Abstract
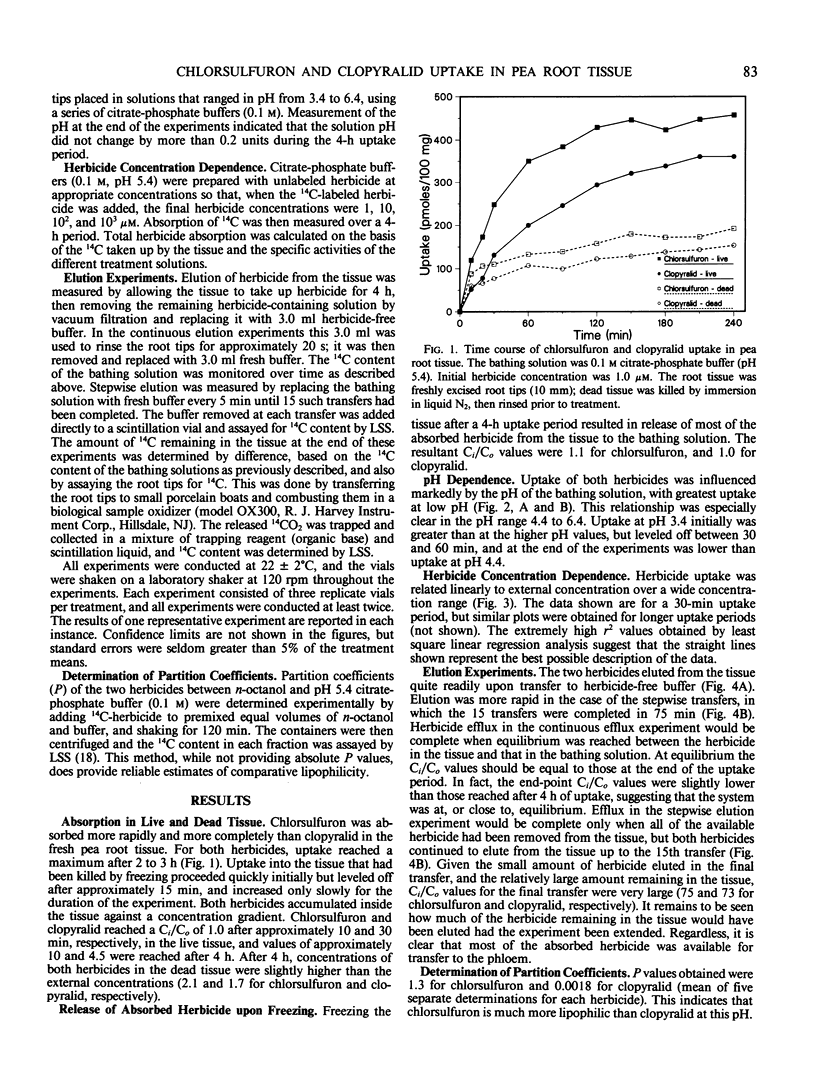
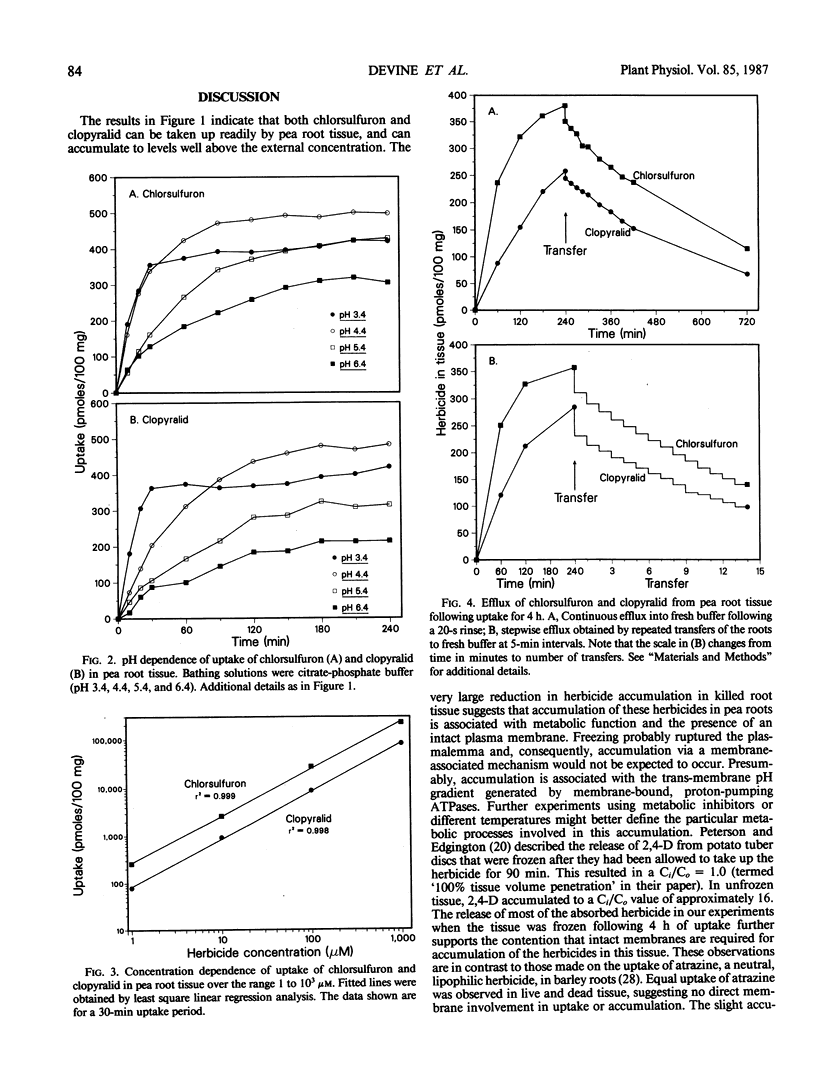
The herbicides chlorsulfuron and clopyralid were taken up rapidly by excised pea root tissue and accumulated in the tissue to concentrations ten and four times those in the external medium, respectively. Uptake was related linearly to external herbicide concentration over a wide concentration range, implying that transport across the membrane is by nonfacilitated diffusion. Uptake of both compounds was influenced by pH, with greatest uptake at low pH. The pH dependence of uptake suggests that the herbicides (both of which are weak acids) are transported across the plasma membrane in the undissociated form, and accumulate in the cytoplasm by an ion trap mechanism. Most of the absorbed herbicide effluxed from the tissue when it was transferred to herbicide-free buffer, indicating that the accumulation was not due to irreversible binding. Consequently, both herbicides remain available for transfer to the phloem. These results can explain the high reported phloem mobility of clopyralid in intact plants. The low phloem mobility of chlorsulfuron must be accounted for by factors that override its ability to accumulate in the symplast.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Donaldson T. W., Bayer D. E., Leonard O. A. Absorption of 2,4-Dichlorophenoxyacetic Acid and 3-(p-Chlorophenyl)-1, 1-dimethylurea (Monuron) by Barley Roots. Plant Physiol. 1973 Dec;52(6):638–645. doi: 10.1104/pp.52.6.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougler J. A., Geiger D. R. Uptake and distribution of N-phosphonomethylglycine in sugar beet plants. Plant Physiol. 1981 Sep;68(3):668–672. doi: 10.1104/pp.68.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W. M., Hartung W. Uptake and Release of Abscisic Acid by Isolated Photoautotrophic Mesophyll Cells, Depending on pH Gradients. Plant Physiol. 1981 Jul;68(1):202–206. doi: 10.1104/pp.68.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtner F. T. Amitrole Absorption by Bean (Phaseolus vulgaris L. cv ;Red Kidney') Roots : Mechanism of Absorption. Plant Physiol. 1983 Feb;71(2):307–312. doi: 10.1104/pp.71.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'neill S. D., Keith B., Rappaport L. Transport of gibberellin a(1) in cowpea membrane vesicles. Plant Physiol. 1986 Apr;80(4):812–817. doi: 10.1104/pp.80.4.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. R., McDaniel C. N. Transport of the herbicide 3-amino-1,2,4-triazole by cultured tobacco cells and leaf protoplasts. Plant Physiol. 1982 Jun;69(6):1382–1386. doi: 10.1104/pp.69.6.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree M. T. A simple theory regarding ambimobility of xenobiotics with special reference to the nematicide, oxamyl. Plant Physiol. 1979 Feb;63(2):367–374. doi: 10.1104/pp.63.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya M. K., Noodén L. D. Mode of Dinitroaniline Herbicide Action: II. CHARACTERIZATION OF [C]ORYZALIN UPTAKE AND BINDING. Plant Physiol. 1980 Dec;66(6):1048–1052. doi: 10.1104/pp.66.6.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]


