Abstract
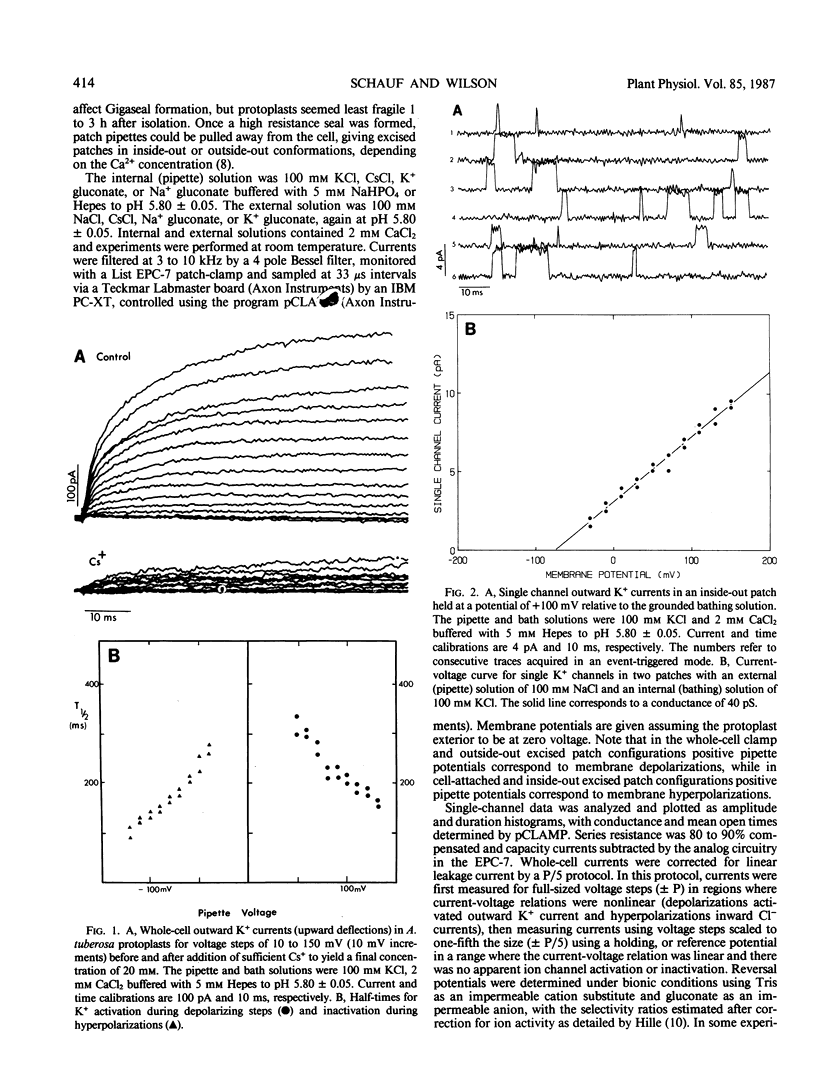
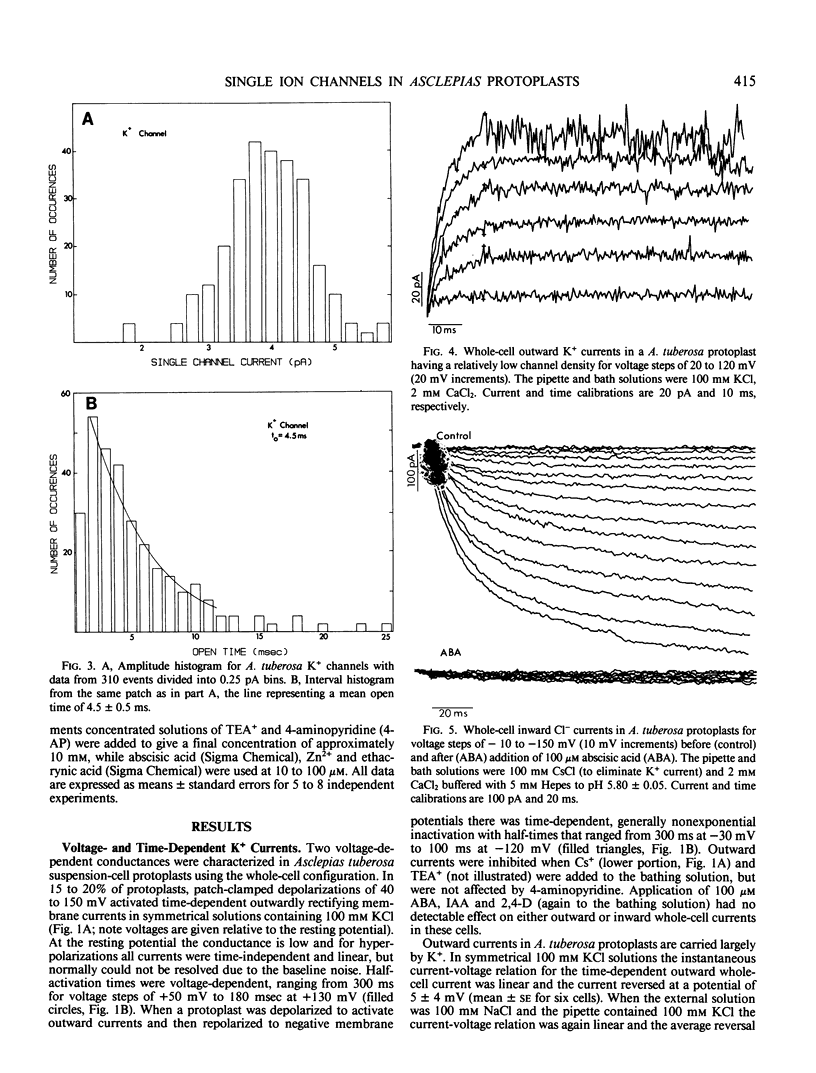
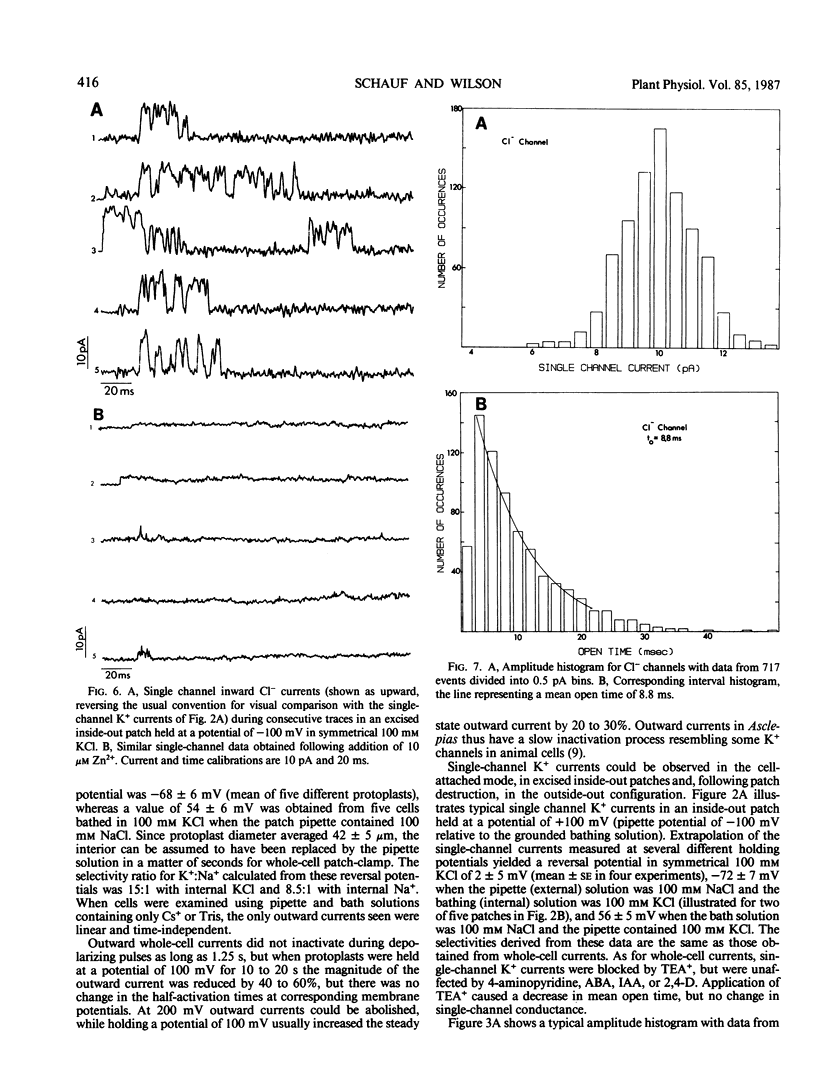
Potassium and chloride channels were characterized in Asclepias tuberosa suspension cell derived protoplasts by patch voltage-clamp. Whole-cell currents and single channels in excised patches had linear instantaneous current-voltage relations, reversing at the Nernst potentials for K+ and Cl−, respectively. Whole cell K+ currents activated exponentially during step depolarizations, while voltage-dependent Cl− channels were activated by hyperpolarizations. Single K+ channel conductance was 40 ± 5 pS with a mean open time of 4.5 milliseconds at 100 millivolts. Potassium channels were blocked by Cs+ and tetraethylammonium, but were insensitive to 4-aminopyridine. Chloride channels had a single-channel conductance of 100 ± 17 picosiemens, mean open time of 8.8 milliseconds, and were blocked by Zn2+ and ethacrynic acid. Whole-cell Cl− currents were inhibited by abscisic acid, and were unaffected by indole-3-acetic acid and 2,4-dichlorophenoxyacetic acid. Since internal and external composition can be controlled, patch-clamped protoplasts are ideal systems for studying the role of ion channels in plant physiology and development.
Full text
PDF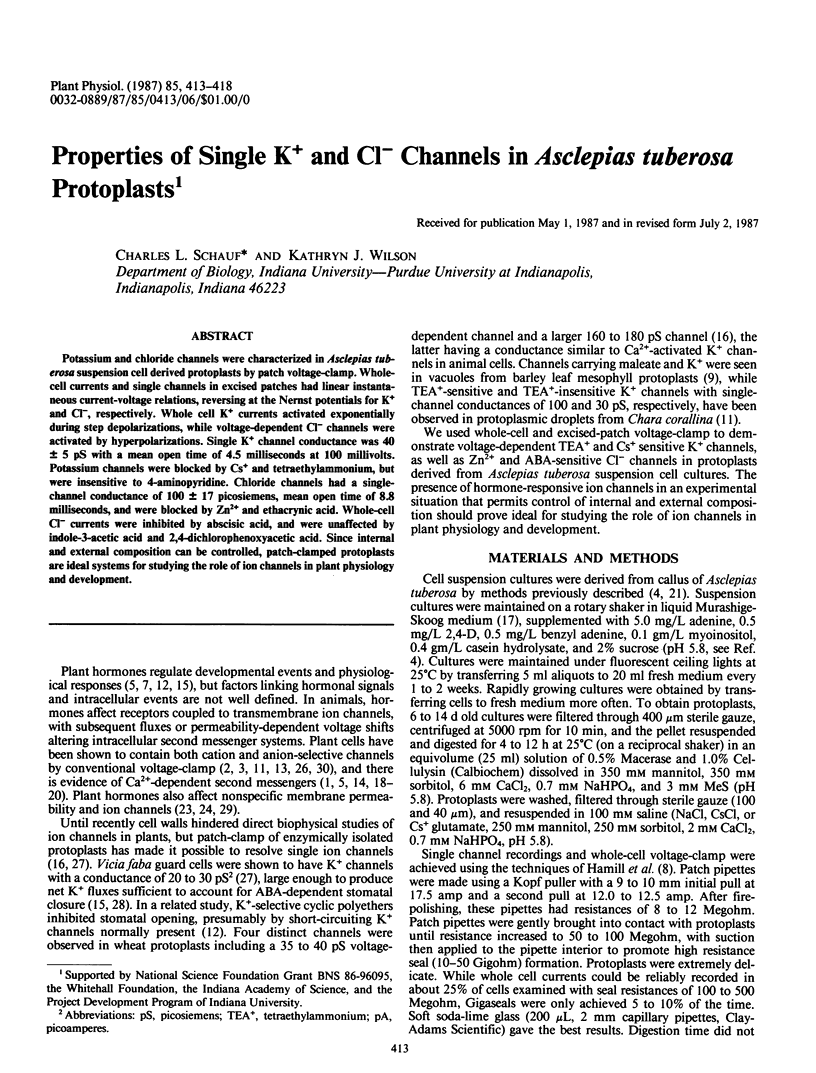

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrejauskas E., Hertel R., Marmé D. Specific binding of the calcium antagonist [3H]verapamil to membrane fractions from plants. J Biol Chem. 1985 May 10;260(9):5411–5414. [PubMed] [Google Scholar]
- Elliott D. C., Batchelor S. M., Cassar R. A., Marinos N. G. Calmodulin-binding drugs affect responses to cytokinin, auxin, and gibberellic Acid. Plant Physiol. 1983 May;72(1):219–224. doi: 10.1104/pp.72.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Z. Abscisic Acid effect on root exudation related to increased permeability to water. Plant Physiol. 1973 Jan;51(1):217–219. doi: 10.1104/pp.51.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Homblé F., Ferrier J. M., Dainty J. Voltage-Dependent K-Channel in Protoplasmic Droplets of Chara corallina: A Single Channel Patch Clamp Study. Plant Physiol. 1987 Jan;83(1):53–57. doi: 10.1104/pp.83.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N., Ehrenstein G., Iwasa K., Bare C., Mischke C. Ion channels in plasmalemma of wheat protoplasts. Science. 1984 Nov 16;226(4676):835–838. doi: 10.1126/science.6093255. [DOI] [PubMed] [Google Scholar]
- Schauf C. L., Bringle B., Stillwell W. Membrane-directed effects of the plant hormones abscisic acid, indole-3-acetic acid and 2,4-dichlorophenoxyacetic acid. Biochem Biophys Res Commun. 1987 Mar 30;143(3):1085–1091. doi: 10.1016/0006-291x(87)90363-9. [DOI] [PubMed] [Google Scholar]



