Abstract
In recent years, nanoparticles derived from cellular membranes have been increasingly explored for the prevention and treatment of human disease. With their flexible design and ability to interface effectively with the surrounding environment, these biomimetic nanoparticles can outperform their traditional synthetic counterparts. As their popularity has increased, researchers have developed novel ways to modify the nanoparticle surface to introduce new or enhanced capabilities. Moving beyond naturally occurring materials derived from wild-type cells, genetic manipulation has proven to be a robust and flexible method by which nanoformulations with augmented functionalities can be generated. In this review, an overview of genetic engineering approaches to expressing novel surface proteins is provided, followed by a discussion on the various biomedical applications of genetically modified cellular nanoparticles.
Keywords: biomimetic nanoparticle, cell membrane coating, genetic engineering, immunotherapy, drug delivery, detoxification
Graphical Abstract
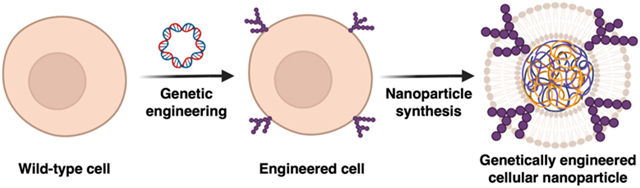
1. Introduction
Recent advances in nanotechnology have overcome many challenges associated with traditional medicine by improving drug localization, retention, and bioavailability [1-4]. With the wide range of nanomaterials that are available, it is possible to develop custom nanotherapeutics that can effectively accomplish specific tasks. Each individual component of a nanoparticle plays an important role in its overall functionality [5]. For example, the nanoparticle core can be used to carry and release various payloads, while the primary responsibility of the nanoparticle surface is to interact with the surrounding environment. Traditional approaches to functionalizing the nanoparticle surface include the introduction of polymers such as polyethylene glycol, poly(amino acids), and polysaccharides, as well as individual ligands such as peptides and aptamers [6, 7].
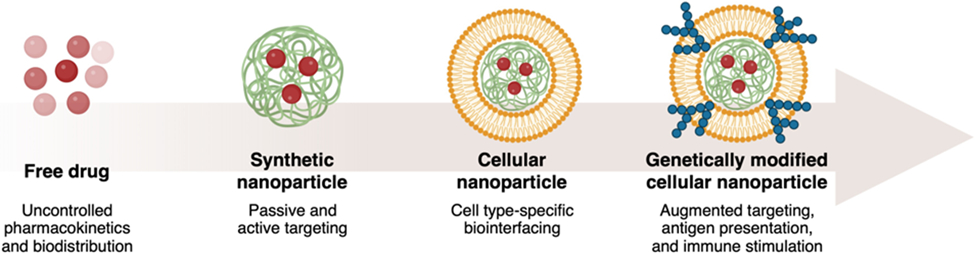
In recent years, researchers have begun to develop biomimetic nanoparticles with enhanced biointerfacing capabilities by taking inspiration from living cells [8-10]. As cells have evolved to interact effectively with their surroundings to accomplish certain tasks, they possess a unique array of surface markers that assist them in carrying out their specific roles. By leveraging cell-derived materials such as the plasma membrane, it is possible to fabricate cellular nanoparticle formulations that mimic the capabilities of the original source cell [11, 12]. For example, red blood cells (RBCs) are capable of prolonged circulation in the body, and accordingly RBC membrane-coated nanoparticles have demonstrated drastically extended blood residence times [13, 14]. Platelet membrane can be used for immune evasion while bestowing additional properties such as an enhanced affinity for damaged vasculature, cancer cells, and pathogens [15-18]; these abilities result from surface display of various markers, including selectins, integrins, and von Willebrand factor receptors, among others [19-21]. Platelet membrane-coated nanoparticles have been leveraged to deliver therapeutic payloads for the treatment of restenosis, atherosclerotic plaques, strokes, tumors, and drug-resistant infections [22-25]. White blood cells are another attractive membrane source, and they offer properties such as the recognition of pathogen associated molecular patterns and localization to sites of inflammation [26-28]. As such, white blood cell membrane-coated nanoparticles have been developed for the treatment of autoimmune disorders, primary and metastatic tumors, and bacterial infections [29-33]. Beyond membranes derived from blood cells, cellular nanoparticles constructed with cancer cell membrane are well-equipped for tumor treatment applications due to their inherent capability for immune evasion and homotypic binding [34-36]. Additional membrane coating sources include stem cells, epithelial cells, bacteria, and many others [37-42]. Overall, cell membrane coating nanotechnology has given rise to a versatile class of biomimetic nanoparticles that are inherently multifunctional and can outperform their traditional synthetic counterparts.
The popularity of cellular nanoparticles has resulted in a demand for methods to fine-tune or further augment their functionalities. The modification of wild-type cells and their derivative materials has allowed researchers to create customized nanoformulations beyond what can be achieved using natural cell membrane. Methods for modification include click chemistry, lipid insertion, membrane hybridization, metabolic engineering, and genetic engineering [43]. Lipid insertion can be used to introduce active targeting properties onto cell membrane by physically anchoring ligands which are not natively expressed [44-46]. This physical process does not expose the cell membrane to damaging chemicals or solvents, thus ensuring the preservation of surface markers [47]. Mechanical methods such as extrusion can be employed to create hybrid membranes, which merge the functionalities of two different cells in order to combine their advantages [48, 49]. Hybrid membranes can also be generated by first fusing live cells together using chemical methods, followed by membrane derivation [50, 51]. Metabolic engineering leverages various pathways within living cells to incorporate modified carbohydrate residues that subsequently allow for the straightforward attachment of external ligands [52, 53]. In this review, we will focus on the genetic engineering strategy for modifying cellular nanoparticles (Fig. 1). Through the use of well-established gene manipulation approaches, new capabilities can be effectively and facilely integrated onto biomimetic nanoformulations that can be leveraged to better address critical areas of need in biomedicine.
Fig. 1. Evolution of genetically modified cellular nanoparticles.
With their flexible design and the ability to custom-tailor their functionality, genetically modified cellular nanoparticles offer improvements over conventional therapeutic approaches for biomedical applications. Created with BioRender.
2. Synthesis of genetically modified cellular nanoparticles
The modification of source cells via genetic engineering provides a facile methodology by which cellular nanoparticles can be functionalized for biomedical applications (Table 1). Many approaches have been developed over the years [54], and they can largely be categorized based on their use of viral vectors, nonviral vectors, or physical disruption (Fig. 2). Following modification, cellular material such as the plasma membrane can be isolated and used to fabricate biomimetic nanoparticles with unique properties.
Table 1. Examples of genetically engineered cellular nanoparticle platforms.
Wild-type cells can be genetically engineered to modulate their protein expression. Nanoparticles derived from these modified cells exhibit unique functionalities that can be leveraged for various biomedical applications.
| Cell type | Modification | Engineering method |
Application | Ref. |
|---|---|---|---|---|
| Nanodelivery | ||||
| Primary T cell | Anti-glypican 3 CAR | Lentiviral transduction | Delivery of photothermal agent to hepatocellular carcinoma cells | [67] |
| C1498 (acute myeloid leukemia) | VLA-4 | Retroviral transduction | Targeting VCAM-1 in inflammation sites and delivery of dexamethasone | [59] |
| Adipose-derived stem cell | CXCR4 | Retroviral transduction | Targeting SDF-1 in inflammation sites and delivery of VEGF | [148] |
| Neural stem cell | CXCR4 | Lentiviral transduction | Targeting SDF-1 in inflammation sites and delivery of VEGF through the blood–brain barrier | [149] |
| B16F10 (melanoma) | Influenza HA | Lipid-based transfection | Improved endosomal escape for increased cytosolic delivery | [98] |
| Dendritic cell | IL-4 | Adenovirus transduction | Delivery of IL-4 to suppress inflammation in arthritis | [68] |
| Cancer immunotherapy | ||||
| E. coli (OMV) | Cytolysin A-SpyCatcher | Bacterial transformation | Presentation of tumor antigens alongside immunostimulatory OMVs | [66] |
| Cytolysin A-SnoopCatcher | ||||
| B16F10 (melanoma) | CD80 | Lipid-based transfection | Direct activation of tumor-targeting T cells | [166] |
| HEK293T | PD-1 | Lipid-based transfection | Targeting to PD-L1 on melanoma cells and delivery of anti-immunosuppressive molecules | [60] |
| Platelet | PD-1 | Lentiviral transduction | Reversal of T cell exhaustion in post-surgical cancer sites | [183] |
| Vaccination against pathogens | ||||
| S. aureus | Deletion of agr locus | Bacterial transformation | Broad-spectrum protection against dengue virus | [193] |
| Dengue virus antigens | ||||
| E. coli (OMV) | Glycan epitope associated with N. meningitidis infection | Bacterial transformation | Protection against N. meningitidis | [198] |
| E. coli (OMV) | F. tularensis O-antigen polysaccharide | Bacterial transformation | Protection against F. tularensis | [199] |
| E. coli (OMV) | Omp22-Cytolysin A | Bacterial transformation | Protection against A. baumannii | [199] |
| Detoxification | ||||
| HEK293T | ACE2 | Lipid-based transfection | Detoxification of SARS-CoV-2 to prevent infection | [57] |
Fig. 2. Fabrication of genetically modified cellular nanoparticles.
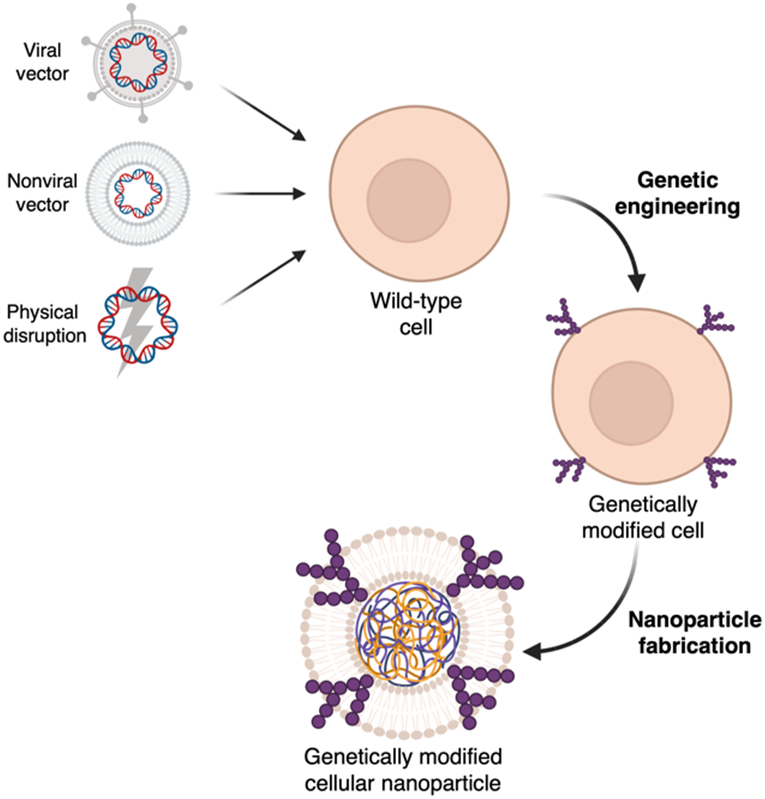
Wild-type cells are genetically engineered using either a viral vector, nonviral vector, or physical disruption. Cellular components such as the plasma membrane can then be harvested to fabricate cellular nanoparticles with enhanced functionalities. Created with BioRender.
2.1. Plasmid design for protein expression on the cell surface
As the synthesis of cellular nanoparticles oftentimes leverages the outermost membrane layer of the source cell, the ability to achieve surface expression of transgenes is highly desirable [55]. When overexpressing naturally occurring surface proteins, the genetic sequence itself will consist of a transmembrane domain to anchor the protein onto the cell surface [56]. This straightforward approach has been used to express receptors for virus neutralization [57], direct interfacing with specific cell types [58-60], improved circulation [61], and macrophage repolarization [62].
In many cases, it is necessary to express a protein that is not naturally bound to the cell surface [63]. To address this challenge, the gene of interest can be genetically fused to a signal peptide along with a transmembrane domain from a different protein. After expression in the target cell, the signal peptide will direct the fusion protein to the cell membrane [64], where the transmembrane domain will anchor the construct [65]. Through this approach, precise control over the presentation of the desired construct, including orientation and distance from the cell membrane, can be achieved [63]. This construction technique has already been widely used for the development of augmented cellular nanoparticles, including to present heterologous antigens for vaccination [66], targeting ligands [67], and immunomodulatory proteins [68].
2.2. Genetic modification using viral vectors
Viruses have evolved to be adept at entering mammalian cells, and in some cases they can effectively integrate their genetic material into the host genome. Taking advantage of this property, viruses can be used to carry and deliver transgenes directly to target cells [69]. With their ability to achieve consistently high protein expression, viral vectors are a well-established tool for genetic engineering [70]. To date, a vast repertoire of viral vectors exists, including those based on retroviruses, lentiviruses, adenoviruses, and adeno-associated viruses (AAVs), and each has its own advantages [71]. Notably, transduction can be made safer through the use of self-inactivating viral particles, which allows for delivery of the target gene without the danger of viral replication in the host [70].
Retroviruses are RNA-based and contain essential genes that encode for structural proteins, transcription and integration enzymes, and outer envelope glycoproteins [72]. These vector systems leverage the natural mechanisms of retroviral replication in order to stably express transgenes in transduced cells [73]. After cellular entry, the viral RNA is transcribed to produce double-stranded DNA, which is subsequently incorporated into the host genome with the help of integrase enzymes [74]. While standard retroviral vectors can induce high expression of a transduced construct, they cannot be used to modify quiescent cells [75]. A subclass of retroviruses known as lentiviruses provide a powerful tool for gene transfer that can be used with both non-dividing and dividing cells [76]. Overall, retroviral vectors can be used to genetically alter a wide range of cells, including primary neuronal and immune cells, and they typically have a packaging capacity of approximately 8 kb [77]. With their ability to induce stable transgene expression, retroviral and lentiviral vectors have been commonly used to develop cellular nanoparticle-based platforms. Through the production of stable cell lines, it is possible to decrease batch-to-batch variability and improve scalability. Notably, retroviral and lentiviral techniques have also been used to successfully modify cells for therapies currently being investigated in the clinic [78].
Adenoviruses and AAVs comprise a useful class of viral vectors that can infect a broad range of cell types [79]. Adenoviruses contain double-stranded DNA and possess a genome approximately 40 kb in length. On the other hand, AAVs contain a significantly smaller single-stranded DNA genome of 5 kb [80]. Between these two systems, there are several key differences, including their packaging capabilities, expression level, and duration of gene expression. Adenoviruses typically have a carrying capacity of 8 kb and can induce high expression of the target protein; however, the gene expression is transient, with high production starting as early as 16 hours post-transfection [81]. While AAVs have a lower packaging capacity of approximately 5 kb and elicit comparatively lower protein expression levels, they have the ability to integrate into the host cell genome for stable expression [82]. Compared to retroviral vectors, adenoviruses and AAVs are less favorable for the development of cellular nanoparticles due to their transient expression and lower packaging capabilities, respectively. However, AAVs also have a lower mutagenesis rate compared to retroviruses, thus making them a potentially safer alternative in clinical settings, where various therapeutic platforms based on the technology are being investigated [83].
Other viral vector systems have been developed using the herpes simplex virus (HSV) [84]. HSV comprises of an enveloped viral capsid containing a double-stranded DNA genome of approximately 150 kb in length. As a neurotropic virus, HSV vectors have significant potential towards the genetic engineering of neuron cells [85]. In order to improve their safety, HSV vectors are designed with partial deletions in their base genome. While they have a high packaging capacity of over 30 kb, HSV vectors can only be used for transient protein expression [86].
2.3. Genetic modification using nonviral vectors
Without assistance, it is difficult for free genetic material to pass through the negatively charged membrane of a cell due to electrostatic repulsion forces. However, cellular entry can be facilitated through a process known as transfection using nonviral vectors [87]. This strategy generally employs positively charged reagents that interact efficiently with the genetic material, and the resulting complexes are often used to induce temporary gene expression [88]. Transfection is scalable and can be used to elicit high protein expression across many cell types in vitro, although many traditional platforms are limited by unfavorable toxicity profiles [89]. In the development of cellular nanoparticles, nonviral vectors are oftentimes restricted by transient gene expression, which can significantly increase the workload required for large-scale synthesis and negatively affect batch-to-batch variability. While time-consuming, it is possible to employ transfection to establish stable expression using an appropriate selection strategy [90].
Calcium phosphate coprecipitation represents one of the earliest methods of transfection [91]. By mixing DNA with calcium chloride and adding this mixture to a buffered phosphate solution in a controlled manner, a precipitate of DNA and calcium phosphate is formed. When added to cultured cells, these DNA-containing complexes can gain entry via phagocytosis. While this approach is facile, broadly applicable amongst different cell types, and inexpensive, transfection using calcium phosphate coprecipitates has a relatively low efficiency compared to other approaches [92]. This approach is also sensitive to changes in pH, temperature, and salt concentrations [93].
In recent years, lipid-based and polymer-based reagents have become more popular due to their higher transfection efficiency and improved batch-to-batch consistency compared to methods like calcium phosphate coprecipitation [94]. In each case, the positively charged groups on the lipid or polymer complex with the negatively charged nucleic acids. With a net positive charge that enhances their cellular interactions, the resulting nanocomplexes can achieve high transfection efficiencies [95]. Entry into the cell may be facilitated by endocytosis or by direct fusion with the plasma membrane [96]. Once the nucleic acids reach the cytosol, they are translated into their encoded proteins. Positively charged transfection reagents can be used to deliver nucleic acids of all sizes. While this approach is applicable across a broad range of cells, the efficiency can vary significantly depending on the targeted cell type, cell health, and the specific reagent that is employed [97]. Lipid-based and polymer-based transfection approaches have been successfully employed to genetically modify various cellular nanoparticle platforms [58, 60, 98].
2.4. Genetic modification by physical disruption
Rather than using a viral or nonviral carrier, cellular entry of free nucleic acids can be mediated by physically disrupting the cell membrane [99]. While the transformation of bacteria with foreign genetic material by this type of methodology is facile and well-established [100], the modification of eukaryotic cells by physical disruption is a more specialized process. This general approach, which relies on transient pore formation on the cell surface, can achieve high transfection efficiencies regardless of cell type, but its scalability is limited. One example is microinjection, a physical method that relies on the use of a needle to efficiently transfer DNA into the nucleus of a target cell [101]. Once within the nucleus, the exogenous DNA can integrate into the host genome, thus generating a stably modified cell. This technique offers impressive control over nucleic acid delivery and promotes efficient integration into the host genome, but it is time-consuming and expensive with a very low throughput [102]. Due to its inherently low throughput, in addition to the availability and simplicity of alternative approaches, microinjection has not been used thus far in the fabrication of cellular nanoparticles.
Another genetic engineering approach based on the physical disruption of cell membrane is electroporation [103]. During this process, a mixture of cells and exogenous DNA is pulsed with a strong electric field. This destabilizes the plasma membrane, allowing for passage of DNA from the surrounding environment into the cells. After the pulsing is completed, the membrane stabilizes with the genetic material trapped inside, where it can be expressed by endogenous cellular machinery [104]. This method can be used to achieve high transfection efficiency and is not limited by cell type; however, the main drawbacks of electroporation are the high rate of cell death and potential degradation of the nucleic acid material [105]. Although electroporation has not been leveraged to synthesize cellular nanoparticles, it could eventually prove to be useful due to its high efficiency and potential for genomic integration. For cell-based therapies, electroporation has been used to introduce a CAR construct and programmed cell death protein 1 (PD-1) onto T cells in a preclinical setting [106].
2.5. Cellular nanoparticle synthesis
While biomimetic nanoparticles can be constructed solely from cell-derived components [107-110], cell membrane-coated nanoparticles have gained popularity over the past decade due to their ability to combine the advantages of both natural and synthetic nanomaterials into a single platform [11, 12]. Their synthesis typically comprises of three distinct steps: isolation of membrane from cells, nanoparticle core preparation, and the coating of the membrane onto the nanoparticle core. The process of membrane extraction requires cells to be lysed, followed by purification of the membrane material from intracellular components. For anucleate cells such as RBCs and platelets, lysis can be achieved using either hypotonic treatment or repeated freeze and thaw cycles [111]. From here, centrifugation is used to isolate the membrane and remove soluble proteins. Nucleated cells are more difficult to process due to the presence of intracellular organelles. Various forms of membrane disruption, including homogenization, mechanical shearing, or nitrogen cavitation, have been employed [112]. Traditional homogenization techniques use shear force to physically break cells apart. They can be optimized for a variety of cell types, are relatively scalable, and do not require the introduction of additives [113]. Nitrogen cavitation relies on the formation of gas bubbles for cellular disruption [114]. Without friction and the resulting heat, nitrogen cavitation is a gentler process that may help to better preserve cell membrane proteins. From here, differential or gradient centrifugation techniques can be applied to isolate the plasma membrane and remove unwanted cellular debris.
Cell membrane coating nanotechnology has been applied to a wide range of nanomaterials, resulting in the development of cell-mimicking nanoformulations with unique functionalities for biomedical applications [11, 12, 115]. Biodegradable organic cores, including those based on polymers such as poly(lactic-co-glycolic acid) (PLGA), have been widely used for their biocompatibility and ability to enhance to bioavailability of various drug payloads [13, 116]. Inorganic and metallic cores have also been popular due to their cargo loading capabilities, inherent bioactivity, and unique functions that can be employed for various therapeutic and diagnostic purposes. Examples of such platforms include mesoporous silica nanoparticles, upconversion nanoparticles, gold and silver nanoparticles, iron oxide nanoparticles, and metal–organic frameworks, among many others [24, 117-122].
After successfully obtaining the purified cell membrane and prefabricated nanoparticulate cores, a membrane coating process is required to form the final nanostructure. Currently, the two most common approaches for coating are either by physical extrusion or the application of ultrasonic energy [123]. For extrusion, the two components are mixed together and passed repeatedly through a porous membrane [13]. This mechanical process facilitates the disruption of the membrane structure, enabling it to assemble around the nanoparticle substrate and form a core–shell structure. Sonication similarly provides energy to drive the cell membrane coating process and represents a facile alternative to extrusion with the additional benefit of reduced material loss [124]. However, its disruptive nature may render it less suitable in situations where maintaining integrity of the membrane constituents is critical.
3. Applications of genetically engineered cellular nanoparticles
3.1. Nanodelivery
Nanomedicine has provided important solutions to many of the issues associated with traditional drug formulations. By overcoming poor bioavailability and unfavorable biodistribution, nanocarriers have been utilized to significantly improve the therapeutic index of drug payloads [125-127]. Various strategies can be employed to reduce clearance by mononuclear phagocytes and organs of the reticuloendothelial system [128, 129], therefore lengthening blood circulation time and enabling nanoparticles to take advantage of passive targeting effects based on properties such as charge, size, shape, and hydrophobicity [130-133]. Through optimized pharmacokinetics, nanodrugs have a higher chance of being utilized at their intended site of action, thus leading to improved bioactivity. Nanomedicine also offers various tools for active targeting to further improve drug delivery specificity [134, 135]. This is generally achieved by attaching ligands onto the nanoparticle surface to take advantage of their affinity to unique or overexpressed biomarkers, including those found on sites of inflammation, tumors, or pathogens [136-138]. In traditional nanoparticle platforms, various ligands based on small molecules, peptides, aptamers, and antibodies have been widely used [139-143].
Cellular nanoparticles have proven to be highly effective as drug carriers with their ability to simultaneously achieve long circulation times and targeted delivery through the display of different surface markers [46, 59, 115, 144]. Genetically engineered cellular nanoparticles can be designed with additional functionality to further improve their utility for treating cancer, inflammation, and stroke, among others. One strategy has been to use engineered source cells that express ligands with a strong affinity to specific disease-relevant markers. For example, hepatocellular carcinoma, which overexpresses glypican-3, can be targeted by lentivirus-transduced chimeric antigen receptor (CAR)-T cells specific for the protein [145, 146]. Such CAR-T cell membrane has been coated onto mesoporous silica nanoparticles containing IR780, a photothermal and imaging agent (Fig. 3) [67]. The engineered nanoparticles demonstrated significant advantages for both photothermal therapy and imaging purposes when compared to their noncoated counterparts. Notably, the genetically modified formulation demonstrably increased local temperatures when exposed to near-infrared radiation, and this effect was used to suppress tumor growth. Much like cancer, sites of inflammation upregulate numerous biomarkers which can be targeted by the appropriate ligands [136, 147]. Taking advantage of this, C1498 cells were engineered by retroviral transduction to overexpress very late antigen 4 (VLA-4) to create cellular nanoparticles capable of specifically accumulating at sites of inflammation with high levels of vascular cell adhesion molecule 1 (VCAM-1) (Fig. 4) [59]. The nanoformulation was used to deliver the anti-inflammatory drug dexamethasone to treat a murine model of acute lung inflammation induced by the instillation of lipopolysaccharide.
Fig. 3. CAR-T cell membrane-coated nanoparticles for anticancer phototherapy.
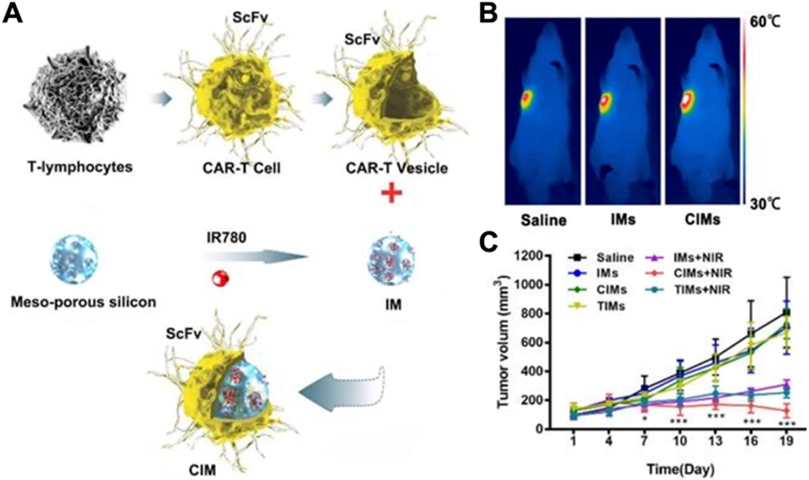
(A) T lymphocytes are engineered to express a CAR construct specific for an overexpressed hepatocarcinoma antigen. Membrane derived from these cells is then used to coat IR780-loaded mesoporous silica nanoparticles (IM), yielding the final CAR-T cell membrane-coated nanoformulation (CIM). (B) After intravenous injection, the CIM nanoformulation significantly increases intratumoral temperatures when exposed to near-infrared irradiation (NIR). (C) Intravenous administration of CIM with subsequent exposure to NIR significantly suppresses tumor growth. Adapted with permission [67]. Copyright 2020, Ivyspring International Publisher.
Fig. 4. Engineered cell membrane-coated nanoparticles for inflammation targeting.
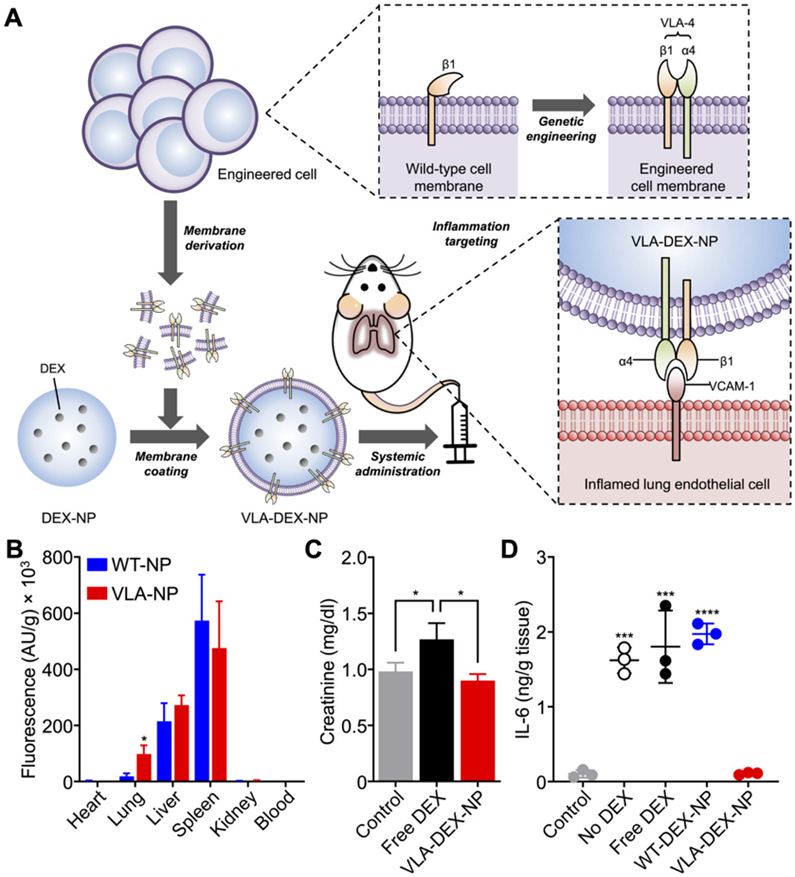
(A) Membrane derived from wild-type cells engineered to express VLA-4 is coated onto polymeric nanoparticles carrying dexamethasone (DEX). The resulting nanoparticles target the inflammatory marker VCAM-1 on vascular endothelial cells, thereby reducing local inflammation. (B) When administered intravenously, the VLA-4 expressing-nanoparticles (VLA-NP) target the lungs more efficiently than wild-type nanoparticles (WT-NP). (C) The toxicity of DEX is reduced upon encapsulation into VLA-NP (VLA-DEX-NP). (D) VLA-DEX-NP reduces proinflammatory cytokine production in an animal model of acute lung inflammation. Adapted with permission [59]. Copyright 2021, American Association for the Advancement of Science.
Genetically engineered stem cell membrane-coated nanoparticles have also been used to target ischemia of the brain and extremities [148, 149]. Stromal cell-derived factor-1 (SDF-1) is significantly upregulated by ischemic cells, and the marker can be targeted using C-X-C chemokine receptor type 4 (CXCR4) [150]. To take advantage of this, cellular nanoparticles were made using membrane from human adipose-derived stem cells retrovirally transduced to overexpress CXCR4 (Fig. 5) [148]. The membrane was coated around PLGA nanocores loaded with vascular endothelial growth factor (VEGF) in order to counteract the effects of ischemia. The engineered nanoparticles retained characteristics of the source cells such as immune evasion and endothelial penetration, while drastically increasing VEGF accumulation the site of ischemia in a murine model. This improved retention resulted in superior blood reperfusion, muscle repair, and limb salvage. Another work harnessed the SDF-1 and CXCR4 interaction for the treatment and imaging of stroke within the ischemic brain [149]. This platform utilized membrane from CXCR4-overexpressing neural stem cells, which naturally possess tropism for the ischemic brain and are capable of penetrating the blood–brain barrier. The membrane was used to coat PLGA nanoparticles loaded with either the fluorescent agent IR780 or glyburide for imaging or stroke treatment, respectively. Compared to their wild-type counterparts, the engineered cellular nanoparticles demonstrated more favorable accumulation at the target site, enabling better imaging while enhancing mouse survival, reducing infarct volumes, and improving neurological scores.
Fig. 5. Engineered stem cell membrane-coated nanoparticles for ischemia treatment.
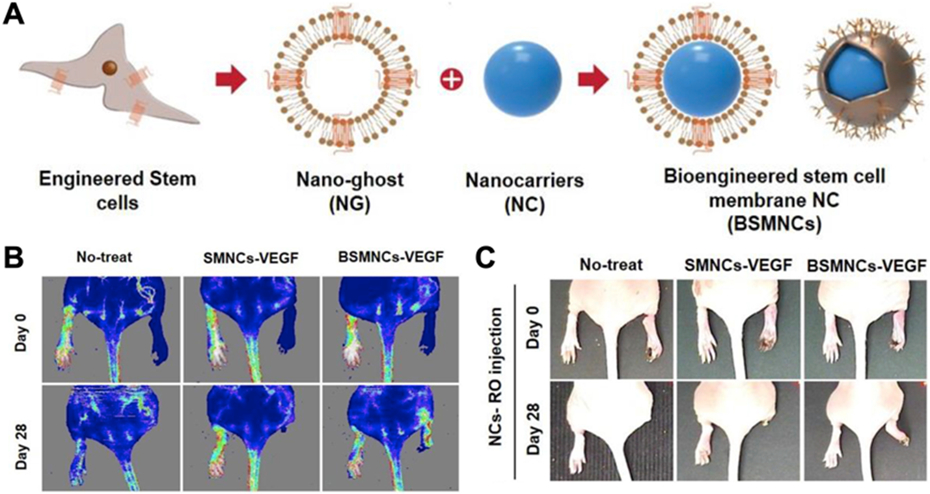
(A) The membrane from stem cells genetically engineered to express surface CXCR4 for ischemia targeting is used to coat VEGF-loaded nanoparticulate cores. (B) Intravenous administration of the engineered nanoparticles improves perfusion of ischemic limbs. (C) The improved perfusion due to the nanoparticles results in higher degrees of limb salvage. Adapted with permission [148]. Copyright 2018, Elsevier.
Upon reaching their target cells, some therapeutic agents may require localization within a specific intracellular compartment in order to exert activity [151]. Along these lines, cellular nanoparticles have been successfully engineered for the cytosolic delivery of mRNA by taking advantage of the influenza hemagglutinin (HA) protein (Fig. 6) [98]. Usually, endosomal entrapment of nanoparticles after the cellular uptake poses a significant challenge due to harsh conditions that can destroy the therapeutic payload before it reaches the appropriate intracellular target [152, 153]. However, influenza HA has evolved to enable viral entry into the cytosol upon being activated by the low pH of the endosomes [154]. By engineering the protein onto the surface of mRNA-loaded cellular nanoparticles via lipid-based transfection, endosomal escape functionality was successfully introduced, thus enabling cytosolic delivery of the payload [98]. Compared with wild-type membrane-coated nanoparticle controls, the HA-expressing nanoformulation was able to enhance mRNA transfection efficiency when administered via intranasal and intravenous routes into mice.
Fig. 6. Engineered cell membrane-coated nanoparticles for cytosolic mRNA delivery.
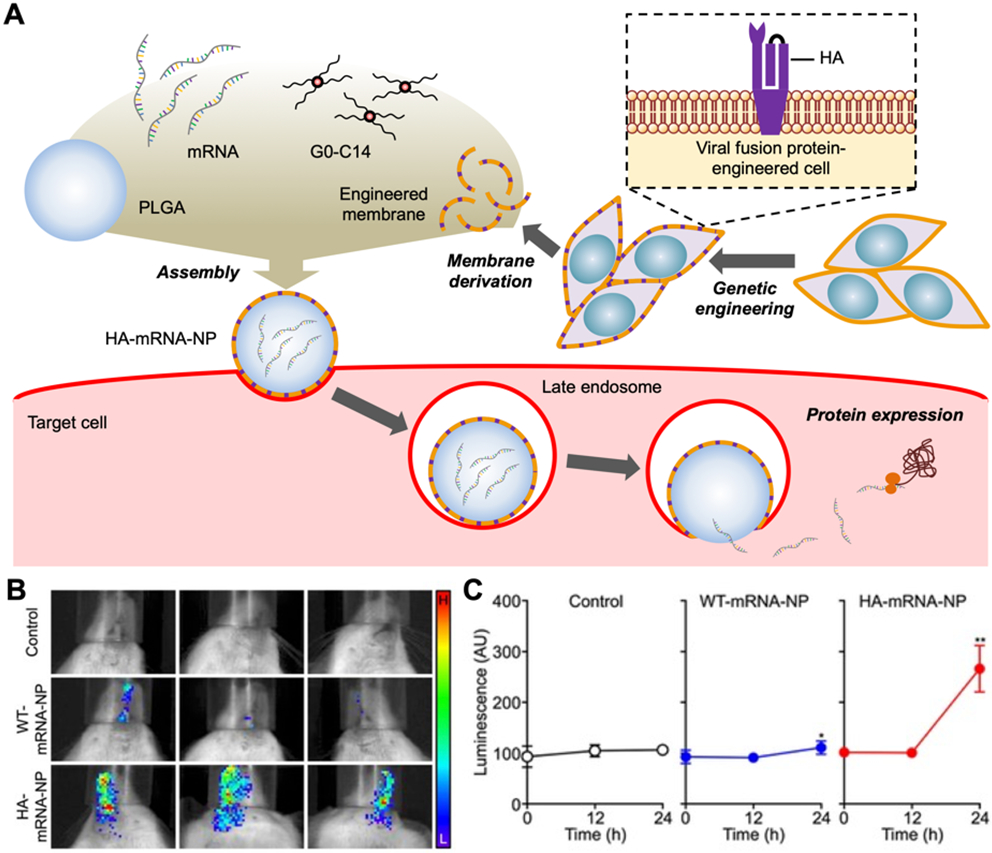
(A) Cells are genetically modified for the surface expression of viral hemagglutinin (HA), which enables mRNA-loaded nanoparticles coated with the HA-expressing membrane (HA-mRNA-NP) to achieve endosomal escape after cellular uptake. (B,C) HA-mRNA-NP promote in vivo expression of a Cypridina luciferase-encoding payload after intranasal (B) or intravenous (C) administration. Adapted with permission [98]. Copyright 2021, Wiley-VCH.
Beyond cell membrane-coated nanoparticles with a core–shell structure, genetic engineering has also been applied to cell membrane vesicle-based targeted delivery platforms, illustrating the broad applicability of this functionalization approach [155]. Genetically engineered membrane vesicles can either be harvested directly from cellular secretions such as exosomes, or they can be fabricated using whole cells as the starting material [156-159]. Similar to cell membrane-coated nanoparticles, these engineered vesicles can be loaded with therapeutics and applied towards many of the same applications [160-162].
Inflammation is a natural response to injury and infection that serves as a means for the body to protect itself. Inflammatory processes are essential in healing and maintaining tissue homeostasis; however, chronic inflammation can oftentimes lead to deleterious effects [163]. Many anti-inflammatory drugs suffer from low bioavailability and toxicity issues [164], which has motivated the development alternative treatments using biomimetic nanoparticle platforms [136, 165, 166]. Leveraging the fact that genetic engineering can be used to introduce anti-inflammatory markers onto the cell surface, a cellular nanoparticle formulation was developed by using an adenovirus to introduce interleukin 4 (IL-4) onto dendritic cell (DC) exosomes [68]. IL-4 can modulate immunity along the T helper 1 (Th1)/Th2 axis, inhibiting IL-2 and interferon γ (IFN-γ) to suppress macrophage activation and reduce inflammatory responses [167]. Previous studies demonstrated that DCs expressing IL-4 could reverse the disease phenotype in a murine autoimmune arthritis model [168]. In the present example, administration of the IL-4-expressing exosomes helped to suppress inflammation in delayed-type hypersensitivity and collagen-induced arthritis murine models [68].
3.2. Cancer immunotherapy
From its ability to evade and suppress the immune system to its heterogeneity, cancer poses a significant challenge for current medical research [169, 170]. Traditional cancer therapeutic strategies, including surgery, chemotherapy, and radiotherapy, work to eradicate tumor cells, but they are oftentimes unable to prevent tumor recurrence [171]. More recently, immunotherapies have been developed that utilize the body’s immune system to fight malignant growths while encouraging the development of durable antitumor immunity. The approach can vary greatly, from the use of monoclonal antibodies and small molecule immunomodulators to the adoptive transfer of engineered T cells and therapeutic vaccination [172-175]. To overcome some of the challenges associated with traditional immunotherapeutic payloads, including limited tumor penetration, off-target toxicities, and low immunogenicity, a wide range of nanomedicine platforms have been developed [176-178], including various cellular nanoparticle formulations [179-182].
Utilizing a genetic engineering approach, it was demonstrated that bacterial outer membrane vesicles (OMVs) can be modified to facilely present different antigens on their surface (Fig. 7) [66]. Escherichia coli was genetically modified for the surface expression of cytolysin A fused with either SpyCatcher or SnoopCatcher. The engineered OMVs could then be easily conjugated with tumor antigens fused respectively to the SpyTag or SnoopTag peptides via the spontaneous formation of an isopeptide bond. In an MC38 colon cancer model, administration of OMVs labeled with a tagged Adpgk neoantigen peptide strongly suppressed tumor growth, with 60% of treated mice showing complete tumor regression. Overall, this versatile and modular design approach can enable the quick development of new vaccine formulations without the need to completely reengineer the source bacteria every time.
Fig. 7. Engineered OMVs as a versatile antigen display platform.
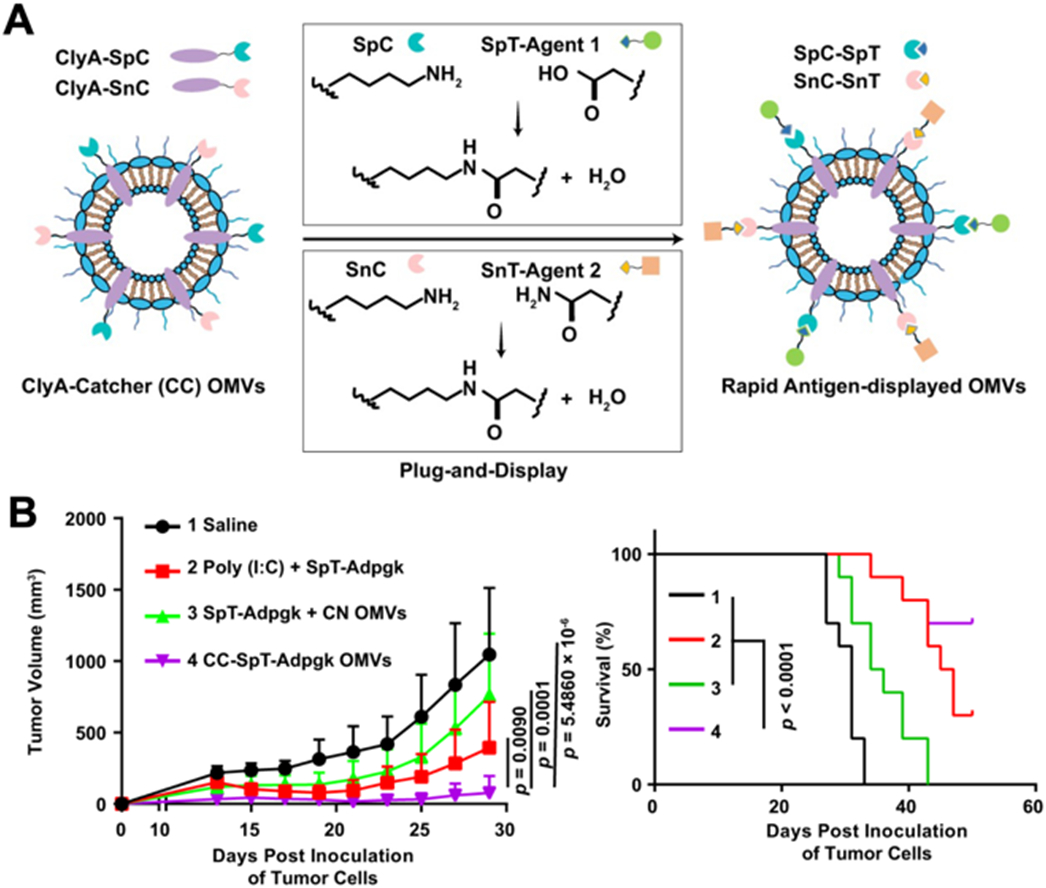
(A) SpyCatcher (SpC) and SnoopCatcher (SnC) are genetically introduced onto the surface of OMVs via fusion with cytolysin A (ClyA). Antigens labeled with SpyTag (SpT) or SnoopTag (SnT) can then be readily conjugated onto the surface of the OMVs. (B) Engineered OMVs expressing the MC38-related antigen Adpgk are able to significantly suppress tumor growth and improve survival in an MC38 tumor model. Adapted with permission [66]. Copyright 2020, Springer Nature.
Instead of delivering antigenic materials that need to subsequently be processed by antigen-presenting cells (APCs), it is also possible to engineer cellular nanoparticles to directly stimulate antigen-specific T cells. In a notable example, a nanoscale artificial APC platform was developed to activate tumor-targeting T cells (Fig. 8) [58]. In order to provide an immunostimulatory signal, the costimulatory marker CD80 was engineered onto the surface of cancer cells, and the membrane from these engineered cells was subsequently used to coat PLGA nanoparticle cores. By displaying CD80 along with native tumor epitopes presented via major histocompatibility complex I, the nanoformulation was able to significantly activate antigen-specific T cells. Mice treated with the artificial APC nanoparticles exhibited reduced tumor growth and extended survival rates.
Fig. 8. Engineered cell membrane-coated nanoparticles for direct antigen presentation.
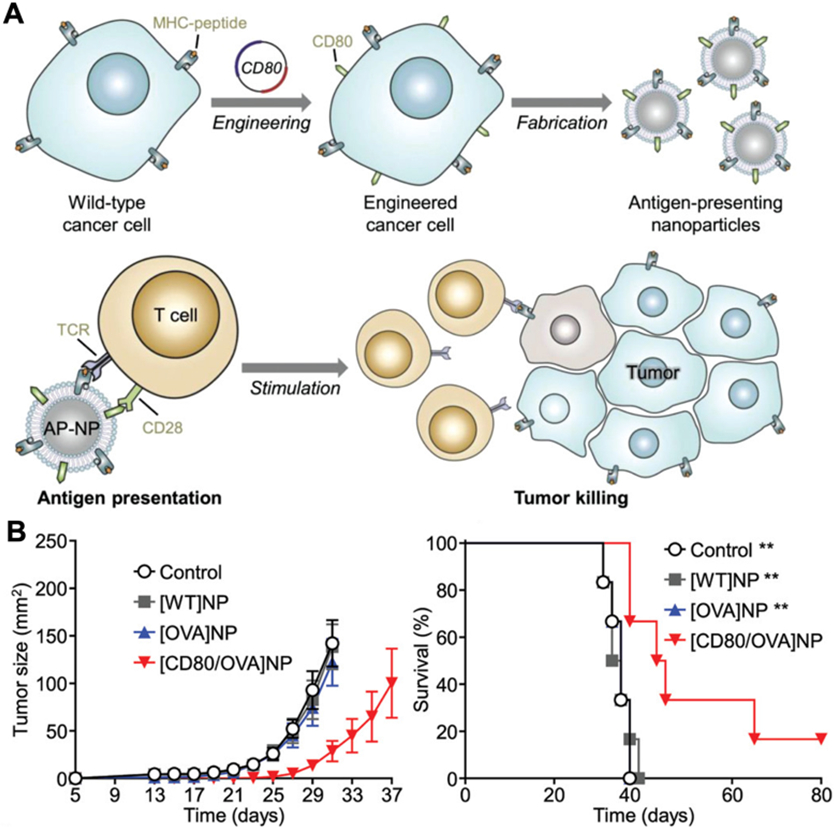
(A) Wild-type cancer cells are engineered to express a costimulatory signal alongside their native MHC-I antigens. Nanoparticles coated with the membrane derived from these engineered cells can generate anticancer immunity by directly activating tumor-specific T cells. (B) Prophylactic immunization with the engineered nanoparticles significantly slows tumor growth and improves survival in a murine cancer model. Adapted with permission [58]. Copyright 2020, Wiley-VCH.
Nanovesicles displaying checkpoint blockade molecules have also been developed using genetic engineering approaches in order to enhance antitumor immune responses. In an example, HEK293T cells were manipulated to constitutively express PD-1 (Fig. 9) [60]. Nanovesicles were then fabricated by extruding the PD-1-expressing membrane from the engineered cells. The formulation was also loaded with an indoleamine 2,3-dioxygenase-1 inhibitor as a means to further overcome the immunosuppressive tumor microenvironment. Mice treated with the nanovesicles demonstrated significant tumor regression and improved survival. In another study, PD-1-expressing platelets were engineered from megakaryocyte progenitors [183]. This platform utilized the natural homing of platelets to post-surgical tumor sites in order to maximize the ability of the PD-1 to reverse T cell exhaustion. In vivo, the engineered cellular nanoparticles were able to delay tumor growth with no systemic toxicities. In conjunction with the chemotherapeutic agent cyclophosphamide, the nanoformulation was able to eradicate residual tumor cells and significantly reduce the incidence of relapse.
Fig. 9. Engineered cell vesicles for cancer immunotherapy via PD-1 blockade.
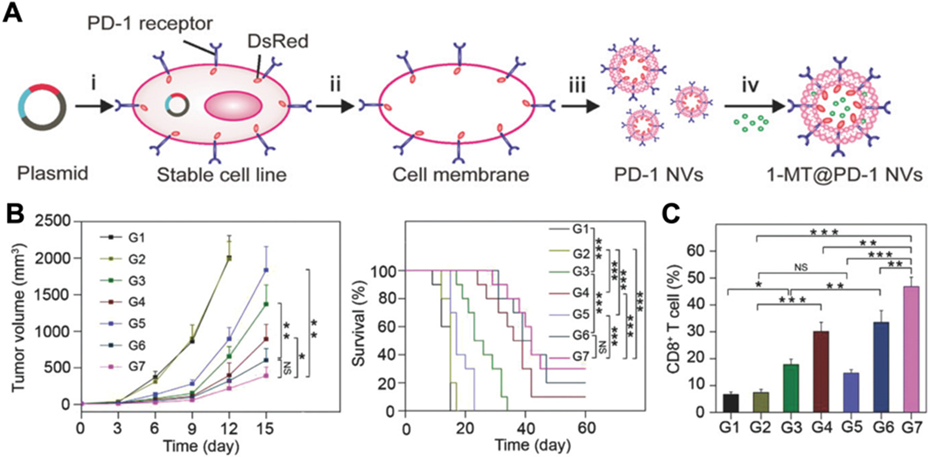
(A) The membrane from HEK293T cells engineered to express PD-1 is used to form nanovesicles, which are then loaded with 1-methyl-tryptophan (1-MT), an indoleamine 2,3-dioxygenase-1 inhibitor. (B) Therapeutic treatment of tumor-bearing mice with the genetically engineered nanovesicles carrying 1-MT (G7) suppresses tumor growth and improves survival compared to controls of saline (G1), wild-type nanovesicles (G2), free 1-MT (G3), empty engineered nanovesicles (G4), free 1-MT inhibitor with wild-type nanovesicles (G5), and free 1-MT with anti-PD-L1 (G6). (C) Treatment with the engineered nanovesicles carrying 1-MT generates a high intratumoral percentage of CD8+ T cells. Adapted with permission [60]. Copyright 2018, Wiley-VCH.
3.3. Vaccination against pathogens
Historically, vaccines have helped to control the severity and spread of many deadly and harmful pathogens [184]. Unfortunately, not all infectious diseases are preventable by vaccination [185], which has resulted in the development of novel vaccine nanoformulations with the potential to promote sterilizing immunity against various bacteria and viruses [186]. Along these lines, cellular nanoparticles have the ability to excel due to their inherently multiantigenic nature, unique biointerfacing properties, and design flexibility [187].
The use of bacterial outer membrane vesicles (OMVs) represents a promising approach for delivering multiantigenic material to the immune system for eliciting antibacterial immune responses [40, 187, 188]. OMVs naturally possess many of the same biochemical, immunogenic, and antigenic properties as their source bacteria. When used as a vaccine, OMVs derived from Acinetobactor baumannii were able to generate strong cytokine and antibody responses in immunized mice that protected them against sepsis [189]. Highlighting the potential of OMV platforms, the United States Food and Drug Administration approved Bexsero, which contains meningococcal group B OMVs, as a vaccine to protect against Neisseria meningitidis [190]. While wild-type OMVs can mediate strong protective immune responses [188, 191], their potent immunostimulatory properties may present safety issues when administered into healthy recipients. Genetic engineering has thus been employed as a method to decrease the potential toxicities associate with the in vivo use of bacterial components [192]. In one example, a Staphylococcus aureus strain was genetically modified to delete the agr locus, which reduced virulence factor production and improved their safety profile [193]. Extracellular vesicles from the bacteria were also genetically engineered to carry heterologous antigens derived from the dengue virus, and the resulting vaccine formulation was shown to be safe and elicited broad protection against four dengue strains. Different studies explored deletion of the lpxL1 and lpxL2 genes, which encode for lipid enzymes, in N. meningitidis to reduce lipopolysaccharide expression and thus reduce the toxicity of the bacteria’s OMVs [194, 195]. This platform was still able to successfully induce proinflammatory cytokines such as IL-6 and IL-1β, as well as promote dendritic cell maturation. Building on this approach, another study functionalized OMVs derived from E. coli to display the influenza M2 matrix protein ectodomain [196]. These OMVs were also genetically remodeled to express a safe form of lipopolysaccharide unable to activate human Toll-like receptor pathways. Prophylactic studies using these modified OMVs showed higher antibody responses, which resulted in complete survival in an influenza challenge model. The platform was able to elicit protection against multiple strains of influenza, thereby demonstrating it broad protective capabilities.
Genetically modified OMVs expressing specific glycans or other bacterial antigens have also demonstrated potential for vaccine applications. Studies have shown that OMV glycan modifications can significantly enhance innate immune responses [197-199], thus reducing the need to work with larger amounts of bacterial material. Glycoengineered OMVs expressing capsular polysaccharides from Streptococcus pneumoniae or a heptasaccharide derived from Campylobacter jejuni both induced significant antigen-specific antibody responses [197]. The OMVs from one bacterial species can be used to boost the immune responses against antigens from different bacteria using engineering approaches. For example, OMVs derived from E. coli were engineered to express a glycan epitope associated with N. meningitidis infection, enabling strong IgG responses to be generated against the antigen [198]. In a serum bactericidal activity assay, the antibodies generated by this nanovaccine formulation potently killed N. meningitidis. This platform was also used to express the O-antigen polysaccharide from F. tularensis, resulting in complete protection against challenge with the bacteria [199]. In a similar type of concept, E. coli OMVs were genetically modified to express Omp22, an outer membrane protein of A. baumannii, conjugated with cytolysin A for membrane presentation (Fig. 10) [200]. When immunized with these genetically modified OMVs, mice displayed high Omp22-specific titers and had greatly reduced bacterial burden in their major organs after challenge with A. baumannii.
Fig. 10. Engineered E. coli OMVs expressing heterologous A. baumannii antigens for vaccination.
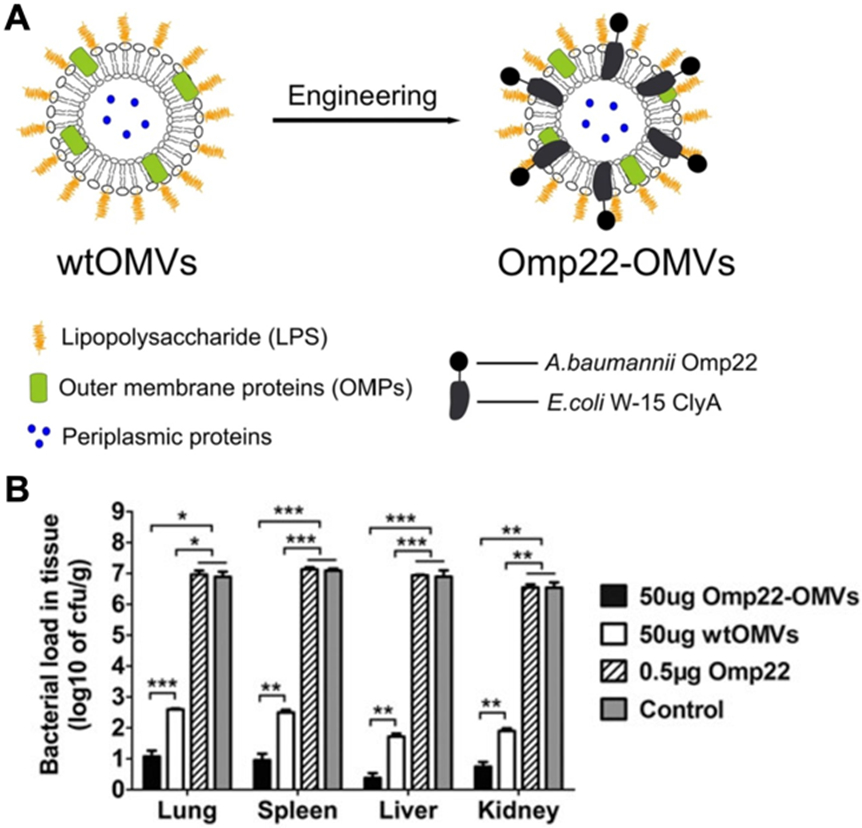
(A) A. baumannii Omp22 is introduced onto the surface of E. coli OMVs through genetic fusion with the cytolysin A (ClyA) protein. (B) Vaccination with the engineered OMVs protects against A. baumannii infection, leading to reduced bacterial burden. Adapted with permission [200]. Copyright 2016, Springer Nature.
3.4. Detoxification
Due to their ability to effectively interact with various biological moieties, cellular nanoparticles can be leveraged for biodetoxification applications. One example is for antivirulence therapy, where the neutralization of toxins secreted by pathogens can enhance the body’s ability to fight infection [201]. Along these lines, cell membrane-coated nanosponges have been developed as decoys to bind and neutralize pore-forming toxins (PFTs) [202]. The nanosponge concept is powerful because it can be generalized to many toxins, pathogens, and pathological antibodies or cytokines based on their mechanism of action [202-207], which is in stark contrast to antibodies that can only bind to a single structural epitope [208]. On top of various strategies to enhance toxin binding, including augmenting the membrane by physically inserting molecular attractants [209], the specificity of nanosponges can be improved by genetic engineering. A study leveraged this approach to formulate a decoy nanoparticle to protect against COVID-19 [57]. The cell membrane from HEK293T cells transfected to express angiotensin converting enzyme II (ACE2), a receptor for severe acute respiratory syndrome coronavirus 2, was fused with the membrane from THP-1 monocytes. By expressing ACE2, the resulting nanodecoys were able to compete for virus binding with source cells to help prevent infection. At the same time, the monocyte membrane component was able to neutralize inflammatory cytokines such as IL-6 and granulocyte-macrophage colony-stimulating factor to reduce lung injury.
4. Conclusions
As cellular nanoparticles have been increasingly explored for various biomedical applications, novel approaches for further enhancing their functionality have emerged. As we have discussed in this review, genetic engineering is a powerful method for generating cell-derived nanomaterials that can offer additional benefits beyond what can be provided by wild-type cells. Engineered cellular nanoparticles can outperform traditional nanomedicine platforms through properties such as improved targeting to disease sites, enhanced immune activity, and reduced toxicity. The strategies discussed in this review highlight the exceptional flexibility offered by genetic engineering in the design of novel nanotherapeutics and nanovaccines. Moving forward, there are some particular challenges that must be taken into consideration. First, since cellular nanoparticles are derived from living cultures, there is inherent batch-to-batch variability. Accordingly, the expression level of genetically engineered markers must be carefully monitored, and the use of low passage number cell stocks may be helpful in ensuring consistency. Additionally, the expression of exogenous proteins on cellular nanoparticles can provoke immune responses upon in vivo administration. While this is often beneficial for immune modulation applications such as vaccination, carrier-specific immunity can reduce the performance of nanodelivery vehicles over time. This may be mitigated by expressing ligands that naturally are lowly immunogenic or by applying more sophisticated techniques to remove any immunodominant epitopes on the proteins of interest [210]. As more research is conducted on genetically engineered platforms, these challenges will eventually be overcome. This is supported by the fact that there has been a sharp rise in genetically engineered cellular platforms reaching the clinic in recent years [211-213]. Notably in 2017, the United States Food and Drug Administration approved a CAR-T cell therapy that targets B cell lymphoma [78]. Subsequently, CAR-T cells have been increasingly explored for the treatment of other cancers, including solid tumors expressing epidermal growth factor receptor, mesothelin, and epithelial cellular adhesion molecule [214-216]. Following these trends, with their great design flexibility, enhanced functionality, and biocompatibility, engineered cellular nanoparticles present an attractive option for addressing some of the most important challenges facing the biomedical field.
Acknowledgements
This work is supported by the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense under Grant Number HDTRA1-21-1-0010 and the National Institutes of Health under Award Number R21AI159492.
References
- [1].Blanco E, Shen H, Ferrari M, Principles of nanoparticle design for overcoming biological barriers to drug delivery, Nat. Biotechnol 33 (9) (2015) 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Farokhzad OC, Langer R, Impact of nanotechnology on drug delivery, ACS Nano 3 (1) (2009) 16–20. [DOI] [PubMed] [Google Scholar]
- [3].Farokhzad OC, Langer R, Nanomedicine: Developing smarter therapeutic and diagnostic modalities, Adv. Drug Deliv. Rev 58 (14) (2006) 1456–1459. [DOI] [PubMed] [Google Scholar]
- [4].Wang AZ, Langer R, Farokhzad OC, Nanoparticle delivery of cancer drugs, Annu. Rev. Med 63 (2012) 185–198. [DOI] [PubMed] [Google Scholar]
- [5].Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R, Engineering precision nanoparticles for drug delivery, Nat. Rev. Drug Discov 20 (2) (2021) 101–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Diez-Pascual AM, Surface engineering of nanomaterials with polymers, biomolecules, and small ligands for nanomedicine, Materials 15 (9) (2022) 3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mout R, Moyano DF, Rana S, Rotello VM, Surface functionalization of nanoparticles for nanomedicine, Chem. Soc. Rev 41 (7) (2012) 2539–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yoo JW, Irvine DJ, Discher DE, Mitragotri S, Bio-inspired, bioengineered and biomimetic drug delivery carriers, Nat. Rev. Drug Discov 10 (7) (2011) 521–535. [DOI] [PubMed] [Google Scholar]
- [9].Hu CM, Fang RH, Zhang L, Erythrocyte-inspired delivery systems, Adv. Healthc. Mater 1 (5) (2012) 537–547. [DOI] [PubMed] [Google Scholar]
- [10].Fang RH, Hu CMJ, Zhang LF, Nanoparticles disguised as red blood cells to evade the immune system, Expert Opin. Biol. Ther 12 (4) (2012) 385–389. [DOI] [PubMed] [Google Scholar]
- [11].Fang RH, Kroll AV, Gao W, Zhang L, Cell membrane coating nanotechnology, Adv. Mater 30 (23) (2018) 1706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fang RH, Jiang Y, Fang JC, Zhang L, Cell membrane-derived nanomaterials for biomedical applications, Biomaterials 128 (2017) 69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L, Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform, Proc. Natl. Acad. Sci. U. S. A 108 (27) (2011) 10980–10985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Luk BT, Fang RH, Hu CM, Copp JA, Thamphiwatana S, Dehaini D, Gao W, Zhang K, Li S, Zhang L, Safe and immunocompatible nanocarriers cloaked in RBC membranes for drug delivery to treat solid tumors, Theranostics 6 (7) (2016) 1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lievens D, von Hundelshausen P, Platelets in atherosclerosis, Thromb. Haemost 106 (5) (2011) 827–838. [DOI] [PubMed] [Google Scholar]
- [16].Nieswandt B, Pleines I, Bender M, Platelet adhesion and activation mechanisms in arterial thrombosis and ischaemic stroke, J. Thromb. Haemost 9 (Suppl 1) (2011) 92–104. [DOI] [PubMed] [Google Scholar]
- [17].Buergy D, Wenz F, Groden C, Brockmann MA, Tumor-platelet interaction in solid tumors, Int. J. Cancer 130 (12) (2012) 2747–2760. [DOI] [PubMed] [Google Scholar]
- [18].Hamzeh-Cognasse H, Damien P, Chabert A, Pozzetto B, Cognasse F, Garraud O, Platelets and infections - Complex interactions with bacteria, Front. Immunol 6 (2015) 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kappelmayer J, Nagy B, The interaction of selectins and PSGL-1 as a key component in thrombus formation and cancer progression, Biomed. Res. Int 2017 (2017) 6138145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Arman M, Krauel K, Tilley DO, Weber C, Cox D, Greinacher A, Kerrigan SW, Watson SP, Amplification of bacteria-induced platelet activation is triggered by FcγRIIA, integrin αIIbβ3, and platelet factor 4, Blood 123 (20) (2014) 3166–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].O'Seaghdha M, van Schooten CJ, Kerrigan SW, Emsley J, Silverman GJ, Cox D, Lenting PJ, Foster TJ, Staphylococcus aureus protein A binding to von Willebrand factor A1 domain is mediated by conserved IgG binding regions, FEBS J. 273 (21) (2006) 4831–4841. [DOI] [PubMed] [Google Scholar]
- [22].Song YA, Huang ZY, Liu X, Pang ZQ, Chen J, Yang HB, Zhang N, Cao ZL, Liu M, Cao JT, Li CG, Yang XD, Gong H, Qian JY, Ge JB, Platelet membrane-coated nanoparticle-mediated targeting delivery of rapamycin blocks atherosclerotic plaque development and stabilizes plaque in apolipoprotein E-deficient (ApoE−/−) mice, Nanomedicine 15 (1) (2019) 13–24. [DOI] [PubMed] [Google Scholar]
- [23].Xu JP, Wang XQ, Yin HY, Cao X, Hu QY, Lv W, Xu QW, Gu Z, Xin HL, Sequentially site-specific delivery of thrombolytics and neuroprotectant for enhanced treatment of ischemic stroke, ACS Nano 13 (8) (2019) 8577–8588. [DOI] [PubMed] [Google Scholar]
- [24].Zhuang J, Gong H, Zhou J, Zhang Q, Gao W, Fang RH, Zhang L, Targeted gene silencing in vivo by platelet membrane-coated metal-organic framework nanoparticles, Sci. Adv 6 (13) (2020) eaaz6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hu CM, Fang RH, Wang K, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen C, Kroll AV, Carpenter C, Ramesh M, Qu V, Patel S, Zhu J, Shi W, Hofman FM, Chen T, Gao W, Zhang K, Chien S, Zhang L, Nanoparticle biointerfacing by platelet membrane cloaking, Nature 526 (7571) (2015) 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vestweber D, How leukocytes cross the vascular endothelium, Nat. Rev. Immunol 15 (11) (2015) 692–704. [DOI] [PubMed] [Google Scholar]
- [27].Zindel J, Kubes P, DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation, Annu. Rev. Pathol 15 (2020) 493–518. [DOI] [PubMed] [Google Scholar]
- [28].Langer HF, Chavakis T, Leukocyte-endothelial interactions in inflammation, J. Cell. Mol. Med 13 (7) (2009) 1211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fontana F, Albertini S, Correia A, Kemell M, Lindgren R, Makila E, Salonen J, Hirvonen JT, Ferrari F, Santos HA, Bioengineered porous silicon nanoparticles@macrophages cell membrane as composite platforms for rheumatoid arthritis, Adv. Funct. Mater 28 (22) (2018) 1801355. [Google Scholar]
- [30].Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, Brown BS, Khaled SZ, Yazdi IK, Vittoria Enzo M, Isenhart L, Ferrari M, Tasciotti E, Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions, Nat. Nanotechnol 8 (2013) 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Krishnamurthy S, Gnanasammandhan MK, Xie C, Huang K, Cui MY, Chan JM, Monocyte cell membrane-derived nanoghosts for targeted cancer therapy, Nanoscale 8 (13) (2016) 6981–6985. [DOI] [PubMed] [Google Scholar]
- [32].Wang C, Wang YL, Zhang LL, Miron RJ, Liang JF, Shi MS, Mo WT, Zheng SH, Zhao YB, Zhang YF, Pretreated macrophage-membrane-coated gold nanocages for precise drug delivery for treatment of bacterial infections, Adv. Mater 30 (46) (2018) 1804023. [DOI] [PubMed] [Google Scholar]
- [33].Thamphiwatana S, Angsantikul P, Escajadillo T, Zhang Q, Olson J, Luk BT, Zhang S, Fang RH, Gao W, Nizet V, Zhang L, Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management, Proc. Natl. Acad. Sci. U. S. A 114 (43) (2017) 11488–11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jin JF, Bhujwalla ZM, Biomimetic nanoparticles camouflaged in cancer cell membranes and their applications in cancer theranostics, Front. Oncol 9 (2020) 1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fang RH, Hu CM, Luk BT, Gao W, Copp JA, Tai Y, O'Connor DE, Zhang L, Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery, Nano Lett. 14 (4) (2014) 2181–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sun HP, Su JH, Meng QS, Yin Q, Chen LL, Gu WW, Zhang PC, Zhang ZW, Yu HJ, Wang SL, Li YP, Cancer-cell-biomimetic nanoparticles for targeted therapy of homotypic tumors, Adv. Mater 28 (43) (2016) 9581–9588. [DOI] [PubMed] [Google Scholar]
- [37].Narain A, Asawa S, Chhabria V, Patil-Sen Y, Cell membrane coated nanoparticles: Next-generation therapeutics, Nanomedicine 12 (21) (2017) 2677–2692. [DOI] [PubMed] [Google Scholar]
- [38].Jakaria MG, Sorkhdini P, Yang D, Zhou Y, Meenach SA, Lung cell membrane-coated nanoparticles capable of enhanced internalization and translocation in pulmonary epithelial cells, Int. J. Pharm 613 (2022) 121418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang Y, Chen Y, Lo C, Zhuang J, Angsantikul P, Zhang Q, Wei X, Zhou Z, Obonyo M, Fang RH, Gao W, Zhang L, Inhibition of pathogen adhesion by bacterial outer membrane-coated nanoparticles, Angew. Chem. Int. Ed. Engl 58 (33) (2019) 11404–11408. [DOI] [PubMed] [Google Scholar]
- [40].Krishnan N, Kubiatowicz LJ, Holay M, Zhou J, Fang RH, Zhang L, Bacterial membrane vesicles for vaccine applications, Adv. Drug Deliv. Rev 185 (2022) 114294. [DOI] [PubMed] [Google Scholar]
- [41].Angsantikul P, Thamphiwatana S, Zhang Q, Spiekermann K, Zhuang J, Fang RH, Gao W, Obonyo M, Zhang L, Coating nanoparticles with gastric epithelial cell membrane for targeted antibiotic delivery against Helicobacter pylori infection, Adv. Ther 1 (2) (2018) 1800016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wei X, Ran D, Campeau A, Xiao C, Zhou J, Dehaini D, Jiang Y, Kroll AV, Zhang Q, Gao W, Gonzalez DJ, Fang RH, Zhang L, Multiantigenic nanotoxoids for antivirulence vaccination against antibiotic-resistant Gram-negative bacteria, Nano Lett. 19 (7) (2019) 4760–4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ai X, Wang S, Duan Y, Zhang Q, Chen M, Gao W, Zhang L, Emerging approaches to functionalizing cell membrane-coated nanoparticles, Biochem. 60 (13) (2021) 941–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fang RH, Hu CM, Chen K, Luk BT, Carpenter C, Gao W, Li S, Zhang DE, Lu W, Zhang L, Lipid-insertion enables targeting functionalization of erythrocyte membrane-cloaked nanoparticles, Nanoscale 5 (19) (2013) 8884–8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ak G, Yilmaz H, Gunes A, Sanlier SH, In vitro and in vivo evaluation of folate receptor-targeted a novel magnetic drug delivery system for ovarian cancer therapy, Artif. Cells Nanomed. Biotechnol 46 (sup1) (2018) S926–S937. [DOI] [PubMed] [Google Scholar]
- [46].Chai Z, Ran D, Lu L, Zhan C, Ruan H, Hu X, Xie C, Jiang K, Li J, Zhou J, Wang J, Zhang Y, Fang RH, Zhang L, Lu W, Ligand-modified cell membrane enables the targeted delivery of drug nanocrystals to glioma, ACS Nano 13 (5) (2019) 5591–5601. [DOI] [PubMed] [Google Scholar]
- [47].Zhang MH, Cheng SS, Jin Y, Zhang N, Wang Y, Membrane engineering of cell membrane biomimetic nanoparticles for nanoscale therapeutics, Clin. Transl. Med 11 (2) (2021) e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhao YA, Li AX, Jiang LD, Gu YW, Liu JY, Hybrid membrane-coated biomimetic nanoparticles (HM@BNPs): A multifunctional nanomaterial for biomedical applications, Biomacromolecules 22 (8) (2021) 3149–3167. [DOI] [PubMed] [Google Scholar]
- [49].Dehaini D, Wei X, Fang RH, Masson S, Angsantikul P, Luk BT, Zhang Y, Ying M, Jiang Y, Kroll AV, Gao W, Zhang L, Erythrocyte-platelet hybrid membrane coating for enhanced nanoparticle functionalization, Adv. Mater 29 (16) (2017) 1606209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Liu WL, Zou MZ, Liu T, Zeng JY, Li X, Yu WY, Li CX, Ye JJ, Song W, Feng J, Zhang XZ, Expandable immunotherapeutic nanoplatforms engineered from cytomembranes of hybrid cells derived from cancer and dendritic cells, Adv. Mater 31 (18) (2019) 1900499. [DOI] [PubMed] [Google Scholar]
- [51].Liu WL, Zou MZ, Liu T, Zeng JY, Li X, Yu WY, Li CX, Ye JJ, Song W, Feng J, Zhang XZ, Cytomembrane nanovaccines show therapeutic effects by mimicking tumor cells and antigen presenting cells, Nat. Commun 10 (2019) 3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Agatemor C, Buettner MJ, Ariss R, Muthiah K, Saeui CT, Yarema KJ, Exploiting metabolic glycoengineering to advance healthcare, Nat. Rev. Chem 3 (10) (2019) 605–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ai X, Wang D, Honko A, Duan Y, Gavrish I, Fang RH, Griffiths A, Gao W, Zhang L, Surface glycan modification of cellular nanosponges to promote SARS-CoV-2 inhibition, J. Am. Chem. Soc 143 (42) (2021) 17615–17621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Nowakowski A, Andrzejewska A, Janowski M, Walczak P, Lukomska B, Genetic engineering of stem cells for enhanced therapy, Acta Neurobiol. Exp 73 (1) (2013) 1–18. [DOI] [PubMed] [Google Scholar]
- [55].Fang RH, Gao W, Zhang L, Targeting drugs to tumours using cell membrane-coated nanoparticles, Nat. Rev. Clin. Oncol 20 (1) (2023) 33–48. [DOI] [PubMed] [Google Scholar]
- [56].Eisenberg D, Three-dimensional structure of membrane and surface proteins, Annu. Rev. Biochem 53 (1984) 595–623. [DOI] [PubMed] [Google Scholar]
- [57].Rao L, Xia S, Xu W, Tian R, Yu G, Gu C, Pan P, Meng QF, Cai X, Qu D, Lu L, Xie Y, Jiang S, Chen X, Decoy nanoparticles protect against COVID-19 by concurrently adsorbing viruses and inflammatory cytokines, Proc. Natl. Acad. Sci. U. S. A 117 (44) (2020) 27141–27147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jiang Y, Krishnan N, Zhou J, Chekuri S, Wei X, Kroll AV, Yu CL, Duan Y, Gao W, Fang RH, Zhang L, Engineered cell-membrane-coated nanoparticles directly present tumor antigens to promote anticancer immunity, Adv. Mater 32 (30) (2020) 2001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Park JH, Jiang Y, Zhou J, Gong H, Mohapatra A, Heo J, Gao W, Fang RH, Zhang L, Genetically engineered cell membrane-coated nanoparticles for targeted delivery of dexamethasone to inflamed lungs, Sci. Adv 7 (25) (2021) eabf7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhang X, Wang C, Wang J, Hu Q, Langworthy B, Ye Y, Sun W, Lin J, Wang T, Fine J, Cheng H, Dotti G, Huang P, Gu Z, PD-1 blockade cellular vesicles for cancer immunotherapy, Adv. Mater 30 (22) (2018) 1707112. [DOI] [PubMed] [Google Scholar]
- [61].Lv Q, Cheng L, Lu Y, Zhang X, Wang Y, Deng J, Zhou J, Liu B, Liu J, Thermosensitive exosome-liposome hybrid nanoparticle-mediated chemoimmunotherapy for improved treatment of metastatic peritoneal cancer, Adv. Sci 7 (18) (2020) 2000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Rao L, Zhao SK, Wen C, Tian R, Lin L, Cai B, Sun Y, Kang F, Yang Z, He L, Mu J, Meng QF, Yao G, Xie N, Chen X, Activating macrophage-mediated cancer immunotherapy by genetically edited nanoparticles, Adv. Mater 32 (47) (2020) 2004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ren E, Liu C, Lv P, Wang JQ, Liu G, Genetically engineered cellular membrane vesicles as tailorable shells for therapeutics, Adv. Sci 8 (21) (2021) 2100460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Voorhees RM, Hegde RS, Toward a structural understanding of co-translational protein translocation, Curr. Opin. Cell Biol 41 (2016) 91–99. [DOI] [PubMed] [Google Scholar]
- [65].Spear TT, Nagato K, Nishimura MI, Strategies to genetically engineer T cells for cancer immunotherapy, Cancer Immunol. Immunother 65 (6) (2016) 631–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cheng K, Zhao R, Li Y, Qi Y, Wang Y, Zhang Y, Qin H, Qin Y, Chen L, Li C, Liang J, Li Y, Xu J, Han X, Anderson GJ, Shi J, Ren L, Zhao X, Nie G, Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via Plug-and-Display technology, Nat. Commun 12 (2021) 2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ma WJ, Zhu DM, Li JH, Chen X, Xie W, Jiang X, Wu L, Wang GG, Xiao YS, Liu ZS, Wang FB, Li A, Shao D, Dong WF, Liu W, Yuan YF, Coating biomimetic nanoparticles with chimeric antigen receptor T cell-membrane provides high specificity for hepatocellular carcinoma photothermal therapy treatment, Theranostics 10 (3) (2020) 1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD, Effective treatment of inflammatory disease models with exosomes derived from dendritic cells genetically modified to express IL-4, J. Immunol 179 (4) (2007) 2242–2249. [DOI] [PubMed] [Google Scholar]
- [69].Nayerossadat N, Maedeh T, Ali PA, Viral and nonviral delivery systems for gene delivery, Adv. Biomed. Res 1 (2012) 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Bouard D, Alazard-Dany D, Cosset FL, Viral vectors: From virology to transgene expression, Br. J. Pharmacol 157 (2) (2009) 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Bulcha JT, Wang Y, Ma H, Tai PWL, Gao G, Viral vector platforms within the gene therapy landscape, Signal Transduct. Target Ther 6 (2021) 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Balvay L, Lopez Lastra M, Sargueil B, Darlix JL, Ohlmann T, Translational control of retroviruses, Nat. Rev. Microbiol 5 (2) (2007) 128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Vargas JE, Chicaybam L, Stein RT, Tanuri A, Delgado-Canedo A, Bonamino MH, Retroviral vectors and transposons for stable gene therapy: Advances, current challenges and perspectives, J. Transl. Med 14 (2016) 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Nowrouzi A, Glimm H, von Kalle C, Schmidt M, Retroviral vectors: Post entry events and genomic alterations, Viruses 3 (5) (2011) 429–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Palu G, Parolin C, Takeuchi Y, Pizzato M, Progress with retroviral gene vectors, Rev. Med. Virol 10 (3) (2000) 185–202. [DOI] [PubMed] [Google Scholar]
- [76].Poletti V, Mavilio F, Designing lentiviral vectors for gene therapy of genetic diseases, Viruses 13 (8) (2021) 1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kalidasan V, Ng WH, Ishola OA, Ravichantar N, Tan JJ, Das KT, A guide in lentiviral vector production for hard-to-transfect cells, using cardiac-derived c-kit expressing cells as a model system, Sci. Rep 11 (2021) 19265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Stiff PJ, Friedberg JW, Flinn IW, Goy A, Hill BT, Smith MR, Deol A, Farooq U, McSweeney P, Munoz J, Avivi I, Castro JE, Westin JR, Chavez JC, Ghobadi A, Komanduri KV, Levy R, Jacobsen ED, Witzig TE, Reagan P, Bot A, Rossi J, Navale L, Jiang Y, Aycock J, Elias M, Chang D, Wiezorek J, Go WY, Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma, N. Engl. J. Med 377 (26) (2017) 2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lee CS, Bishop ES, Zhang R, Yu X, Farina EM, Yan S, Zhao C, Zheng Z, Shu Y, Wu X, Lei J, Li Y, Zhang W, Yang C, Wu K, Wu Y, Ho S, Athiviraham A, Lee MJ, Wolf JM, Reid RR, He TC, Adenovirus-mediated gene delivery: Potential applications for gene and cell-based therapies in the new era of personalized medicine, Genes Dis. 4 (2) (2017) 43–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Naso MF, Tomkowicz B, Perry WL 3rd, Strohl WR, Adeno-associated virus (AAV) as a vector for gene therapy, BioDrugs 31 (4) (2017) 317–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Li C, Lieber A, Adenovirus vectors in hematopoietic stem cell genome editing, FEBS Lett. 593 (24) (2019) 3623–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Grieger JC, Samulski RJ, Packaging capacity of adeno-associated virus serotypes: Impact of larger genomes on infectivity and postentry steps, J. Virol 79 (15) (2005) 9933–9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Au HKE, Isalan M, Mielcarek M, Gene therapy advances: A meta-analysis of AAV usage in clinical settings, Front. Med 8 (2021) 809118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Lachmann R, Herpes simplex virus-based vectors, Int. J. Exp. Pathol 85 (4) (2004) 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].de Silva S, Bowers WJ, Targeting the central nervous system with herpes simplex virus / Sleeping Beauty hybrid amplicon vectors, Curr. Gene Ther 11 (5) (2011) 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zemskova MY, Fodor I, Transient expression of deletion mutants of the herpes simplex virus thymidine kinase-encoding gene in mouse fibroblast cells, Gene 106 (2) (1991) 249–253. [DOI] [PubMed] [Google Scholar]
- [87].Chong ZX, Yeap SK, Ho WY, Transfection types, methods and strategies: A technical review, PLoS One 9 (2021) e11165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ramamoorth M, Narvekar A, Non viral vectors in gene therapy- An overview, J. Clin. Diagnostic Res 9 (1) (2015) GE01–GE06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Masotti A, Mossa G, Cametti C, Ortaggi G, Bianco A, Grosso ND, Malizia D, Esposito C, Comparison of different commercially available cationic liposome-DNA lipoplexes: Parameters influencing toxicity and transfection efficiency, Col. Surf. B. Biointerfaces 68 (2) (2009) 136–144. [DOI] [PubMed] [Google Scholar]
- [90].Fus-Kujawa A, Prus P, Bajdak-Rusinek K, Teper P, Gawron K, Kowalczuk A, Sieron AL, An overview of methods and tools for transfection of eukaryotic cells in vitro, Front. Bioeng. Biotechnol 9 (2021) 701031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Calcium phosphate-mediated transfection of eukaryotic cells, Nat. Methods 2 (4) (2005) 319–320. [Google Scholar]
- [92].Goetze B, Grunewald B, Baldassa S, Kiebler M, Chemically controlled formation of a DNA/calcium phosphate coprecipitate: Application for transfection of mature hippocampal neurons, J. Neurobiol 60 (4) (2004) 517–255. [DOI] [PubMed] [Google Scholar]
- [93].Kumar P, Nagarajan A, Uchil PD, Transfection of mammalian cells with calcium phosphate-DNA coprecipitates, Cold Spring Harb. Protoc 2019 (10) (2019) 10. [DOI] [PubMed] [Google Scholar]
- [94].Rahimi P, Mobarakeh VI, Kamalzare S, SajadianFard F, Vahabpour R, Zabihollahi R, Comparison of transfection efficiency of polymer-based and lipid-based transfection reagents, Bratisl. Lek. Listy 119 (11) (2018) 701–705. [DOI] [PubMed] [Google Scholar]
- [95].Zuhorn IS, Engberts JB, Hoekstra D, Gene delivery by cationic lipid vectors: Overcoming cellular barriers, Eur. Biophys. J 36 (4-5) (2007) 349–362. [DOI] [PubMed] [Google Scholar]
- [96].Cui S, Wang B, Zhao Y, Chen H, Ding H, Zhi D, Zhang S, Transmembrane routes of cationic liposome-mediated gene delivery using human throat epidermis cancer cells, Biotechnol. Lett 36 (2014) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kiefer K, Clement J, Garidel P, Peschka-Suss R, Transfection efficiency and cytotoxicity of nonviral gene transfer reagents in human smooth muscle and endothelial cells, Pharm. Res 21 (6) (2004) 1009–1017. [DOI] [PubMed] [Google Scholar]
- [98].Park JH, Mohapatra A, Zhou J, Holay M, Krishnan N, Gao W, Fang RH, Zhang L, Virus-mimicking cell membrane-coated nanoparticles for cytosolic delivery of mRNA, Angew. Chem. Int. Ed. Engl 61 (2) (2022) e202113671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Stewart MP, Langer R, Jensen KF, Intracellular delivery by membrane disruption: Mechanisms, strategies, and concepts, Chem. Rev 118 (16) (2018) 7409–7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Chen I, Dubnau D, DNA uptake during bacterial transformation, Nat. Rev. Microbiol 2 (3) (2004) 241–249. [DOI] [PubMed] [Google Scholar]
- [101].Chow Y, Chen S, Wang R, Liu C, Kong C, Li R, Cheng S, Sun D, Single cell transfection through precise microinjection with quantitatively controlled injection volumes, Sci. Rep 6 (2016) 24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Shen YM, Hirschhorn RR, Mercer WE, Surmacz E, Tsutsui Y, Soprano KJ, Baserga R, Gene transfer: DNA microinjection compared with DNA transfection with a very high efficiency, Mol. Cell. Biol 2 (9) (1982) 1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Tsong TY, Electroporation of cell membranes, Biophys. J 60 (2) (1991) 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Yarmush ML, Golberg A, Sersa G, Kotnik T, Miklavcic D, Electroporation-based technologies for medicine: Principles, applications, and challenges, Annu. Rev. Biomed. Eng 16 (2014) 295–320. [DOI] [PubMed] [Google Scholar]
- [105].Shi J, Ma Y, Zhu J, Chen Y, Sun Y, Yao Y, Yang Z, Xie J, A review on electroporation-based intracellular delivery, Molecules 23 (11) (2018) 3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zhang J, Hu Y, Yang J, Li W, Zhang M, Wang Q, Zhang L, Wei G, Tian Y, Zhao K, Chen A, Tan B, Cui J, Li D, Li Y, Qi Y, Wang D, Wu Y, Li D, Du B, Liu M, Huang H, Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL, Nature 609 (7926) (2022) 369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Li A, Zhao Y, Li Y, Jiang L, Gu Y, Liu J, Cell-derived biomimetic nanocarriers for targeted cancer therapy: Cell membranes and extracellular vesicles, Drug Deliv. 28 (1) (2021) 1237–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Qin M, Du G, Sun X, Biomimetic cell-derived nanocarriers for modulating immune responses, Biomater. Sci 8 (2) (2020) 530–543. [DOI] [PubMed] [Google Scholar]
- [109].Ma Y, Dong S, Li X, Kim BYS, Yang Z, Jiang W, Extracellular vesicles: An emerging nanoplatform for cancer therapy, Front. Oncol 10 (2020) 606906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].de la Torre P, Perez-Lorenzo MJ, Alcazar-Garrido A, Flores AI, Cell-based nanoparticles delivery systems for targeted cancer therapy: Lessons from anti-angiogenesis treatments, Molecules 25 (3) (2020) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Chugh V, Vijaya Krishna K, Pandit A, Cell membrane-coated mimics: A methodological approach for fabrication, characterization for therapeutic applications, and challenges for clinical translation, ACS Nano 15 (11) (2021) 17080–17123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Shehadul Islam M, Aryasomayajula A, Selvaganapathy PR, A review on macroscale and microscale cell lysis methods, Micromachines 8 (3) (2017) 83. [Google Scholar]
- [113].Goldberg S, Mechanical/physical methods of cell disruption and tissue homogenization, Methods Mol. Biol 424 (2008) 3–22. [DOI] [PubMed] [Google Scholar]
- [114].Simpson RJ, Disruption of cultured cells by nitrogen cavitation, Cold Spring Harb. Protoc 2010 (11) (2010) 1219–1222. [DOI] [PubMed] [Google Scholar]
- [115].Kroll AV, Fang RH, Zhang L, Biointerfacing and applications of cell membrane-coated nanoparticles, Bioconjug. Chem 28 (1) (2017) 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Aryal S, Hu CM, Fang RH, Dehaini D, Carpenter C, Zhang DE, Zhang L, Erythrocyte membrane-cloaked polymeric nanoparticles for controlled drug loading and release, Nanomedicine 8 (8) (2013) 1271–1280. [DOI] [PubMed] [Google Scholar]
- [117].Zhang F, Mundaca-Uribe R, Askarinam N, Li Z, Gao W, Zhang L, Wang J, Biomembrane-functionalized micromotors: Biocompatible active devices for diverse biomedical applications, Adv. Mater 34 (5) (2022) 2107177. [DOI] [PubMed] [Google Scholar]
- [118].Zhang F, Zhuang J, Esteban Fernandez de Avila B, Tang S, Zhang Q, Fang RH, Zhang L, Wang J, A nanomotor-based active delivery system for intracellular oxygen transport, ACS Nano 13 (10) (2019) 11996–12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Gao W, Hu CM, Fang RH, Luk BT, Su J, Zhang L, Surface functionalization of gold nanoparticles with red blood cell membranes, Adv. Mater 25 (26) (2013) 3549–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Xuan M, Shao J, Zhao J, Li Q, Dai L, Li J, Magnetic mesoporous silica nanoparticles cloaked by red blood cell membranes: Applications in cancer therapy, Angew. Chem. Int. Ed. Engl 57 (21) (2018) 6049–6053. [DOI] [PubMed] [Google Scholar]
- [121].Wang S, Kai M, Duan Y, Zhou Z, Fang RH, Gao W, Zhang L, Membrane cholesterol depletion enhances enzymatic activity of cell-membrane-coated metal-organic-framework nanoparticles, Angew. Chem. Int. Ed. Engl 61 (24) (2022) e202203115. [DOI] [PubMed] [Google Scholar]
- [122].Zhuang J, Duan Y, Zhang Q, Gao W, Li S, Fang RH, Zhang L, Multimodal enzyme delivery and therapy enabled by cell membrane-coated metal-organic-framework nanoparticles, Nano Lett. 20 (5) (2020) 4051–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Liu L, Bai X, Martikainen MV, Karlund A, Roponen M, Xu W, Hu G, Tasciotti E, Lehto VP, Cell membrane coating integrity affects the internalization mechanism of biomimetic nanoparticles, Nat. Commun 12 (2021) 5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Copp JA, Fang RH, Luk BT, Hu CM, Gao W, Zhang K, Zhang L, Clearance of pathological antibodies using biomimetic nanoparticles, Proc. Natl. Acad. Sci. U. S. A 111 (37) (2014) 13481–13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Hoshyar N, Gray S, Han HB, Bao G, The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction, Nanomedicine 11 (6) (2016) 673–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Petschauer JS, Madden AJ, Kirschbrown WP, Song G, Zamboni WC, The effects of nanoparticle drug loading on the pharmacokinetics of anticancer agents, Nanomedicine 10 (3) (2015) 447–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Onoue S, Yamada S, Chan HK, Nanodrugs: Pharmacokinetics and safety, Int. J. Nanomedicine 9 (2014) 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Tang YX, Wang XY, Li J, Nie Y, Liao GJ, Yu Y, Li C, Overcoming the reticuloendothelial system barrier to drug delivery with a "don't-eat-us" strategy, ACS Nano 13 (11) (2019) 13015–13026. [DOI] [PubMed] [Google Scholar]
- [129].Mills JA, Liu FF, Jarrett TR, Fletcher NL, Thurecht KJ, Nanoparticle based medicines: Approaches for evading and manipulating the mononuclear phagocyte system and potential for clinical translation, Biomater. Sci 10 (12) (2022) 3029–3053. [DOI] [PubMed] [Google Scholar]
- [130].Perry JL, Reuter KG, Luft JC, Pecot CV, Zamboni W, DeSimone JM, Mediating passive tumor accumulation through particle size, tumor type, and location, Nano Lett. 17 (5) (2017) 2879–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Zhao ZM, Ukidve A, Krishnan V, Mitragotri S, Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers, Adv. Drug Deliv. Rev 143 (2019) 3–21. [DOI] [PubMed] [Google Scholar]
- [132].Kalyane D, Raval N, Maheshwari R, Tambe V, Kalia K, Tekade RK, Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer, Mater. Sci. Eng. C 98 (2019) 1252–1276. [DOI] [PubMed] [Google Scholar]
- [133].Lazarovits J, Chen YY, Sykes EA, Chan WCW, Nanoparticle-blood interactions: The implications on solid tumour targeting, Chem. Comm 51 (14) (2015) 2756–2767. [DOI] [PubMed] [Google Scholar]
- [134].Dehaini D, Fang RH, Zhang L, Biomimetic strategies for targeted nanoparticle delivery, Bioeng. Transl. Med 1 (1) (2016) 30–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Manzari MT, Shamay Y, Kiguchi H, Rosen N, Scaltriti M, Heller DA, Targeted drug delivery strategies for precision medicines, Nat. Rev. Mater 6 (4) (2021) 351–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Jin K, Luo ZM, Zhang B, Pang ZQ, Biomimetic nanoparticles for inflammation targeting, Acta Pharm. Sin. B 8 (1) (2018) 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Bazak R, Houri M, El Achy S, Kamel S, Refaat T, Cancer active targeting by nanoparticles: A comprehensive review of literature, J. Cancer Res. Clin. Oncol 141 (5) (2015) 769–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Yoo J, Park C, Yi G, Lee D, Koo H, Active targeting strategies using biological ligands for nanoparticle drug delivery systems, Cancers 11 (5) (2019) 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Richards DA, Maruani A, Chudasama V, Antibody fragments as nanoparticle targeting ligands: A step in the right direction, Chem. Sci 8 (1) (2017) 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Chinen AB, Guan CM, Ferrer JR, Barnaby SN, Merkel TJ, Mirkin CA, Nanoparticle probes for the detection of cancer biomarkers, cells, and tissues by fluorescence, Chem. Rev 115 (19) (2015) 10530–10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Jeong WJ, Bu J, Kubiatowicz LJ, Chen SS, Kim Y, Hong S, Peptide-nanoparticle conjugates: A next generation of diagnostic and therapeutic platforms?, Nano Converg. 5 (2018) 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Guan B, Zhang X, Aptamers as versatile ligands for biomedical and pharmaceutical applications, Int. J. Nanomedicine 15 (2020) 1059–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Zhang XX, Eden HS, Chen X, Peptides in cancer nanomedicine: Drug carriers, targeting ligands and protease substrates, J. Control. Release 159 (1) (2012) 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Luk BT, Zhang L, Cell membrane-camouflaged nanoparticles for drug delivery, J. Control. Release 220 (2015) 600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Prieto J, Melero I, Sangro B, Immunological landscape and immunotherapy of hepatocellular carcinoma, Nat. Rev. Gastroenterol. Hepatol 12 (12) (2015) 681–700. [DOI] [PubMed] [Google Scholar]
- [146].Sterner RC, Sterner RM, CAR-T cell therapy: Current limitations and potential strategies, Blood Cancer J. 11 (4) (2021) 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Sands BE, Biomarkers of inflammation in inflammatory bowel disease, Gastroenterology 149 (5) (2015) 1275–1285. [DOI] [PubMed] [Google Scholar]
- [148].Bose RJC, Kim BJ, Arai Y, Han IB, Moon JJ, Paulmurugan R, Park H, Lee SH, Bioengineered stem cell membrane functionalized nanocarriers for therapeutic targeting of severe hindlimb ischemia, Biomaterials 185 (2018) 360–370. [DOI] [PubMed] [Google Scholar]
- [149].Ma JN, Zhang SQ, Liu J, Liu FY, Du F, Li M, Chen AT, Bao YM, Suh HW, Avery J, Deng G, Zhou Y, Wu P, Sheth K, Wang HJ, Zhou JB, Targeted drug delivery to stroke via chemotactic recruitment of nanoparticles coated with membrane of engineered neural stem cells, Small 15 (35) (2019) 1902011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ, CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion, J. Mol. Histol 35 (3) (2004) 233–245. [DOI] [PubMed] [Google Scholar]
- [151].Barua S, Mitragotri S, Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects, Nano Today 9 (2) (2014) 223–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Hou XC, Zaks T, Langer R, Dong YZ, Lipid nanoparticles for mRNA delivery, Nat. Rev. Mater 6 (12) (2021) 1078–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Rennick JJ, Johnston APR, Parton RG, Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics, Nat. Nanotechnol 16 (3) (2021) 266–276. [DOI] [PubMed] [Google Scholar]
- [154].Hamilton BS, Whittaker GR, Daniel S, Influenza virus-mediated membrane fusion: Determinants of hemagglutinin fusogenic activity and experimental approaches for assessing virus fusion, Viruses 4 (7) (2012) 1144–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Fan ZJ, Jiang C, Wang YC, Wang KY, Marsh J, Zhang D, Chen X, Nie LM, Engineered extracellular vesicles as intelligent nanosystems for next-generation nanomedicine, Nanoscale Horiz. 7 (7) (2022) 682–714. [DOI] [PubMed] [Google Scholar]
- [156].Li YJ, Wu JY, Liu J, Xu W, Qiu X, Huang S, Hu XB, Xiang DX, Artificial exosomes for translational nanomedicine, J. Nanobiotechnology 19 (2021) 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Wu PP, Zhang B, Ocansey DKW, Wenrong H Qian, Extracellular vesicles: A bright star of nanomedicine, Biomaterials 269 (2021) 120467. [DOI] [PubMed] [Google Scholar]
- [158].Yang BW, Chen Y, Shi JL, Exosome biochemistry and advanced nanotechnology for next-generation theranostic platforms, Adv. Mater 31 (2) (2019) 1802896. [DOI] [PubMed] [Google Scholar]
- [159].Wang F, Cerione RA, Antonyak MA, Isolation and characterization of extracellular vesicles produced by cell lines, STAR Protoc. 2 (1) (2021) 100295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Susa F, Limongi T, Dumontel B, Vighetto V, Cauda V, Engineered extracellular vesicles as a reliable tool in cancer nanomedicine, Cancers 11 (12) (2019) 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Tang TT, Wang B, Lv LL, Liu BC, Extracellular vesicle-based nanotherapeutics: Emerging frontiers in anti-inflammatory therapy, Theranostics 10 (18) (2020) 8111–8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Yang YS, Hong YS, Nam GH, Chung JH, Koh E, Kim IS, Virus-mimetic fusogenic exosomes for direct delivery of integral membrane proteins to target cell membranes, Adv. Mater 29 (13) (2017) 1605604. [DOI] [PubMed] [Google Scholar]
- [163].Medzhitov R, Origin and physiological roles of inflammation, Nature 454 (7203) (2008) 428–435. [DOI] [PubMed] [Google Scholar]
- [164].Brusini R, Varna M, Couvreur P, Advanced nanomedicines for the treatment of inflammatory diseases, Adv. Drug Deliv. Rev 157 (2020) 161–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Molinaro R, Pasto A, Taraballi F, Giordano F, Azzi JA, Tasciotti E, Corbo C, Biomimetic nanoparticles potentiate the anti-inflammatory properties of dexamethasone and reduce the cytokine storm syndrome: An additional weapon against COVID-19?, Nanomaterials 10 (11) (2020) 2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Wang H, Liu Y, He R, Xu D, Zang J, Weeranoppanant N, Dong H, Li Y, Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery, Biomater. Sci 8 (2) (2020) 552–568. [DOI] [PubMed] [Google Scholar]
- [167].Mitchell RE, Hassan M, Burton BR, Britton G, Hill EV, Verhagen J, Wraith DC, IL-4 enhances IL-10 production in Th1 cells: Implications for Th1 and Th2 regulation, Sci. Rep 7 (2017) 11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Morita Y, Yang J, Gupta R, Shimizu K, Shelden EA, Endres J, Mule JJ, McDonagh KT, Fox DA, Dendritic cells genetically engineered to express IL-4 inhibit murine collagen-induced arthritis, J. Clin. Investig 107 (10) (2001) 1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].de Visser KE, Eichten A, Coussens LM, Paradoxical roles of the immune system during cancer development, Nat. Rev. Cancer 6 (2006) 24–37. [DOI] [PubMed] [Google Scholar]
- [170].Loeb KR, Loeb LA, Significance of multiple mutations in cancer, Carcinogenesis 21 (3) (2000) 379–385. [DOI] [PubMed] [Google Scholar]
- [171].Mahvi DA, Liu R, Grinstaff MW, Colson YL, Raut CP, Local cancer recurrence: The realities, challenges, and opportunities for new therapies, CA Cancer J. Clin 68 (6) (2018) 488–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Kalos M, June CH, Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology, Immunity 39 (1) (2013) 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Saxena M, van der Burg SH, Melief CJM, Bhardwaj N, Therapeutic cancer vaccines, Nat. Rev. Cancer 21 (6) (2021) 360–378. [DOI] [PubMed] [Google Scholar]
- [174].Murphy AG, Zheng L, Small molecule drugs with immunomodulatory effects in cancer, Hum. Vaccin. Immunother 11 (10) (2015) 2463–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Weiner LM, Surana R, Wang S, Monoclonal antibodies: Versatile platforms for cancer immunotherapy, Nat. Rev. Immunol 10 (5) (2010) 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Debele TA, Yeh CF, Su WP, Cancer immunotherapy and application of nanoparticles in cancers immunotherapy as the delivery of immunotherapeutic agents and as the immunomodulators, Cancers 12 (12) (2020) 3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Zheng Y, Tang L, Mabardi L, Kumari S, Irvine DJ, Enhancing adoptive cell therapy of cancer through targeted delivery of small-molecule immunomodulators to internalizing or noninternalizing receptors, ACS Nano 11 (3) (2017) 3089–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Yoo YJ, Lee CH, Park SH, Lim YT, Nanoparticle-based delivery strategies of multifaceted immunomodulatory RNA for cancer immunotherapy, J. Control. Release 343 (2022) 564–583. [DOI] [PubMed] [Google Scholar]
- [179].Yang R, Xu J, Xu L, Sun X, Chen Q, Zhao Y, Peng R, Liu Z, Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination, ACS Nano 12 (6) (2018) 5121–5129. [DOI] [PubMed] [Google Scholar]
- [180].Kroll AV, Fang RH, Jiang Y, Zhou J, Wei X, Yu CL, Gao J, Luk BT, Dehaini D, Gao W, Zhang L, Nanoparticulate delivery of cancer cell membrane elicits multiantigenic antitumor immunity, Adv. Mater 29 (47) (2017) 1703969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Johnson DT, Zhou J, Kroll AV, Fang RH, Yan M, Xiao C, Chen X, Kline J, Zhang L, Zhang DE, Acute myeloid leukemia cell membrane-coated nanoparticles for cancer vaccination immunotherapy, Leukemia 36 (4) (2022) 994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Chen Q, Zhang L, Li L, Tan M, Liu W, Liu S, Xie Z, Zhang W, Wang Z, Cao Y, Shang T, Ran H, Cancer cell membrane-coated nanoparticles for bimodal imaging-guided photothermal therapy and docetaxel-enhanced immunotherapy against cancer, J. Nanobiotechnology 19 (2021) 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Zhang X, Wang J, Chen Z, Hu Q, Wang C, Yan J, Dotti G, Huang P, Gu Z, Engineering PD-1-presenting platelets for cancer immunotherapy, Nano Lett. 18 (9) (2018) 5716–5725. [DOI] [PubMed] [Google Scholar]
- [184].Plotkin SA, Vaccines: Past, present and future, Nat. Med 11 (Suppl 4) (2005) S5–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Plotkin SA, Updates on immunologic correlates of vaccine-induced protection, Vaccine 38 (9) (2020) 2250–2257. [DOI] [PubMed] [Google Scholar]
- [186].Pati R, Shevtsov M, Sonawane A, Nanoparticle vaccines against infectious diseases, Front. Immunol 9 (2018) 2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Angsantikul P, Thamphiwatana S, Gao W, Zhang L, Cell membrane-coated nanoparticles as an emerging antibacterial vaccine platform, Vaccines 3 (4) (2015) 814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Kaparakis-Liaskos M, Ferrero RL, Immune modulation by bacterial outer membrane vesicles, Nat. Rev. Immunol 15 (6) (2015) 375–387. [DOI] [PubMed] [Google Scholar]
- [189].Huang W, Yao Y, Long Q, Yang X, Sun W, Liu C, Jin X, Li Y, Chu X, Chen B, Ma Y, Immunization against multidrug-resistant Acinetobacter baumannii effectively protects mice in both pneumonia and sepsis models, PLoS One 9 (6) (2014) e100727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Vernikos G, Medini D, Bexsero® chronicle, Pathog. Glob. Health 108 (7) (2014) 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Jang SC, Kim SR, Yoon YJ, Park KS, Kim JH, Lee J, Kim OY, Choi EJ, Kim DK, Choi DS, Kim YK, Park J, Di Vizio D, Gho YS, In vivo kinetic biodistribution of nano-sized outer membrane vesicles derived from bacteria, Small 11 (4) (2015) 456–461. [DOI] [PubMed] [Google Scholar]
- [192].Qing G, Gong N, Chen X, Chen J, Zhang H, Wang Y, Wang R, Zhang S, Zhang Z, Zhao X, Luo Y, Liang X, Natural and engineered bacterial outer membrane vesicles, Biophys. Rep 5 (4) (2019) 184–198. [Google Scholar]
- [193].Yuan J, Yang J, Hu Z, Yang Y, Shang W, Hu Q, Zheng Y, Peng H, Zhang X, Cai X, Zhu J, Li M, Hu X, Zhou R, Rao X, Safe staphylococcal platform for the development of multivalent nanoscale vesicles against viral infections, Nano Lett. 18 (2) (2018) 725–733. [DOI] [PubMed] [Google Scholar]
- [194].Zariri A, Beskers J, van de Waterbeemd B, Hamstra HJ, Bindels TH, van Riet E, van Putten JP, van der Ley P, Meningococcal outer membrane vesicle composition-dependent activation of the innate immune response, Infect. Immun 84 (10) (2016) 3024–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].van de Waterbeemd B, Streefland M, van der Ley P, Zomer B, van Dijken H, Martens D, Wijffels R, van der Pol L, Improved OMV vaccine against Neisseria meningitidis using genetically engineered strains and a detergent-free purification process, Vaccine 28 (30) (2010) 4810–4816. [DOI] [PubMed] [Google Scholar]
- [196].Watkins HC, Rappazzo CG, Higgins JS, Sun X, Brock N, Chau A, Misra A, Cannizzo JPB, King MR, Maines TR, Leifer CA, Whittaker GR, DeLisa MP, Putnam D, Safe recombinant outer membrane vesicles that display M2e elicit heterologous influenza protection, Mol. Ther 25 (4) (2017) 989–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Price N, Goyette-Desjardins G, Nothaft H, Valguarnera E, Szymanski C, Segura M, Feldman M, Glycoengineered outer membrane vesicles: A novel platform for bacterial vaccines, Sci. Rep 6 (2016) 24931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Valentine JL, Chen L, Perregaux EC, Weyant KB, Rosenthal JA, Heiss C, Azadi P, Fisher AC, Putnam D, Moe GR, Merritt JH, DeLisa MP, Immunization with outer membrane vesicles displaying designer glycotopes yields class-switched, glycan-specific antibodies, Cell Chem. Biol 23 (6) (2016) 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Chen L, Valentine JL, Huang CJ, Endicott CE, Moeller TD, Rasmussen JA, Fletcher JR, Boll JM, Rosenthal JA, Dobruchowska J, Wang Z, Heiss C, Azadi P, Putnam D, Trent MS, Jones BD, DeLisa MP, Outer membrane vesicles displaying engineered glycotopes elicit protective antibodies, Proc. Natl. Acad. Sci. U. S. A 113 (26) (2016) E360918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Huang W, Wang S, Yao Y, Xia Y, Yang X, Li K, Sun P, Liu C, Sun W, Bai H, Chu X, Li Y, Ma Y, Employing Escherichia coli-derived outer membrane vesicles as an antigen delivery platform elicits protective immunity against Acinetobacter baumannii infection, Sci. Rep 6 (2016) 37242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Fleitas Martinez O, Cardoso MH, Ribeiro SM, Franco OL, Recent advances in antivirulence therapeutic strategies with a focus on dismantling bacterial membrane microdomains, toxin neutralization, quorum-sensing interference and biofilm inhibition, Front. Cell Infect. Microbiol 9 (2019) 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Hu CM, Fang RH, Copp J, Luk BT, Zhang L, A biomimetic nanosponge that absorbs pore-forming toxins, Nat. Nanotechnol 8 (5) (2013) 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Wang F, Gao W, Thamphiwatana S, Luk BT, Angsantikul P, Zhang Q, Hu CM, Fang RH, Copp JA, Pornpattananangkul D, Lu W, Zhang L, Hydrogel retaining toxin-absorbing nanosponges for local treatment of methicillin-resistant Staphylococcus aureus infection, Adv. Mater 27 (22) (2015) 3437–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Coburn PS, Miller FC, LaGrow AL, Land C, Mursalin H, Livingston E, Amayem O, Chen Y, Gao W, Zhang L, Callegan MC, Disarming pore-forming toxins with biomimetic nanosponges in intraocular infections, mSphere 4 (3) (2019) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Pang Z, Hu CM, Fang RH, Luk BT, Gao W, Wang F, Chuluun E, Angsantikul P, Thamphiwatana S, Lu W, Jiang X, Zhang L, Detoxification of organophosphate poisoning using nanoparticle bioscavengers, ACS Nano 9 (6) (2015) 6450–6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Chen Y, Zhang Y, Zhuang J, Lee JH, Wang L, Fang RH, Gao W, Zhang L, Cell-membrane-cloaked oil nanosponges enable dual-modal detoxification, ACS Nano 13 (6) (2019) 7209–7215. [DOI] [PubMed] [Google Scholar]
- [207].Zhang Q, Dehaini D, Zhang Y, Zhou J, Chen X, Zhang L, Fang RH, Gao W, Zhang L, Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis, Nat. Nanotechnol 13 (12) (2018) 1182–1190. [DOI] [PubMed] [Google Scholar]
- [208].Fang RH, Luk BT, Hu CM, Zhang L, Engineered nanoparticles mimicking cell membranes for toxin neutralization, Adv. Drug Deliv. Rev 90 (2015) 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Zhang Q, Fang RH, Gao W, Zhang L, A biomimetic nanoparticle to “lure and kill” phospholipase A2, Angew. Chem. Int. Ed. Engl 59 (26) (2020) 10461–10465. [DOI] [PubMed] [Google Scholar]
- [210].Mazor R, King EM, Pastan I, Strategies to reduce the immunogenicity of recombinant immunotoxins, Am. J. Pathol 188 (8) (2018) 1736–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Naldini L, Ex vivo gene transfer and correction for cell-based therapies, Nat. Rev. Genet 12 (5) (2011) 301–315. [DOI] [PubMed] [Google Scholar]
- [212].Fodor WL, Tissue engineering and cell based therapies, from the bench to the clinic: The potential to replace, repair and regenerate, Reprod. Biol. Endocrinol. 1 (2003) 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Raffin C, Vo LT, Bluestone JA, Treg cell-based therapies: Challenges and perspectives, Nat. Rev. Immunol 20 (3) (2020) 158–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Schaft N, The landscape of CAR-T cell clinical trials against solid tumors—A comprehensive overview, Cancers 12 (9) (2020) 2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Zhang Y, Zhang Z, Ding Y, Fang Y, Wang P, Chu W, Jin Z, Yang X, Wang J, Lou J, Qian Q, Phase I clinical trial of EGFR-specific CAR-T cells generated by the piggyBac transposon system in advanced relapsed/refractory non-small cell lung cancer patients, J. Cancer Res. Clin. Oncol 147 (12) (2021) 3725–3734. [DOI] [PubMed] [Google Scholar]
- [216].Adusumilli PS, Zauderer MG, Riviere I, Solomon SB, Rusch VW, O’Cearbhaill RE, Zhu A, Cheema W, Chintala NK, Halton E, Pineda J, Perez-Johnston R, Tan KS, Daly B, Araujo Filho JA, Ngai D, McGee E, Vincent A, Diamonte C, Sauter JL, Modi S, Sikder D, Senechal B, Wang X, Travis WD, Gonen M, Rudin CM, Brentjens RJ, Jones DR, Sadelain M, A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab, Cancer Discov. 11 (11) (2021) 2748–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]


