Abstract
The parasitoid wasp Venturia canescens is an important biological control agent of stored products moth pests and serves as a model to study the function and evolution of domesticated endogenous viruses (DEVs). The DEVs discovered in V. canescens are known as virus-like particles (VcVLPs), which are produced using nudivirus-derived components and incorporate wasp-derived virulence proteins instead of packaged nucleic acids. Previous studies of virus-derived components in the V. canescens genome identified 53 nudivirus-like genes organized in six gene clusters and several viral pseudogenes, but how VcVLP genes are organized among wasp chromosomes following their integration in the ancestral wasp genome is largely unknown. Here, we present a chromosomal scale genome of V. canescens consisting of 11 chromosomes and 56 unplaced small scaffolds. The genome size is 290.8 Mbp with a N50 scaffold size of 24.99 Mbp. A high-quality gene set including 11,831 protein-coding genes were produced using RNA-Seq data as well as publicly available peptide sequences from related Hymenoptera. A manual annotation of genes of viral origin produced 61 intact and 19 pseudogenized nudivirus-derived genes. The genome assembly revealed that two previously identified clusters were joined into a single cluster and a total of 5 gene clusters comprising of 60 intact nudivirus-derived genes were located in three chromosomes. In contrast, pseudogenes are dispersed among 8 chromosomes with only 4 pseudogenes associated with nudivirus gene clusters. The architecture of genes encoding VcVLP components suggests it originates from a recent virus acquisition and there is a link between the processes of dispersal and pseudogenization. This high-quality genome assembly and annotation represents the first chromosome-scale assembly for parasitoid wasps associated with VLPs, and is publicly available in the National Center for Biotechnology Information Genome and RefSeq databases, providing a valuable resource for future studies of DEVs in parasitoid wasps.
Keywords: Venturia canescens, domesticated endogenous virus, parasitoid, nudivirus
Graphical Abstract
Graphical Abstract.
Introduction
Venturia canescens (Hymenoptera: Ichneumonidae) is a synovigenic solitary koinobiont endoparasitoid wasp that plays an important role in integrated pest management programs (Salt 1976; Schöller et al. 1997). It is a parasitoid of stored-product pest insects and can reproduce in sexual and asexual reproductive modes, both of which have made it attractive for studies on physiology, behavior, sex determination, life history traits and trade-offs, and genetic variability (Harvey et al. 2001; Desouhant et al. 2005; Pelosse et al. 2007; Mateo Leach et al. 2012; Amat et al. 2017), and as a model to study the function and evolution of domesticated viruses (Reineke et al. 2006; Pichon et al. 2015; Leobold et al. 2018). V. canescens is an effective parasitoid of the larvae of various lepidopteran species including some of the world's most widespread moth pests of stored products, such as Plodia interpunctella and Ephestia kuehniella (Heinlein et al. 2002; Ozkan et al. 2004).
During parasitism, V. canescens lays eggs into moth larvae and uses virus-like particles (VcVLPs) to protect eggs against host defenses (Feddersen et al. 1986; Beck et al. 2000). VcVLPs are produced in the calyx cells during the wasp pupal stage and are released into the calyx lumen, where they attach to egg surfaces during maturation. The VLPs are 100–150 nm electron-dense particles surrounded by viral envelopes (Feddersen et al. 1986). VcVLPs together with bracoviruses (BVs) and Fopius arisanus Endogenous Nudivirus represent three types of nudivirus-derived beneficial domesticated endogenous viruses (DEVs) found in parasitoid wasps (Burke 2019). DEVs represent cases in which a virus genome has become permanently integrated into a wasp genome and viral genes are used to produce viruses or VLPs. Virus-derived genes are inherited by all individual wasps and retained when wasps undergo speciation. VcVLPs are produced using nudivirus-derived components, including envelope proteins, of which genes are hypothesized to be transcribed by an ancestrally viral RNA polymerase (Pichon et al. 2015; Cerqueira de Araujo et al. 2022). Contrary to the well-known BVs with circular double-stranded DNAs packaged into virions, VcVLPs contain virulence proteins of wasp origins and are devoid of packaged nucleic acids (Pichon et al. 2015). The wasp-derived proteins are hypothesized to help cloak the egg against the host's immune system (Feddersen et al. 1986). So far, three wasp derived proteins have been identified as associated with the VcVLPs: VLP1, VLP2, and VLP3. VLP1 is a putative transmembrane, phospholipid-hydroperoxide glutathione peroxidase-like protein, which may protect against oxidative damage in the wasp (Li et al. 2003). VLP2 is a RhoGap-like protein, which is hypothesized to interfere with the cytoskeleton of hemocytes (Labrosse et al. 2005; Du et al. 2020). VLP3 has a neprilysin-like domain. Neprilysin is a metalloendopeptidase that is responsible for the regulation of peptide signaling on the cell surface (Turner et al. 2001), and has been found in many venom-producing wasp species (Colinet et al. 2013; Undheim et al. 2013; Yang et al. 2020).
To date, V. canescens is the only wasp in the family Ichneumonidae known to produce nudivirus-derived VLPs, while several ichneumonid wasps are associated with ichnoviruses, indicating VcVLPs might have evolved from an ichnovirus-for-nudivirus replacement event (Pichon et al. 2015). Previous phylogenetic analyses using nudivirus genes from DEVs and pathogenic nudiviruses showed that VcVLPs are derived from Alphanudivirus, a distinct genus from the ancestor of BVs (Betanudivirus) (Pichon et al. 2015; Leobold et al. 2018; Drezen et al. 2022). Unlike BVs, whose genomic architecture has been investigated comparatively in several wasp species with high-quality genome assemblies (Burke et al. 2014; Gauthier et al. 2021; Mao et al. 2022), the genomic architecture of the VcVLP type of DEVs has only been studied in a single species, V. canescens. Fifty-three nudivirus-like genes and several viral pseudogenes have been identified previously in the V. canescens genome (Pichon et al. 2015; Leobold et al. 2018; Drezen et al. 2022). However, the current V. canescens genome assembly is fragmented and gene annotations were mainly generated for VcVLPs. To gain a better insight into how VcVLPs have evolved after their ancestor was integrated into wasp genomes, we announce a chromosomal scale genome of V. canescens with an annotated gene set for both wasp and viral genes. This publicly available genome assembly will continue to facilitate research on the evolution of VLPs, V. canescens and comparative genomic studies of wasps.
Materials and methods
Wasp samples
Wasps used for genome sequencing and assembly were maintained at the University of Georgia (isolate “UGA”) on its host, P. interpunctella. P. interpunctella larvae were kept in 32-ounce plastic containers with diet composed of chick starter feed, cornmeal, and glycerol in a 2:2:1 weight ratio (Phillips and Strand 1994). Fourteen-day old P. interpunctella larvae were parasitized by V. canescens wasps and kept in diet until emergence. Emerged adult wasps were kept in cages and fed with honey and water agar. All cultures were maintained at 26°C, 40–62% humidity, with a 12 h light: 12 h dark photoperiod. Voucher specimens have been deposited in the UGA Collection of Arthropods, University of Georgia, Athens, GA.
Whole genome sequencing and assembly
High molecular weight DNA was extracted from a single female wasp derived from the colony using the Qiagen MagAttract HMW DNA Kit. Genomic DNA quantity, quality, and purity were assessed using a combination of fluormetric, spectrophoetric, and electrophoretic methods using the Denovix DS-11 and Agilent Genomics FemtoPulse Capillary electrophoresis systems. Once sample size and purity were confirmed to be suitable for library prep, a PacBio SMRTBell library was prepared using the SMRTBell Express Template Prep Kit 2.0. DNA was first sheared to ∼15–20 kb using a Diagenode Megaruptor 2 and then the library prep methods were followed as the manufacturer specifies, with the exception of using a bead-based size selection (3 kb molecule cutoff) using modified SPRI beads in lieu of electrophoresis-based size selection due to low DNA input amount. The prepared library was bound and sequenced at the USDA-ARS Genetics and Animal Breeding Research Unit in Clay Center, Nebraska, USA on a Pacific Biosciences 8M SMRT Cell on a Sequel II system (Pacific Biosciences, Menlo Park, California, USA) beginning with a 2-hour pre-extension followed by a 30-hour movie collection time. After sequencing, consensus sequences from the PacBio Sequel II subreads were obtained by running ccs from pb-bioconda anaconda package. In addition to the HiFi sequencing, a HiC library was prepared from a pool of wasps to ensure sufficient tissue was present to generate high quality proximity ligations. The Arima Genomics HiC kit was used for preparation of the library from formaldehyde-fixed tissue. After HiC proximity ligation, the library was sheared using a Bioruptor Pico and adapted for Illumina sequencing using the Swift Accel 2S Plus library preparation kit. The library was sequenced on a fraction of a lane of Illumina iSeq 100, using 150 base pair paired-end sequencing.
Prior to genome assembly, HiFi reads (SRA accession number: SRR24234441) containing artifact adapter sequences were removed from the HiFi read pool using the program HiFiAdapterFilt (Sim et al. 2022). This filtered read set was assembled into a contig assembly using HiFiASM (Cheng et al. 2021) using the default parameters. The primary contig assembly was scaffolded using the HiC reads (SRA accession number: SRR24234440) generated from the wasp pool. Briefly, HiC reads were mapped to the primary contig assembly using BWA with the -5SP flags to allow for the unique mapping characteristics of the chimeric HiC reads. PCR duplicates were filtered using samblaster, and the subsequent bam file, along with the contig assembly were reformatted to allow visualization and editing in Juicebox using scripts available from Phase Genomics (https://github.com/phasegenomics/juicebox_scripts). The resulting .hic and .assembly files were loaded into Juicebox and manually edited to order and orient contigs into chromosome scale scaffolds. The resulting edited assembly file was used to then output a scaffolded fasta file using juicebox_assembly_converter.py from the same script repository listed above. Blobtools analysis was performed to identify any non-wasp and non-chromosomal contigs and remove them (Laetsch and Blaxter 2017). This was considered the final chromosome set that was then submitted to NCBI for curation and annotation.
RNA purification and sequencing
RNA was extracted from larval and pupal stages, and ovaries, venom gland, head, thorax, and abdomen from adult females (Table 1). Samples were extracted using the Quick-RNA tissue/insect kit with on-column DNAse treatment (Zymo Research). Samples were then treated with the Ambion TURBO DNA-free kit (Invitrogen). Standard strand-specific Illumina-compatible RNA-Seq libraries were constructed for each sample, and sequenced (2 × 150 bp reads) by Novogene Co. Inc. (CA, USA) using the NovaSeq 6000 system with read yields for each library noted in Table 1.
Table 1.
RNA-Seq reads from V. canescens samples used for annotation.
| Sample type | SRA accession | Raw reads sequenced |
|---|---|---|
| Adult female head (N = 10) | SRR14772634 | 21.2 million |
| Adult female thorax (N = 10) | SRR14772633 | 20.8 million |
| Adult female abdomen (N = 10) | SRR14772632 | 19.0 million |
| Pupae (mixed stages, N = 5) | SRR14772630 | 20.2 million |
| Larvae (mixed stages, N = 5) | SRR14772628 | 23.2 million |
| Ovaries from adult females (N = 50) | SRR14772631 | 20.8 million |
| Venom glands from adult females (N = 50) | SRR14772629 | 21.2 million |
Genome annotation
Genome annotation was performed using the NCBI Eukaryotic Genome Annotation Pipeline (https://www.ncbi.nlm.nih.gov/genome/annotation_euk/process/). This automated pipeline utilized the RNA-Seq data from this study and existing data in GenBank for V. canescens (Pichon et al. 2015), in addition to protein sequences for V. canescens, NCBI RefSeq protein sets for Bombus impatiens, Diachasma alloeum, Chelonus insularis, Harpegnathos saltator, Tribolium castaneum, and Apis mellifera, 39,085 other Insecta RefSeq proteins, and 112,623 protein sequences from GenBank derived from the Insecta for gene prediction. Statistics and the evidence used for annotation are available at https://www.ncbi.nlm.nih.gov/genome/annotation_euk/Venturia_canescens/100/. The completeness of the annotated gene set was analyzed by BUSCO v.4.0.5 with the insecta_odb10 lineage dataset (Simão et al. 2015).
Nudivirus gene annotation
Open Reading Frames (ORFs) from the V. canescens genome assembly and annotations generated by NCBI were first searched against the published nudivirus proteins of V. canescens as well as a database of nudivirus-like proteins from C. insularis, Microplitis demolitor, and Cotesia congregata using BLASTP (e = 0.01) (Burke et al. 2014; Pichon et al. 2015; Gauthier et al. 2021; Mao et al. 2022). ORFs were then searched against the NCBI viral protein database (downloaded November 2021). In addition, previously published pseudogenized nudivirus genes of V. canescens were annotated by aligning pseudogene sequences to the genome assembly using MAFFT (Katoh et al. 2002; Leobold et al. 2018; Drezen et al. 2022). All of the identified ORFs or gene annotations were then manually converted into annotated gene models with the V. canescens jBrowse/Apollo instance on the i5k workspace (https://i5k.nal.usda.gov/available-genome-browsers). A bigWig formatted coverage blot generated from the transcriptome data of ovaries was used to define nudivirus-derived and hypothetical gene transcription boundaries.
VLP protein identification
To identify each VLP protein sequence in the genome, the amino acid sequences were extracted from the papers in which they were identified (Hellers et al. 1996 for VLP1; Reineke et al. 2002 for VLP2; Asgari et al. 2002 for VLP3). These sequences were searched against the assembled genome using TBLASTN. To look for paralogous genes, BLASTN was used with each VLP predicted mRNA sequence and those with the highest nucleotide identity were annotated as described for nudivirus-derived genes.
Expression analysis of nudivirus genes and VLP genes in ovaries
Raw reads from ovaries of adult females (Table 1) were adapter-trimmed and quality filtered with Trimmomatic v0.36 (program settings: ILLUMINACLIP:2:20:10:1 LEADING:20 TRAILING:20 SLIDINGWINDOW:4:20 MINLEN:36) (Bolger et al. 2014). Expressed genes in ovaries of female adults were determined by read mapping to the assembled genome with HISAT2 (Kim et al. 2015). Transcript assembly and abundance estimation were performed using StringTie and expression value (FPKM) per gene was obtained using Ballgown (Pertea et al. 2016).
Results and discussion
The V. canescens genome assembly using PacBio HiFi reads yielded 11 chromosomes and 56 unplaced scaffolds with an N50 scaffold length of 24.99 Mbp (Supplementary Fig. 1). The overall length of the assembly is 290.8 Mbp (genome assembly coverage = 40×) with only 0.001% of the assembly comprised of sequencing gaps. The G + C content of the genome is 39.6%, which is similar to other parasitoid genomes (Table 2). The BlobPlot also revealed that all the chromosomes and scaffolds have similar coverage and GC content (Supplementary Fig. 2). When compared to other parasitoids with a chromosomal scale genome, the assembly statistics are similar in both genome size and N50 length of scaffolds except Alloplasta piceator with a larger genome size (549.8 Mbp) and C. congregata with a smaller N50 length (1.12 Mb) (Table 2). The chromosome sizes range from 17.33 to 41.1 Mbp with the G + C contents ranging from 38.6 to 40.4% (Table 3). Genome annotation with the NCBI Eukaryotic Annotation Pipeline yielded 14,009 genes or pseudogenes, including 11,831 containing protein-coding regions, and 23,831 annotated mRNA transcripts (Table 4). Gene coding densities vary among chromosomes (Table 3). Evidence for gene annotations were derived from RNA-Seq data in this study (Table 1) and those existing in GenBank as of August 2021, proteins from related species, or ab initio evidence predicted by GNOMON. A large proportion of transcripts [22,626 of 23,831 (94.9%)] were fully supported with experimental evidence. A total of 2,028 noncoding genes, 197 tRNAs, 2,167 lncRNAs and other genome components were also identified (Table 4). Details of the annotation are presented in Tables 3 & 4 as well as online at https://www.ncbi.nlm.nih.gov/genome/annotation_euk/Venturia_canescens/100/.
Table 2.
Assembly summary statistics compared to other parasitoid genomes.
| Species | NCBI BioProject | Contig count (N50 Mb) | Scaffold count (N50 Mb) | Total length (Mb) | GC (%) |
|---|---|---|---|---|---|
| Venturia canescens* | PRJNA736740 | 100 (11.2) | 67 (24.99) | 290.8 | 39.6 |
| Alloplasta piceator* | PRJEB55792 | 1,462 (1.12) | 377 (56.99) | 549.8 | 36 |
| Campoletis raptor* | PRJEB58800 | 177 (2.19) | 21 (18.62) | 218.6 | 36.5 |
| Amblyteles armatorius* | PRJEB51578 | 157 (4.38) | 104 (17.13) | 227.1 | 44.3 |
| Ichneumon xanthorius* | PRJEB48052 | 374 (4.08) | 151 (20.14) | 315 | 42.9 |
| Buathra laborator* | PRJEB51796 | 142 (10.41) | 110 (17.03) | 329.9 | 42.6 |
| Microplitis demolitor | PRJNA251518 | 27,508 (0.014) | 1,794 (1.14) | 241.2 | 33.1 |
| Chelonus insularis | PRJNA624215 | 457 (1.16) | 455 (1.16) | 135.7 | 30.5 |
| Cotesia congregata* | - | - (0.049) | 3,140 (1.12) | 206.9 | 28.2 |
| Cotesia vestalis | PRJNA271135 | 9,156 (0.046) | - | 186.1 | 30.6 |
| Fopius arisanus | PRJNA258104 | 8,510 (0.052) | 1,042 (0.98) | 153.6 | 39.4 |
| Diachasma alloeum | PRJNA306876 | 25,534 (0.044) | 3,968 (0.65) | 388.8 | 39.1 |
| Nasonia vitripennis* | PRJNA13660 | 25,484 (0.019) | 6,169 (0.71) | 295.8 | 40.6 |
assembled at a chromosomal scale.
Table 3.
Annotation statistics of each chromosome.
| Chrom | Accession | Size (Mb) | GC% | Protein | rRNA | tRNA | Other RNA | Gene | Pseudo |
|---|---|---|---|---|---|---|---|---|---|
| Chrom 1 | NC_057421.1 | 41.1 | 38.9 | 3,811 | - | 35 | 509 | 2,138 | 8 |
| Chrom 2 | NC_057422.1 | 34.83 | 39.6 | 2,678 | 1 | 11 | 342 | 1,536 | 12 |
| Chrom 3 | NC_057423.1 | 30.39 | 39.3 | 1,723 | - | 13 | 291 | 1,113 | 9 |
| Chrom 4 | NC_057424.1 | 25.1 | 40.4 | 2,674 | 34 | 33 | 349 | 1,583 | 15 |
| Chrom 5 | NC_057425.1 | 24.99 | 40.1 | 2,097 | 9 | 26 | 251 | 1,247 | 8 |
| Chrom 6 | NC_057426.1 | 23.18 | 39.4 | 1,797 | - | 5 | 189 | 964 | 8 |
| Chrom 7 | NC_057427.1 | 22.67 | 39.8 | 2,312 | - | 10 | 241 | 1,256 | 6 |
| Chrom 8 | NC_057428.1 | 22.63 | 39.8 | 2,013 | - | 30 | 196 | 1,118 | 12 |
| Chrom 9 | NC_057429.1 | 21.67 | 39.9 | 1,892 | 38 | 14 | 178 | 1,094 | 5 |
| Chrom 10 | NC_057430.1 | 18.87 | 40.1 | 1,523 | - | 6 | 136 | 878 | 10 |
| Chrom 11 | NC_057431.1 | 17.33 | 38.6 | 1,279 | - | 14 | 193 | 828 | 16 |
Table 4.
Gene annotation summary statistics.
| Feature | Count | Mean length (bp) | Median length (bp) | Min length (bp) | Max length (bp) |
|---|---|---|---|---|---|
| Genes | 13,859 | 14,044 | 3,980 | 70 | 2,962,486 |
| protein-coding | 11,831 | ||||
| non-coding | 2,028 | ||||
| All transcripts | 27,171 | 3,724 | 2,697 | 70 | 59,128 |
| mRNA | 23,831 | 3,971 | 2,900 | 243 | 56,128 |
| misc_RNA | 673 | 3,700 | 2,560 | 150 | 21,243 |
| tRNA | 197 | 74 | 73 | 71 | 84 |
| lncRNA | 2,167 | 1,734 | 1,226 | 91 | 12,367 |
| CDSs | 23,831 | 2,174 | 1,548 | 213 | 57,756 |
| Exons | 99,793 | 465 | 223 | 2 | 19,327 |
| Introns | 83,888 | 2,543 | 253 | 30 | 535,078 |
An assessment of the completeness of gene annotation using BUSCO shows that 97.3% of the Insecta BUSCOs are present in the V. canescens genome assembly. Only 0.3% of those BUSCOs were detected as fragmented. We compared the BUSCO results with those of the braconid genome of C. congregata which was also assembled at the chromosomal scale (Gauthier et al. 2021). The comparison shows that the percentage of complete BUSCO genes in the V. canescens genome is comparable but marginally higher than that of the C. congregata genome, indicating the assembly is highly complete (Table 5).
Table 5.
BUSCO analysis of the V. canescens and C. congregata gene annotation completeness.
| Species | Gene count | Complete (%) | Fragmented (%) | Missing (%) | |
|---|---|---|---|---|---|
| Single-copy | Duplicated | ||||
| V. canescens | 13,859 | 96.6 | 0.6 | 0.3 | 2.5 |
| C. congregata | 14,140 | 88.5 | 4.5 | 3.3 | 3.7 |
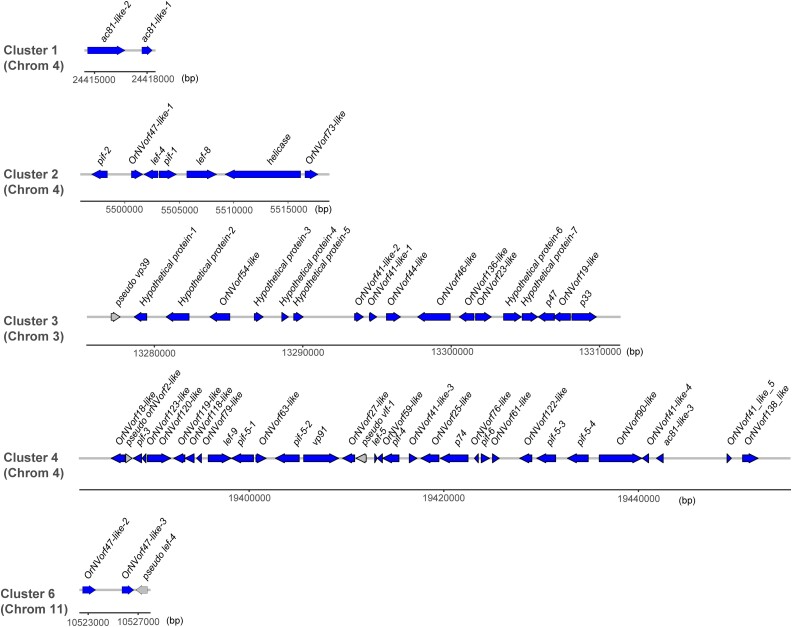
Previous characterization of virus-derived components in the V. canescens genome revealed that 53 nudivirus genes were organized into six clusters surrounded by wasp genes (Pichon et al. 2015). We identified all 53 genes in the newly assembled genome (Table 6; Fig. 1). In addition, one more copy of the OrNVorf47-like gene family (OrNVorf47-like-6) was also identified based upon homology with the other five previously annotated copies. Unlike other nudivirus genes, OrNVorf47-like-6 is not located in any of the nudivirus-derived gene clusters. We further found seven ORFs interspersed within cluster 3 with no similarity to known genes in the NCBI nr database. However, an examination of sequence read coverage from the transcriptome data of ovaries shows that they are transcribed with similar coverage and boundaries compared with the neighboring nudivirus-derived genes in cluster 3. Therefore, we annotated them as hypothetical proteins that are likely of nudivirus origin (Table 6; Fig. 1). None of these newly annotated hypothetical genes had introns except hypothetical protein 6. In addition to intact nudivirus-derived genes, a recent study also identified 19 pseudogenized nudivirus-derived genes, of which homologous genes in baculoviruses have predicted functions that have been lost in VLPs (e.g. capsid components) (Leobold et al. 2018; Drezen et al. 2022). All 19 previously characterized pseudogenes were identified in the current genome assembly.
Table 6.
Nudivirus genes and pseudogenes identified in the V. canescens genome.
| Chrom (length, Mbp) | Cluster | Start | End | Gene | Accession | Function | Expression in ovaries (FPKM) |
|---|---|---|---|---|---|---|---|
| C1_NC_057421.1 (41.1) | 13,046,202 | 13,046,478 | pseudo-OrNVorf139-like | N/A | Unknown | 0 | |
| 25,445,192 | 25,445,817 | pseudo-OrNVorf117-like | N/A | Nucleocapsid | 0 | ||
| C2_NC_057422.1 (34.83) | 22,501,670 | 22,502,290 | pseudo-38K | N/A | Nucleocapsid | 0 | |
| C3_NC_057423.1 (30.39) | 7,915,392 | 7,915,643 | pseudo-OrNVorf128.1-like | N/A | Nucleocapsid | 0 | |
| 3 | 13,277,052 | 13,277,652 | pseudo-vp39 | N/A | Nucleocapsid | 1 | |
| 13,278,601 | 13,279,450 | Hypothetical protein-1 | KAI5630585.1 | Unknown | 189 | ||
| 13,280,751 | 13,282,300 | Hypothetical protein-2 | KAI5630586.1 | Unknown | 93 | ||
| 13,283,701 | 13,285,050 | OrNVorf54-like | KAI5630587.1 | Unknown | 212 | ||
| 13,286,701 | 13,287,300 | Hypothetical protein-3 | KAI5630588.1 | Unknown | 623 | ||
| 13,288,551 | 13,289,000 | Hypothetical protein-4 | KAI5630589.1 | Unknown | 966 | ||
| 13,289,351 | 13,289,999 | Hypothetical protein-5 | KAI5630590.1 | Unknown | 772 | ||
| 13,293,451 | 13,294,050 | OrNVorf41-like-2 | KAI5630591.1 | Unknown | 474 | ||
| 13,294,451 | 13,294,950 | OrNVorf41-like-1 | KAI5630592.1 | Unknown | 1099 | ||
| 13,295,601 | 13,296,550 | OrNVorf44-like | KAI5630593.1 | Unknown | 486 | ||
| 13,297,701 | 13,299,900 | OrNVorf46-like | KAI5630594.1 | Unknown | 39 | ||
| 13,300,501 | 13,301,500 | OrNVorf136-like | KAI5630595.1 | Unknown | 97 | ||
| 13,301,601 | 13,302,670 | OrNVorf23-like | KAI5630596.1 | Unknown | 14 | ||
| 13,303,496 | 13,304,700 | Hypothetical protein-6 | KAI5630597.1 | Unknown | 298 | ||
| 13,304,751 | 13,305,800 | Hypothetical protein-7 | KAI5630598.1 | Unknown | 935 | ||
| 13,305,828 | 13,306,926 | p47 | KAI5630599.1 | Transcription | 24 | ||
| 13,306,838 | 13,308,000 | OrNVorf19-like | KAI5630600.1 | Unknown | 27 | ||
| 13,308,101 | 13,309,750 | p33 | KAI5630601.1 | Nucleocapsid/Envelope | 120 | ||
| 14,394,309 | 14,394,566 | pseudo-OrNVorf128.2-like | N/A | Nucleocapsid | 0 | ||
| C4_NC_057424.1 (25.1) | 4,383,268 | 4,383,801 | pseudo-OrNVorf99.2-like | N/A | Unknown | 0 | |
| 2 | 5,496,951 | 5,498,400 | pif-2 | KAI5630602.1 | Envelope | 43 | |
| 5,500,601 | 5,501,650 | OrNVorf47-like-1 | KAI5630603.1 | Unknown | 69 | ||
| 5,501,751 | 5,503,050 | lef-4 | KAI5630604.1 | Transcription | 9 | ||
| 5,503,151 | 5,504,750 | pif-1 | KAI5630605.1 | Envelope | 79 | ||
| 5,505,701 | 5,508,450 | lef-8 | KAI5630606.1 | Transcription | 6 | ||
| 5,509,201 | 5,516,150 | helicase | KAI5630607.1 | Replication | 3 | ||
| 5,516,551 | 5,517,750 | OrNVorf73-like | KAI5630608.1 | Unknown | 1 | ||
| 5,557,092 | 5,557,427 | pseudo-OrNVorf62-like | N/A | Unknown | 23 | ||
| 5,593,624 | 5,595,560 | pseudo-fen-1 | N/A | Replication | 0 | ||
| 5,702,918 | 5,703,779 | pseudo-OrNVorf9-like | N/A | Unknown | 0 | ||
| 7,841,691 | 7,843,015 | pseudo-pif-1 | N/A | Envelope | 0 | ||
| 14,125,555 | 14,126,938 | pseudo-dnapol | N/A | Replication | 1 | ||
| 17,556,801 | 17,557,550 | OrNVorf41-like-6 | KAI5630609.1 | Unknown | 112 | ||
| 4 | 19,385,877 | 19,387,315 | OrNVorf18-like | KAI5630610.1 | Unknown | 222 | |
| 19,387,367 | 19,388,012 | pseudo-OrNVorf2-like | N/A | Unknown | 45 | ||
| 19,388,151 | 19,389,000 | pif-3 | KAI5630611.1 | Envelope | 56 | ||
| 19,389,050 | 19,389,450 | OrNVorf123-like | KAI5630612.1 | Unknown | 130 | ||
| 19,389,601 | 19,392,000 | OrNVorf120-like | KAI5630613.1 | Unknown | 73 | ||
| 19,392,301 | 19,393,450 | OrNVorf119-like | KAI5630614.1 | Unknown | 320 | ||
| 19,393,455 | 19,394,400 | OrNVorf118-like | KAI5630615.1 | Unknown | 120 | ||
| 19,394,651 | 19,395,150 | OrNVorf79-like | KAI5630616.1 | Unknown | 206 | ||
| 19,395,851 | 19,398,200 | lef-9 | KAI5630617.1 | Transcription | 42 | ||
| 19,398,251 | 19,400,500 | pif-5-1 | KAI5630618.1 | Envelope | 27 | ||
| 19,400,751 | 19,401,800 | OrNVorf63-like | KAI5630619.1 | Unknown | 36 | ||
| 19,402,751 | 19,405,200 | pif-5-2 | KAI5630620.1 | Envelope | 26 | ||
| 19,405,651 | 19,409,250 | vp91 | KAI5630621.1 | Envelope | 55 | ||
| 19,409,650 | 19,410,900 | OrNVorf27-like | KAI5630622.1 | Unknown | 92 | ||
| 19,411,033 | 19,412,084 | pseudo-vlf-1 | N/A | Replication/Nucleocapsid | 7 | ||
| 19,412,951 | 19,413,205 | lef-5 | KAI5630623.1 | Transcription | 26 | ||
| 19,413,229 | 19,413,750 | OrNVorf59-like | KAI5630624.1 | Unknown | 363 | ||
| 19,413,757 | 19,415,450 | pif-4 | KAI5630625.1 | Envelope | 200 | ||
| 19,416,501 | 19,417,300 | OrNVorf41-like-3 | KAI5630626.1 | Unknown | 194 | ||
| 19,417,701 | 19,419,550 | OrNVorf25-like | KAI5630627.1 | Unknown | 90 | ||
| 19,419,701 | 19,422,550 | p74 | KAI5630628.1 | Envelope | 305 | ||
| 19,423,201 | 19,423,600 | OrNVorf76-like | KAI5630629.1 | Unknown | 66 | ||
| 19,423,901 | 19,424,750 | pif-6 | KAI5630630.1 | Envelope | 65 | ||
| 19,425,051 | 19,425,750 | OrNVorf61-like | KAI5630631.1 | Unknown | 105 | ||
| 19,427,851 | 19,429,100 | OrNVorf122-like | KAI5630632.1 | Nucleocapsid | 19 | ||
| 19,429,600 | 19,431,550 | pif-5-3 | KAI5630633.1 | Envelope | 39 | ||
| 19,432,751 | 19,434,900 | pif-5-4 | KAI5630634.1 | Envelope | 105 | ||
| 19,436,026 | 19,440,400 | OrNVorf90-like | KAI5630635.1 | Unknown | 20 | ||
| 19,440,451 | 19,441,100 | OrNVorf41-like-4 | KAI5630636.1 | Unknown | 67 | ||
| 19,441,901 | 19,442,600 | ac81-like-3 | KAI5630637.1 | Unknown | 88 | ||
| 19,449,151 | 19,449,601 | OrNVorf41-like-5 | KAI5630638.1 | Unknown | 115 | ||
| 19,450,751 | 19,452,350 | OrNVorf138-like | KAI5630639.1 | Unknown | 82 | ||
| 1 | 24,414,601 | 24,416,750 | ac81-like-2 | KAI5630640.1 | Unknown | 6 | |
| 24,417,701 | 24,418,300 | ac81-like-1 | KAI5630641.1 | Unknown | 40 | ||
| C5_NC_057425.1 (24.99) | 14,619,513 | 14,619,795 | pseudo-OrNVorf130-like | N/A | Unknown | 0 | |
| C7_NC_057427.1 (22.67) | 8,505,478 | 8,506,069 | pseudo-OrNVorf22-like | N/A | Unknown | 27 | |
| 14,311,928 | 14,312,841 | pseudo-integrase | N/A | Replication/Nucleocapsid | 0 | ||
| C9_NC_057429.1 (21.67) | 19,449,204 | 19,449,601 | pseudo-OrNVorf99.1-like | N/A | Unknown | 0 | |
| C11_NC_057431.1 (17.33) | 6 | 10,522,551 | 10,523,600 | OrNVorf47-like-2 | KAI5630645.1 | Unknown | 7 |
| 10,525,701 | 10,526,651 | OrNVorf47-like-3 | KAI5630646.1 | Unknown | 50 | ||
| 10,526,823 | 10,527,731 | pseudo-lef-4 | N/A | Transcription | 1 |
Fig. 1.
Locations of nudivirus gene clusters in V. canescens chromosomes.
Having catalogued all previously characterized intact and pseudogenized nudivirus-derived genes in the genome, we next examined where these elements were located in chromosomes. Clusters 1, 2, 4, and 5 were all located in chromosome 4 (Figs. 1 & 2), while clusters 3 and 6 were located in chromosome 3 and 11, respectively. We further detected that clusters 4 and 5 are adjacent and form a single cluster that we named cluster 4. We did not re-name cluster 6 to be consistent with how it had been named earlier (Pichon et al. 2015). Four pseudogenes (pseudo-vp39, pseudo-OrNVorf2-like, pseudo-vlf-1, and pseudo-lef-4) were located in clusters 3, 4, and 6, and the remainder were dispersed among 8 chromosomes (Fig. 2).
Fig. 2.
V. canescens chromosome map with the distribution of intact and pseudogenized nudivirus-derived genes and VLP genes. Numbers of genes in 1 Mbp-length loci are indicated by vertical bars with coloration and scale indicated in the figure legend. Gene clusters are labeled with numbers.
Genes for VLP1-3 are located far from the nudivirus-derived gene containing clusters. VLP1 and VLP3 are in chromosomes with no nudivirus-derived gene clusters. While VLP2 is located in chromosome 3, which also contains nudivirus-derived gene cluster 3, VLP2 is located about 4Mb away from that cluster. VLP2 and 3 each have paralogs located next to them (Table 7; Fig. 2). In V. canescens ovaries, VLP2 is expressed about twice as much as its paralog, and VLP3 is expressed about 160 times as much as its neighboring paralog. Additionally, other genes similar to VLP3 were identified in chromosome 1, but these were expressed at low levels in the ovaries.
Table 7.
VLP genes identified in the V. canescens genome.
| Chrom (length, Mbp) | Start | End | Gene | Accession | Predicted function | Expression in ovaries (FPKM) |
|---|---|---|---|---|---|---|
| C1_NC_057421.1 (41.1) | 36,504,557 | 36,524,198 | VLP3p-2 | KAI5630565.1 | neprilysin-like | 30 |
| 36,526,558 | 36,533,880 | VLP3p-3 | KAI5630568.1 | neprilysin-like | 10 | |
| 36,534,085 | 36,543,403 | VLP3p-4 | KAI5630573.1 | neprilysin-like | 23 | |
| 36,552,978 | 36,556,446 | VLP3p-5 | KAI5630579.1 | neprilysin-like | 0 | |
| 36,560,156 | 36,566,977 | VLP3p-6 | KAI5630581.1 | neprilysin-like | 0 | |
| C3_NC_057423.1 (30.39) | 9,332,883 | 9,338,326 | VLP2p-1 | KAI5630583.1 | rac GTPase-activating protein 1-like | 414 |
| 9,339,477 | 9,345,184 | VLP2 | KAI5630584.1 | rac GTPase-activating protein 1-like | 876 | |
| C8_NC_057428.1 (22.63) | 13,692,502 | 13,697,475 | VLP3p-1 | KAI5630642.1 | neprilysin-like | 11 |
| 13,700,752 | 13,705,772 | VLP3 | KAI5630643.1 | neprilysin-like | 1763 | |
| C10_NC_057430.1 (18.87) | 8,910,988 | 8,913,043 | VLP1 | KAI5630644.1 | glutathione peroxidase 1-like | 726 |
Knowledge about the gene content of nudivirus-derived genes in wasp genomes can help ascertain how they arrived there. Dispersed viral genes could be the product of integration of the ancestral viral genome in a single location followed by dispersal, or alternatively integration of multiple copies of the ancestral genome into several locations (Strand and Burke 2013). In both cases, genes may remain intact or become pseudogenized due to a lack of functional constraint if they are not necessary for virion or VLP production. The likely number of ancestral genome copies in the wasp genome can be determined by counting whether most virus-derived genes are present as single or multiple copies; however, this is only possible for cases in which degradation of inactivated (pseudogenized) genes has not proceeded to a point of being unrecognizable. In V. canescens, the presence of numerous pseudogenes makes estimation of ancestral genome copies possible. Most nudivirus-derived genes and pseudogenes in the V. canescens genome are present as single copy genes except pif-5, ac81-like, OrNVorf41-like, OrNVorf47-like, lef-4, and pif-1 gene families. Many of these gene family members most likely represent localized duplications, as paralogs are often located in clusters, e.g. pif-5 and OrNVorf41-like paralogs in cluster 4. Other paralogs are present in multiple locations, for example, the OrNVorf47-like and pseudo-lef-4 genes in cluster 6 are repeated in the same order and orientation in cluster 2, in which OrNVorf47-like was duplicated again and maintained while lef-4 became pseudogenized. Despite these exceptions, it appears that VcVLPs arose from a single integration event from an ancestral genome because the majority of virus-derived genes are single copy in V. canescens.
Examination of the introduction of a set of foreign genes simultaneously into a eukaryotic genome provides the opportunity to study patterns of gene movement and maintenance over time. The V. canescens genome assembly at a chromosomal scale provides a comprehensive architectural view of the virus-derived components that produce VcVLPs in the wasp genome, allowing us to investigate the origin and fate of nudivirus-derived genes among wasp chromosomes. If the nudivirus-derived genes in the V. canescens genome arose from a single integration event, it follows that the ancestral circular double-stranded DNA viral genome must have become linearized and integrated into a single locus in the wasp genome originally. Our chromosome-scale assembly confirms the previous finding that V. canescens nudivirus genes are not widely distributed throughout the wasp genome (Pichon et al. 2015). Currently, all of the nudivirus-derived genes are distributed among 3 chromosomes (out of 11 total), with 3 gene clusters comprising 68% of nudivirus-derived genes located on a single chromosome (chromosome 4). The extent of dispersal of nudivirus-derived genes in wasp genomes can be an indicator of the relative age of viral integration events. Nudivirus-derived gene dispersal in V. canescens can be put into context by comparison with other, independently derived DEVs in parasitoid wasps. Fopius arisanus is an opiine braconid wasp that has an alphanudivirus-derived DEV that produces VLPs (Burke et al. 2018). It is not known when the alphanudivirus ancestor integrated into the ancestor of F. arisanus, but given that nudivirus-derived genes could only be detected in 4/6 species in the genus Fopius, it seems likely that this virus is a relatively recent acquisition (Burke et al. 2018). In F. arisanus, the nudivirus-derived genes are thought to have arisen from a single integration event and are located in nine clusters throughout the F. arisanus genome (Burke et al. 2018).
Fossil evidence suggests BVs have an ∼100my history with parasitoid wasps in the “microgastroid complex” of braconid wasps, representing ∼50,000 extant species (Whitfield 2002; Bézier et al. 2009; Thézé et al. 2011). The nudivirus-derived genes that produce CcBV identified in the C. congregata genome have spread among all ten wasp chromosomes and only a single virus gene cluster remains (Gauthier et al. 2021). In another BV-producing species, C. insularis, no large clusters (>5 genes) of virus-derived genes remain (Mao et al. 2022). Overall, the organization of nudivirus genes in the V. canescens chromosomes suggests that the acquisition of the viral ancestor in this species happened within a more recent or similar time frame to the DEV of F. arisanus, and much more recently when compared to the age of BVs in microgastroid wasps (Drezen et al. 2017; Burke et al. 2021).
A chromosomal assembly of the V. canescens genome now also makes it possible to examine the maintenance or loss of nudivirus-derived genes over time in relation to their locations in the wasp genome. Unlike BV-producing wasps with few recognizable pseudogenized nudivirus-derived genes, several nudivirus-derived genes encoding nucleocapsid components and genes with unknown function were pseudogenized in the V. canescens genome (Leobold et al. 2018). Due to their presence in clusters with other, intact, nudivirus-derived genes, it is likely that three genes (pseudo-vp39, pseudo-OrNVorf2-like, and pseudo-vlf-1) were pseudogenized in place. In contrast, pseudo-lef-4 and pseudo-pif-1 seem to have duplicated and dispersed in chromosomes 4 and 11, followed by pseudogenization (with an intact copy of both genes remaining in cluster 2). The remaining 14 pseudogenes are dispersed among eight chromosomes and are not associated with nudivirus gene clusters. As most do not have paralogs in the V. canescens genome (intact or pseudogenized), this is suggestive of a link between the processes of dispersal and pseudogenization. While the pseudogenization of genes in place is most likely related to a lack of functional constraint for their role in making components lacking in VLPs (such as nucleocapsid components), pseudogenization via dispersal could sometimes be a random process unrelated to functional constraint early after the acquisition of an ancestral viral genome. The random dispersal and pseudogenization of a gene encoding one of the essential nucleocapsid components (e.g. 38K) could be the event that resulted in V. canescens producing VLPs rather than nucleic-acid containing virions. The early dispersal of nudivirus-derived genes in wasp genomes could have important functional consequences, making the reconstruction of rearrangement events between related species that produce VLPs or viruses of interest for future studies.
Finally, it has been suggested previously that the VLPs produced by V. canescens replaced an ichnovirus association because V. canescens was assumed to belong to a clade of wasps that all produce ichnoviruses (Pichon et al. 2015). However, evidence for the remnants of ichnovirus genes in the V. canescens genome is weak (Pichon et al. 2015) and this species may not belong to an ichnovirus-producing clade of wasps (Burke et al. 2021). The high-quality genome assembly for V. canescens will allow for future comparative genomic studies of closely related species to elucidate the nudivirus acquisition and domestication process and any potential ichnovirus replacement in this wasp species and relatives.
Supplementary Material
Acknowledgements
We thank Monica Poelchau and the i5k project for providing a genome browser and facilitating manual gene annotation. We also thank Dr. Steven M Sait (School of Biology, University of Leeds, UK) for V. canescens identification.
Contributor Information
Meng Mao, Department of Entomology, University of Georgia, Athens, GA 30602, USA.
Tyler J Simmonds, Tropical Pest Genetics and Molecular Biology Research Unit, USDA-ARS Daniel K Inouye U.S. Pacific Basin Agricultural Research Center, USDA-ARS, Hilo, HI 96720, USA; Oak Ridge Institute for Science and Education, Oak Ridge Associated Universities, Oak Ridge, TN 37830, USA.
Corinne M Stouthamer, Department of Entomology, University of Georgia, Athens, GA 30602, USA.
Tara M Kehoe, Department of Entomology, University of Georgia, Athens, GA 30602, USA.
Scott M Geib, Tropical Pest Genetics and Molecular Biology Research Unit, USDA-ARS Daniel K Inouye U.S. Pacific Basin Agricultural Research Center, USDA-ARS, Hilo, HI 96720, USA.
Gaelen R Burke, Department of Entomology, University of Georgia, Athens, GA 30602, USA.
Data availability
All raw HiFi, HiC, and RNA-Seq read datasets are available from the NCBI SRA database (see Materials and Methods, and Table 1 for accessions). The genome assembly, WGS Project JAHMHP01, is represented as BioProject PRJNA736740 and BioSample SAMN19659032 with identical records in GenBank as accession GCA_019457755.1 and RefSeq as accession GCF_019457755.1 named ASM1945775v1. An FTP site for data download is at ftp.ncbi.nlm.nih.gov/genomes/all/annotation_releases/32260/100/. NCBI's Genome Data Viewer can be accessed at https://www.ncbi.nlm.nih.gov/genome/gdv/browser/genome/?id=GCF_019457755.1 and an overview of release 100 annotations can be accessed at https://www.ncbi.nlm.nih.gov/genome/annotation_euk/Venturia_canescens/100/. Curation of this assembly and consolidated sequence-based resources are hosted by the i5k Workspace (https://i5k.nal.usda.gov/) allowing visualization within jBrowse, manual curation with Apollo and other tools.
Supplemental material available at G3 online.
Funding
This work was supported by the US Department of Agriculture—Agricultural Research Service (to S.M.G.), an appointment to the Agricultural Research Service Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and the U.S. Department of Agriculture (USDA). ORISE is managed by ORAU under DOE contract number DE-SC0014664 (supporting T.J.S.), and the U.S. National Science Foundation (DEB-1916788 to G.R.B.). The US Department of Agriculture—Agricultural Research Service is an equal opportunity/affirmative action employer and all agency services are available without discrimination.
Literature cited
- Amat I, van Alphen JJ, Kacelnik A, Desouhant E, Bernstein C. Adaptations to different habitats in sexual and asexual populations of parasitoid wasps: a meta-analysis. PeerJ. 2017;5:e3699. doi: 10.7717/peerj.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari S, Reineke A, Beck M, Schmidt O. Isolation and characterization of a neprilysin-like protein from Venturia canescens virus-like particles. Insect Mol Biol. 2002;11(5):477–485. doi: 10.1046/j.1365-2583.2002.00356.x. [DOI] [PubMed] [Google Scholar]
- Beck M, Theopold U, Schmidt O. Evidence for serine protease inhibitor activity in the ovarian calyx fluid of the endoparasitoid Venturia canescens. J. Insect Physiol. 2000;46(9):1275–1283. doi: 10.1016/S0022-1910(00)00048-2. [DOI] [PubMed] [Google Scholar]
- Bézier A, Annaheim M, Herbinière J, Wetterwald C, Gyapay G, Bernard-Samain S, Wincker P, Roditi I, Heller M, Belghazi M, et al. Polydnaviruses of braconid wasps derive from an ancestral nudivirus. Science. 2009;323(5916):926–930. doi: 10.1126/science.1166788. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke GR. Common themes in three independently derived endogenous nudivirus elements in parasitoid wasps. Curr Opin Insect Sci. 2019;32:28–35. doi: 10.1016/j.cois.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Burke GR, Hines HM, Sharanowski BJ. The presence of ancient core genes reveals endogenization from diverse viral ancestors in parasitoid wasps. Genome Biol Evol. 2021;13(7):evab105. doi: 10.1093/gbe/evab105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke GR, Simmonds TJ, Sharanowski BJ, Geib SM. Rapid viral symbiogenesis via changes in parasitoid wasp genome architecture. Mol Biol Evol. 2018;35(10):2463–2474. doi: 10.1093/molbev/msy148. [DOI] [PubMed] [Google Scholar]
- Burke GR, Walden KK, Whitfield JB, Robertson HM, Strand MR. Widespread genome reorganization of an obligate virus mutualist. PLoS Genet. 2014;10(9):e1004660. doi: 10.1371/journal.pgen.1004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira de Araujo A, Leobold M, Bézier A, Musset K, Uzbekov R, Volkoff AN, Drezen JM, Huguet E, Josse T. Conserved viral transcription plays a key role in virus-like particle production of the parasitoid wasp Venturia canescens. J Virol. 2022;96(13):e0052422. doi: 10.1128/jvi.00524-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Concepcion GT, Feng X, Zhang H, Li H. Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat Methods. 2021;18(2):170–175. doi: 10.1038/s41592-020-01056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colinet D, Deleury E, Anselme C, Cazes D, Poulain J, Azema-Dossat C, Belghazi M, Gatti J-L, Poirié M. Extensive inter-and intraspecific venom variation in closely related parasites targeting the same host: the case of Leptopilina parasitoids of Drosophila. Insect Biochem Mol Biol. 2013;43(7):601–611. doi: 10.1016/j.ibmb.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Desouhant E, Driessen G, Amat I, Bernstein C. Host and food searching in a parasitic wasp Venturia canescens: a trade-off between current and future reproduction? Anim Behav. 2005;70(1):145–152. doi: 10.1016/j.anbehav.2004.10.015. [DOI] [Google Scholar]
- Drezen J-M, Bézier A, Burke GR, Strand MR. Bracoviruses, ichnoviruses, and virus-like particles from parasitoid wasps retain many features of their virus ancestors. Curr Opin Insect Sci. 2022;49:93–100. doi: 10.1016/j.cois.2021.12.003. [DOI] [PubMed] [Google Scholar]
- Drezen J-M, Leobold M, Bézier A, Huguet E, Volkoff A-N, Herniou EA. Endogenous viruses of parasitic wasps: variations on a common theme. Curr Opin Virol. 2017;25:41–48. doi: 10.1016/j.coviro.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Du J, Lin Z, Volovych O, Lu Z, Zou Z. A RhoGAP venom protein from Microplitis mediator suppresses the cellular response of its host Helicoverpa armigera. Dev Comp Immunol. 2020;108:103675. doi: 10.1016/j.dci.2020.103675. [DOI] [PubMed] [Google Scholar]
- Feddersen I, Sander K, Schmidt O. Virus-like particles with host protein-like antigenic determinants protect an insect parasitoid from encapsulation. Experientia. 1986;42(11–12):1278–1281. doi: 10.1007/BF01946422. [DOI] [Google Scholar]
- Gauthier J, Boulain H, van Vugt JJ, Baudry L, Persyn E, Aury J-M, Noel B, Bretaudeau A, Legeai F, Warris S, et al. Chromosomal scale assembly of parasitic wasp genome reveals symbiotic virus colonization. Commun Biol. 2021;4(1):104. doi: 10.1038/s42003-020-01623-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey JA, Harvey IF, Thompson DJ. Lifetime reproductive success in the solitary endoparasitoid, Venturia canescens. J. Insect Behav. 2001;14(5):573–593. doi: 10.1023/A:1012219116341. [DOI] [Google Scholar]
- Heinlein G, Scholler M, Prozell S, Reichmuth C. Oviposition of Venturia canescens (Gravenhorst) (Hymenoptera: Ichneumonidae) parasitizing the Indian meal moth Plodia interpunctella (Hubner) (Lepidoptera: Pyralidae). IOBC WPRS Bull. 2002;25(3):109–114. [Google Scholar]
- Hellers M, Beck M, Theopoid U, Kamei M, Schmidt O. Multiple alleles encoding a virus-like particle protein in the ichneumonid endoparasitoid Venturia canescens. Insect Mol Biol 1996;5(4):239–249. doi: 10.1111/j.1365-2583.1996.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30(14):3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrosse C, Eslin P, Doury G, Drezen J, Poirié M. Haemocyte changes in D. melanogaster in response to long gland components of the parasitoid wasp Leptopilina boulardi: a rho-GAP protein as an important factor. J Insect Physiol. 2005;51(2):161–170. doi: 10.1016/j.jinsphys.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Laetsch DR, Blaxter ML. Blobtools: interrogation of genome assemblies. F1000Res. 2017;6:1287. doi: 10.12688/f1000research.12232.1. [DOI] [Google Scholar]
- Leobold M, Bézier A, Pichon A, Herniou EA, Volkoff A-N, Drezen J-M. The domestication of a large DNA virus by the wasp Venturia canescens involves targeted genome reduction through pseudogenization. Genome Biol Evol. 2018;10(7):1745–1764. doi: 10.1093/gbe/evy127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Blasevich F, Theopold U, Schmidt O. Possible function of two insect phospholipid-hydroperoxide glutathione peroxidases. J. Insect Physiol. 2003;49(1):1–9. doi: 10.1016/S0022-1910(02)00189-0. [DOI] [PubMed] [Google Scholar]
- Mao M, Strand MR, Burke GR. The complete genome of Chelonus insularis reveals dynamic arrangement of genome components in parasitoid wasps that produce bracoviruses. J Virol. 2022;96(5):e0157321. doi: 10.1128/jvi.01573-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo Leach I, Ferber S, van de Zande L, Beukeboom LW. Genetic variability of arrhenotokous and thelytokous Venturia canescens (hymenoptera). Genetica. 2012;140(1–3):53–63. doi: 10.1007/s10709-012-9657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkan C, Gokcek N, Tunca H. Development time, fecundity and longevity of Venturia canescens (Gravenhorst) (Hymenoptera: Ichneumonidae) with Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) as host. Bulletin OILB/SROP. 2004;27(9):173–175. [Google Scholar]
- Pelosse P, Bernstein C, Desouhant E. Differential energy allocation as an adaptation to different habitats in the parasitic wasp Venturia canescens. Evol Ecol. 2007;21(5):669–685. doi: 10.1007/s10682-006-9145-5. [DOI] [Google Scholar]
- Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-Seq experiments with HISAT, StringTie and Ballgown. Nat Protoc. 2016;11(9):1650–1667. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TW, Strand MR. Larval secretions and food odors affect orientation in female Plodia interpunctella. Entomol Exp Appl. 1994;71(3):185–192. doi: 10.1111/j.1570-7458.1994.tb01785.x. [DOI] [Google Scholar]
- Pichon A, Bézier A, Urbach S, Aury J-M, Jouan V, Ravallec M, Guy J, Cousserans F, Thézé J, Gauthier J, et al. Recurrent DNA virus domestication leading to different parasite virulence strategies. Sci Adv. 2015;1(10):e1501150. doi: 10.1126/sciadv.1501150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke A, Asgari S, Ma G, Beck M, Schmidt O. Sequence analysis and expression of a virus-like particle protein, VLP2, from the parasitic wasp Venturia canescens. Insect Mol Biol. 2002;11(3):233–239. doi: 10.1046/j.1365-2583.2002.00330.x. [DOI] [PubMed] [Google Scholar]
- Reineke A, Asgari S, Schmidt O. Evolutionary origin of Venturia canescens virus-like particles. Arch Insect Biochem Physiol. 2006;61(3):123–133. doi: 10.1002/arch.20113. [DOI] [PubMed] [Google Scholar]
- Salt G. The hosts of Nemeritis canescens a problem in the host specificity of insect parasitoids. Ecol Entomol. 1976;1(1):63–67. doi: 10.1111/j.1365-2311.1976.tb01205.x. [DOI] [Google Scholar]
- Schöller M, Prozell S, Al-Kirshi A-G, Reichmuth C. Towards biological control as a major component of integrated pest management in stored product protection. J Stored Prod Res. 1997;33(1):81–97. doi: 10.1016/S0022-474X(96)00048-3. [DOI] [Google Scholar]
- Sim SB, Corpuz RL, Simmonds TJ, Geib SM. Hifiadapterfilt, a memory efficient read processing pipeline, prevents occurrence of adapter sequence in PacBio HiFi reads and their negative impacts on genome assembly. BMC Genomics. 2022;23(1):1–7. doi: 10.1186/s12864-022-08375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(19):3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- Strand MR, Burke GR. Polydnavirus-wasp associations: evolution, genome organization, and function. Curr Opin Virol. 2013;3(5):587–594. doi: 10.1016/j.coviro.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Thézé J, Bézier A, Periquet G, Drezen J-M, Herniou EA. Paleozoic origin of insect large dsDNA viruses. Proc Natl Acad Sci USA. 2011;108(38):15931–15935. doi: 10.1073/pnas.1105580108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AJ, Isaac RE, Coates D. The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. Bioessays. 2001;23(3):261–269. doi:. [DOI] [PubMed] [Google Scholar]
- Undheim EA, Sunagar K, Herzig V, Kely L, Low D, Jackson T, Jones A, Kurniawan N, King G, Ali S, et al. A proteomics and transcriptomics investigation of the venom from the barychelid spider Trittame loki (brush-foot trapdoor). Toxins (Basel). 2013;5(12):2488–2503. doi: 10.3390/toxins5122488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield JB. Estimating the age of the polydnavirus/braconid wasp symbiosis. Proc Natl Acad Sci USA. 2002;99(11):7508–7513. doi: 10.1073/pnas.112067199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Yang Y, Liu M-M, Yan Z-C, Qiu L-M, Fang Q, Wang F, Werren JH, Ye G-Y. Identification and comparative analysis of venom proteins in a pupal ectoparasitoid, Pachycrepoideus vindemmiae. Front Physiol. 2020;11:9. doi: 10.3389/fphys.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw HiFi, HiC, and RNA-Seq read datasets are available from the NCBI SRA database (see Materials and Methods, and Table 1 for accessions). The genome assembly, WGS Project JAHMHP01, is represented as BioProject PRJNA736740 and BioSample SAMN19659032 with identical records in GenBank as accession GCA_019457755.1 and RefSeq as accession GCF_019457755.1 named ASM1945775v1. An FTP site for data download is at ftp.ncbi.nlm.nih.gov/genomes/all/annotation_releases/32260/100/. NCBI's Genome Data Viewer can be accessed at https://www.ncbi.nlm.nih.gov/genome/gdv/browser/genome/?id=GCF_019457755.1 and an overview of release 100 annotations can be accessed at https://www.ncbi.nlm.nih.gov/genome/annotation_euk/Venturia_canescens/100/. Curation of this assembly and consolidated sequence-based resources are hosted by the i5k Workspace (https://i5k.nal.usda.gov/) allowing visualization within jBrowse, manual curation with Apollo and other tools.
Supplemental material available at G3 online.