Abstract
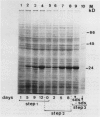
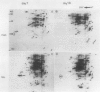
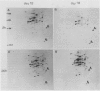
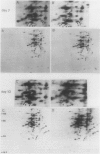
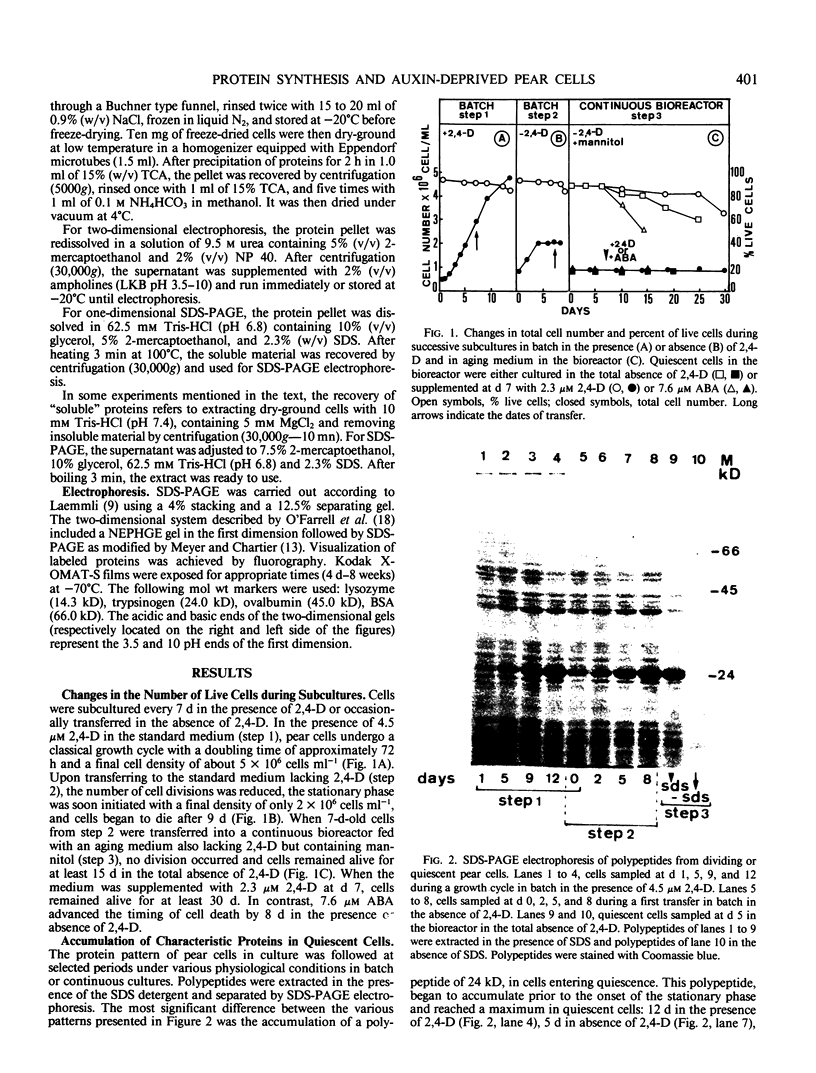
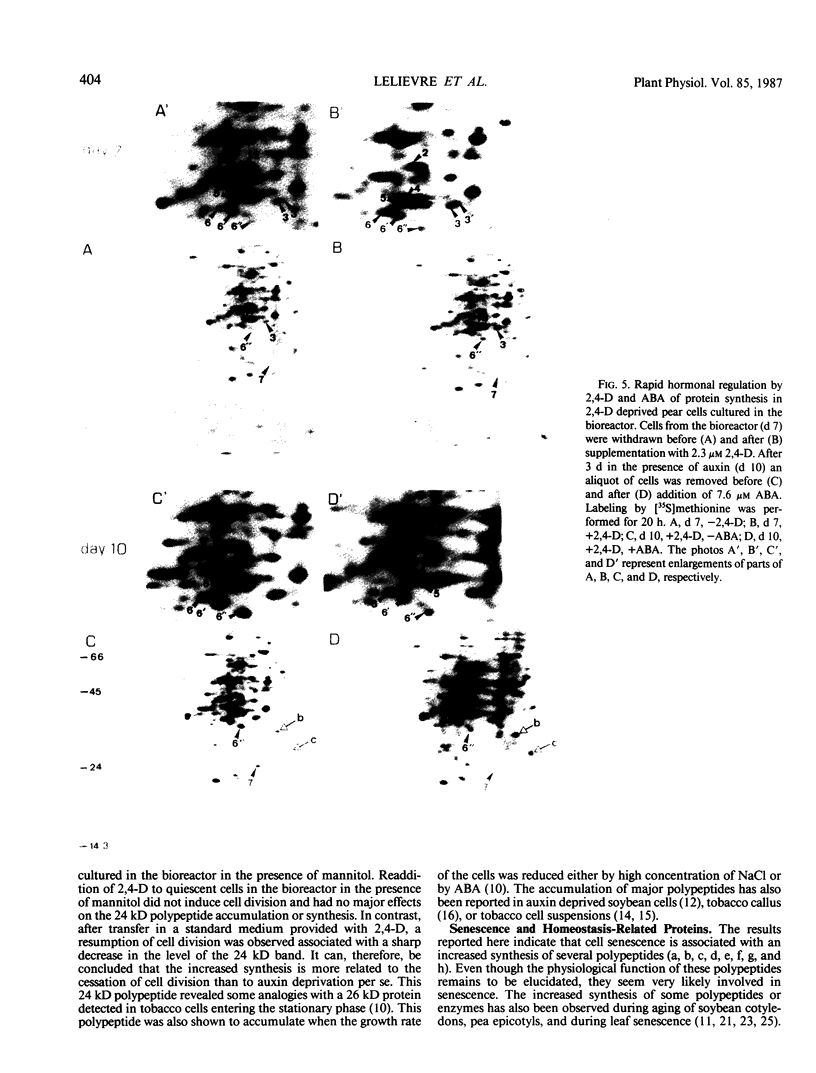
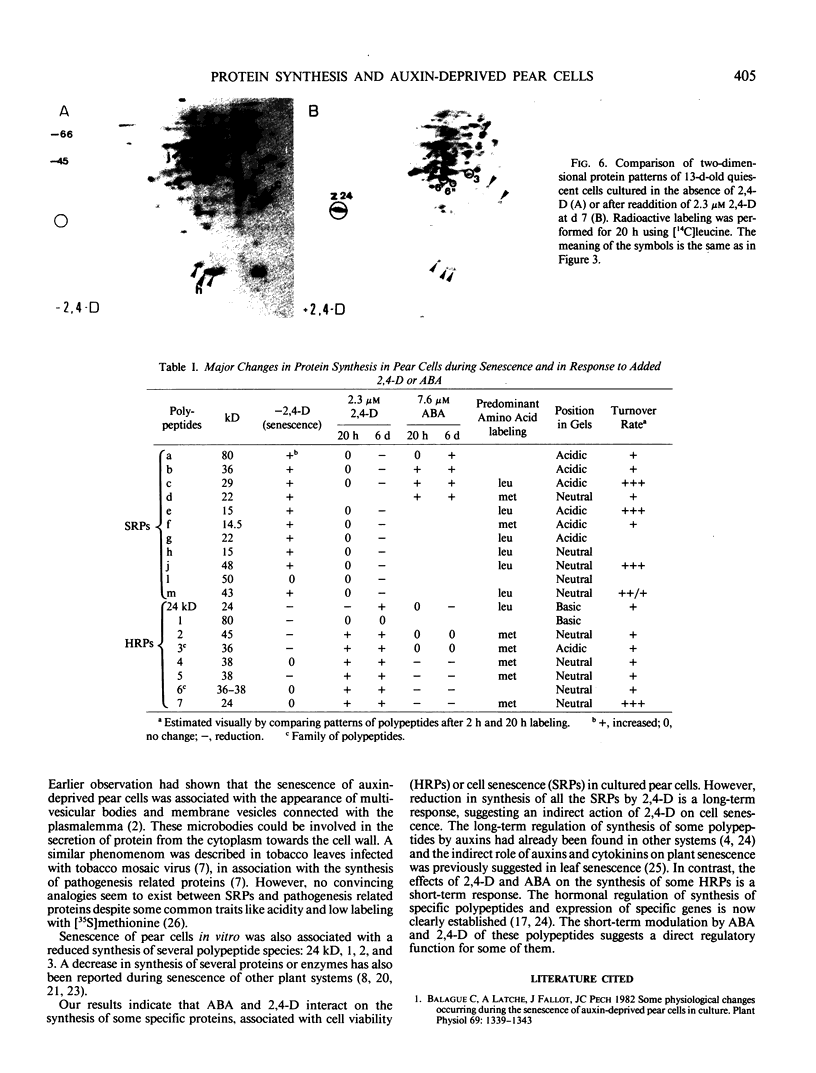
Pear fruit cells (Pyrus communis L. cv Passe Crassane) stopped dividing when subcultured in a bioreactor under auxin starvation in the presence of 0.37 molar mannitol. The cessation of cell division was preceded by the accumulation of a specific basic polypeptide of 24 kilodalton. Readdition of 2.3 micromolar 2,4-dichlorophenoxyacetic acid (2,4-D) neither caused a resumption of cell division nor depressed the accumulation of this polypeptide. Under complete auxin starvation, cells began to die at day 18. In vivo radioactive labeling of proteins followed by two-dimensional electrophoresis showed that during auxin starvation the synthesis of some polypeptides including the 24 kilodalton one (referred to as homeostasis-related proteins, HRPs) was decreased while the synthesis of some others (referred as senescence-related proteins, SRPs) was increased. Readdition of 2.3 micromolar 2,4-D postponed the onset of cell death by 10 to 15 days while supplementation with 7.6 micromolar abscisic acid advanced cell death by 8 days. Two-dimensional analysis of protein synthesis indicated that both hormones interact on the synthesis of these two groups of polypeptides. The levels of most HRPs were maintained or increased in the presence of auxin, while the levels of the SRPs were decreased by auxin and increased by abscisic acid. Short and long-term effects of 2,4-D and abscisic acid on the synthesis of specific polypeptides were observed, allowing a discrimination between the direct and indirect effect of both hormones on the development of cell senescence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balagué C., Latché A., Fallot J., Pech J. C. Some Physiological Changes Occurring during the Senescence of Auxin-Deprived Pear Cells in Culture. Plant Physiol. 1982 Jun;69(6):1339–1343. doi: 10.1104/pp.69.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G., Kochert G. Protein synthesis in a new system for the study of senescence. Exp Cell Res. 1980 Jun;127(2):451–457. doi: 10.1016/0014-4827(80)90452-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larosa P. C., Handa A. K., Hasegawa P. M., Bressan R. A. Abscisic Acid accelerates adaptation of cultured tobacco cells to salt. Plant Physiol. 1985 Sep;79(1):138–142. doi: 10.1104/pp.79.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Y., Aspart L., Chartier Y. Auxin-Induced Regulation of Protein Synthesis in Tobacco Mesophyll Protoplasts Cultivated In Vitro: II. Time Course and Level of Auxin Control. Plant Physiol. 1984 Aug;75(4):1034–1039. doi: 10.1104/pp.75.4.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Y., Chartier Y., Alibert G. Auxin reduces the synthesis of major vacuolar proteins in tobacco mesophyl protoplast. Plant Physiol. 1987 Mar;83(3):713–718. doi: 10.1104/pp.83.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Y., Chartier Y. Hormonal Control of Mitotic Development in Tobacco Protoplasts: TWO-DIMENSIONAL DISTRIBUTION OF NEWLY-SYNTHESIZED PROTEINS. Plant Physiol. 1981 Dec;68(6):1273–1278. doi: 10.1104/pp.68.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohnen D., Shinshi H., Felix G., Meins F. Hormonal regulation of beta1,3-glucanase messenger RNA levels in cultured tobacco tissues. EMBO J. 1985 Jul;4(7):1631–1635. doi: 10.1002/j.1460-2075.1985.tb03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Pech J. C., Romani R. J. Senescence of Pear Fruit Cells Cultured in a Continuously Renewed, Auxin-deprived Medium. Plant Physiol. 1979 Nov;64(5):814–817. doi: 10.1104/pp.64.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster A. M., Davies E. Ribonucleic Acid and protein metabolism in pea epicotyls : I. The aging process. Plant Physiol. 1983 Nov;73(3):809–816. doi: 10.1104/pp.73.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster A., Davies E. Ribonucleic Acid and Protein Metabolism in Pea Epicotyls : III. Response to Auxin in Aged Tissue. Plant Physiol. 1983 Nov;73(3):822–827. doi: 10.1104/pp.73.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skadsen R. W., Cherry J. H. Quantitative changes in in vitro and in vivo protein synthesis in aging and rejuvenated soybean cotyledons. Plant Physiol. 1983 Apr;71(4):861–868. doi: 10.1104/pp.71.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]