Highlights
-
•
The tumor mutation burden (ΤΜΒ) is the leading and most promising predictive biomarker for the efficacy of immune checkpoint inhibition (ICI) therapies in non-small cell lung cancer (NSCLC).
-
•
The overall efficiency of anti-PD-1/PD-L1 and anti-CTLA-4 monoclonal antibodies remains unsatisfactory, urging for the investigation of novel inhibitors, such as those targeting LAG-3, TIM-3, TIGIT and VISTA, which could be used either as a monotherapy or synergistically with the PD-1/PD-L1 or CTLA-4 blockers.
-
•
PD-L1+/IHC, MSI/dMMR, tumor infiltrating lymphocytes (TILs), as well as the role of the microbiome and circulating tumor DNA (ctDNA) can be used as further biomarkers that can be used in lung cancer response to immunotherapy.
Keywords: Tumor mutation burden (TMB), Immune checkpoint inhibition therapy, Patient response, Tumor-infiltrating lymphocytes, Tumor microenvironment
Abstract
Immune checkpoint inhibition (ICI) therapies have reshaped the therapeutic landscape in lung cancer management, providing first-time improvements in patient response, prognosis, and overall survival. Despite their clinical effectiveness, variability in treatment responsiveness, as well as drug resistance, have led to a compelling need for predictive biomarkers facilitating the individualized selection of the most efficient therapeutic approach. Significant progress has been made in the identification of such biomarkers, with tumor mutation burden (ΤΜΒ) appearing as the leading and most promising predictive biomarker for the efficacy of ICIs in non-small cell lung cancer (NSCLC) among other tumors. Anti-PD-1/PD-L1 and anti-CTLA-4 antibodies have been extensively studied and clinically utilized. However, the overall efficiency of these drugs remains unsatisfactory, urging for the investigation of novel inhibitors, such as those targeting LAG-3, TIM-3, TIGIT and VISTA, which could be used either as a monotherapy or synergistically with the PD-1/PD-L1 or CTLA-4 blockers. Here, we investigate the role of TMB and cancer neoantigens as predictive biomarkers in the response of lung cancer patients to different ICI therapies, specifically focusing on the most recent immune checkpoint inhibitors, against LAG-3, TIM-3, TIGIT and VISTA. We further discuss the new trends in immunotherapies, including CAR T-cell therapy and personalized tumor vaccines. We also review further potential biomarkers that could be used in lung cancer response to immunotherapy, such as PD-L1+ IHC, MSI/dMMR, tumor infiltrating lymphocytes (TILs), as well as the role of the microbiome and circulating tumor DNA (ctDNA). Finally, we discuss the limitations and challenges of each.
Graphical abstract
Introduction
Lung cancer is the leading cause of tumor-related mortality worldwide with its poor prognosis attributed to lack of adequate screening methods and subsequent diagnosis of the disease in advanced stages [1,2].
Based on morphology, lung cancer is traditionally classified into non-small cell lung cancer (NSCLC) (80–85 % of the cases) and small cell lung cancer (SCLC)(15–20 %). In terms of histological classification, the most common subtype of NSCLC is adenocarcinoma (LUAD) with a 40 % reported incidence, while squamous cell lung carcinoma (LUSC) comprises ∼20–30 % of the cases [3]. The majority of NSCLC cases (47 %) are diagnosed at advanced stages (stages III/IV), when lymph node invasion and distant metastasis to other organs is usually present and the median survival is ∼18 months. Only < 20 % of the tumors are diagnosed at an early stage, when the tumor is still localized without regional lymph node involvement. The histological classification of lung cancer is crucial due to the different therapeutic approaches that can be followed based on the histological type. Specifically, for LUSC, some treatment options, such as bevacizumab, might have potential side effects [4]. The WHO classification of lung tumors was revised in 2015 and sub-classifications, were incorporated, based on specific genetic and immunohistochemical aspects [5]. Significant revisions were further made for LUAD and large cell carcinoma, with large cell carcinoma diagnosis being established as lacking of morphological and immunohistochemical signs of differentiation from resected tumors. Furthermore, LUSC is classified as keratinizing, non-keratinizing and basaloid, while lymphoepithelial carcinoma is also considered to be squamous cell carcinoma. LUSC belongs to the category of neuroendocrine tumors and comprises sarcomatous carcinomas, including pleomorphic carcinoma, pulmonary blastoma and carcinosarcoma. Finally, the diagnosis of lung adenocarcinoma and large cell carcinoma should not be made, but these cases are not specified in NSCLC.
In respect to the epidemiological and demographic characteristics, adenocarcinomas are more prevalent among women, especially the epidermal growth factor (EGFR) mutations (mut)-positive adenocarcinomas. Conversely, squamous cell carcinomas are more commonly associated with males. Risk factors highly associated with the etiology of lung cancer include tobacco smoking, occupational and environmental exposure to carcinogens, genetic predisposition (family history) and other risk factors, such as pulmonary scarring, previous irradiation through radiation therapy or other, pulmonary fibrosis and chronic infections (e.g., HIV, tuberculosis). Most NSCLC patients display advanced disease at the time of diagnosis, and they consequently appear with poor prognosis [6]. Tumor growth and invasiveness in NSCLC is highly acquired by driver genes mutations. The pathophysiology behind the onset of lung carcinogenesis lies in molecular and cellular components of the lung tumor microenvironment (TME) [7]. Altogether, both exogenous (tobacco smoke, UV light and iatrogenic and prior radiotherapy exposure) and endogenous factors, such as deficiency in the mismatch repair system (dMMR), homologous recombination (HR) and base excision repair (BER), can cause the accumulation of further DNA damage and subsequently lead to the accumulation of more mutations in the tumor cells that contribute to lung carcinogenesis. It is well established that lung cancers have the highest somatic mutation burden among all solid tumors, due to a continuous direct exposure to the above-mentioned mutagenic agents [7].
The therapeutic modalities used for lung cancer treatment range from conventional chemotherapy, radiotherapy, surgical removal to novel target therapies. The success of lung cancer management depends on the stage of cancer at diagnosis (TNM staging). In addition, the TME has been the target for novel therapeutic approaches by identifying molecular pathways and specific cell types to find potential mechanisms for attacking tumor cells. Various drugs targeting TME components in lung cancer are currently undergoing clinical trials for their use as potential targeted therapies. The complex and heterogeneous lung tumor microenvironment components can serve as targets for immunotherapy.
Immunogenicity and tumor microenvironment in lung cancer
The cellular composition of the TME is responsible for the generation of crucial carcinogenetic hallmarks, which include tumor proliferation, angiogenesis, invasion and metastasis. The TME also offers a significant resource of immunotherapeutic targets. The complex microenvironment of lung tumors favors the development of primary lung cancer, as well as metastasis from intrathoracic neoplasms. In primary lung carcinoma, the altered TME landscape has been extensively reported as a significant determinant of tumor generation and progression, due to its increased heterogeneity of varying cellular components that interact with the cancer cells. The physiological lung microenvironment is composed of smooth muscle cells, endothelial cells, fibroblasts, as well as alveolar macrophages and dendritic cells (DCs) [7].
The alveolar cell walls responsible for gas exchange and immune cell infiltration are lined by vascular capillaries consisting of mostly smooth muscle cells and endothelial cells. A variety of cellular features is observed in each anatomical structure of the lungs; with the proximal airways consisting of secretory club cells, mucus-producing cells, neuroendocrine cells and undifferentiated basal cells. Furthermore, the distal airways are composed mainly of alveolar type I and type II cells [8]. Immunological homeostasis in the lung microenvironment is mostly maintained by alveolar macrophages. However, these can also be responsible for the development of inflammatory and pre-malignant processes, as demonstrated in various in vivo primary studies [9]. In addition, inflammatory states, including chronic obstructive pulmonary disease (COPD), have been associated with increased risk of lung cancer development, due to the remodeling of the airway epithelium landscape in combination with the increased expression of inflammatory mediators, leading to altered inflammatory cells [10]. Physiologically, once pathogens invade through the lung mucosal barriers, tissue-resident memory T cells residing in the lung airways are activated and provide immunity to potential infections [11]. The inactivation of cytotoxic CD8+ cells through inhibitory immunological pathways serves as a significant hallmark of immunosuppression in the TME.
Tumor cells lead to the stimulation of molecular and cellular alterations in the host's healthy tissues leading to an emerging tumor microenvironment which continuously changes. The TME is comprised of heterogeneous cell types such as immune cells, stromal cells, fibroblasts, endothelial cells, and the extracellular matrix (ECM). The composition of each TME varies according to the tumor type, while it also can promote cancer progression and act dynamically in conjunction with the cancer cells during the earlier tumor growth stages. Angiogenesis is enabled by the TME to overcome the acidic and hypoxic environment, which does not favor further tumor growth [7]. Therefore, with the promotion of angiogenesis the TME ensures the restoration of oxygen and nutrient supply, as well as the removal of metabolic waste. The tumor cells become infiltrated with a wide variety of innate and adaptive immune cells, which have a significant anti-cancerogenic effect able to either suppress tumor formation or on the contrary promote tumor growth. The factor determining whether the immune cells in the TME will be pro- or anti-tumorigenic, is mostly the tumor type and the context of the microenvironment [12].
The TME is composed of both innate and adaptive immune cells, which appear to be tumor-suppressing but also tumor-promoting. Innate immunity operates based on the cellular receptors identifying broad pathogen-related patterns and signals. The innate immune cells carrying out the response to the identified antigens, are granulocytes, including neutrophils, eosinophils, and basophils, Natural Killer (NK) cells, mast cells, antigen presenting cells (APCs), dendritic cells (DCs) expressing MHC class II and Fc receptors, as well as monocytes and macrophages [12]. The innate immune system also comprises from other host defense mechanisms, such as physical and biochemical barriers including skin or mucous membranes and most importantly humoral defenses associated with acute phase proteins (e.g., CRP, ESR, transferrin, albumin, and antithrombin) and the complement system [7].
Finally, the innate immunity is developed in-utero and there is no genomic imprinting or adaptation required to specific antigens. In contrast, adaptive (or acquired) immunity provides an antigen-specific response against the detected pathogen and involves mostly B cells, T cells and antibodies that specifically target pathogens. Immunologic memory is a mechanism of adaptive immunity by which memory B and T cells are formed. These are antigen-specific cells that have a rapid and more efficient response than typical lymphocytes when re-exposed to an antigen. Another important component determining carcinogenesis is the extracellular matrix (ECM), composed of collagen, proteoglycans and glycosaminoglycans. The ECM promotes carcinogenesis through the mediation of interactions between cancer cells and stromal cells. As a result, targeting the ECM would potentially have therapeutic importance, by promoting T cell infiltration into the TME, thus facilitating anti-tumor activity [7].
Effector and inhibitory immune cells
Effector immune cells
CD8+ T cells
The TME is comprised of an extensive population that plays a deterministic role in tumorigenesis (Fig. 1). Each T cell has a specific T cell receptor (TCR) able to recognize a specific pathogen. Cytotoxic T cells (CD8+) are responsible for the detection of tumor antigens expressed on cancer cells, which target them leading to their destruction. CD8+ T cells play a critical role in the adaptive immune reactions directed against immune responses towards tumors. In order to exhibit their cytokine-secreting and cell-killing agent activities, they undergo a complex series of pathways [7,13]. This process involves the activation of CD8+ T cells (CTLs) precursors, which rapidly divide and differentiate into lymphoblasts. These lymphoblasts express Fas ligand (FasL) and contain lytic granules containing perforins and granzymes, which enable them to execute their cytotoxic functions. The destructive impact exerted by CD8+ T cells is primarily attributed to the combined effect of perforin and granzymes. These molecules are released simultaneously upon recognition of specific targets, leading to the formation of an immune synapse, and thus resulting in targeted cell destruction. The perforin/granzyme apoptosis pathway serves as the main signaling route employed by cytotoxic lymphocytes to eradicate cells that are either infected by viruses or have undergone transformation due to malignancy [13,14].
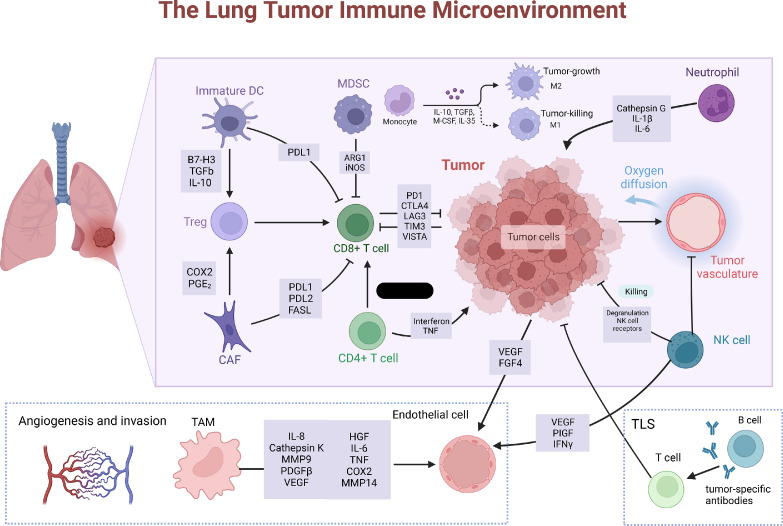
Fig. 1.
Composition of the heterogenous lung tumor microenvironment (TME): reprogrammed stromal cells contributing to immunosuppression. CD8+ T cell inactivation is achieved through diverse pathways. Immature dendritic cells (DCs) produce TGF-β, a protein leading to the expansion of immunosuppressive T-reg cells, which inhibit the activity of CD8+ T cells. DCs express PD-L1, which directly suppresses C8+ T cells. MDSCs express ARG1 and iNOS, contributing to T cell inhibition. Cancer-associated fibroblasts (CAFs) can suppress CD8+ T cells via T-reg induction or expression of PD-L1, PD-L2 and FAS ligand (FASL). Additionally, tumor cells have the ability to generate inhibitory molecules hindering CD8+ tumor-infiltrating lymphocytes, which include cyclooxygenase 2 (COX2), prostaglandin E2 (PGE2), programmed death ligand 1 (PD-L1), and indoleamine 2,3-dioxygenase (IDO). These inhibitory molecules can diminish the efficacy of these lymphocytes in their ability to target and eliminate tumor cells. The induction of angiogenesis is facilitated by the response of endothelial cells to vascular endothelial growth factor (VEGF) originating from various sources, including tumor cells, natural killer (NK) cells, and tumor-associated macrophages (TAMs). TAMs also activate other pathways by achieving this by secreting factors such as interleukin-6 (IL-6), cyclooxygenase 2 (COX2), matrix metalloproteinase 9 (MMP9), and MMP14. NK cells contribute to immune suppression and downregulation of NK receptors, decreased degranulation potential, and reduced expression of interferon-gamma (IFNγ). (Created with BioRender.com).
Natural killer (NK) cells
NK cells elicit an anti-tumor response by naturally killing cancer cells. This destruction is mediated through the perforin/granzyme apoptosis pathway too, similar to the CD8+ T cells. Granzyme B is crucial for this process. NK cells have specific activatory and inhibitory membrane receptors, the balance and mediation of which, determines the outcome of their function. Due to the combination of their very potent anti-tumor activity and anti-inflammatory role, NK cells have a specific research interest in developing immune-oncologic treatments. Their participation in anti-tumor immune responses, includes the destruction of target cells and cytokine production. Within the TME, NK cells interact with tumor cells and other immune cells that control the growth of the tumor. Researchers have reached the conclusion that only a small percentage of NK cells infiltrate the tumors. In addition, the methods used to identify tumor-infiltrating NK cells are disputable [15]. Their presence in the TME, mostly in the stroma of the tumor, may be linked to a better prognosis. In the case of lung cancer, the number of infiltrating NK cells is significantly smaller and thus, it has a poorer prognosis [15]. These results have great interest in the anti-tumor research and have led to the investigation of treatments, which primarily target NK cells and T cells, as most tissues with NK-cell infiltration also show a marked infiltration of T cells. However, within an immunosuppressive tumor microenvironment, there are also inhibitory cells, which may induce the transformation of normal cells and finally lead to dysfunctional NK cells, and tumor escape. The dysfunctional NK cells are supposed to derive from changes in the vascularization in the TME, which restrict access to nutrients and oxygen, resulting in the presence of regions with either transient or permanent hypoxia. This hypoxic environment is considered to be linked with limitations in the functionality of NK cells, by fragmenting their mitochondria. Other studies have associated decreased NK activity with an abundant production of H2O2 in the TME, which leads to a marked apoptosis, having a direct impact on their cytotoxicity [15].
Dendritic cells (DCs)
Dendritic cells appear in three different subsets infiltrating the tumor microenvironment, plasmacytoid DC (pDC), conventional DC (cDC) and inflammatory DC (inf-DC) [16], which either promote the anti-tumor immune response or act in favor of tumorigenesis. The latter happens because tumors frequently develop strategies to alter the development and functions of DCs in the TME [16].
The multiple and complex DC subtypes, as well as their interactions, suggest that many complementary strategies are needed to eradicate cancer in patients going through DC-mediated anti-cancer therapies. Dendritic cells act as tumor presenting cells in the TME. They have a critical anti-tumor role, which relies upon their ability to present tumor-derived antigens to naïve T cells, while infiltrating tumors. The fact that these cells are able to prime anti-tumor T cell immunity, is the major target for breakthrough cancer immunotherapies, which exploit the capacity of DCs to develop and enhance effective anti-tumor immunity. However, as previously mentioned, suppressive signals elicited by the tumors are able to impede the immune-presenting actions of the DCs. Moreover, excessive DC recruitment in the TME can cause T cell tolerance and lead to progressive tumor growth [16].
Chemotherapy and radiotherapy are two major strategies used to combat cancer. Chemotherapy enhances their function of dendritic cells through different mechanisms, most of which have to do with immunogenic cell death (ICD), increasing the availability of antigen and depleting the suppressive immune cell subsets. Radiotherapy on the other, mimics somehow the mechanisms of chemotherapy, and enhances the role of dendritic cells by inducing ICD and the destruction of tumor cells. The goal of DC-targeted therapies is to enhance DC function in TME and send signals to effector T cells, in order to infiltrate the tumor [16].
However, as DCs in the TME often have an impaired function as a result of the suppressive signals of the tumor, it is of major importance to develop therapies that facilitate their activation and maturation. One such therapy includes their ex-vivo activation using the mRNA-based delivery of numerous co-stimulatory molecules (a cocktail named TriMix). The vaccination with this cocktail promotes the function of DCs in TME. This, may also be assisted by therapies that target epigenetic dysregulations in the TME. Epigenetic dysfunction can be a result of cytokines, such as TGFβ, which induces chromatin modifications, which in turn have a negative effect on the differentiation and functions of DCs. All in all, both DC-mediated therapies and others, need to be carefully controlled and their impact on the function of dendritic cells should always be evaluated [16].
Inhibitory cells
Regulatory T cells (T-regs)
Regulatory T cells (or suppressor T-cells), are a specialized subpopulation of T-cells with the role of suppressing the immune response though the inhibition of T cell proliferation and cytokine production, in order to maintain tolerance to self-antigens and prevent autoimmunity. T-regs are formed in the bone marrow and mature in the thymus gland, before being released into the bloodstream. While the most specific marker for their identification is intracellular FoxP3, certain surface markers, such as CD25high or CD127low may also help identify this cellular subpopulation [17]. According to Knochelmann et al., it is also important to maintain a healthy homeostasis between immune response enhancing Th17 cells and T-regs, however, the role of their relationship on carcinogenesis is yet to be fully understood [18].
Myeloid-derived suppressor cells (MDSCs)
As their name suggests, MDSCs originate from the myeloid lineage of bone marrow stem cells. They closely resemble monocytes (monocytic MDSCs) or immature neutrophils (polymorphonuclear MDSCs) and are not normally present in a healthy individual but can rather be found in a large variety of chronic conditions, such as obesity, cancer, and autoimmune diseases [19], [20], [21]. In a classical infection, myeloid cells are activated via strong signals, usually through toll-like receptors (TLR), damage-associated molecular patterns (DAMP), or pathogen-associated molecular patterns (PAMP), which leads to the immediate mobilization of myeloid cells from the bone marrow, and therefore a rapid increase in phagocytosis. In conditions such as chronic inflammations or cancer, however, the activating signals are rather exerted by inflammatory mediators and growth factors and they are often weak and long-lived. The so-formed MDSCs have been ascertained to have a less potent phagocytic activity than physiologic neutrophils and monocytes and suppress the immune response, which may result in a positive effect on tumor progression and metastasis [20].
The most important mechanisms facilitating the immunosuppressive activity of MDSCs include arginase, nitric oxide (NO), up-regulation of reactive oxygen species (ROS) and the production of prostaglandin E2 (PGE2) and anti-inflammatory cytokines, such as Interleukin-6 (IL-6) and Transforming Growth Factor-beta (TGF-β), leading to T- and NK-cell inhibition, neo-angiogenesis, and the promotion of immunotherapy resistance [19,20,22]. The endoplasmic reticulum (ER) stress response has also been identified as an important factor for the prediction of immunosuppression through MDSCs, as recent studies showed that mouse MDSCs deficient of the transcription factor CCAAT-enhancer-binding protein homologous protein (CHOP), an important mediator of the protein kinase RNA-like ER kinase (PERK) pathway, sensing ER stress, had an unusually low production of arginase and IL-6 [23]. Furthermore, it has been shown that the primary energy source of tumor infiltrating MDSCs is through fatty acid beta-oxidation, which may allow for another target in enhancing immunotherapy efficacy [23]. Due to their important role in maintaining the immunosuppressive tumor microenvironment, it can be appreciated, that targeting one or multiple of the suppressive mechanisms of MDSCs may significantly facilitate the breakdown of the TME and allow for the access and activation of immune effector cells [21]. There are already various preclinical and clinical drugs, including gemcitabine, sunitinib, or sildenafil, which aim to target the development, differentiation, and suppressive activity of MDSCs [24]
M1 and M2 macrophages
Macrophage activation usually results in the formation of either one of two different sub-categories of these cells. While both M1 and M2 macrophages are involved in the inflammatory response, the former (M1) mainly promote inflammation, whereas the latter (M2) are rather related to the anti-inflammatory response [25]. The polarization of undifferentiated macrophages to the M2 type is driven by microbial components, such as the anti-inflammatory cytokine interleukin-4 (IL-4) and results in an elevated expression of factors including arginase-1 (Arg-1), interleukin-10 (IL-10) and chemokines CCL17 and CCL22. During an infection, M2 macrophage polarization takes place later in time than that of M1 macrophages, as they function in tissue repair, angiogenesis, and metabolism, rather than protection against pathogens. Studies have shown that an acidic or hypoxic tumor microenvironment may drive macrophage polarization towards the M2 type [25]. As M1 macrophages are considered to be tumor-killing and immune-promoting, whereas M2 macrophages generally promote tumor growth and immune escape, angiogenesis, and metastasis, a possible therapeutic target could be the conversion of macrophages from the M2 to the M1 type. Studies have emphasized that agents including calcium carbonate nanoparticles and chloroquine can increase the pH in the tumor microenvironment and the lysosomal compartment of macrophages. This illustrates the possible role of these agents as add-on drugs, as the increased pH leads to the promotion of a phenotypic shift towards the M1 type [25]. The lung TME has served as a significant target for immunotherapy, anti-angiogenic and anti-inflammatory therapies by identifying molecular pathways and specific cell types to find potential mechanisms for attacking tumor cells. Various drugs targeting TME components are currently undergoing clinical trials.
Tumor mutational burden (TMB), neoantigens and immune system response
Cancer is regarded as a genomic disorder influenced by the accumulation of germline and somatic mutations, while recent advanced molecular techniques have enabled the examination of and the tumor's mutational status and how it impacts on cancer risk, patient prognosis and treatment response. There is a considerable variation in the tumor mutation frequency among distinct cancer types and even between tumors of the same histotype. Tumor mutational burden has been defined in various ways and there are multiple methods for its analysis [26]. Initially, TMB was determined through whole genome sequencing (WGS) on DNA from both the tumor and it's corresponding normal tissue, with the aim to exclude common inherited variations in the DNA sequence. Subsequently, TMB was characterized as the total count of somatic or coding mutations (i.e., substitutions, insertions, and depletions) per megabase (Mb) of DNA present in the cancer cells. The measurement excluded synonymous or ‘silent’ mutations [26]. Therefore, TMB characterises the overall burden of nonsynonymous tumor antigens found in the genome and varies according to the tumor type and among different patients. Importantly, TMB has been extensively studied for its potential as a predictive biomarker for patient response to immune checkpoint inhibition therapy. Recent evidence demonstrated the significant role of high TMB (TMB-H) for an improved immunotherapy response compared to tumors with low TMB levels (TMB-L) [26].The higher the TMB in a tumor, the greater the recognition of the tumor cells by the immune system, will be. Thus, TMB-H tumors are usually associated with an improved prognosis and better efficacy of ICI treatment [26]. High mutational burden is typically associated with cancers. The most noticeable responses to immune checkpoint inhibitors are reported in NSCLC and melanoma. These tumor types often exhibit a substantial mutation burden caused by the mutagenic influence of tobacco smoke and exposure to ultraviolet (UV) light, respectively [27]. TMB is a key determinant in the generation of immunogenic cancer neoantigens that are presented through major histocompatibility complexes (MHC) on the surface of tumor cells [28]. Neoantigen-containing peptides rise from somatic mutations in the tumor DNA after transcription and translation of these mutated genes has occurred, and through major histocompatibility complexes (MHC) are presented on the surface of tumor cells. However, it should be noted that not all mutations produce neoantigens, while a minority is expressed on cell surfaces from MHC complexes and a small proportion of these could be detected by T cells [29,30]. There is a positive correlation between the somatic mutation burden of a tumor and the neoantigen production. Specifically, the higher the number of the somatic mutations within the tumor genome, there is a concomitant higher chance in the abundance of neoantigens. Consequently, TMB becomes a more representative estimation of the tumor's neoantigen load.
TMB as a biomarker in immune checkpoint inhibition therapy
The efficacy of the TMB as a biomarker was recently discussed by Moeckel and Bakhl [31]. The rationale that TMB can serve as a predictive biomarker for cancer immunotherapy is originating from the hypothesis that tumor mutation-specific antigens can be recognized by tumor-infiltrating T cells [28], [29], [30]. Therefore, a higher TMB will lead to the generation of more neoantigens able to activate more intratumorally T cells, which in turn, will attack and kill tumor cells with the help of ICIs. Novel cancer therapies have been based on the expression of cancer neoantigens, including personalized neoantigen vaccines, adoptive T cell therapy, and immune checkpoint inhibitors [32]. The high immunogenicity of cancer antigens in combination with their lack of expression in healthy tissues have become the key targets for tumor immunotherapy. Importantly in ICI therapies, an increased cancer neoantigen burden is key to achieving an effective treatment response. The occurrence of an increased number of somatic mutations in the tumor DNA results in more emerging neoantigens, which will subsequently through transcription and translation. This influences how tumor cells respond to ICI therapies and since NSCLC is characterized by an increased somatic tumor mutational load, thus in theory this leads to a significant generation of neoantigens and promising response from ICIs [32,33]. Finally, tumor neoantigens could also facilitate the prediction of immunotherapy efficacy as additional predictive biomarkers alongside TMB. Conclusively, various studied investigated the correlation between high TMB and the probability of displaying a favorable response to immunotherapy as its increased mutational load would lead to a greater recognition by cancer neoantigen reactive T cells [33,34].
Specifically, studies have demonstrated an association between high TMB and anti-CTLA therapy in skin melanoma, as well as between high TMB and PD-1 in NSLC. The first study investigating TMB as a predictive biomarker in immune checkpoint inhibition therapy was in 2014 by Snyder et al, in melanoma patients [34]. Later on, the KEYNOTE-001 study demonstrated an association between TMB and a durable clinical benefit in patients with NSCLC during their second-line treatment phase [35]. The KEYNOTE-158 was the first study to introduce TMB as a predictive biomarker of ICI therapy using pembrolizumab (anti-PD-1 monoclonal antibody), in a multi-cohort phase II clinical trial investigating ten types of malignancy [35]. Pembrolizumab was then approved for all solid tumors in patients with ≥ 10 mutations/MB, as measured with the Foundation One CDx platform. According to the KEYNOTE-158 study results TMB-H tumors demonstrated the most meaningful responses in cancer immunotherapy with pembrolizumab. Considering the limitations of the KEYNOTE-158 study, concerns were raised for the determination of a single TMB-H threshold across different cancer types. The main limitation of the study was the lack of demonstration of the clinical activity across multiple tumor histologies, as well as the variability in the TMB threshold in certain tumor types with different tumor histology, which were not extensively investigated. Specifically, from the TMB-H cohort 90 % of participants suffered from small cell lung cancer, as well as cervical, endometrial, vulvar or anal cancer [34,35].
The favorable responses were varying across tumor types, suggesting that both the tumor origin, histopathology and TME may play a significant role in clinical benefit from immunotherapy. Furthermore, common tumor histologic types, such as breast, prostate and microsatellite stable colorectal cancers, were not adequately represented in the study. Importantly, the TMB-H threshold in the KEYNOTE-158 study was investigated in prior studies for NSCLC [35]. In the CheckMate 227 study, the same TMB threshold was used for the efficacy comparison of nivolumab plus ipilimumab compared with chemotherapy alone in stage IV NSCLC patients [36]. The results of the CheckMate-227 clinical trial demonstrated that patients with a high TMB (≥ 10 mutations/Mb) and combined immunotherapy treatment as a first-line treatment leads to the prolongation of patients’ progression-free survival (PFS) [36,37]. Thus, TMB could be implemented as a potential predictive biomarker for NSCLC immunotherapy response, however this threshold has not been validated across other tumor types.
TMB detection methods and definition variability
Different platforms can be used to determine TMB, including WGS, whole-exome sequencing (WES), or targeted panel sequencing. Nevertheless, a main issue is that the analytical and bioinformatic methods employed for the analysis of TMB have not been standardized across clinical studies. Regarding WGS, both coding and non-coding sequences are analyzed. However, since TMB assessment focuses only on coding sequences, WES, which covers the entire exome (protein-coding regions of the genome), and gene panels that target specific regions are considered suitable alternatives. TMB assessment is most accurately performed by WES, which allows for the detection of non-synonymous somatic mutations within the entire exome, while targeting ∼30 Mb of the coding regions, which comprise ∼1 % of the entire genome [28]. Despite the good accuracy of this method, it is not clinically utilized due to impracticality reasons, including high cost, demand of larger tumor samples than most current clinical methods, larger DNA input, and increased time needed to produce results. In the last decade, WES has been mostly utilized in research for tumor profiling.
Panel-based sequencing of tumor tissue is mostly used in clinical practice, with multiple panel sizes and increased variability in regard to the input sample requirements, mutation types, number or identity of genes included, workflow followed and bioinformatic platforms. Utilized. There are multiple gene panels available for TMB measurement and they cover between 0.80 to 2.40 Mb which represent < 5 % of the whole coding sequence [38]. The NGS panels that are approved by the U.S Food and Drug Administration (FDA) and are commercially available, consist of the Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) and the Foundation One CDx assay. The latter determines TMB by counting both synonymous and non-synonymous variants present at 5 % or greater of gene frequency [39]. Importantly, other NGS panel tests, namely FoundationOne, Foundation Medicine bTMB assay, Thermo Fisher Scientific Oncomine Tumor Mutation Load Assay, NEO New Oncology NEOplus, have emerged and have demonstrated robust agreement with TMB assessment derived from WES data, which was confirmed by computational analysis and empirical comparisons [39], [40], [41], [42]. As a result, gene panels present a potentially valuable and efficient method for TMB estimation in clinical settings. Importantly, it has been continuously questioned whether a single TMB-H threshold for all cancer types would accurately predict immunotherapy responses since the immunogenicity and TME of each tumor type has great variability thus affecting TMB levels.
Existing and novel immune checkpoint inhibition (ICI) therapies
The immune system can exert an antitumor response, but this is usually compromised by immune checkpoints. These are proteins found on the surface of tumor cells within the TME and can inhibit or stimulate pathways assisting an immune response. The rationale behind these immunological pathways is to prevent the destruction of normal cells along with the identified tumor cells. ICI therapies act by blocking or stimulating these immunological pathways and thus strengthening the body's anti-tumor activity [43]. The most clinically recognized and utilized immune checkpoint pathways include the programmed cell death receptor -1 (PD-1) / programmed cell death ligand-1 (PD-L1) axis, and that of cytotoxic T lymphocyte-antigen-4 (CTLA-4). The development of drugs blocking these pathways has demonstrated significant anti-tumor effects and has been utilized in the clinical setting for the treatment of various malignancies, especially non-small cell lung cancer and skin melanoma [43].
Anti-PD1/PD-L1
The PD-1/PD-L1 pathway is one of the key mechanisms of immunomodulation, since the activation of PD-1, through its binding by PD-L1, prevents the excessive activity of PD-1-positive immune cells, such as T lymphocytes, making it an essential factor in the prevention of autoimmunity and immune-mediated tissue damage. PD-L1 overexpression is also considered as one of the most vital immune evasion mechanisms exploited by various malignancy types, including lung carcinomas [44]. In malignant tissues, the PD-1/PD-L1 interaction has shown to result in various effects on tumor-infiltrating T lymphocytes, such as the negative regulation on proliferation and cytokine secretion by antigen-specific T cells, and the promotion of their apoptosis, while inhibiting the apoptosis of immunosuppressive T-regs [45,46]. The increased tumor cell expression of PD-L1 may be the result of either primary oncogenic signaling, or secondarily through the induction by inflammatory mediators, such as IFN-gamma [47].
This information can help us appreciate the diagnostic value of targeting the PD-1/PD-L1 pathway, as the blockade of either one of the molecules, and the resulting disruption of their interaction, may lead to increased T cell proliferation and cytokine secretion, as well a decrease in their apoptosis, and therefore larger anti-tumor effects. Within recent years, anti-PD-1 and anti-PD-L1 monoclonal antibodies (mabs) have increasingly gained more and more recognition in the clinical practice. Among them, the PD-1 inhibitors pembrolizumab and nivolumab, have been approved by the FDA for first-line treatment for patients with metastatic NSCLC [48]. The effect of anti-PD-1 and anti-PD-L1 mabs on the prognosis of small cell- and non-small cell lung cancers is controversial, as many studies have shown positive outcomes of the combination therapy with traditional chemotherapies. However, many patient groups develop resistance to the drugs, due to different etiologies [49]. PD-1/ PD-L1 immune checkpoint inhibitors have been approved as either monotherapy for metastatic NSCLC or in combination with other treatment modalities for synergistically eliciting more potent immunological responses. Specifically, in 2015, nivolumab (PD-1 inhibitor) was the first immune checkpoint inhibitor to become approved for the treatment of metastatic NSCLC patients. The approval of nivolumab was established after the results of two important phase III clinical trials; CheckMate-017, investigating its effect in tumors with squamous histology, and CheckMate-057 focusing on non-squamous histology tumors [50]. Both studies enrolled patients with advanced NSCLC during or after conventional therapy, which were allocated randomly to the groups of nivolumab or a standard second-line chemotherapy regimen (docetaxel) [51]. The results of both clinical trials indicated that overall survival (OS), the primary outcome of the study, was superior with nivolumab compared with docetaxel treatment. Furthermore, other PD-1 and PDL-1 mabs have gained approval for metastatic NSCLC monotherapy, including pembrolizumab and atezolizumab. In the KEYNOTE-024 phase III clinical trial, patients with untreated NSCLC with PD-1 expression in > 50 % of their tumor cells and no EGFR or ALK mutations, were randomized with pembrolizumab or platinum-doublet chemotherapy treatment. The results showed that OS, progression-free survival (PFS) and response rates were higher in the pembrolizumab-treated group. This led to the approval of pembrolizumab as a standard of care monotherapy for NSCLC patients without specific histopathology, expressing PD-L1 in ≥ 50 % of cancer cells [52]. Atezolizumab is another PD-L1 mab approved as monotherapy treatment, with the difference that the patient population it targets, includes patients with previously treated NSCLC [53].
The OAK trial demonstrated higher overall survival with atezolizumab compared to docetaxel, regardless of PD-L1 expression levels or tumor infiltrating cells present. More trials are in progress assessing the other PD-L1 antibodies efficacy for NSCLC treatment, with the most significant one being the approval of durvalumab for localized stage III NSCLC by the FDA. Undoubtedly, immunotherapy with ICIs as a second-line treatment is being introduced as a standard of care for patients who have not responded to the first-line chemotherapy according to the Eastern Cooperative Oncology Group (ECOG) performance status.
Combination therapies have been extensively investigated especially since the anti-PD-1/PD-L1 therapy demonstrated such tremendous responses. Synergistic effects of ICIs with cytotoxic therapies, such as chemotherapy and radiotherapy or combination treatment of different ICIs have been studied and showed more potent immunological responses compared to monotherapy. The landmark trial KEYNOTE-189, which included untreated patients with non-squamous NSCLC without taking PD-L1 expression into consideration, randomized them in a group of standard chemotherapy plus pembrolizumab or standard chemotherapy alone. The results of this study pointed out the superiority of combination therapy of chemotherapy with immunotherapy as all clinical outcomes were higher regardless of PD-L1 expression level [54].
Furthermore, the CheckMate-227 clinical trial demonstrated the effectiveness of combination therapy with two immune checkpoint inhibitors. Specifically, ipilimumab (anti-CTLA-4) and nivolumab (anti-PD1) were investigated in a large phase III trial of randomized stage IV or recurrent NSCLC patients who were not previously treated with chemotherapy.Results with minimum 4 years’ follow-up, reported increases in the OS during the administration of ipilimumab and nivolumab combination therapy as compared to chemotherapy alone, in both groups with a tumor PD-L1 expression of greater or equal to, or less than 1 % [54].
In March of 2019 and 2020, the FDA also granted approval for the use of atezolizumab and durvalumab in combination with conventional chemotherapeutic agents for the first-line treatment of patients with extensive-stage small cell lung carcinoma (ES-SCLC), following the outcomes of the IMpower133 and CASPIAN clinical trials, respectively. The IMpower133 trial showed an increased OS of 12.3 months in subjects receiving a combination therapy of atezolizumab with etoposide and carboplatin, as compared to 10.3 months in those receiving etoposide and carboplatin alone. CASPIAN showed similar outcomes, as the additional administration of durvalumab to the standard treatment with etoposide and either cisplatin or carboplatin showed a prolongation in OS from 10.3 months to 13 months. ICI monotherapy, however, has so far failed to show any improvement in the OS of patients with SCLC [55,56].
Anti-CTLA-4
CTLA-4 (or CD152) is another inhibitor of T-cell function. Once expressed, it antagonistically binds to the B7 protein (CD80 or CD86), a surface receptor on antigen-presenting cells (APCs) that normally binds to the CD28 receptor on naive T cells. CTLA-4 is expressed mainly by T-regs; however, its upregulation can also be achieved by other T cells, mostly activated CD4+ T cells [57]. CTLA-4 is mainly found in intracellular vesicles and is only expressed when activated in the immunological synapse, right before its endocytosis occurs [7]. Using this mechanism, T-regs suppress the immunological function of non-activated tumor-infiltrating T cells. The development of anti-CTLA mabs was firstly achieved in 1996 and showed significant results of tumor regression in animal models [58]. In 2011, ipilimumab(anti-CTLA-4), was the first checkpoint inhibitor to be tested and approved by the FDA for patients suffering from advanced stage IV melanoma [59], as it showed tremendous results in improving overall survival [60]. Ipilimumab's mechanism of action focuses on the stimulation of the effector T cell proliferation, without inducing any T-cell suppression. This anti-CTLA-4 antibody, together with nivolumab, has gained approval for metastatic colorectal cancer treatment in patients with high microsatellite instability (MSI-H) or mismatch repair deficiency (dMMR) [61,62]. The combination of ipilimumab and nivolumab has been indicated as the first-line treatment for NSCLCs expressing PD-L1 > 1 %. According to trials recorded at ClinicalTrials.gov, approximately 600 studies have investigated the effectiveness of ipilimumab against several types of cancer [61].
Past studies showed that treatment with anti-CTLA-4 mab alone did not have a significant effect on the prognosis of lung cancer, which is why most recent investigations focus on combining CTLA-4 inhibition therapy with either other ICIs, or traditional regimens (e.g., etoposide and platinum chemotherapies) in SCLC and NSCLC [63]. A preclinical mouse trial, conducted by Bates et al., for example, indicates anti-CTLA-4 as a possible adjunct to bempegaldesleukin (a CD122-preferential IL-2 pathway agonist), as a secondary treatment after surgical resection or hypo-fractionated radiation therapy of primary Lewis Lung Carcinoma (LLC), a specific type of NSCLC. This could be attributed to elevated CD8 T and NK cell levels and increased NK-cell degranulation, as well as depressed T-reg levels within the primary tumor microenvironment, upon combinatory treatment. The specific response of T-reg levels to the treatment could probably be attributed to surface-CTLA-4 overexpression of NSCLC-infiltrating T-regulatory cells [64,65]. Furthermore, it has been demonstrated, that responses of NSCLC to ipilimumab could be enhanced by focal tumor irradiation, possibly to the radiation-induced transcriptional upregulation of neo-antigens, which in reverse results in the expansion of CD8 T cells [66,67].
In contrast to NSCLC, there are limited data available to indicate significant responses of SCLC to anti-CTLA-4 treatment. However, a clinical trial, conducted by Hellmann et al., identified a positive correlation between efficacy of nivolumab (PD-1 blocker) and ipilimumab (CTLA-4 blocker) combination treatment and a high TMB. Additionally, the study also showed an increased clinical benefit of the combinatory treatment in relation to nivolumab monotherapy [68,69].
Novel immune checkpoint inhibitors
Despite the impressive advances of PD-1/PDL-1 and CTLA-4 immunotherapies, the vast majority of NSCLC patients either have poor response to them or after the initial successful response, they usually develop resistance; while just a fraction of ∼12 % of them will benefit from ICI treatment [70]. Therefore, this unsatisfactory performance of the aforementioned drugs for multiple patient populations and tumor types, has led to the need to investigate emerging immune checkpoint inhibitors, shifting the focus towards targeting alternative inhibitory receptors within the TME. Furthermore, with the FDA-approval of different drugs targeting the PD-1/PD-L1 and CTLA-4 pathways, the attention was then focused on other immune checkpoints, including lymphocyte activation gene-3 (LAG-3), T-cell immunoglobulin and mucin domain-containing molecule 3 (TIM-3) and T cell immunoglobulin and ITIM domain (TIGIT) [71] (Fig. 2 and Table 1). Furthermore, ongoing clinical trials are aiming to determine the optimal synergistic combinations of immunotherapies, since most evidence underlines possible synergistic effects of new ICIs with the existing anti-PD-1/PDL-1 and anti-CTLA-4 blockers. Especially in the treatment of NSCL, a large portion of clinical trials are investigating the combination of the traditional ICIs with novel ones or with other treatment modalities such as chemotherapy, radiation therapy and cancer vaccines [72].
Fig. 2.
Emerging targets for immune-checkpoint inhibition therapy and their connection to DNA repair mechanisms. Lymphocyte activation gene-3 (LAG-3) regulates T-cell function and acts in synergy with PD-1 inhibitors; T-cell Immunoglobulin and Mucin Domain-containing Molecule 3 (TIM-3) inhibits T-cell Immunoglobulin and ITIM Domain (TIGIT) competes with CD226 and also synergizes with PD-1/PD-L1 inhibitors; V-domain Immunoglobulin Suppressor of T-cell Activation (VISTA) maintains T-cell function; CD73 suppresses T-cell activation by producing adenosine and its inhibition synergizes with PD-1/PD-L1 blockade; CD27 expressed in naïve CD4 T cells, promotes T-cell activation and has a promising effect in combination with PD-1 inhibitors (Created with BioRender.com).
Table 1.
Characteristics of some NGS gene panels used for the definition of TMB.
| TMB Assay | Number of Genes | Comments | TMB region coverage (Mb) |
TMB Definition | Ref. |
|---|---|---|---|---|---|
| WES | ∼22 gene coding regions | Subtraction utilizing patient-matched normal samples | 30 Mb | No. somatic mutations in the tumor genome sequence | [73] |
| Foundation Medicine FoundationOne CDx™ | 324 tumor-related genes | Estimation through bioinformatic algorithms followed by subtraction. Does not distinguish between somatic and germline mutations Gene variants include: single nucleotide variants, indels, CNAs, TMB and dMMR/MSI analysis |
0.8 Mb | No. of synonymous and non-synonymous mutations, short indels per Mb of cancer genome | https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170019B.pdf |
|
MSKCC NGS (MSK-IMPACT) |
468 tumor-related genes | Subtracted utilizing patient-matched blood samples. FDA-approved and diagnostic assay authorization Gene variants include: |
1.22 Mb | No. of synonymous and non-synonymous mutations, short indels per Mb of cancer genome | [41] |
| FoundationOne Liquid CDx Medicine (bTMB assay) | 311 tumor-related genes (exonic coding), 15 genes with select intronic regions |
Liquid biopsy cfDNA. Especially for ALK mutations in NSCLC patients with ALK-targeted therapy indication Detection of bTMB, MSI and %Tumor Fraction |
1.1 Mb | No. of synonymous and non-synonymous mutations SNVs |
[74] |
|
Thermo Fisher Scientific Oncomine Tumor Mutation Load Assay |
409 tumor-related genes | Library generation with reagents from two pools of multiplex PCR primers sufficient for 24 samples Automated library preparation option | 1.20 Mb | No. of somatic mutations in the tumor genome sequence | https://assets.thermofisher.com/TFSAssets/LSG/manuals/MAN0017042_TumorMutLoad_UG.pdf |
| NEO New OncologyNEOplus v2 RUO | >340 tumor-related genes | Gene variants detected include single, nucleotide variants, indels, fusions, CNAs. Analyses TMB, MSI and driver mutations. | 1.1 Mb | No. of somatic mutations in the tumor genome | http://www.newoncology.com/en/assays |
Mb, megabase; CNA, copy number alteration; dMMR, mismatch repair deficiency.
Anti-LAG-3
Lymphocyte activation gene-3 (LAG-3; CD223) has been extensively reported as a potential tumor immunotherapeutic target due to its physiological function as a cell surface inhibitory receptor responsible for T-cell function regulation. Its negative regulatory role in controlling T cell activation and its ability to mediate T cell exhaustion, synergistically with PD-1/PD-L1 antibodies has placed LAG-3 in the center of investigation as a target of immunotherapy [72]. Similarly to PD-1 and CTLA-4 blockers, LAG-3 is also expressed on the surface of activated T and NK cells, T-regs, active B cells and pDCs, while it is known that LAG-3 is frequently subjected to epigenetic alterations, mostly methylation [75]. Upon binding of LAG-3 to MHC-II with higher affinity than CD4, there is a disruption of between the interactions CD4 and MHC-II [[75], [76], [77]] and an induction of signal transduction in DCs, which subsequently activates protein kinases and phospholipases. Gal-3 is also of significant importance due to its increased expression on activated T cells and tumor cells, as well as its contribution in suppressing CD8+ T-cells and pDCs.
It is also speculated that LAG-3 impacts CD8+ T cell function and interaction with APCs. In regards to the clinical utility of LAG-3 inhibitors, there are currently several LAG-3 modulating immunotherapies at different stages of clinical and preclinical development. Specifically, there are 97 clinical trials testing 16 LAG-3 targeted therapies conducted by various pharmaceutical industries, including Bristol-Myers Squibb (BMS-986016), Regeneron Pharmaceuticals (REGN3767 and 89Zr-DFO-REGN3767), Merck (MK-4280), Novartis (LAG525), Tesaro (GSK) (TSR-033), Symphogen (Sym022), GlaxoSmith (GSK2831781), Incyte Biosciences International Sàrl (INCAGN02385), Prima BioMed/Immutep (IMP321), MacroGenics (MGD013), F-Star (FS118), Hoffmann-La Roche (RO7247669), Shanghai EpimAb Biotherapeutics (EMB-02), Xencor (XmAb841) and Innovent Biologics (IBI323) [75]. The therapeutic modalities under investigation can be categorized into anti-LAG3 mabs, soluble LAG-3 immunoglobulin (Ig) fusion proteins and anti-LAG-3 bispecific drugs.
Anti-TIM-3
T-cell immunoglobulin and mucin domain-containing molecule 3 (TIM-3) is a co-inhibitory cell surface receptor, which is primarily expressed on the plasma membrane of IFN-gamma-producing T-cells, but also on other immune cells, including T-regs, macrophages, and DCs, as well as leukemic stem cells [76,77]. The main activator of TIM-3 is considered to be galectin-9, however, recent studies have also recognized other ligands, carcinoembryonic antigen-associated cell adhesion molecule (Ceacam-1), and High Mobility Group Box 1 (HMGB1) [78].
Upon ligand-mediated activation of TIM-3 in T-cells, it can exhibit its immunosuppressive function by the inhibition of T-cell activation. One example of this mechanism is the overexpression of a long-non-coding RNA that binds TIM-3 (lnc-TIM-3) in dysfunctional CD8+ T-cells in hepatocellular carcinoma, which diminishes anti-tumor immunity through the inhibition of T-cell activation [79]. Furthermore, the importance of TIM-3 in dendritic cells was emphasized by Dixon et al., as the loss of TIM-3 on DCs alone showed significant promotion of anti-tumor immunity. Without TIM-3, DCs did not have the ability to express a regulatory program, thereby facilitating the maintenance of CD8+ effector and stem-like T cells [80]. Additionally, TIM-3 has been used as a potential predictor for immune checkpoint inhibition therapy. In regard to its role as a predictive biomarker, a study by Limagne et al. demonstrated that increased levels of TIM-3 expression in the peripheral lymphoid cells after nivolumab treatment leads to a low response to PD-1 inhibition. Disease progression reaching advanced stages was observed in patients with high TIM-3 expression compared to the group of patients with lower TIM-3 levels who were more responsive to PD-1 therapy.
From these data, it can be concluded that the TIM-3 system is yet to be understood to serve as an optimized target of therapy. According to data of an early clinical trial conducted by Borate et al., TIM-3 inhibition by the high-affinity humanized anti-TIM-3 IgG4 antibody MBG453 (sabatolimab), showed remarkable efficacy in combination with decitabine (chemotherapy drug) in the treatment of myelodysplastic syndrome (MDS) and acute myelogenous leukemia (AML) [79,81].A further clinical trial investigating the co-inhibitory effect of TIM-3 alongside with PD-1 by the administration of sabatolimab in combination with spartalizumab (anti-PD-1) in advanced solid tumors, also showed positive results in the treatment of NSCLC and SCLC, as well as colorectal cancer and malignant perianal melanoma [82].
The effect of co-therapy using anti-TIM-3 antibodies alongside with adoptive cell transfer and anti-PD1 therapies was also investigated during a study by Martinez et al., which showed that the prognosis of mice with non-squamous NSCLC, significantly improved upon addition of anti-TIM-3 antibodies to the treatment regimen. This may be the result of a significant increase in tumor infiltrating CD8+ T lymphocytes (TILs) during the combination therapy, including adoptive cell transfer and anti-PD1 and anti-TIM-3 antibodies, in contrast to only adoptive cell transfer with or without anti-PD1 antibodies, or only PD-1 blockade, which was also demonstrated during the study [83]. On the contrary, anti-TIM-3 treatment alone showed no significant improvement in the prognosis, which may be attributed to TIM-3 overexpression, resulting from PD-1 blockade therapy [83].
Anti-TIGIT
T cell immunoglobulin and ITIM domain (TIGIT) was firstly discovered in 2009 as an immune checkpoint that also suppresses T cell activation [84]. The TIGIT gene is located on the chromosome 3q13.31, encoding a 244 amino-acid glycoprotein which structurally comprises of an extracellular IgV region, an immunoglobulin tail tyrosine (ITT) -like phosphorylation motif, a cytoplasmic tail and a transmembrane domain [84]. TIGIT is strictly expressed on T-regs and memory T cells, as well as NK cells, while it binds CD155 (PVR or Necl-5) and CD112 (nectin-2/PRR2 or PVRL2), without having the same affinity with each of them. TIGIT can suppress T cell activation by competing with CD226 (DNAM-1) or CD96 [85]. These protein molecules function as a group with co-stimulatory effects similar to that of the CTLA-4 B7 and CD28 pathway mentioned above. Its role is to bind nectin and nectin-like proteins, which are most observed in APCs, T cells and tumor cells. TIGIT, by being expressed in CD8+ tumor infiltrating lymphocytes, has an important regulatory role in human cancers and specifically, in anti-tumor responses. Anti-TIGIT in association with anti-PD-L1 has a synergistic anti-tumor response, which is principally mediated via cytotoxic T cells [85]. The inhibition of TIGIT either on its own or synergistically with PD-L1, is being thoroughly studied, as the combination of these two molecules seems to have an important role in cancer therapy.
As the preclinical research conducted on TIGIT appears very promising, this novel immune checkpoint has become the target of cancer immunotherapy and has thus gained significant attention by the pharmaceutical industry. Dual blockade of the PD-1/PD-L1 and TIGIT immune checkpoints co-inhibitory receptors on T cells has demonstrated promising results [86]. Although PD-1/PD-L1 and TIGIT are responsible for the regulation of different costimulatory receptors, CD28 and CD226, decreased effectiveness has been reported in preclinical studies when CD226 is absent. Therefore, CD226 is speculated to be a requirement to anti-PD-1/PD-L1 or anti-TIGIT immunotherapy. Clinical trials have mostly focused on combining anti-TIGIT with anti-PD-1/PD-L1 mabs for the treatment of NSCLC. These efforts led to the development of 3 anti-TIGIT agents which have currently entered phase III clinical trials. The immunosuppressive effect of anti-TIGIT mabs is attributed to the inhibition of T cell proliferation both directly and indirectly.
Anti-VISTA
VISTA (V-domain immunoglobulin suppressor of T-cell activation) has a pivotal role as an immunomodulatory molecule orchestrating the regulation of T-cell and myeloid cell functionality, ensuring the maintenance of cellular quiescence. VISTA represents a distinctive co-stimulatory molecule, that exhibits expression within the immune independent of prior T-cell activation [87]. Specifically, VISTA is found in resting T cells and nonactivated macrophages, where it plays a crucial role in maintaining immune system quiescence. Unlike other immune checkpoint inhibitors, which regulate the immune response after T-cell activation, VISTA acts as an early checkpoint regulator for peripheral T-cell tolerance. Its primary function is to enforce quiescence in naïve T lymphocytes when exposed to self-antigens [88]. Regarding its molecular structure, VISTA harbors an extracellular domain that shares homology with PD-L1, a ligand of the B7 family [88]. Acting as a ligand, VISTA-Ig fusion protein has demonstrated its capacity to restrain CD4-positive and CD8-positive T cells, as well as reduce T-cell cytokine production, comprising IL-2, IL-10, TNF-α, and IFN-γ in mice and humans. Furthermore, VISTA expression on T cells hinders their proliferation in mouse models following antigen presentation, unlike the condition without VISTA [89]. In comparison with CTLA-4 and PD-1, which are confined to activated T cells, VISTA's broader potential as a target is attributed to its presence on various cells in the TME. Myeloid-derived suppressor cells, comprising microglia and neutrophils, tumor-associated macrophages, and dendritic cells all expressing VISTA, regulate the function of the TME by exerting suppressive effects [90]. A phase I clinical trial is currently investigating an anti-human VISTA antibody in patients with NSCLC. Importantly, in mouse models with human VISTA knock-in, the surrogate antibody showed tumor growth inhibition by modulating the myelomonocytic and T cell components [90,91].
Anti-NK group 2 member A (Anti- NKG2A)
NKG2A is a surface molecule found on cells, primarily on NK cells, although it can also be induced on T cells, particularly CD8+ T cells [91]. An upregulation of HLA-E on tumor cells leading to its overexpression is associated with a poor response to ICIs [92]. Furthermore, a monoclonal antibody that targets NKG2A, called monalizumab has exhibited encouraging anti-tumor effects. A recent phase II clinical trial demonstrated improved objective response rate (ORR) and progression-free survival (PFS) when monalizumab was used in combination with durvalumab compared to the former drug alone. This was observed in patients with unresectable stage III NSCLC with no tumor progression after receiving concurrent chemoradiation therapy [92,93].
Anti-CD73
CD73, also known as ecto-5′-nucleotidase (NT5E), acts as an immune checkpoint by producing adenosine, which dampens immune activation through the A2A receptor. Increased levels of CD73 have been observed in various tumor types, including NSLC and SCLC as well as increased expression of CD73 in tumor tissue has been linked to poor prognosis [94,95]. A lot of observational studies exist have investigated the potential combination of CD73 inhibition with PD-1/PD-L1 blockade, revealing a synergistic anti-tumor effect through enhanced infiltration of CD8+ tumor specific T cells into the tumor [94]. A recent phase III clinical trial assessing oleclumab, a monoclonal antibody targeting CD73 in combination with durvalumab after chemoradiation in advanced unrespectable stage III NSLC, have shown improved progression- free survival (PFS) compared to durvalumab alone [94]. Despite the aforementioned immune checkpoint targets there are more under study including B7-H3 (CD276), IDO-1, glucocorticoid-induced TNFR-related receptor (GITR) and CD47. A variety of ongoing clinical trials are investigating the inhibition of these targets either as monotherapy or combined with PD-1/PD-L1 or CTLA-4 inhibition therapy.
New trends and future directions
CAR T cell therapy
Since 2017, chimeric antigen receptor (CAR) T cell therapies have emerged as highly promising immunotherapies for hematological tumors [96]. This innovative approach involves the modification of T lymphocytes to enable them to selectively target and eliminate tumor cells. This is achieved by the recognition of surface markers such as CD19, CD20, CD22 and B cell maturation antigen (BCMA). CAR T cell therapies have demonstrated notable efficacy in hematologic malignancies and have also exhibited significant potential for the treatment of solid tumors such as lung cancer [31,97]. Specifically, in the management of solid tumors recent evidence suggests that they show rapid tumor eradication and durable responses [97]. However, there are certain challenges regarding the effectiveness of this innovative therapeutic approach. The immunosuppressive nature of the tumor microenvironment presents significant challenges for effective CAR T Cell therapy in solid tumors, including difficulties in CAR T cell infiltration into the dense fibrotic tissue as well as limited activation due to a lack of chemokine expression, and subsequent exhaustion of the CAR T cells [97] (Fig. 3A). However, innovative approaches to overcome these challenges have been deployed including targeting specific tumor-associated antigens such as mucin-1, B7H3, human epidermal growth factor receptor 2 (HER2), epidermal growth factor receptor (EGFR), and carcinoembryonic antigen. By engineering CAR T cells to recognize these antigens, they can be utilized for solid tumors treatment (Fig. 3A). Furthermore, the use of tumor neoantigens shows significant promise, such as anti-EGFRvIII-CAR T cells showing positive outcomes in treating high-grade glioblastoma cells [97]. Additionally, CAR T cell therapy has been associated with significant toxicities with systemic administration including high levels of cytokines, that could also potentially be lethal. In order to prevent this and reduce the levels of cytokine release engineered CAR T cells and NK cells have been utilized.
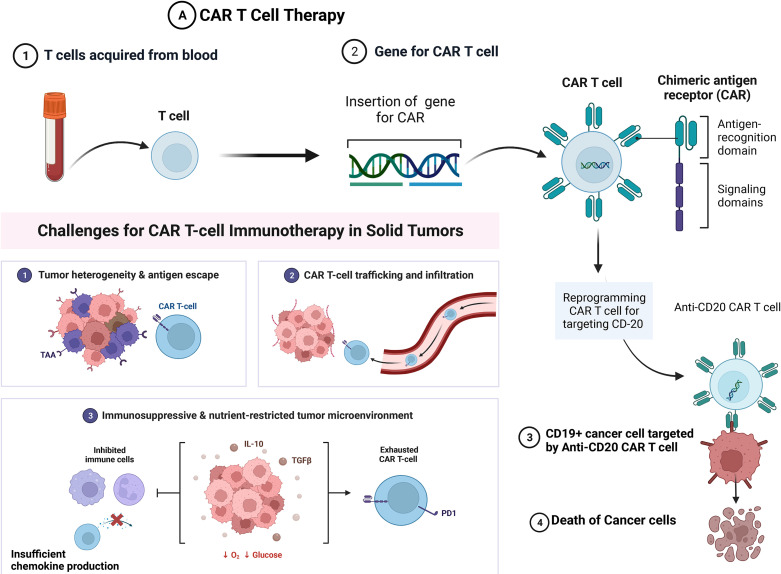
Fig. 3.
Future direction in the field. (A) CAR T cell therapy involves the transfer of T lymphocytes that have been genetically modified to target tumor cells, particularly those expressing antigens like CD-20, CD-22, CD-19. However, the application of CAR T cell therapies in solid tumors faces significant challenges, mainly due to difficulty infiltrating the dense tumor stroma and the immunosuppressive TME, which reduces chemokine expression. Potential solution to this challenge is the utilization of specific tumor-associated antigens (TAAs) enhancing the effectiveness of CAR T cell therapies. (B) Personalized vaccines with specific anti-tumor antigens designed to elicit an immune response and induce apoptosis in tumor cells. These tumor vaccines are designed to each patient's specific tumor features (Created with BioRender.com).
Finally, genetic modification of T or NK cells has led to an improvement in targeting lung cancer cells by immune recognition enhancement and modification of the immune cells behavior to be more functional [97]. Apart from CAR T cell therapy, there is a growing interest in exploring the potential of allogeneic NK cells as a safer alternative to CAR-T cell infusion. Engineered cell-based therapies until recently have been designed to target epidermal growth factor receptor (EGFR) and variant III (EGFRviii), human epidermal growth factor receptor 2 (HER2), erythropoietin-producing hepatocellular carcinoma A2 (EphA2), mesothelin (MSLN), prostate stem cell antigen (PSCA), mucin 1 (MUC1), carcinoembryonic antigen (CEA), natural killer group 2D (NKG2D), tyrosine kinase-like orphan receptor 1 (ROR1), and programmed cell death ligand 1 (PD-L1) [96,97].
Personalized tumor vaccines
Another innovative approach employs tumor neoantigens in personalized tumor vaccines to stimulate the patient's immune system and elicit a targeted anti-tumor response [33]. These vaccines are specifically designed to stimulate the activation of T cells that specifically recognize and attack tumor cells, while not affecting normal healthy cells. This is achieved by utilizing neoantigens that are exclusively present in the patient's tumor. Different types of vaccines, including those based on synthetic long peptides, RNA, DNA, and dendritic cells, have undergone extensive clinical testing to confirm their efficacy [31]. Specifically, the development of neoantigen peptide vaccine tailored for patients with NSCLC and EGFR mutations has been a recent breakthrough in this field [98].
Other potential biomarkers in lung cancer immunotherapy
The investigation of further biomarkers of immunotherapy that could be used in lung cancer has been the target of most recent studies. The identification of such biomarkers will provide a more precise approach to NSCLC cancer immunotherapy (precision oncology). So far, tissue-based biomarkers with promising effects have been reported to be the following: TMB (discussed above), PD-L1 expression, MSI-H/dMMR, mutations in cancer driver genes, including EGFR, ALK, STK11/LKB1, KRAS/TP53 co-mutation, as well as MDM2/MDM4 and EGFR amplification. Most evidence suggests that PD-L1 expression and TMB-H are the most promising predictive biomarkers for NSCLC immune checkpoint inhibition therapy.
PD-L1+ IHC
PD-L1 protein expression has been identified as a biomarker with significant clinical utility for the treatment decisions regarding immunotherapy. Numerous studies have demonstrated the importance of this biomarker and thus PD-L1 expression gained approval from regulatory agencies. Specifically, in the CheckMate-227, KEYNOTE-024 and KEYNOTE-042 studies, PD-L1 expression was approved as a predictive biomarker with a cut-off threshold of ≥ 1 % for the treatment of advanced or metastatic NSCLCs. The immunotherapies used in the trials were a combination of nivolumab and ipilimumab (CheckMate-227) and pembrolizumab monotherapy (KEYNOTE-024 and -042 trials). Although PD-L1 immunohistochemistry (IHC) has been used and approved as a diagnostic tool for anti-PD-1/PD-L1 inhibition therapy, there was initially increased concern on how different PD-L1 IHC assays used in clinical trials could be compared [54,99,100]. This concern was addressed with the “PD-L1 Blueprint Project” which focused on the comparison of multiple assays and concluded that three assays appeared to have similar patterns and be most reproducible; including 22C3 (Dako), 28-8 (Dako) and SP 263 (Ventana) [101]. These assays have now been established in the clinical practice and used interchangeably, depending on the analysis done prior to their use. The FDA approved the use of PD-L1 expression (≥ 50 % tumor proportion score [TPS] or in ≥ 50 % of tumor cells or PD-L1–stained tumor-infiltrating immune cells in ≥ 10 % of the tumor tissue area) as a biomarker for immunotherapy against advanced NSCLC. Specifically, pembrolizumab, cemiplimab and atezolizumab, were approved as a first-line treatment for NSCLC with high PD-1 expression of ≥ 50 % TPS. Recent evidence suggests that monotherapy with pembrolizumab in PD-L1-high NSCLC patients with is the preferred treatment choice as retrospective analysis of controls revealed that there is no outcome variation between immunotherapy alone or combined with chemotherapy.
Microsatellite instability (MSI)/mismatch repair deficiency (dMMR)
The DNA mismatch repair system (MMR) is responsible for maintaining genome integrity and stability since it provides a repair mechanism by overlooking the nucleotide sequence and trying to detect any insertions or deletions of DNA microsatellites. MMR deficiency (dMMR) leads to genetic instability and the buildup of DNA mutations. Germline mutations or epigenetic effects, such as DNA methylation of key genes are responsible for the deficiency of the DNA mismatch repair system (dMMR). The mismatch repair genes undergoing alterations, mainly comprise MLH 1, MSH 2, MSH6 and PMS2, leading to dMMR. Consequently, these alterations accumulate, leading to DNA mutations being able to be passed on to the next generation [102]. Mismatch repair deficiency (dMMR) in cancers often leads to microsatellite instability-high (MSI-H) phenotype, characterized by high mutation rates in microsatellite regions. Microsatellites are short, repeated DNA segments prone to replication errors. dMMR-induced accumulation of mutations in coding regions generates neoantigens, abnormal proteins that can trigger an immune response. MSI-H cancers exhibit a favorable immune environment and high neoantigen loads, making them responsive to immune checkpoint blockade therapy. However, approximately 50% of patients do not respond, suggesting the presence of resistance mechanisms. Despite early dMMR, high mutation rate and immune response may not control tumor growth. Additional driver mutations in genes like APC, KRAS, PI3K, PTEN, BRAF, or p53 contribute to malignancy. Studying immune responses in early tumors can provide insights into resistance mechanisms during tumor progression and therapy response [102].
Specifically, MSI-H is usually due to MLH1 methylation or an epigenetic inactivation of MSH2 gene, the most important of the MMR system genes. MSI-H or dMMR is present in multiple cancer types, including endometrial carcinoma, colorectal cancer, gastric cancer and hepatocellular carcinoma. Most evidence about the predictive role of MSI-H/dMMR is observed in those cancer types, with MSI-H-type of colorectal cancer and endometrial carcinoma having a better prognosis than MSS tumors. In June 2020, pembrolizumab was approved for the treatment of metastatic colorectal carcinoma with MSI-H or dMMR status. This placed the MSI/MMR status in the spotlight of possible predictive biomarkers among other tumor types, too. However, in NSCLC MSI is not common (< 1 %), thus making this predictive biomarker less valuable for lung carcinoma [103,104]. Further research is needed to assess its potential as an alternative predictive biomarker.
T-Cell-related biomarkers: Tumor infiltrating lymphocytes (TILs)
The efficacy of anti-PD-1/PD-L1 inhibition therapy has been associated with the composition of effector T cells, including CD4+ T cell, CD8+ T cell, T-regs, composition of tumor-infiltrating lymphocytes (TILs) and TCR sequencing diversity [105]. Especially, TILs comprise a component of the tumor microenvironment expressing activated antigens. TILs have gained significant attention for their predictive role in cancer treatment, due to the ability of tumor cells to inactivate TILs and subsequently surpass the immune surveillance leading to carcinogenesis [106,107]. In the Chen et al. study [105], TILs were investigated in NSCLC patients with early and advanced stage and showed that a new group of TILs accumulated in the tumor microenvironment, called CD8+ TILs. CD8+ TILs correlated well with NSCLC patient resistance to ICI therapy. Subsequently, the suggestion of overcoming ICI resistance was proposed by measuring the levels of CD8+ TILs in NSCLC patients as a predictor of their treatment response [108]. Furthermore, a phase I clinical trial was conducted based on TILs in advanced NSCLC patients treated with nivolumab. After disease progression, tumor lesions were extracted from the participants and TILs amplified in vitro were infused to the patients. Results showed an average reduction of 38% of tumor diameter on chest CT examination, proving that TILs are promising in their use as treatment enhancing options, as well as predictive biomarkers for the resistance to ICI therapy [109]. However, there are limited studies showing the role of TILs as a predictive biomarker, due to the lack of standardized methods for measuring TIL and effectively scoring the abundance of these cell subpopulations. The current TIL techniques, including IHC and flow cytometry have several limitations; thus, the need for more effective and repeatable methods are needed in order to further investigate them as predictive biomarkers in clinical trials settings.
NSCLC driver gene mutations
Acquired genetic alterations in cancer driver genes play a pivotal role in NSCLC growth and invasiveness. These mutations have extensively been the key component of targeted drugs, such as tyrosine kinase inhibitors (TKI) used for the management of the disease, either as an alternative therapy or combined with chemotherapy and immunotherapy. One of the major oncogenic driver mutations in NSCLC is found on the gene encoding for the epidermal growth factor receptor (EGFR), which has been mostly correlated with low response to ICI therapy. Additionally, anaplastic lymphoma kinase (ALK), is also associated with a low ICI response. Furthermore, other significant driver mutations in NSCLC have been detected in c-ROS oncogene 1 (ROS1), and v-raf murine sarcoma viral oncogene homolog B (BRAF) and therefore, comprise further targets of novel therapies [110].
Less commonly mutated genes not listed in the guidelines, could also predict the response of NSCLC patients to ICIs. A rare gene mutation indicating a low response to ICI treatment is phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), whereas other less common mutations demonstrating a positive response to ICI therapy are found in KRAS, TP53, and mesenchymal-to-epithelial transition factor (MET) genes. However, further research needs to be conducted for the exploration of these driver mutations as potential predictive biomarkers for ICI efficacy [111]. Finally, epigenetic effects can also potentially affect ICI efficacy, mainly by promoter methylation of genes encoding immune checkpoints and prevent the presentation of antigens and the migration of T cells. Importantly, EPIMMUNE is a DNA methylation signature that has been associated with ICI efficacy and overall survival in NSCLC patients [112].
Cancer neoantigens
Cancer neoantigens have gained attention as potential predictive biomarkers, since both their quantity and quality could add in the prediction of ICI efficacy. Firstly, it has been established that increased levels of high-quality tumor neoantigens may improve the prediction of immune checkpoint inhibition therapy, while low levels of neoantigens have been associated with ICI resistance, especially in squamous cell lung carcinoma [113]. Furthermore, adoptive T-cell therapies and tumor neoantigen-based vaccines are developed. One significant limitation of the use of cancer neoantigens as predictive biomarkers, is the absence of a standardized method for their identification and measurement. Current methods being used include HLA typing, inference of neopeptides, MHC-binding prediction and candidate neoantigen selection, which are all based on bioinformatic pipeline methods [113,114]. The median number of cancer neoantigens per NSCLC tumor has been predicted to range between 63-214. As expected, higher numbers of cancer neoantigens are linked to high PD-L1 expression and tobacco smoking-related genomic mutations [115,116] Therefore, this correlation could add in the potential predictive role of cancer neoantigens for ICIs response.
The microbiome
The microbiome consists of organisms that normally inhabit different sites of the human body. These microorganisms have been found to be linked with anticancer immunity and response to immunotherapy [115]. The mechanism through which the microbiome shields us from pathogens involves communication with the immune cells in the mucosa through multiple receptors. As a result, the innate immune system that is initially activated through the receptors by the commensal bacteria, regulates the inflammatory responses [117]. However, the mechanism through which the microbiome interferes with immunotherapy is still under investigation. Patients with NSCLC, when treated with antibiotics, whose mechanism of action alters the gut microbiome, had as reported, reduced survival rates when treated with PD-1 inhibitors [117]. On the other hand, evidence supports that higher proportions of “good” bacteria, also known as “eubiosis”, had improved response to treatment with PD-1 inhibitors. Microbiome studies focus mostly on gut microbiota, because of their large numbers and overall significance. However, studies with preclinical mice have shown that microbiome population in the lower respiratory tract could be proven of utmost importance for immunotherapy in lung cancer and even be more predictive than the gut microbiome for the same purpose [118]. The method used up to date for the evaluation of abundance of bacterial microbiomes involves shotgun sequencing, 16S rRNA gene sequencing and PCR techniques. Nevertheless, the optimal method that should be used for this evaluation has not yet been discovered. All in all, the microbiome has shown evidence that supports its significant effect on immune response, with a “healthy” microbiome favoring response to ICIs [115,119]. As a result, both gut and lung microbiome could possibly be considered as predictive biomarkers of immunotherapy response [92,117].
Circulating tumor DNA (ctDNA)
“Liquid Biopsy” which includes the analysis of tumor-derived materials present in the bloodstream, has gained significant attention as a predictive biomarker too. However, in the context of ICI therapy for lung cancer, the current role of ctDNA analysis is primarily focused on identifying specific genomic alterations [120]. While several commercial platforms offer blood-based tumor mutation burden (TMB) assays, their clinical usefulness is yet to be confirmed through extensive prospective trials. The utility of ctDNA analysis in the context of ICI for lung cancer is mostly focused on detecting genomic alterations and driver mutations mentioned above [121]. Preliminary findings from studies examining the consecutive assessment of ctDNA as a potential biomarker for response and survival in lung cancer patients treated with ICIs reveal encouraging associations between molecular and radiographic responses [31,121].
Immune checkpoint inhibition therapy and small cell lung cancer
Despite the profound advances of ICI therapy, genomic and clinicopathologic predictive biomarkers, the efficacy of ICIs remains undefinable and elusive. Current approaches to overcoming resistance to ICIs were recently discussed [31]. Undoubtedly, a unifying feature of multiple solid malignant tumors for which immune checkpoint inhibition therapy has been responsive is a high somatic mutation burden [121]. Pre-clinical and clinical trial data support the use of TMB as a predictive biomarker in NSCLC, while further investigation is required for the implementation of TMB as a predictor for SCLC immunotherapy response too, a more aggressive malignancy type compared to NSCLC, which mostly carries a dismal prognosis. While the treatment of non-small cell lung cancer (NSCLC) has seen numerous paradigm changes, there has been little progress in the treatment of SCLC [121]. The clinical effectiveness of ICIs in managing SCLC is currently confined to first-line treatment of extensive stage disease in combination with chemotherapy with modest impact on overall survival [92]. SCLC has been found to have genetic alterations, such as inactivation of tumor suppressor genes like TP53, RB1, and Notch, as well as amplification of the MYC oncogene [122]. These changes result in rapid cell proliferation and replication stress, making SCLC cells heavily reliant on a defective DNA damage response and repair pathway (DDR) for their survival [121,122]. This could potentially make the tumor cells more vulnerable to DNA-damaging agents like chemotherapy and radiotherapy, explaining the initial high response rates of SCLC to platinum drugs like cisplatin and carboplatin.
Specifically, the study by Park et al. investigates the role of DNA damage response and repair pathway (DDR) alterations in SCLC and how it is associated TMB and chemotherapy outcomes. Dysregulation of DNA damage response mechanisms leads to the emergence of genomic instability, the creation of neoantigens, the enhancement of PD-L1 expression, and the modulation of various signaling pathways, including cyclic GMP-AMP synthase-stimulator of interferon genes (cGAS-STING) signaling [121,123]. The Park et al. study investigated a large cohort of SCLC patients who received platinum-based chemotherapy and found that more than 60 % of patients with SCLC have DDR pathway alterations. However, the study also demonstrated that DDR alterations did not correlate with chemotherapy survival outcomes in SCLC. A positive correlation was found between TMB and DDR pathway mutations in four out of six DDR genes and that TMB was associated with improved progression-free survival and overall survival in patients with limited-stage SCLC. The study suggests that future studies with PARP inhibitors in SCLC should consider stratifying for DDR pathway alterations to potentially improve treatment outcomes [121].
Furthermore, SCLC is typically linked with tobacco smoking, which leads to widespread genetic mutation and a high tumor mutation burden (TMB). Consequently, employing ICIs is a compelling approach to consider in this context, given the association between ICI activity and high TMB observed in NSCLC. Results from the CheckMate 032 phase I/II trial, a multitumor, nonrandomized study, showed a 10 % objective response rate with nivolumab and 23 % with nivolumab/ipilimumab in the SCLC cohort. Two phase III clinical trials with extensive-stage SCLC (ES-SCLC) patients, Impower [123] and CASPIAN trial [55] have provided evidence supporting the use of atezolizumab and durvalumab, respectively, in combination with chemotherapy as a first-line treatment for ES-SCLC. Therefore, these checkpoint inhibitors have gained approval for use in this specific setting. However, the effectiveness of PD-L1 inhibitors in SCLC is relatively modest when compared to their impact in NSCLC. This emphasizes the critical need to deepen our understanding of SCLC phenotypes and immune signatures, which could offer a more rational and targeted approach to selecting patients for treatment [124].
Blood TMB as a predictive biomarker in lung cancer
The incorporation of blood-based biomarker testing and particularly the utility of NGS-based panel testing would be optimal for mutation detection in cell-free DNA. Extracting cell-free DNA (cfDNA) from liquid biopsies for the analysis of circulating tumor DNA (ctDNA) has gained tremendous attention due to its less invasive nature of sample collection and the potential for frequent sampling. There are advantages offered by utilizing cfDNA for the assessment of TMB over traditional tissue biopsies, particularly for monitoring the initial responses to immune checkpoint inhibition therapy. Multiple assays have been developed and validated to measure TMB using cfDNA and there are continuous endeavors to align the findings with TMB data acquired from tissue samples. A systematic review comparing ICIs to chemotherapy with subgroup analysis on different levels of blood TMB (bTMB) also evaluated the clinical benefit of NSCLC patients with varying TMB levels for the validation of bTMB as a predictive biomarker. The clinical benefit of immunotherapy was analyzed in terms of overall survival, progression-free survival, and objective response rates, compared to chemotherapy. The results revealed that there were no meaningful differences between NSCLC patients with low and high bTMB. Consequently, blood based TMB did not demonstrate efficacy in screening NSCLC patients for ICIs [125]. Tissue and blood-based biomarker testing of tumor mutational burden have considerable differences regarding the sample collection method, the sensitivity of detecting a mutation, the TMB concordance between the two methods as well as a difference in detecting tumor heterogeneity. Regarding sample collection, liquid biopsies involve the isolation of cfDNA from blood samples thus they offer a less invasive method compared to the traditional tissue biopsy approach [126]. Therefore, this makes liquid biopsies and blood TMB utility a more practical and attractive method for future use, especially for obtaining frequent samples during the treatment course of solid tumor patients. However, another crucial difference is related to the sensitivity each method has for the detection of the mutations under investigation.
Liquid biopsies including cfDNA analysis exhibit a decreased sensitivity in the detection of mutations compared to solid tumor samples, regardless of the TMB assay method, WES or NGS gene panel, used to analyze each specimen type [126,127]. Thus, liquid biopsies may be unable to detect certain mutations leading to an underestimation of the true TMB burden in solid tumor types. On the contrary, a potential benefit of liquid biopsies and ctDNA use as a clinical biomarker, is the ability of this method to reflect the heterogeneity of various tumor locations, compared to tissue biopsies which provide a more localized representation of the tumor under investigation [128]. The variability in tumor heterogeneity could have an impact on the concordance between TMB values obtained from tissue samples and liquid biopsies, thus from tissue tumor DNA measurement and ctDNA. The concordance of TMB identification between these two methods has been a subject of debate, investigated by various studies [128]. Some studies report that the concordance between the two methods is sufficient and thus the values obtained from liquid biopsies provide similar results to the ones from tissue biopsies. However, there are also conflicting reports underlying that TMB values from liquid biopsies may not always align with those derived from tissue samples [129].
TMB limitations and challenges as a clinical biomarker
Further investigation is necessary to the clinical utility of TMB due to the variability of outcomes observed across studies and across evaluating the efficacy of this biomarker. Such inconsistencies may stem from TMB's predictive potential being confined to specific cancer types. Additionally, a consensus regarding which mutations should be included when determining mutation rates has not been reached. A divergence in different approaches is observed across different studies: certain authors encompass all mutations, whereas others selectively incorporate non-synonymous mutations [130,131]. A subset of other studies focuses exclusively on mutations that induce modifications in the protein sequence, particularly missense mutations and indels occurring within exons. These specific mutations exhibit a substantial range of prevalence, encompassing a wide spectrum from as low as 0.01 mutations per megabase pair (Mbp) to a remarkably high value of 400 Mbp [132]. Furthermore, the optimal TMB threshold for a broad range of malignancies remains undetermined, as diverse cutoff values have been implemented. While most studies have adopted similar high TMB cutoff values ranging from 5 to 10 Mbp, they are inadequate in establishing discriminating criteria for categorizing patients into low, intermediate, and high TMB groups. As a result, research has been directed towards patients with intermediate TMB, as their clinical management is poorly understood. Although TMB is being studied in various cancer types, further research is needed to refine the respective cutoff values in each case.
In addition to the variability in cutoff values for TMB, the utilization of targeted next-generation sequencing (NGS) has complicated research on this biomarker, primarily due to the high cost and complexity of whole exome sequencing (WES). The FDA has approved two non-genome sequencing techniques thus far, namely MSK-IMPACT® and FoundationOne CDx assay, but disparities exist between gene panels used in different studies [132]. Therefore, it is crucial to consider methodological differences when interpreting TMB biomarker studies.
It is also pertinent to acknowledge the potential of circulating tumor DNA (ctDNA) sequencing as a promising, noninvasive approach for tailoring cancer therapy. ctDNA profiling offers advantages over tissue biopsy, as it allows the avoidance of biopsy-related complications and provides more comprehensive insights into tumor heterogeneity, particularly in metastatic disease [132]. This technology has clinical implications for routinely monitoring or detecting cancer, identifying biomarkers of ICI efficacy, including TMB, and subsequently matching patients with targeted therapies, particularly when used with a large gene panel [133].
Resistance to immune checkpoint inhibition therapy
Even though immune checkpoint inhibition therapy is a major advancement in the management of lung cancer, only small proportion of patients exhibit a meaningful therapeutic response. The vast majority of patients treated present with resistance to ICIs, which could either be primary resistance; not showing any response to the treatment throughout the whole treatment duration or acquired meaning that there was an initial response to ICIs unable to subsequently prevent malignancy progression [130]. Tumor resistance to immunotherapy arises from intrinsic mechanisms within the tumor cells as well as external factors. Intrinsic mechanisms involve changes in pathways responsible for effective antitumor immune responses and alterations in signaling pathways within tumor cells, which create an immunosuppressive environment [130,132]. These mechanisms also include the loss of antigen presentation capacity and the altered response or prolonged exposure of IF-γ [134]. A considerable number of patients demonstrate resistance to ICIs due to insufficient antigen presentation, leading to the investigation of the association between impaired expression of major histocompatibility complex class I (MHC-I) and resistance to ICI therapy [134]. Notably, interferon-gamma (IFN-γ), found within tumors, can augment the expression of MHC-I genes through the JAK-STAT pathway, thereby enhancing the immune response against tumor cells by stimulating CD8+ T cells [134]. However, IFN- γ can also exhibit immunosuppressive effects by promoting the expression of CD274, encoding PD-L1.
Extrinsic factors involve the interactions among tumor cells, the TME, and host-related elements, such as age, gender, body fat composition and gut microbiome. Factors of TME influencing resistance include immunosuppressive cells such as MDSCs, T-regs, TAM as well as coinhibitory receptors (e.g., PD-L1, CTLA-4, TIM-3 and TIGIT) [135]. Specifically, the abnormal neovascularization the TME exhibits caused by tumor-associated macrophages (TAMs) infiltration around tumor cells leads to chronic inflammatory carcinogenesis by the release of proinflammatory mediators (i.e., IL-6 and IL-1β), followed by an increasing tumor angiogenesis which promotes tumor invasion and metastasis [135,136]. Furthermore, new research has demonstrated that tumors characterized by a high persistent tumor mutation burden (pTMB) exhibit a more inflamed TME [131,132]. As stated before, the TME plays a significant role in the immunotherapy response and according to the interaction between the two, solid tumors have been categorized into hot and cold tumors; with the former having immunostimulatory function to the TME and thus being more responsive to ICIs, whereas the latter exhibiting immunosuppressive properties and therefore result in a worse immunotherapy response [131,132]. By transforming "cold" tumors into "hot" ones, combination immunotherapies exhibit greater clinical advantages compared to using a single therapy approach in cancer patients. This field has exhibited tremendous research potential with cells of the TME serving as targets for therapy. One of these cells are the myeloid-derived suppressor cells (MDSCs), which possess mechanisms to hinder the activity of T cells and NK cells, thereby facilitating tumor growth, and immunotherapy resistance [137]. Growing evidence suggests that targeting MDSCs therapeutically can attenuate their tumor-promoting functions and immunosuppressive activities, thus enhancing the effectiveness of checkpoint inhibitors [137]. Furthermore, investigations have also directed their attention towards interventions aimed at restoring the vascular state within the TME. Notably, endoglin endoglin (CD105) has been identified as a promising target due to its involvement in important processes such as angiogenesis, inflammation, and the accumulation of CAFs [131,137].
Most ICIs resistance documented is acquired leading to the need of examining another biomarker called persistent tumor mutation burden (pTMB), which can potentially predict the efficacy of ICI therapy better than tissue or blood TMB [131,135]. pTMB refers to the number of mutations in both single-copy and multiple-copy regions within a cell. It thus serves as an indicator of how distinct the tumor is from healthy cells and remains unaffected by the loss of neoantigens during the tumor's progression to an advanced stage [131,135]. Despite the effect of immunotherapy, certain mutations persist throughout the tumor's evolutionary process. Consequently, a hypothesis has emerged suggesting that these mutations may be responsible for sustaining immune responses driven by neoantigens [131] (Table 3).
Table 3.
The table organizes the ongoing clinical trials based on their respective phases. Phase I trials encompass the evaluation of VISTA and B7-H3 immune targets (CA-170 and Enoblituzumab); Phase II trials assess drugs targeting LAG-3, TIM-3, TIGIT NKG2A and CD733; TIGIT is the novel immune target under Phase III clinical trial investigation.
| Phase | Immune Checkpoint Target | Drug | Mechanism of Action | ClinicalTrials.gov Identifier |
|---|---|---|---|---|
| Phase I | VISTA | CA-170 (oral PD-1, PD-L2, and VISTA antagonist) | Suppression of T cell activity and effector functions. | NCT02812875 |
| Phase I | B7-H3 | Enoblituzumab (anti B7-H3 mAb) | Inhibition of CD8+ and CD4+ cells. Reduction of inflammatory mediators. | NCT02381314 |
| Phase II | LAG-3 | Eftilagimod alpha (LAG-3 soluble fusion protein) | Inhibition of T-cell co-receptor during activation. | NCT036255323 |
| Phase II | LAG-3 | Relatlimab (anti-LAG3 mAb) | Inhibition of T-cell co-receptor during activation. | NCT04623775 |
| Phase II | LAG-3 | BI 754111 (anti-LAG3 mAb) | Inhibition of T-cell co-receptor during activation. | NCT03156114 |
| Phase II | TIM-3 | MGB453 (anti-TIM-3 mAb) | Inhibition of receptor on CD8+ and CD4+ cells. | NCT03708328 |
| Phase II | TIM-3 | TSR-022 (anti-TIM-3 mAb) | Inhibition of receptor on CD8+ and CD4+ cells. | NCT03307785 |
| Phase II | TIGIT | Tiragolumab (anti-TIGIT mAb) | Inhibition of T-cell receptors. Binding to CD155 and CD112 on cancer cells. | NCT04264210 |
| Phase II | TIGIT | Domavanalimab (anti-TIGIT mAb) | Inhibition of T-cell receptors. Binding to CD155 and CD112 on cancer cells. | NCT042994810 |
| Phase II | TIGIT | Domavanalimab (anti-TIGIT mAb) | Inhibition of T-cell receptors. Binding to CD155 and CD112 on cancer cells. | NCT04736173 |
| Phase II | TIGIT | Domavanalimab (anti-TIGIT mAb) | Inhibition of T-cell receptors. Binding to CD155 and CD112 on cancer cells. | NCT04262856 |
| Phase II | NKG2A | Monalizumab (anti-NKG2A mAb) | Inhibition of receptors on NK and CD8+ cells. | NCT03822351 |
| Phase II | NKG2A | Monalizumab (anti-NKG2A mAb) | Inhibition of receptors on NK and CD8+ cells. | NCT03833440 |
| Phase II | CD73 | Oleclumab (anti CD73 mAb) | Generation of adenosine leading to suppression of immune response. | NCT05061550 |
| Phase II | CD73 | Oleclumab (anti CD73 mAb) | Generation of adenosine leading to suppression of immune response. | NCT03822351 |
| Phase III | TIGIT | Tiragolumab (anti-TIGIT mAb) | Inhibition of T-cell receptors. Binding to CD155 and CD112 on cancer cells. | NCT04264210 |
| Phase III | TIGIT | Domavanalimab (anti-TIGIT mAb) | Inhibition of T-cell receptors. Binding to CD155 and CD112 on cancer cells. | NCT042994810 |
| Phase III | TIGIT | Domavanalimab (anti-TIGIT mAb) | Inhibition of T-cell receptors. Binding to CD155 and CD112 on cancer cells. | NCT04736173 |
| Phase III | TIGIT | Domavanalimab (anti-TIGIT mAb) | Inhibition of T-cell receptors. Binding to CD155 and CD112 on cancer cells. | NCT04262856 |
Conclusion
Immune checkpoint inhibitors (ICIs) emerging as the gold standard therapeutic approach for patients diagnosed with metastatic, locally advanced, and resectable NSCLC, yielding remarkable improvements in overall survival. However, the clinical utility of ICIs in SCLC is currently restricted to the initial treatment of extensive-stage disease, resulting in modest improvements in overall survival. TMB is a rapidly developing biomarker for immune checkpoint inhibition therapy response, with multiple ongoing clinical trials listed in Table 2. TMB could potentially serve as a biomarker for assessing the effectiveness of ICIs as it represents the number of somatic mutations in a tumor and is associated with the generation of neoantigens. TMB levels vary among patients depending on factors like cancer type, tumor subtype, age, environmental factors, and inactivation of specific genes in the germline, while it can be influenced by factors such as MSI and dMMR. . Notably, resistance to immunotherapy remains a significant challenge which might be overcome with novel immune targets, such as LAG-3, TIGIT, TIM-3 and CD73. Cellular therapy is promising in enhancing immunotherapeutic strategies for lung cancer, but challenges such as lack of tumor-specific antigens and an unfavorable TME, also need to be overcome.
Table 2.
Novel Immune Checkpoint Inhibitors and Ongoing Clinical Trials.
| Immune Checkpoint Target | Drug | Mechanism of Action | Phase | ClinicalTrials.gov Identifier |
|---|---|---|---|---|
| LAG-3 | Eftilagimod alpha (LAG-3 soluble fusion protein) Relatlimab (anti-LAG3 mAb) BI 754111 (anti-LAG3 mAb) |
Inhibition of T-cell co-receptor during activation | II II I |
NCT036255323 NCT04623775 NCT03156114 |
| TIM-3 | MGB453 (anti-TIM-3 mAb) TSR-022 (anti-TIM-3 mAb) |
Inhibition of receptor on CD8+ and CD4+ cells with subsequent Th1 cells suppression | I I |
NCT03708328 NCT03307785 |
| TIGIT | Tiragolumab (anti-TIGIT mAb) Domavanalimab (anti-TIGIT mAb) |
Inhibition of T-cells receptors that have been activated. Binding to CD155 and CD112 on cancer cells. | III III III II |
NCT0426421 NCT042994810 NCT04736173 NCT04262856 |
| VISTA | CA-170 (oral PD-1, PD-L2 and VISTA checkpoint antagonist) | Suppression of T cell activity and effector functions. Inhibition of T cell proliferation, cytokine production and cytotoxicity. Homologous to PD-L1. | I | NCT02812875 |
| NKG2A | Monalizumab (anti-NKG2A mAb) | Inhibition of receptors on NK and CD8+ cells | II II |
NCT03822351 NCT03833440 |
| CD73 | Oleclumab (anti-CD73 mAb) | Generation of adenosine leading to suppression of immune response through the A2 receptor | II II |
NCT05061550 NCT03822351 |
| B7-H3 | Enoblituzumab (anti-B7-H3 mAb) | Inhibition of CD8+ and CD4+ cells. Reduction of inflammatory mediators such as IL-2, IFN-gamma production | I | NCT02381314 |
Funding statement
No funding was received.
Informed consent statement
Not applicable.
CRediT authorship contribution statement
Paraskevi Vryza: Methodology, Investigation, Writing – review & editing, Visualization, Writing – original draft, Data curation, Software. Timo Fischer: Data curation, Writing – review & editing, Investigation, Visualization, Writing – original draft. Elena Mistakidi: Methodology, Visualization, Investigation, Writing – original draft, Data curation, Writing – review & editing, Software. Apostolos Zaravinos: Conceptualization, Writing – review & editing, Supervision, Methodology, Software.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Bibliography
- 1.Luo G., Zhang Y., Etxeberria J., Arnold M., Cai X., Hao Y., et al. Projections of lung cancer incidence by 2035 in 40 countries worldwide: population-based study. JMIR Public Health Surveill. 2023;9:e43651. doi: 10.2196/43651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA A Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson A.G., Tsao M.S., Beasley M.B., Borczuk A.C., Brambilla E., Cooper W.A., et al. The 2021 WHO classification of lung tumors: impact of advances since 2015. J. Thorac. Oncol. 2022;17:362–387. doi: 10.1016/j.jtho.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Johnson D.H., Fehrenbacher L., Novotny W.F., Herbst R.S., Nemunaitis J.J., Jablons D.M., et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J. Clin. Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 5.Osmani L., Askin F., Gabrielson E., Li Q.K. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin. Cancer Biol. 2018;52:103–109. doi: 10.1016/j.semcancer.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Travis W.D., Brambilla E., Nicholson A.G., Yatabe Y., Austin J.H.M., Beasley M.B., et al. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 7.Altorki N.K., Markowitz G.J., Gao D., Port J.L., Saxena A., Stiles B., et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat. Rev. Cancer. 2019;19:9–31. doi: 10.1038/s41568-018-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogan B.L.M., Barkauskas C.E., Chapman H.A., Epstein J.A., Jain R., Hsia C.C.W., et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morales-Nebreda L., Misharin A.V., Perlman H., Budinger G.R.S. The heterogeneity of lung macrophages in the susceptibility to disease. Eur. Respir. Rev. 2015;24:505–509. doi: 10.1183/16000617.0031-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houghton A.M. Mechanistic links between COPD and lung cancer. Nat. Rev. Cancer. 2013;13:233–245. doi: 10.1038/nrc3477. [DOI] [PubMed] [Google Scholar]
- 11.Takamura S. Niches for the long-term maintenance of tissue-resident memory T cells. Front. Immunol. 2018;9:1214. doi: 10.3389/fimmu.2018.01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson N.M., Simon M.C. The tumor microenvironment. Curr. Biol. 2020;30:R921–R925. doi: 10.1016/j.cub.2020.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farhood B., Najafi M., Mortezaee K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: a review. J. Cell. Physiol. 2019;234:8509–8521. doi: 10.1002/jcp.27782. [DOI] [PubMed] [Google Scholar]
- 14.Cao X., Cai S.F., Fehniger T.A., Song J., Collins L.I., Piwnica-Worms D.R., et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Cózar B., Greppi M., Carpentier S., Narni-Mancinelli E., Chiossone L., Vivier E. Tumor-infiltrating natural killer cells. Cancer Discov. 2021;11:34–44. doi: 10.1158/2159-8290.CD-20-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wylie B., Macri C., Mintern J.D., Waithman J. Dendritic cells and cancer: from biology to therapeutic intervention. Cancers. 2019;11:521. doi: 10.3390/cancers11040521. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondĕlková K., Vokurková D., Krejsek J., Borská L., Fiala Z., Ctirad A. Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Medica. 2010;53:73–77. doi: 10.14712/18059694.2016.63. (Hradec. Kralove) [DOI] [PubMed] [Google Scholar]
- 18.Knochelmann H.M., Dwyer C.J., Bailey S.R., Amaya S.M., Elston D.M., Mazza-McCrann J.M., et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell Mol. Immunol. 2018;15:458–469. doi: 10.1038/s41423-018-0004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabrilovich D.I. Myeloid-derived suppressor cells. Cancer Immunol. Res. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar V., Patel S., Tcyganov E., Gabrilovich D.I. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37:208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veglia F., Perego M., Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018;19:108–119. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Law A.M.K., Valdes-Mora F., Gallego-Ortega D. Myeloid-derived suppressor cells as a therapeutic target for Cancer. Cells. 2020;9:561. doi: 10.3390/cells9030561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chesney J.A., Mitchell R.A., Yaddanapudi K. Myeloid-derived suppressor cells-a new therapeutic target to overcome resistance to cancer immunotherapy. J. Leukoc. Biol. 2017;102:727–740. doi: 10.1189/jlb.5VMR1116-458RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dysthe M., Parihar R. Myeloid-derived suppressor cells in the tumor microenvironment. Adv. Exp. Med. Biol. 2020;1224:117–140. doi: 10.1007/978-3-030-35723-8_8. [DOI] [PubMed] [Google Scholar]
- 25.Yunna C., Mengru H., Lei W., Weidong C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020;877 doi: 10.1016/j.ejphar.2020.173090. [DOI] [PubMed] [Google Scholar]
- 26.Huang T., Chen X., Zhang H., Liang Y., Li L., Wei H., et al. Prognostic role of tumor mutational burden in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.706652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galuppini F., Dal Pozzo C.A., Deckert J., Loupakis F., Fassan M., Baffa R. Tumor mutation burden: from comprehensive mutational screening to the clinic. Cancer Cell Int. 2019;19:209. doi: 10.1186/s12935-019-0929-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sha D., Jin Z., Budczies J., Kluck K., Stenzinger A., Sinicrope F.A. Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discov. 2020;10:1808–1825. doi: 10.1158/2159-8290.CD-20-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coulie P.G., Van den Eynde B.J., van der Bruggen P., Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat. Rev. Cancer. 2014;14:135–146. doi: 10.1038/nrc3670. [DOI] [PubMed] [Google Scholar]
- 30.Carreno B.M., Magrini V., Becker-Hapak M., Kaabinejadian S., Hundal J., Petti A.A., et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348:803–808. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moeckel C., Bakhl K. Georgakopoulos-Soares I, Zaravinos A. the efficacy of tumor mutation burden as a biomarker of response to immune checkpoint inhibitors. Int. J. Mol. Sci. 2023;24:6710. doi: 10.3390/ijms24076710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borden E.S., Buetow K.H., Wilson M.A., Hastings K.T. Cancer neoantigens: challenges and future directions for prediction, prioritization, and validation. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.836821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng M., Mo Y., Wang Y., Wu P., Zhang Y., Xiong F., et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol. Cancer. 2019;18:128. doi: 10.1186/s12943-019-1055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snyder A., Makarov V., Merghoub T., Yuan J., Zaretsky J.M., Desrichard A., et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Malley D.M., Bariani G.M., Cassier P.A., Marabelle A., Hansen A.R., De J., Acosta A., et al. Pembrolizumab in patients with microsatellite instability-high advanced endometrial cancer: results from the KEYNOTE-158 study. J. Clin. Oncol. 2022;40:752–761. doi: 10.1200/JCO.21.01874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hellmann M.D., Ciuleanu T.E., Pluzanski A., Lee J.S., Otterson G.A., Audigier-Valette C., et al. Nivolumab plus Ipilimumab in lung cancer with a high tumor mutational burden. N. Engl. J. Med. 2018;378:2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su Z. Different interpretations of the CheckMate-227 trial in non-small-cell lung cancer. Contemp. Clin. Trials Commun. 2020;18 doi: 10.1016/j.conctc.2020.100578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frampton G.M., Fichtenholtz A., Otto G.A., Wang K., Downing S.R., He J., et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat. Biotechnol. 2013;31:1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strickler J.H., Hanks B.A., Khasraw M. Tumor mutational burden as a predictor of immunotherapy response: is more always better? Clin. Cancer Res. 2021;27:1236–1241. doi: 10.1158/1078-0432.CCR-20-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chalmers Z.R., Connelly C.F., Fabrizio D., Gay L., Ali S.M., Ennis R., et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zehir A., Benayed R., Shah R.H., Syed A., Middha S., Kim H.R., et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017;23:703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carbone D.P., Reck M., Paz-Ares L., Creelan B., Horn L., Steins M., et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N. Engl. J. Med. 2017;376:2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marin-Acevedo J.A., Dholaria B., Soyano A.E., Knutson K.L., Chumsri S., Lou Y. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J. Hematol. Oncol. 2018;11:39. doi: 10.1186/s13045-018-0582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kythreotou A., Siddique A., Mauri F.A., Bower M., Pinato D.J. PD-L1. J. Clin. Pathol. 2018;71:189–194. doi: 10.1136/jclinpath-2017-204853. [DOI] [PubMed] [Google Scholar]
- 45.Han Y., Liu D., Li L. PD-1/PD-L1 pathway: current researches in cancer. Am. J. Cancer Res. 2020;10:727–742. [PMC free article] [PubMed] [Google Scholar]
- 46.Ghosh C., Luong G., Sun Y. A snapshot of the PD-1/PD-L1 pathway. J. Cancer. 2021;12:2735–2746. doi: 10.7150/jca.57334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ai L., Xu A., Xu J. Roles of PD-1/PD-L1 pathway: signaling, cancer, and beyond. Adv. Exp. Med. Biol. 2020;1248:33–59. doi: 10.1007/978-981-15-3266-5_3. [DOI] [PubMed] [Google Scholar]
- 48.Tsoukalas N., Kiakou M., Tsapakidis K., Tolia M., Aravantinou-Fatorou E., Baxevanos P., et al. PD-1 and PD-L1 as immunotherapy targets and biomarkers in non-small cell lung cancer. J. BUON. 2019;24:883–888. [PubMed] [Google Scholar]
- 49.Qu J., Mei Q., Liu L., Cheng T., Wang P., Chen L., et al. The progress and challenge of anti-PD-1/PD-L1 immunotherapy in treating non-small cell lung cancer. Ther. Adv. Med. Oncol. 2021;13 doi: 10.1177/1758835921992968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brahmer J., Reckamp K.L., Baas P., Crinò L., Eberhardt W.E.E., Poddubskaya E., et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borghaei H., Paz-Ares L., Horn L., Spigel D.R., Steins M., Ready N.E., et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 53.Rittmeyer A., Barlesi F., Waterkamp D., Park K., Ciardiello F., von Pawel J., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paz-Ares L.G., Ramalingam S.S., Ciuleanu T.E., Lee J.S., Urban L., Caro R.B., et al. First-line nivolumab plus ipilimumab in advanced NSCLC: 4-year outcomes from the randomized, open-label, phase 3 CheckMate 227 part 1 trial. J. Thorac. Oncol. 2022;17:289–308. doi: 10.1016/j.jtho.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 55.Goldman J.W., Dvorkin M., Chen Y., Reinmuth N., Hotta K., Trukhin D., et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22:51–65. doi: 10.1016/S1470-2045(20)30539-8. [DOI] [PubMed] [Google Scholar]
- 56.Melosky B., Cheema P.K., Brade A., McLeod D., Liu G., Price P.W., et al. Prolonging survival: the role of immune checkpoint inhibitors in the treatment of extensive-stage small cell lung cancer. Oncologist. 2020;25:981–992. doi: 10.1634/theoncologist.2020-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan D.V., Gibson H.M., Aufiero B.M., Wilson A.J., Hafner M.S., Mi Q.S., et al. Differential CTLA-4 expression in human CD4+ versus CD8+ T cells is associated with increased NFAT1 and inhibition of CD4+ proliferation. Genes Immun. 2014;15:25–32. doi: 10.1038/gene.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leach D.R., Krummel M.F., Allison J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 59.Hodi F.S., Mihm M.C., Soiffer R.J., Haluska F.G., Butler M., Seiden M.V., et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hodi F.S., O'Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naimi A., Mohammed R.N., Raji A., Chupradit S., Yumashev A.V., Suksatan W., et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun. Signal. 2022;20:44. doi: 10.1186/s12964-022-00854-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sur D., Havasi A., Cainap C., Samasca G., Burz C., Balacescu O., et al. Chimeric antigen receptor T-cell therapy for colorectal cancer. J. Clin. Med. 2020;9:182. doi: 10.3390/jcm9010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abunasser A.A.A., Xue J., Balawi E.J.A., Zhu Y. Combination of the EP and anti-PD-1 pathway or anti-CTLA-4 for the phase III trial of small-cell lung cancer: a meta-analysis. J. Oncol. 2021;2021 doi: 10.1155/2021/6662344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bates A.M., Brown R.J., Pieper A.A., Zangl L.M., Arthur I., Carlson P.M., et al. Combination of bempegaldesleukin and anti-CTLA-4 prevents metastatic dissemination after primary resection or radiotherapy in a preclinical model of non-small cell lung cancer. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.645352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanseviero E., O'Brien E.M., Karras J.R., Shabaneh T.B., Aksoy B.A., Xu W., et al. Anti-CTLA-4 activates intratumoral NK cells and combined with IL15/IL15Rα complexes enhances tumor control. Cancer Immunol. Res. 2019;7:1371–1380. doi: 10.1158/2326-6066.CIR-18-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Formenti S.C., Rudqvist N.P., Golden E., Cooper B., Wennerberg E., Lhuillier C., et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 2018;24:1845–1851. doi: 10.1038/s41591-018-0232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilkins A., McDonald F., Harrington K., Melcher A. Radiotherapy enhances responses of lung cancer to CTLA-4 blockade. J. Immunother. Cancer. 2019;7:64. doi: 10.1186/s40425-019-0542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tay R.Y., Heigener D., Reck M., Califano R. Immune checkpoint blockade in small cell lung cancer. Lung Cancer. 2019;137:31–37. doi: 10.1016/j.lungcan.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 69.Hellmann M.D., Callahan M.K., Awad M.M., Calvo E., Ascierto P.A., Atmaca A., et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell. 2018;33:853–861.e4. doi: 10.1016/j.ccell.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meng L., Xu J., Ye Y., Wang Y., Luo S., Gong X. The combination of radiotherapy with immunotherapy and potential predictive biomarkers for treatment of non-small cell lung cancer patients. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.723609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Christodoulou M.I., Zaravinos A. New clinical approaches and emerging evidence on immune-checkpoint inhibitors as anti-cancer therapeutics: CTLA-4 and PD-1 pathways and beyond. Crit. Rev. Immunol. 2019;39:379–408. doi: 10.1615/CritRevImmunol.2020033340. [DOI] [PubMed] [Google Scholar]
- 72.Andrews L.P., Marciscano A.E., Drake C.G., Vignali D.A.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017;276:80–96. doi: 10.1111/imr.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chan T.A., Yarchoan M., Jaffee E., Swanton C., Quezada S.A., Stenzinger A., et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann. Oncol. 2019;30:44–56. doi: 10.1093/annonc/mdy495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gandara D.R., Paul S.M., Kowanetz M., Schleifman E., Zou W., Li Y., et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat. Med. 2018;24:1441–1448. doi: 10.1038/s41591-018-0134-3. [DOI] [PubMed] [Google Scholar]
- 75.Chocarro L., Blanco E., Arasanz H., Fernández-Rubio L., Bocanegra A., Echaide M., et al. Clinical landscape of LAG-3-targeted therapy. Immunooncol. Technol. 2022;14 doi: 10.1016/j.iotech.2022.100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huard B., Prigent P., Tournier M., Bruniquel D., Triebel F. CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur. J. Immunol. 1995;25:2718–2721. doi: 10.1002/eji.1830250949. [DOI] [PubMed] [Google Scholar]
- 77.Long L., Zhang X., Chen F., Pan Q., Phiphatwatchara P., Zeng Y., et al. The promising immune checkpoint LAG-3: from tumor microenvironment to cancer immunotherapy. Genes Cancer. 2018;9:176–189. doi: 10.18632/genesandcancer.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Das M., Zhu C., Kuchroo V.K. Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 2017;276:97–111. doi: 10.1111/imr.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Acharya N., Sabatos-Peyton C., Anderson A.C. Tim-3 finds its place in the cancer immunotherapy landscape. J. Immunother. Cancer. 2020;8 doi: 10.1136/jitc-2020-000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dixon K.O., Tabaka M., Schramm M.A., Xiao S., Tang R., Dionne D., et al. TIM-3 restrains anti-tumour immunity by regulating inflammasome activation. Nature. 2021;595:101–106. doi: 10.1038/s41586-021-03626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Borate U., Esteve J., Porkka K., Knapper S., Vey N., Scholl S., et al. Phase Ib study of the anti-TIM-3 antibody MBG453 in combination with decitabine in patients with high-risk myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) Blood. 2019;134:570. [Google Scholar]
- 82.Curigliano G., Gelderblom H., Mach N., Doi T., Tai D., Forde P.M., et al. Phase I/Ib clinical trial of sabatolimab, an anti-TIM-3 antibody, alone and in combination with spartalizumab, an anti-PD-1 antibody, in advanced solid tumors. Clin. Cancer Res. 2021;27:3620–3629. doi: 10.1158/1078-0432.CCR-20-4746. [DOI] [PubMed] [Google Scholar]
- 83.Martinez M., Kim S., St Jean N., O'Brien S., Lian L., Sun J., et al. Addition of anti-TIM3 or anti-TIGIT antibodies to anti-PD1 blockade augments human T cell adoptive cell transfer. Oncoimmunology. 2021;10 doi: 10.1080/2162402X.2021.1873607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu X., Harden K., Gonzalez L.C., Francesco M., Chiang E., Irving B., et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 85.Le Mercier I., Lines J.L., Noelle R.J. Beyond CTLA-4 and PD-1, the generation Z of negative checkpoint regulators. Front. Immunol. 2015;6:418. doi: 10.3389/fimmu.2015.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Banta K.L., Xu X., Chitre A.S., Au-Yeung A., Takahashi C., O'Gorman W.E., et al. Mechanistic convergence of the TIGIT and PD-1 inhibitory pathways necessitates co-blockade to optimize anti-tumor CD8+ T cell responses. Immunity. 2022;55:512–526.e9. doi: 10.1016/j.immuni.2022.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mulati K., Hamanishi J., Matsumura N., Chamoto K., Mise N., Abiko K., et al. VISTA expressed in tumour cells regulates T cell function. Br. J. Cancer. 2019;120:115–127. doi: 10.1038/s41416-018-0313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang L., Rubinstein R., Lines J.L., Wasiuk A., Ahonen C., Guo Y., et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 2011;208:577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mortezaee K., Majidpoor J., Najafi S. VISTA immune regulatory effects in bypassing cancer immunotherapy: updated. Life Sci. 2022;310 doi: 10.1016/j.lfs.2022.121083. [DOI] [PubMed] [Google Scholar]
- 90.Labadie B.W., Bao R., Luke J.J. Reimagining IDO pathway inhibition in cancer immunotherapy via downstream focus on the tryptophan-kynurenine-aryl hydrocarbon axis. Clin. Cancer Res. 2019;25:1462–1471. doi: 10.1158/1078-0432.CCR-18-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haanen J.B., Cerundolo V. NKG2A, a new kid on the immune checkpoint block. Cell. 2018;175:1720–1722. doi: 10.1016/j.cell.2018.11.048. [DOI] [PubMed] [Google Scholar]
- 92.Mamdani H., Matosevic S., Khalid A.B., Durm G., Jalal S.I. Immunotherapy in lung cancer: current landscape and future directions. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.823618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Herbst R.S., Majem M., Barlesi F., Carcereny E., Chu Q., Monnet I., et al. COAST: an open-label, phase II, multidrug platform study of durvalumab alone or in combination with oleclumab or monalizumab in patients with unresectable, stage III non-small-cell lung cancer. J. Clin. Oncol. 2022;40:3383–3393. doi: 10.1200/JCO.22.00227. [DOI] [PubMed] [Google Scholar]
- 94.Yu M., Guo G., Huang L., Deng L., Chang C.S., Achyut B.R., et al. CD73 on cancer-associated fibroblasts enhanced by the A2B-mediated feedforward circuit enforces an immune checkpoint. Nat. Commun. 2020;11:515. doi: 10.1038/s41467-019-14060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Turcotte M., Spring K., Pommey S., Chouinard G., Cousineau I., George J., et al. CD73 is associated with poor prognosis in high-grade serous ovarian cancer. Cancer Res. 2015;75:4494–4503. doi: 10.1158/0008-5472.CAN-14-3569. [DOI] [PubMed] [Google Scholar]
- 96.Melenhorst J.J., Chen G.M., Wang M., Porter D.L., Chen C., Collins M.A., et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature. 2022;602:503–509. doi: 10.1038/s41586-021-04390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yan T., Zhu L., Chen J. Current advances and challenges in CAR T-Cell therapy for solid tumors: tumor-associated antigens and the tumor microenvironment. Exp. Hematol. Oncol. 2023;12:14. doi: 10.1186/s40164-023-00373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin J., Liu J., Hao S.G., Lan B., Zheng X.B., Xiong J.N., et al. An EGFR L858R mutation identified in 1862 Chinese NSCLC patients can be a promising neoantigen vaccine therapeutic strategy. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1022598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J. Clin. Oncol. 2019;37:537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 100.Mok T.S.K., Wu Y.L., Kudaba I., Kowalski D.M., Cho B.C., Turna H.Z., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 101.Hirsch F.R., McElhinny A., Stanforth D., Ranger-Moore J., Jansson M., Kulangara K., et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J. Thorac. Oncol. 2017;12:208–222. doi: 10.1016/j.jtho.2016.11.2228. [DOI] [PubMed] [Google Scholar]
- 102.Zhao P., Li L., Jiang X., Li Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J. Hematol. Oncol. 2019;12:54. doi: 10.1186/s13045-019-0738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Giustini N., Bazhenova L. Recognizing prognostic and predictive biomarkers in the treatment of non-small cell lung cancer (NSCLC) with immune checkpoint inhibitors (ICIs) Lung Cancer. 2021;12:21–34. doi: 10.2147/LCTT.S235102. (Auckl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Takamochi K., Takahashi F., Suehara Y., Sato E., Kohsaka S., Hayashi T., et al. DNA mismatch repair deficiency in surgically resected lung adenocarcinoma: microsatellite instability analysis using the Promega panel. Lung Cancer. 2017;110:26–31. doi: 10.1016/j.lungcan.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 105.He Q., Liu Z., Liu Z., Lai Y., Zhou X., Weng J. TCR-like antibodies in cancer immunotherapy. J. Hematol. Oncol. 2019;12:99. doi: 10.1186/s13045-019-0788-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kitsou M., Ayiomamitis G.D., Zaravinos A. High expression of immune checkpoints is associated with the TIL load, mutation rate and patient survival in colorectal cancer. Int. J. Oncol. 2020;57:237–248. doi: 10.3892/ijo.2020.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zaravinos A., Roufas C., Nagara M., de L., Moreno B., Oblovatskaya M., Efstathiades C., et al. Cytolytic activity correlates with the mutational burden and deregulated expression of immune checkpoints in colorectal cancer. J. Exp. Clin. Cancer Res. 2019;38:364. doi: 10.1186/s13046-019-1372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen J., He Q., Liu J., Xiao Y., Xiao C., Chen K., et al. CD8+ tumor-infiltrating lymphocytes as a novel prognostic biomarker in lung sarcomatoid carcinoma, a rare subtype of lung cancer. Cancer Manag. Res. 2018;10:3505–3511. doi: 10.2147/CMAR.S169074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Creelan B.C., Wang C., Teer J.K., Toloza E.M., Yao J., Kim S., et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat. Med. 2021;27:1410–1418. doi: 10.1038/s41591-021-01462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ettinger D.S., Wood D.E., Aggarwal C., Aisner D.L., Akerley W., Bauman J.R., et al. NCCN guidelines insights: non-small cell lung cancer, version 1.2020. J. Natl. Compr. Cancer Netw. 2019;17:1464–1472. doi: 10.6004/jnccn.2019.0059. [DOI] [PubMed] [Google Scholar]
- 111.Kauffmann-Guerrero D., Tufman A., Kahnert K., Bollmann B.A., Reu S., Syunyaeva Z., et al. Response to checkpoint inhibition in non-small cell lung cancer with molecular driver alterations. Oncol. Res. Treat. 2020;43:289–298. doi: 10.1159/000506842. [DOI] [PubMed] [Google Scholar]
- 112.Duruisseaux M., Martínez-Cardús A., Calleja-Cervantes M.E., Moran S., Castro de Moura M., Davalos V., et al. Epigenetic prediction of response to anti-PD-1 treatment in non-small-cell lung cancer: a multicentre, retrospective analysis. Lancet Respir. Med. 2018;6:771–781. doi: 10.1016/S2213-2600(18)30284-4. [DOI] [PubMed] [Google Scholar]
- 113.McGranahan N., Furness A.J.S., Rosenthal R., Ramskov S., Lyngaa R., Saini S.K., et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.De Mattos-Arruda L., Vazquez M., Finotello F., Lepore R., Porta E., Hundal J., et al. Neoantigen prediction and computational perspectives towards clinical benefit: recommendations from the ESMO precision medicine working group. Ann. Oncol. 2020;31:978–990. doi: 10.1016/j.annonc.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gopalakrishnan V., Helmink B.A., Spencer C.N., Reuben A., Wargo J.A. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33:570–580. doi: 10.1016/j.ccell.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gettinger S.N., Choi J., Mani N., Sanmamed M.F., Datar I., Sowell R., et al. A dormant TIL phenotype defines non-small cell lung carcinomas sensitive to immune checkpoint blockers. Nat. Commun. 2018;9:3196. doi: 10.1038/s41467-018-05032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Roviello G., Iannone L.F., Bersanelli M., Mini E., Catalano M. The gut microbiome and efficacy of cancer immunotherapy. Pharmacol. Ther. 2022;231 doi: 10.1016/j.pharmthera.2021.107973. [DOI] [PubMed] [Google Scholar]
- 118.Lu Y., Yuan X., Wang M., He Z., Li H., Wang J., et al. Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022;15:47. doi: 10.1186/s13045-022-01273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dong Q., Chen E.S., Zhao C., Jin C. Host-microbiome interaction in lung cancer. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.679829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thompson J.C., Carpenter E.L., Silva B.A., Rosenstein J., Chien A.L., Quinn K., et al. Serial monitoring of circulating tumor DNA by next-generation gene sequencing as a biomarker of response and survival in patients with advanced NSCLC receiving pembrolizumab-based therapy. JCO Precis. Oncol. 2021;5 doi: 10.1200/PO.20.00321. PO.20.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bai R., Lv Z., Xu D., Cui J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark. Res. 2020;8:34. doi: 10.1186/s40364-020-00209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Esposito G., Palumbo G., Carillio G., Manzo A., Montanino A., Sforza V., et al. Immunotherapy in small cell lung cancer. Cancers. 2020;12:2522. doi: 10.3390/cancers12092522. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Catravas J.D., Anthony B.L. In vivo determinations of Ki values for angiotensin converting enzyme inhibitors. Adv. Exp. Med. Biol. 1986;198:453–459. doi: 10.1007/978-1-4684-5143-6_61. Pt A. [DOI] [PubMed] [Google Scholar]
- 124.El Sayed R., Blais N. Immunotherapy in extensive-stage small cell lung cancer. Curr. Oncol. 2021;28:4093–4108. doi: 10.3390/curroncol28050347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bodor J.N., Boumber Y., Borghaei H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC) Cancer. 2020;126:260–270. doi: 10.1002/cncr.32468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang Y., Chang L., Yang Y., Fang W., Guan Y., Wu A., et al. The correlations of tumor mutational burden among single-region tissue, multi-region tissues and blood in non-small cell lung cancer. J. Immunother. Cancer. 2019;7:98. doi: 10.1186/s40425-019-0581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stadler J.C., Belloum Y., Deitert B., Sementsov M., Heidrich I., Gebhardt C., et al. Current and future clinical applications of ctDNA in immuno-oncology. Cancer Res. 2022;82:349–358. doi: 10.1158/0008-5472.CAN-21-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chae Y.K., Davis A.A., Agte S., Pan A., Simon N.I., Iams W.T., et al. Clinical implications of circulating tumor DNA tumor mutational burden (ctDNA TMB) in non-small cell lung cancer. Oncologist. 2019;24:820–828. doi: 10.1634/theoncologist.2018-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang Z., Duan J., Cai S., Han M., Dong H., Zhao J., et al. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol. 2019;5:696–702. doi: 10.1001/jamaoncol.2018.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schrock A.B., Ouyang C., Sandhu J., Sokol E., Jin D., Ross J.S., et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann. Oncol. 2019;30:1096–1103. doi: 10.1093/annonc/mdz134. [DOI] [PubMed] [Google Scholar]
- 131.Samstein R.M., Lee C.H., Shoushtari A.N., Hellmann M.D., Shen R., Janjigian Y.Y., et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019;51:202–206. doi: 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Friedlaender A., Nouspikel T., Christinat Y., Ho L., McKee T., Addeo A. Tissue-plasma TMB comparison and plasma TMB monitoring in patients with metastatic non-small cell lung cancer receiving immune checkpoint inhibitors. Front. Oncol. 2020;10:142. doi: 10.3389/fonc.2020.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang N., Zhang J., Wang G., He X., Mi Y., Cao Y., et al. Predictive efficacy of blood-based tumor mutation burden assay for immune checkpoint inhibitors therapy in non-small cell lung cancer: a systematic review and meta-analysis. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.795933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gu S.S., Zhang W., Wang X., Jiang P., Traugh N., Li Z., et al. Therapeutically increasing MHC-I expression potentiates immune checkpoint blockade. Cancer Discov. 2021;11:1524–1541. doi: 10.1158/2159-8290.CD-20-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Niknafs N., Balan A., Cherry C., Hummelink K., Monkhorst K., Shao X.M., et al. Persistent mutation burden drives sustained anti-tumor immune responses. Nat. Med. 2023;29:440–449. doi: 10.1038/s41591-022-02163-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bai R., Chen N., Li L., Du N., Bai L., Lv Z., et al. Mechanisms of cancer resistance to immunotherapy. Front. Oncol. 2020;10:1290. doi: 10.3389/fonc.2020.01290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang J., Huang D., Saw P.E., Song E. Turning cold tumors hot: from molecular mechanisms to clinical applications. Trends Immunol. 2022;43:523–545. doi: 10.1016/j.it.2022.04.010. [DOI] [PubMed] [Google Scholar]