Abstract
Type 2 diabetes mellitus (T2DM) is characterized by hyperglycemia due to persistent insulin resistance, resulting in elevated blood glucose levels. Metformin is the most prescribed oral drug for lowering high blood glucose levels in T2DM patients. However, it is poorly absorbed and has low bioavailability. Here, we introduce magnesium-based microstirrers to a metformin-containing pill matrix to enhance the glucose-lowering effect of metformin. The resulting microstirring pill possesses a built-in mixing capability by creating local fluid transport upon interacting with biological fluid to enable fast pill disintegration and drug release along with accelerated metformin delivery. In vivo glucose tolerance testing using a murine model demonstrates that the metformin microstirring pill significantly improves therapeutic efficacy, lowering blood glucose levels after a meal more rapidly compared to a regular metformin pill without active stirring. As a result, the microstirrers allow for dose sparing, providing effective therapeutic efficacy at a lower drug dosage than passive metformin pills. These encouraging results highlight the versatility of this simple yet elegant microstirring pill technology, which enhances drug absorption after gastrointestinal delivery to improve therapeutic efficacy.
Keywords: micromotor, microstirring pill, microstirrer, diabetes, metformin, glucose
Graphical Abstract

INTRODUCTION
Diabetes mellitus (DM) is a serious chronic disease characterized by elevated blood glucose levels due to the absence of insulin or increased resistance to insulin. With nearly 500 million people who are living with diabetes globally, the disease creates an enormous economic burden on health systems.1,2 Metformin is commonly the first-choice medication for treating patients with type 2 diabetes mellitus (T2DM). Its glycemic control is attributed primarily to its ability to suppress hepatic glucose production.3-5 As a hydrophilic molecule, it has high solubility and low intestinal and cell membrane permeability, limiting its absorption and thus affecting oral bioavailability, which ranges from 40 to 60%.6 Additionally, metformin has a saturable absorption level and an inverse relationship between the dose being ingested by the patient and its absorption rate.7,8 Consequently, due to its low bioavailability, metformin is administered multiple times a day at an average dosage of 500 mg/day (up to 850 mg/day), which may cause many undesirable side effects, such as vomiting, nausea, abdominal discomfort, diarrhea, and headaches.9,10 To address issues with low absorption, different strategies have been developed to enhance metformin’s bioavailability, including a floating cellulose-based hollow-core tablet,11 a water-in-oil microemulsion system,12 a hydrogel-forming microneedle platform for transdermal delivery,13 and complexation with hydroxypropyl-α-cyclodextrin,14 among others.
In this work, we report on a microstirring pill loaded with metformin to enhance the glucose-lowering effect of the drug for the treatment of T2DM. During the past decade, synthetic micromotors have served as highly efficient platforms for active drug delivery, and major developments have led to important progress that could benefit medicine.15-20 Traditionally in these approaches, synthetic micromotors have been modified and loaded with therapeutic agents. More recently, these synthetic platforms have proven themselves to be quite capable in enhancing local fluid transport and mixing. In 2012, Orozco et al. demonstrated the enhanced transport of tracer particles induced by tubular microengines based on bubble propulsion.21 Moreover, in 2021, Mundaca-Uribe et al. applied this concept toward drug delivery, utilizing synthetic microengines as microstirrers and incorporating them into a pharmaceutical pill.22 Using aspirin as a drug model, the authors verified in murine and porcine animal models that the incorporation of Mg microstirrers into the pill formulation led to enhanced bioavailability of the orally administered drug due to the efficient fluid mixing effect exerted by Mg microstirrers upon reaction with stomach fluids,22 yet the improved therapeutic outcome was not demonstrated.
Based on the results of a previous work,22 we hypothesize here that the incorporation of self-propelled Mg microstirrers into a metformin pill will improve treatment efficacy due to enhanced drug absorption and bioavailability (Figure 1). Unlike the early work, this study is aimed to demonstrate the improved therapeutic outcome associated with the increased drug bioavailability, i.e., lowering the blood glucose concentrations, which represents a major advance in the T2DM management. In this design, metformin is not loaded onto the microstirrers, thus its loading is not limited to microstirrer surface but rather the size of the pill, allowing for high drug loading capacity compared to previous micromotor drug delivery systems.23,24 In vitro characterization studies verify that the effective stirring capabilities of the microstirrers remained unaffected when incorporated into a pill. Upon reaching the intestinal environment after ingestion, the microstirring pill starts to disintegrate and dissolve, releasing the metformin particles and the Mg microstirrers, which react with the local biofluid to expedite the pill dissolution and drug release. Such microstirrers activation takes place due to the reaction between the Mg core and the intestinal fluid (pH 6.8), generating H2 gas bubbles and leading to localized fluid mixing. Furthermore, ions constituents of the intestinal fluid (e.g., phosphate) help to the pitting corrosion of the Mg, allowing for stronger reaction which leads to a powerful propulsion of the released microstirrers. Additionally, the localized hydro-dynamic fluid mixing imparted by the released microstirrers greatly enhances the metformin transport within the intestinal compartment, allowing for improved drug absorption. Ultimately, this results in lower blood sugar concentrations when compared with a regular metformin pill control group after glucose feeding, which is validated in vivo using a murine model. Overall, our study illustrates that this simple, yet elegant microstirring pill platform can be extended to different drug payloads and holds high potential for improving oral therapeutic efficacy.
Figure 1.
Schematic mechanism of action of a metformin microstirring pill that provides self-stirring function to enhance the bioavailability of metformin thus improving the therapeutic efficacy for the management of T2DM.
RESULTS
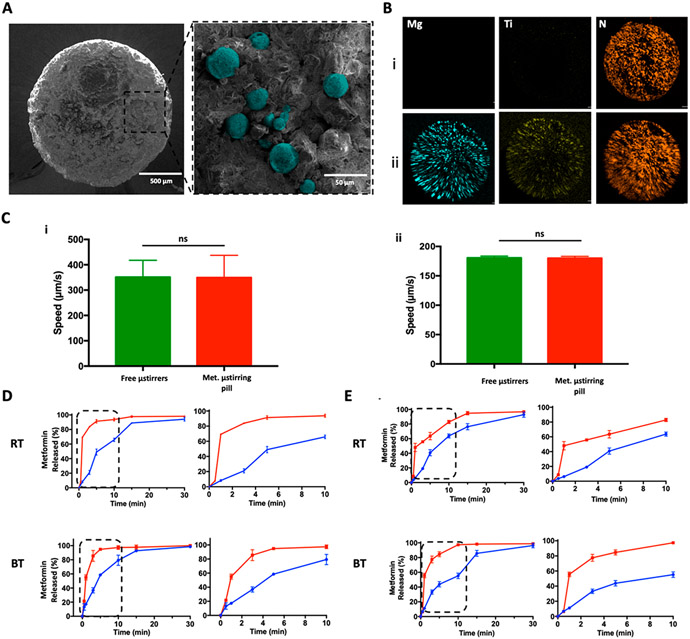
Metformin microstirring pills were prepared by uniformly mixing Mg-based microstirrers, fabricated by asymmetrically coating TiO2 onto ~25 μm Mg microparticles,25 with the drug plus lactose and maltose as excipients in a mortar. The mixture was then compressed in a stainless-steel pill mold, followed by hardening at 60 °C. Finally, the pill was enterically coated with Eudragit L-100 to protect it from the gastric fluid and allow the microstirrers to act only in the small intestine where metformin is absorbed. In order to gain more insight into the metformin microstirring pill structure and morphology, scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDX) was employed. Figure 2A presents side-by-side SEM images of a 2 × 3 mm metformin microstirring pill, which was cut in half to display its cross section as shown in the left image. The same cross section was imaged at higher magnification, shown on the right, in order to investigate the dispersion of the Mg microstirrers, which were incorporated at 10% (w/w). As shown, the Mg microstirrers, pseudocolored in cyan for better visualization, were homogeneously dispersed within the pill matrix. To confirm this finding, EDX analysis was employed to visualize the elemental distribution across metformin pills with or without microstirrers. Figure 2B,i displays a metformin pill without microstirrers indicating only the presence of N from metformin. In comparison, the elemental mapping signals of Mg (cyan), Ti (yellow), and N (orange) in Figure 2B,ii confirmed the presence of the microstirrers (composed of a Mg core and a TiO2 shell) and metformin (containing N from its biguanide chain) in the metformin microstirring pill. To demonstrate that the encapsulation of microstirrers in the pill had no significant impact on their propulsion, a study was conducted to compare their speed to those of free microstirrers, both in simulated gastric fluid (SGF) and simulated intestinal fluid (SIF), prepared according to the United Stated Pharmacopeia (USP) guidelines. The study in SGF is displayed in Figure 2C,i, and no significant difference was found between the groups, with both exhibiting an average speed of ~350 μm/s. Moreover, the lifetime of the microstirrers showed to be ~15 min at body temperature. Figure 2C,ii shows the speed of the microstirrers in SIF, with both groups averaging similar values of ~180 μm/s. These results are consistent with a previous work that demonstrated that neither the formulation excipients nor the encapsulation process played any role in the stirring capability.25
Figure 2.
Characterization of metformin microstirring pills. (A) Left: scanning electron microscopy (SEM) image of a cross section of a metformin microstirring pill. Right: enlarged SEM image of the same pill section showing Mg microstirrers (pseudocolored in cyan) within the pill matrix. (B) Energy-dispersive X-ray spectroscopy (EDX) images displaying the distribution of elemental Mg (cyan), Ti (yellow), and N (orange) of metformin pills without and with microstirrers (i and ii, respectively). (C) Comparison of the speed of free Mg microstirrers and Mg microstirrers released from metformin pills in simulated gastric fluid (i) and simulated intestinal fluid (ii), both supplemented with 1.2% Triton X-100 at 37 °C. Error bars represent the standard deviation calculated from the speed of 10 different microstirrers. Unpaired Student’s t-test, ns = no statistical significance. (D) Comparison of the in vitro dissolution profiles of metformin in simulated gastric fluid, between pills with (red) and without (blue) microstirrers, made with lab excipients, at room and body temperatures (RT and BT, respectively). Left: complete dissolution profile over 30 min. Right: dissolution profiles over the initial 10 min. (E) Comparison of the in vitro dissolution profiles of metformin in simulated intestinal fluid, between pills with (red) and without (blue) microstirrers, made with lab excipients, at room and body temperatures (RT and BT, respectively). Left: complete dissolution profile over 30 min. right: dissolution profiles over the initial 10 min.
Thereafter, the self-mixing effect of the metformin microstirring pills was studied by performing a dissolution study in SGF and SIF. This study was carried out by placing metformin pills loaded with 10% w/w microstirrers into 10 mL of the simulated fluid at room (RT) and body temperature (BT) for 30 min. The dissolution profiles of metformin microstirring pills were then compared to the those obtained from metformin pills without microstirrers, serving as the static control. While conducting this study, we also took into consideration any differences that may result from formulating the pills with lactose and maltose versus what were employed for commercial metformin pills. In the “laboratory prepared excipients” groups (Figure 2D,E), pharmaceutical-grade lactose and maltose were used as excipients in the pill formulations. In contrast, the “commercial excipients” groups (Figure S1,i) employed commercial metformin pills that were triturated in a mortar to form the pill mixture, which was then used to prepare metformin pills with or without microstirrers following the same procedure as for the “laboratory prepared excipients” pills. This control group allowed to study the in vitro behavior of microstirring pills when they were fabricated using the same excipients used by the pharmaceutical industry. After placing the pills to dissolve in SGF, aliquots were taken at different time points for 30 min to quantify the concentration of metformin by ultraviolet spectroscopy with 230 nm as the measuring wavelength. As shown in Figure 2D, higher metformin concentrations were achieved at virtually every time point in all scenarios, both at RT and BT, when microstirrers were part of the pill formulation. This demonstrated that faster release of metformin can be achieved due to the self-mixing effect imparted by the microstirrers, which could be of considerable relevance toward avoiding glucose spikes in T2DM patients. The dissolution tests of metformin pills were also performed by placing them in 10 mL of SIF at RT and BT. As shown in Figure 2E, the dissolution time for the lab-prepared microstirring pills was shorter than the one obtained for pills without microstirrers, reflecting the role of the embedded microstirrers in accelerating the pill dissolution in SIF. As the small intestine is the main compartment where metformin is absorbed, these findings are of great importance and suggest that favorable pill dissolution and metformin release can be achieved in this segment of the GI tract. Similar results were obtained with the “commercial excipient” group, as shown in Figure S1,ii.
After verifying that the microstirring pills provided a faster release of metformin both in SGF and SIF, in vivo studies in a murine model were performed to evaluate therapeutic efficacy. Based on the acute blood sugar lowering effect of metformin,26 the therapeutic efficacy was evaluated by measuring glucose levels after feeding with a bolus of glucose following the administration of metformin microstirring pills (Figure 3A). Mice (n = 3) were administered with pills containing 4.2 mg of metformin with or without microstirrers. Fifteen min after pill administration, a glucose solution was administered to each mouse, and the first blood samples were collected from the tail (t = 0). Additional blood samples were collected at 5, 10, 20, 40, 60, and 120 min post glucose administration. Following each blood sample collection, the glucose levels were measured using a commercial glucometer. A control group that did not receive metformin pills was included in order to determine glucose levels after glucose administration in the absence of any intervention. Figure 3B displays the glucose profiles, in terms of the percent change in glucose, for the “Glucose,” “Glucose + Metformin Bare Pill,” and “Glucose + Metformin Microstirring Pill” groups (glucose profiles in terms of glucose levels (mg/dL) are displayed in Figure S2). After 10 min, ~50% lower glucose levels were obtained for the microstirring pill group compared with the glucose control group, while the bare pill group only exhibited a reduction of ~11%. In all the other evaluated time points, microstirring pill treatment led to lower glucose levels than the controls, demonstrating better therapeutic efficacy, especially between 20 and 40 min, where significant differences were observed. As shown in the inset of Figure 3B, the peak glucose level obtained 20 min after glucose administration demonstrated a substantial enhancement, resulting in ~48% lower glucose levels compared to treatment using pills without microstirrers and ~66% lower levels than the group with no treatment. The area under the curve (AUC), which corresponds to the bioavailability of glucose, was also calculated and compared, as illustrated in Figure 3C. The value was ~30% lower when microstirring pills were utilized compared to metformin pills without microstirrers, and ~50% lower than when no treatment was administered. These results clearly demonstrated the benefits of our microstirring platform, allowing for better absorption of the drug payload and thus providing enhanced therapeutic efficacy.
Figure 3.
In vivo efficacy study of metformin microstirring pills in a murine model. (A) Schematic showing the experimental design, displaying the timeline for pill and glucose administration, and corresponding blood sampling. (B) Blood glucose profiles, in terms of percent change, for the glucose only, glucose plus metformin pill without microstirrers, and glucose plus metformin microstirring pill groups; inset: comparison of peak glucose levels (n = 3). Glucose dose: 1.8 g/kg; metformin dose: 120 mg/kg. Statistical significance was calculated by one-way ANOVA with Tukey’s test. (C) Glucose AUC values for the glucose only, glucose plus metformin pills without microstirrers, and glucose plus metformin microstirring pills group over 2 h (n = 3). Glucose dose: 1.8 g/kg; metformin dose: 120 mg/kg. Statistical significance was calculated by one-way ANOVA with Tukey’s test.
In order to test the versatility of the metformin microstirring pill platform, different conditions were studied. Figure 4A displays the results of the glucose tolerance test, in terms of bioavailability, performed with two different glucose dosages: 0.9 and 1.8 g/kg. In both cases, glucose bioavailability with metformin microstirring pill treatment was lower compared with treatment using metformin pills without microstirrers, demonstrating that the therapeutic efficacy of metformin is improved because of the microstirrer action across different glycemic loads. The glucose tolerance test profiles are displayed in Figure S3. We also studied the performance of the microstirring pills with lower doses of metformin. In this case, we used 80 mg/kg of metformin and the glucose dose was 1.8 g/kg. The resulting trend was similar to that observed when using 120 mg/kg metformin, yielding lower glucose levels and lower AUC values when microstirring pills were utilized (Figures S4 and S5, respectively). Moreover, glucose levels at the peak time point (20 min) were lower with the metformin microstirring pills, confirming the therapeutic benefits of the microstirring pill technology (Figure S6). When the glucose profile obtained with the metformin microstirring pills at a drug dosage of 80 mg/kg was compared with the one obtained with metformin pills without microstirrers at a drug dosage of 120 mg/kg, no significant differences were found, indicating that the incorporation of microstirrers to the metformin pills allowed for ~30% dose sparing. These results are displayed in Figure 4B,C, representing the blood glucose concentrations and AUC, respectively. These studies demonstrate that, by enhancing the therapeutic efficacy of metformin, the microstirring pill platform can reduce the metformin dosage required, which may help to lessen any treatment-related gastrointestinal side effects.
Figure 4.
In vivo oral glucose tolerance test with different glucose and metformin dosages. (A) Glucose AUC values over 2 h for mice receiving glucose plus metformin pills without microstirrers or metformin microstirring pills groups, at two different glucose doses (n = 3). Metformin dose: 120 mg/kg. Statistical significance was calculated by one-way ANOVA with Tukey’s test. (B) Glucose profile, in terms of percent change, comparing the glucose only, glucose plus metformin pill without microstirrers, and glucose plus metformin microstirring pill groups (n = 3). Statistical significance was calculated by one-way ANOVA with Tukey’s test. (C) Glucose AUC values over 2 h for glucose plus metformin pills without microstirrers and metformin microstirring pills (n = 3). Glucose dose: 1.8 g/kg; metformin dose: 80 and 120 mg/kg. Statistical significance was calculated by one-way ANOVA with Tukey’s test.
CONCLUSIONS
We have reported on a microstirring pill platform for delivering metformin, a gold standard drug for treating T2DM. Mg-based microstirrers were incorporated with metformin into a maltose and lactose pill in order to enhance pill disintegration and dissolution, and to improve the absorption of metformin and its glucose-lowering action. We characterized the in vitro dissolution of the microstirring pills in simulated intestinal fluid. Moreover, we evaluated the structure and distribution of the microstirrers within the pill, demonstrating a homogeneous dispersion. The fluid-mixing imparted by the microstirrers allowed for greater drug absorption, ultimately lowering blood glucose levels, as was illustrated in vivo using glucose tolerance tests with a murine animal model. The glucose-lowering effect of the microstirring pills was observed across different glucose dosages. Importantly, it was also demonstrated that the metformin microstirring pill could enable dose sparing, which could allow for lowering the daily dose of metformin required for T2DM patients, thus alleviating common gastrointestinal side effects of the drug, including nausea, vomiting and abdominal discomfort, among others. Effective glucose management is highly important for T2DM patients as there are strong correlations between glycemic peaks and atherosclerosis, endothelial dysfunction, and cardiovascular disorders.27-29 The significance of this work thus lies in the fact that it represents a platform that reduces the glycemic peaks, helping to manage glucose levels more efficiently. Furthermore, the biocompatibility and biosafety of the Mg-based micromotor platform has been demonstrated and discussed in previous studies through the observation of lymphocytic infiltration (inflammation), by the performance of H&E and TUNEL histological assays (microanatomy of tissues and cell DNA damage), as well as by analysis of the appearance of major animal organs.22,24,30 These studies have illustrated that such in vivo operation of the Mg microstirrers has no harmful effects. Aside, Mg is a known micronutrient and TiO2 was approved by the FDA as excipient and food additive.
Thus, this represents a demonstration of the improved therapeutic outcome, i.e., glucose lowering, upon embedding microstirrers within oral pharmaceutical pills. Looking at the future, further large-scale studies are necessary to test the glucose-lowering action, robustness, and biosafety of the metformin microstirring pills using diabetic mice as well as in larger animal models. The ability of oral microstirring pills to enhance the treatment of other diseases should also be examined to further evaluate the generalizability of the platform. With its now proven ability to work in both gastric and intestinal environments, as well as with its proven biocompatibility, the microstirring pill technology could potentially benefit a wide range of clinically relevant applications.
MATERIALS AND METHODS
Microstirrer Fabrication
Mg microstirrers were fabricated using commercial Mg microparticles (catalog no. FMW20, TangShan WeiHao Magnesium Powder Co.; average size, 25 ± 5 μm) as the core. In order to eliminate impurities, the Mg microparticles were initially washed with acetone, followed by a drying process under nitrogen. Then, the Mg microparticles were dispersed onto glass slides (10 mg of Mg microparticles per slide) and coated with a thin layer of TiO2 by atomic layer deposition (ALD) at 100 °C for 3000 cycles using a Beneq TFS 200 system, leaving a small opening at the contact point of the particle to the glass slide. The asymmetric partial TiO2 coating is essential to ensure a bubble tail coming from one side of the microstirrers, allowing for their powerful propulsion and an effective stirring effect.
Pill Preparation
Microstirring pills were fabricated by triturating and mixing lactose (Spectrum Chemical MGF Corp.) and maltose (Spectrum Chemical MGF Corp.) at a 60%/40% ratio. Once this mixture was homogeneous, microstirrers (10% of the total mixture weight) were incorporated, followed by homogenization in a mortar. No changes in the microstirrer structure were observed during this trituration and fabrication process. Metformin hydrochloride (Tokyo Chemical Industry) was also added at this step. In order to obtain a paste-like consistency, an ethanol/water wetting solution (75%/25%) was added to the matrix mixture. Subsequently, the paste was transferred to a cavity plate and all the cavities were filled with the paste by applying enough pressure to guarantee a tight packing. Right after filling each of the cavities, the plate was lowered onto the peg plate until the wet pills were ejected. Lastly, all the pills were allowed to dry and harden over the peg plate for 2 h at 65 °C. Pills without microstirrers were fabricated following the same protocol. After this entire process, the pills were enterically coated with Eudragit L-100 (Evonik Corporations GmbH), 8% v/v, by a dip coating technique.
Metformin Microstirring Pill Characterization
Scanning electron microscopy (SEM) images of metformin microstirring pills were obtained with a FEI Quanta 250 ESEM instrument, using an acceleration voltage of 15 kV. Energy dispersive X-ray analysis (EDX) mapping analysis was performed across the area of the metformin pill to determine the presence of Mg, Ti, and N using an Oxford EDX detector attached to the SEM instrument and operated by Pathfinder software.
In Vitro Metformin Dissolution Analysis
Pills containing metformin were dissolved in 5 mL of diluted gastric fluid simulant or intestinal fluid simulant, both prepared according to the United States Pharmacopeia (USP) specifications, under stirring (100 rpm) at 25 and 37 °C. After the start of the experiment, aliquots of 50 μL were collected at 0, 0.5, 1, 3, 5, 10, 15, and 30 min to investigate the concentration of released metformin at each time point, which was compared to the concentration of metformin from a fully dissolved pill. Metformin was quantified using UV-2450 (Shimadzu Corporation) in accordance with the manufacturer’s instructions. Metformin hydrochloride concentration was determined by measuring the absorbance of ultraviolet light at a wavelength of 230 nm.
In Vivo Glucose Tolerance Test
To perform the in vivo glucose tolerance test, male CD-1 mice (Envigo Laboratories) were fasted overnight prior to the experiment. Then, the mice (n = 3) were intragastrically administered with either metformin microstirring pills or metformin pills without microstirrers using a stainless-steel X-M dosing syringe (Torpac). The metformin dosages were chosen based on the human therapeutic dosages and converted to mice dosage by multiplying it by factor 12.3.31 After 15 min, the mice were orally administered with glucose dissolved in water using an 18G reusable oral gavage needle (PetSurgical). Then, blood samples were obtained at different time points from the tail vein of each mouse by pricking the vein with a syringe needle. Glucose was quantified with an ACCU-CHEK Nano Glucometer (Roche). The collection time points were 30 min prior to administration, immediately after administration (0 min), and 5, 10, 20, 40, 60, and 120 min post glucose administration. Data were normalized to the baseline 30 min prior to administration. Statistics were performed using one-way ANOVA against the metformin microstirring pill. This animal study followed the approved protocol of the IACUC Animal-Care Safety Committee at UC San Diego.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health under Award Number R21AI159492 and the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense under award number HDTRA1-21-1-0010. R.M.-U. acknowledges the support from Universidad de Concepcion. A.A. acknowledges KACST Fellowship from Saudi Arabia. J.S.S.S. acknowledges the UC MEXUS-CONACYT Doctoral Fellowship.
Footnotes
The authors declare no competing financial interest.
Contributor Information
Rodolfo Mundaca-Uribe, Department of Nanoengineering and Chemical Engineering, University of California San Diego, La Jolla, California 92093, United States; Department of Pharmacy, Universidad de Concepcion, Concepcion 4070043, Chile.
Maya Holay, Department of Nanoengineering and Chemical Engineering, University of California San Diego, La Jolla, California 92093, United States.
Amal Abbas, Department of Nanoengineering and Chemical Engineering, University of California San Diego, La Jolla, California 92093, United States.
Nelly Askarinam, Department of Nanoengineering and Chemical Engineering, University of California San Diego, La Jolla, California 92093, United States.
Janna Sofia Sage-Sepulveda, Department of Nanoengineering and Chemical Engineering, University of California San Diego, La Jolla, California 92093, United States.
Luke Kubiatowicz, Department of Nanoengineering and Chemical Engineering, University of California San Diego, La Jolla, California 92093, United States.
Ronnie H. Fang, Department of Nanoengineering and Chemical Engineering, University of California San Diego, La Jolla, California 92093, United States
Liangfang Zhang, Department of Nanoengineering and Chemical Engineering, University of California San Diego, La Jolla, California 92093, United States.
Joseph Wang, Department of Nanoengineering and Chemical Engineering, University of California San Diego, La Jolla, California 92093, United States.
REFERENCES
- (1).Sun H; Saeedi P; Karuranga S; Pinkepank M; Ogurtsova K; Duncan BB; Stein C; Basit A; Chan JCN; Mbanya JC; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract 2022, 183, 109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).World Health Organization. Diabetes, 2022; https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed 2022 December 10).
- (3).LaMoia TE; Shulman GI Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev 2021, 42 (1), 77–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Pernicova I; Korbonits M Metformin—mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol 2014, 10 (3), 143–156. [DOI] [PubMed] [Google Scholar]
- (5).Baker C; Retzik-Stahr C; Singh V; Plomondon R; Anderson V; Rasouli N Should metformin remain the first-line therapy for treatment of type 2 diabetes? Ther. Adv. Endocrinol. Metab 2021, 12, 204201882098022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Jeong Y-S; Jusko WJ Meta-Assessment of Metformin Absorption and Disposition Pharmacokinetics in Nine Species. Pharmaceuticals 2021, 14 (6), 545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Tadesse S. Clinical Pharmacokinetics of Metformin. In Metformin – Pharmacology and Drug Interactions; Juber A, Usama A, Badruddeen, Mohammad Irfan K, Eds.; IntechOpen: London, 2021; Chapter 3. [Google Scholar]
- (8).Graham GG; Punt J; Arora M; Day RO; Doogue MP; Duong JK; Furlong TJ; Greenfield JR; Greenup LC; Kirkpatrick CM; et al. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet 2011, 50 (2), 81–98. [DOI] [PubMed] [Google Scholar]
- (9).Bouriche S; Alonso-García A; Cárceles-Rodríguez CM; Rezgui F; Fernández-Varón E An in vivo pharmacokinetic study of metformin microparticles as an oral sustained release formulation in rabbits. BMC Veterinary Research 2021, 17 (1), 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Kinaan M; Ding H; Triggle CR Metformin: An Old Drug for the Treatment of Diabetes but a New Drug for the Protection of the Endothelium. Med. Princ. Pract 2015, 24 (5), 401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Wook Huh H; Na Y-G; Kang H; Kim M; Han M; Mai Anh Pham T; Lee H; Baek J-S; Lee H-K; Cho C-W Novel self-floating tablet for enhanced oral bioavailability of metformin based on cellulose. Int. J. Pharm 2021, 592, 120113. [DOI] [PubMed] [Google Scholar]
- (12).Li Y; Song J; Tian N; Cai J; Huang M; Xing Q; Wang Y; Wu C; Hu H Improving oral bioavailability of metformin hydrochloride using water-in-oil microemulsions and analysis of phase behavior after dilution. Int. J. Pharm 2014, 473 (1), 316–325. [DOI] [PubMed] [Google Scholar]
- (13).Migdadi EM; Courtenay AJ; Tekko IA; McCrudden MTC; Kearney M-C; McAlister E; McCarthy HO; Donnelly RF Hydrogel-forming microneedles enhance transdermal delivery of metformin hydrochloride. J. Controlled Release 2018, 285, 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Lizy Roselet S; Prema Kumari J. An investigation on host-guest complexation of Metformin hydrochloride with hydroxypropyl-α-cyclodextrin for enhanced oral bioavailability. Mater. Today: Proc 2020, 21, 514–518. [Google Scholar]
- (15).Li J; Esteban-Fernández de Ávila B; Gao W; Zhang L; Wang J. Micro/nanorobots for biomedicine: Delivery, surgery, sensing, and detoxification. Sci. Robot 2017, 2 (4), eaam6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Cai L; Zhao C; Chen H; Fan L; Zhao Y; Qian X; Chai R. Suction-Cup-Inspired Adhesive Micromotors for Drug Delivery. Adv. Sci 2022, 9 (1), 2103384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Zhang F; Mundaca-Uribe R; Askarinam N; Li Z; Gao W; Zhang L; Wang J. Biomembrane-Functionalized Micromotors: Biocompatible Active Devices for Diverse Biomedical Applications. Adv. Mater 2022, 34 (5), 2107177. [DOI] [PubMed] [Google Scholar]
- (18).Choi H; Jeong SH; Kim TY; Yi J; Hahn SK Bioinspired urease-powered micromotor as an active oral drug delivery carrier in stomach. Bioact. Mater 2022, 9, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Zhang F; Zhuang J; Li Z; Gong H; de Ávila BE-F; Duan Y; Zhang Q; Zhou J; Yin L; Karshalev E; et al. Nanoparticle-modified microrobots for in vivo antibiotic delivery to treat acute bacterial pneumonia. Nat. Mater 2022, 21 (11), 1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Peyer KE; Zhang L; Nelson BJ Bio-inspired magnetic swimming microrobots for biomedical applications. Nanoscale 2013, 5 (4), 1259–1272. [DOI] [PubMed] [Google Scholar]
- (21).Orozco J; Jurado-Sánchez B; Wagner G; Gao W; Vazquez-Duhalt R; Sattayasamitsathit S; Galarnyk M; Cortés A; Saintillan D; Wang J. Bubble-Propelled Micromotors for Enhanced Transport of Passive Tracers. Langmuir 2014, 30 (18), 5082–5087. [DOI] [PubMed] [Google Scholar]
- (22).Mundaca-Uribe R; Karshalev E; Esteban-Fernández de Ávila B; Wei X; Nguyen B; Litvan I; Fang RH; Zhang L; Wang J A Microstirring Pill Enhances Bioavailability of Orally Administered Drugs. Adv. Sci 2021, 8 (12), 2100389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).de Ávila BE-F; Angsantikul P; Li J; Angel Lopez-Ramirez M; Ramírez-Herrera DE; Thamphiwatana S; Chen C; Delezuk J; Samakapiruk R; Ramez V; et al. Micromotor-enabled active drug delivery for in vivo treatment of stomach infection. Nat. Commun 2017, 8 (1), 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Karshalev E; Zhang Y; Esteban-Fernández de Ávila B; Beltrán-Gastélum M; Chen Y; Mundaca-Uribe R; Zhang F; Nguyen B; Tong Y; Fang RH; et al. Micromotors for Active Delivery of Minerals toward the Treatment of Iron Deficiency Anemia. Nano Lett. 2019, 19 (11), 7816–7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Karshalev E; Esteban-Fernández de Ávila B; Beltrán-Gastélum M; Angsantikul P; Tang S; Mundaca-Uribe R; Zhang F; Zhao J; Zhang L; Wang J Micromotor Pills as a Dynamic Oral Delivery Platform. ACS Nano 2018, 12 (8), 8397–8405. [DOI] [PubMed] [Google Scholar]
- (26).Horakova O; Kroupova P; Bardova K; Buresova J; Janovska P; Kopecky J; Rossmeisl M Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport. Sci. Rep 2019, 9 (1), 6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Genovese S; De Berardis G; Nicolucci A; Mannucci E; Evangelista V; Totani L; Pellegrini F; Ceriello A. Effect of pioglitazone versus metformin on cardiovascular risk markers in type 2 diabetes. Adv. Ther 2013, 30 (2), 190–202. [DOI] [PubMed] [Google Scholar]
- (28).Hansen M; Palsøe MK; Helge JW; Dela F The effect of metformin on glucose homeostasis during moderate exercise. Diabetes Care 2015, 38 (2), 293–301. [DOI] [PubMed] [Google Scholar]
- (29).Hanefeld M; Temelkova-Kurktschiev T Control of post-prandial hyperglycemia–an essential part of good diabetes treatment and prevention of cardiovascular complications. Nutr. Metab. Cardiovasc. Dis 2002, 12 (2), 98–107. [PubMed] [Google Scholar]
- (30).Li J; Angsantikul P; Liu W; Esteban-Fernandez de Avila B; Thamphiwatana S; Xu M; Sandraz E; Wang X; Delezuk J; Gao W; Zhang L; Wang J Micromotors Spontaneously Neutralize Gastric Acid for pH-Responsive Payload Release. Angew. Chem., Int. Ed. Engl 2017, 56 (8), 2156–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Nair AB; Jacob S A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm 2016, 7 (2), 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.