubMed] [Google Scholar]
- 20. Sarifakioglu N, Sarifakioglu E. Evaluating effects of preservative‐containing saline solution on pain perception during botulinum toxin type‐a injections at different locations: a prospective, single‐blinded, randomized controlled trial. Aesthetic Plast Surg. 2005;29:113‐115. [DOI] [PubMed] [Google Scholar]
- 21. Kwiat DM, Bersani TA, Bersani A. Increased patient comfort utilizing botulinum toxin type a reconstituted with preserved versus nonpreserved saline. Ophthal Plastic Reconstruct Surg. 2004;20:186‐189. [DOI] [PubMed] [Google Scholar]
- 22. Allen SB, Goldenberg NA. Pain difference associated with injection of abobotulinumtoxinA reconstituted with preserved saline and preservative‐free saline: a prospective, randomized, side‐by‐side, double‐blind study. Dermatol Surg. 2012;38:867‐870. [DOI] [PubMed] [Google Scholar]
- 23. Kazim NA, Black EH. Botox: shaken, not stirred. Ophthal Plast Reconstruct Surg. 2008;24:10‐12. [DOI] [PubMed] [Google Scholar]
- 24. Dressler D, Eleopra R. Clinical use of non‐A botulinum toxins: botulinum toxin type B. Neurotoxic Res. 2006;9:121‐125. [DOI] [PubMed] [Google Scholar]
- 25. Dressler D, Bigalke H. Reconstituting botulinum toxin drugs: shaking, stirring or what. J Neural Transm. 2016;123:523‐525. [DOI] [PubMed] [Google Scholar]
- 26. Carruthers A, Bogle M, Carruthers JD, et al. A randomized, evaluator‐blinded, two‐center study of the safety and effect of volume on the diffusion and efficacy of botulinum toxin type A in the treatment of lateral orbital rhytides. Dermatol Surg. 2007;33:567‐571. [DOI] [PubMed] [Google Scholar]
- 27. Carruthers A, Carruthers J, Cohen J. Dilution volume of botulinum toxin type A for the treatment of glabellar rhytides: does it matter. Dermatol Surg. 2007;33:S97‐S104. [DOI] [PubMed] [Google Scholar]
- 28. Yang GC, Chiu RJ, Gillman GS. Questioning the need to use Botox within 4 hours of reconstitution: a study of fresh vs 2‐week‐old Botox. Arch Facial Plast Surg. 2008;10:273‐279. [DOI] [PubMed] [Google Scholar]
- 29. Hexsel DM, De Almeida AT, Rutowitsch M, et al. Multicenter, double‐blind study of the efficacy of injections with botulinum toxin type A reconstituted up to six consecutive weeks before application. Dermatol Surg. 2003;29:523‐529. [DOI] [PubMed] [Google Scholar]
- 30. Hexsel D, Rutowitsch MS, de Castro LCM, do Prado DZ, Lima MM. Blind multicenter study of the efficacy and safety of injections of a commercial preparation of botulinum toxin type A reconstituted up to 15 days before injection. Dermatol Surg. 2009;35:933‐940. [DOI] [PubMed] [Google Scholar]
- 31. Hsu TSJ, Dover JS, Arndt KA. Effect of volume and concentration on the diffusion of botulinum exotoxin A. Arch Dermatol. 2004;140:1351‐1354. [DOI] [PubMed] [Google Scholar]
- 32. Swift A, Green JB, Hernandez CA, et al. Tips and tricks for facial toxin injections with illustrated anatomy. Plast Reconstruct Surg. 2022;149:303e‐312e. [DOI] [PubMed] [Google Scholar]
- 33. Witmanowski H, Błochowiak K. The whole truth about botulinum toxin ‐ a review. Adv Dermatol Allergol. 2020;37:853‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matarasso SL. Complications of botulinum A exotoxin for hyperfunctional lines. Dermatol Surg. 1998;24:1249‐1254. [DOI] [PubMed] [Google Scholar]
- 35. Cox SE, Adigun CG. Complications of injectable fillers and neurotoxins. Dermatol Ther. 2011;24:524‐536. [DOI] [PubMed] [Google Scholar]
- 36. Klein AW. Complications, adverse reactions, and insights with the use of botulinum toxin. Dermatol Surg. 2003;29:549‐556. [DOI] [PubMed] [Google Scholar]
- 37. Nestor MS, Han H, Gade A, Fischer D, Saban Y, Polselli R. Botulinum toxin–induced blepharoptosis: anatomy, etiology, prevention, and therapeutic options. J Cosmet Dermatol. 2021;20:3133‐3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Slonim CB, Foster S, Jaros M, et al. Association of oxymetazoline hydrochloride, 0.1%, solution administration with visual field in acquired ptosis: a pooled analysis of 2 randomized clinical trials. JAMA Ophthalmol. 2020;138:1168‐1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Torres S, Hamilton M, Sanches E, Starovatova P, Gubanova E, Reshetnikova T. Neutralizing antibodies to botulinum neurotoxin type A in aesthetic medicine: five case reports. Clin Cosmet Investig Dermatol. 2014;7:11‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shtefan V, Fletcher J, Duclos OA. Causes of botulinum toxin treatment failure. Clin Cosmet Investig Dermatol. 2022;15:1045‐1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and information reviewed in this article can be accessed through the citation listed below. No independent data was collected by the authors in this review paper.


