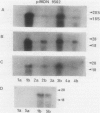
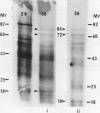
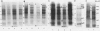Abstract
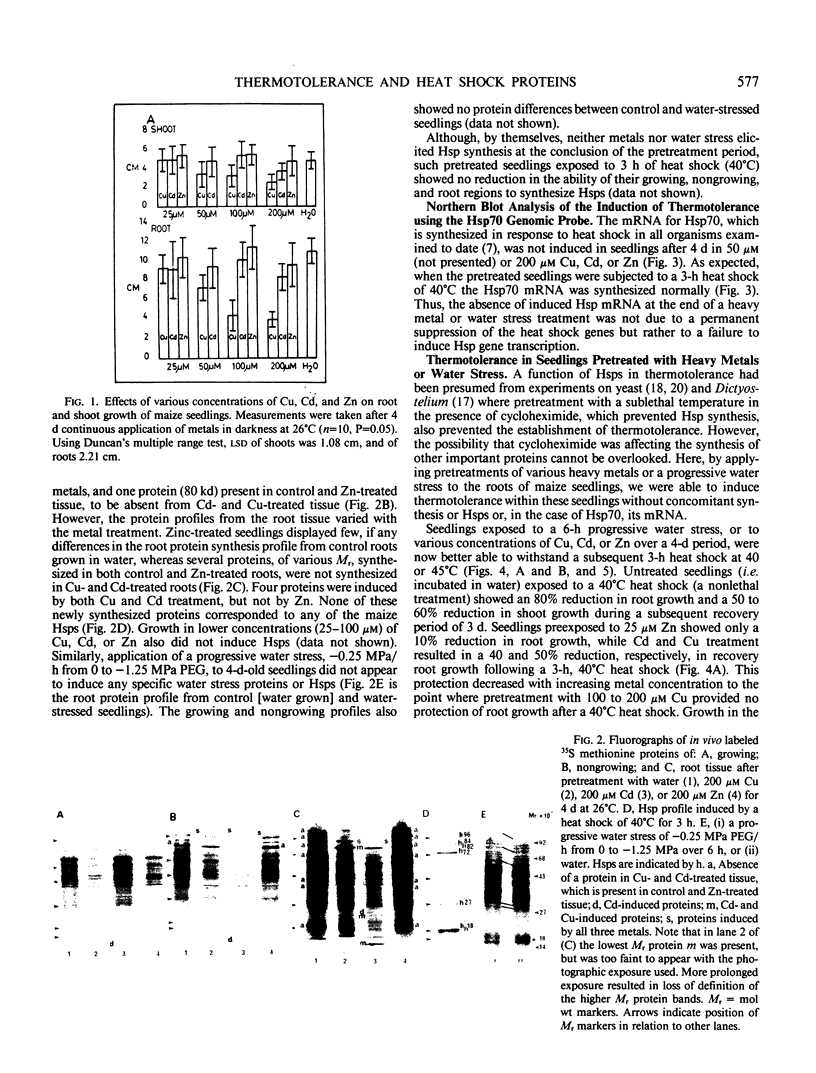
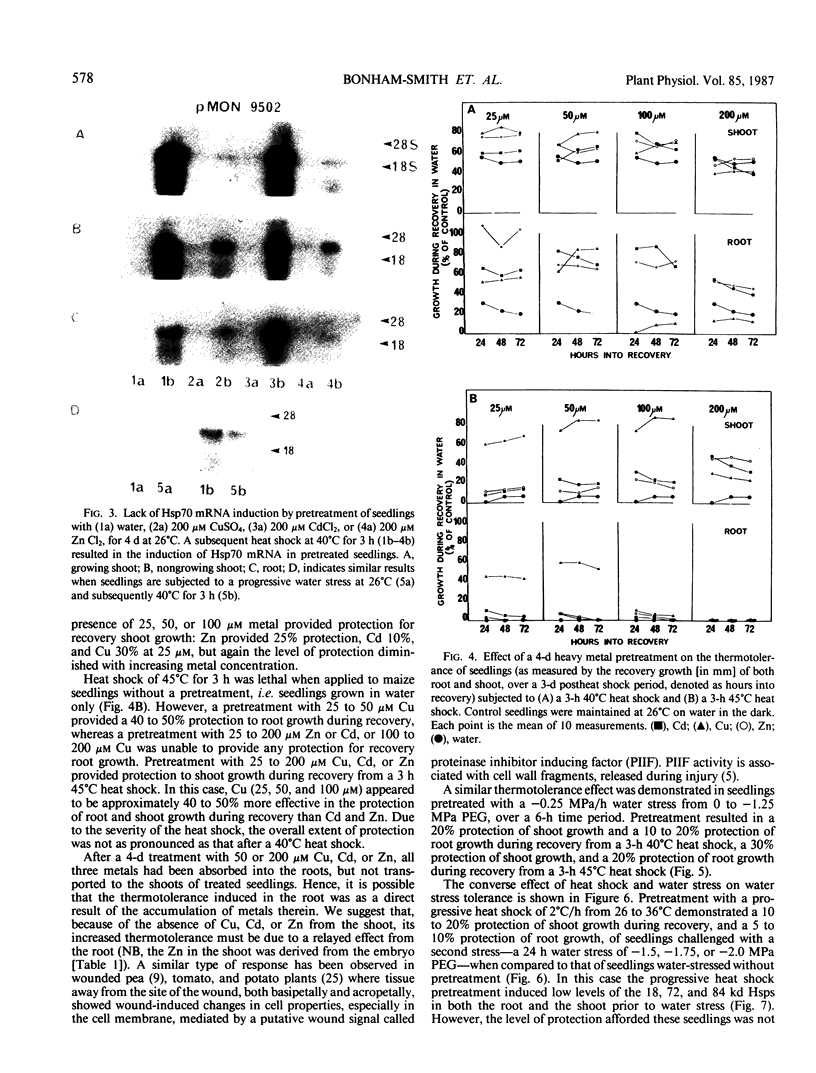
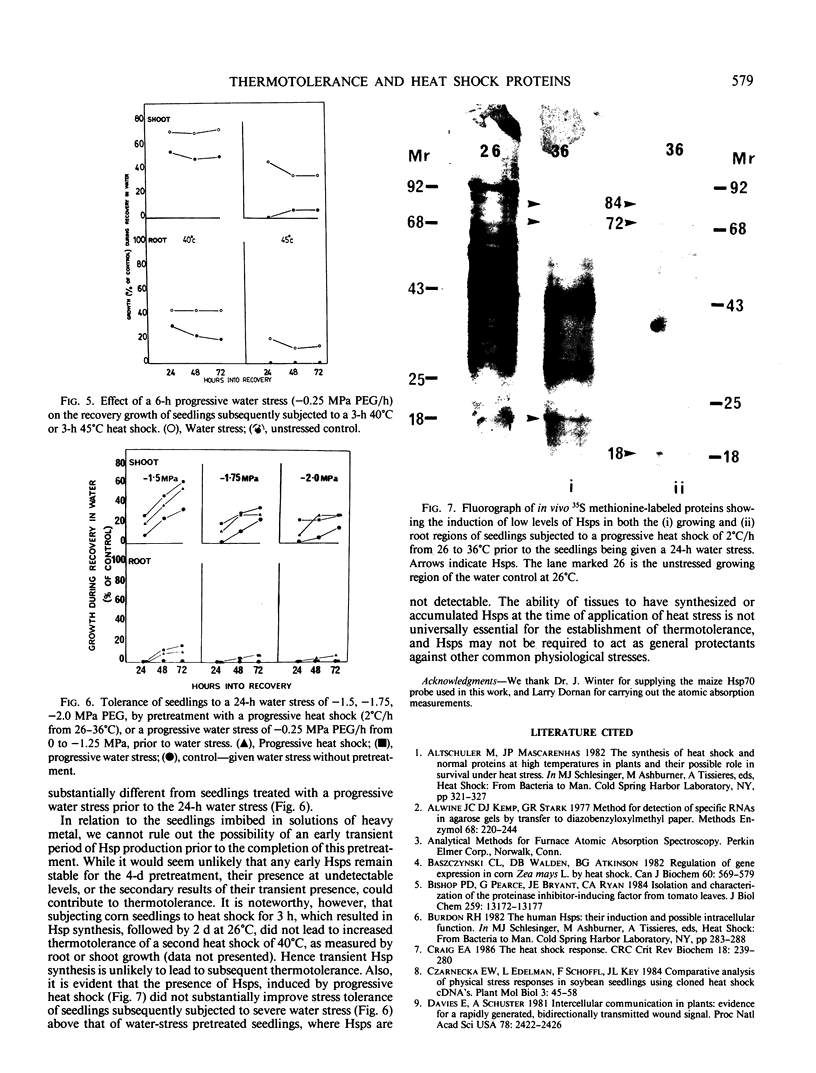
Maize (Zea mays) seedlings were pretreated prior to heat shock with either a progressive water stress of −0.25 megapascal PEG/hour from 0 to −1.25 megapascal over a 6-hour time period, or various concentrations of copper, cadmium, or zinc for 4 days. When the subsequent heat shock of 40 or 45°C was administered for 3 hours, the seedlings showed an induced thermotolerance to these temperatures, which were otherwise lethal to control (water grown) seedlings. Thermotolerance was exhibited by both the root and the shoot of pretreated seedlings, even though the water and heavy metal stresses were applied only to the roots. Neither of these pretreatments had induced the synthesis of detectable levels of heat shock proteins (Hsps) at the time of heat shock. Pretreatment of seedlings with a progressive heat shock of 2°C/hour from 26 to 36°C, which did induce Hsps 18, 70, and 84, resulted in tolerance of a severe water stress of −1.5, −1.75, or −2.0 megapascal for 24 hours. But these seedlings producing Hsps were no better protected against water stress than those pretreated with a progressive water stress which did not produce Hsps. Hsps appear not to act as general stress proteins and their presence is not always required for the establishment of thermotolerance.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Baszczynski C. L., Walden D. B., Atkinson B. G. Regulation of gene expression in corn (Zea Mays L.) by heat shock. Can J Biochem. 1982 May;60(5):569–579. doi: 10.1139/o82-070. [DOI] [PubMed] [Google Scholar]
- Bishop P. D., Pearce G., Bryant J. E., Ryan C. A. Isolation and characterization of the proteinase inhibitor-inducing factor from tomato leaves. Identity and activity of poly- and oligogalacturonide fragments. J Biol Chem. 1984 Nov 10;259(21):13172–13177. [PubMed] [Google Scholar]
- Craig E. A. The heat shock response. CRC Crit Rev Biochem. 1985;18(3):239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Davies E., Schuster A. Intercellular communication in plants: Evidence for a rapidly generated, bidirectionally transmitted wound signal. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2422–2426. doi: 10.1073/pnas.78.4.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner E. W., Schneider M. J. Induced thermal resistance in HeLa cells. Nature. 1975 Aug 7;256(5517):500–502. doi: 10.1038/256500a0. [DOI] [PubMed] [Google Scholar]
- Hallberg R. L. No heat shock protein synthesis is required for induced thermostabilization of translational machinery. Mol Cell Biol. 1986 Jun;6(6):2267–2270. doi: 10.1128/mcb.6.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila J. J., Papp J. E., Schultz G. A., Bewley J. D. Induction of heat shock protein messenger RNA in maize mesocotyls by water stress, abscisic Acid, and wounding. Plant Physiol. 1984 Sep;76(1):270–274. doi: 10.1104/pp.76.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila J. J., Schultz G. A., Iatrou K., Gedamu L. Expression of a set of fish genes following heat or metal ion exposure. J Biol Chem. 1982 Oct 25;257(20):12000–12005. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li G. C. Induction of thermotolerance and enhanced heat shock protein synthesis in Chinese hamster fibroblasts by sodium arsenite and by ethanol. J Cell Physiol. 1983 May;115(2):116–122. doi: 10.1002/jcp.1041150203. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S. Heat shock response of Dictyostelium. Dev Biol. 1980 Oct;79(2):399–408. doi: 10.1016/0012-1606(80)90125-6. [DOI] [PubMed] [Google Scholar]
- McAlister L., Finkelstein D. B. Heat shock proteins and thermal resistance in yeast. Biochem Biophys Res Commun. 1980 Apr 14;93(3):819–824. doi: 10.1016/0006-291x(80)91150-x. [DOI] [PubMed] [Google Scholar]
- Minard D. The Bretton Woods Symposium: physiological characterization of health hazards in man's environment. Environ Res. 1969 Oct;2(5):324–330. doi: 10.1016/0013-9351(69)90002-4. [DOI] [PubMed] [Google Scholar]
- Mitchel R. E., Morrison D. P. Heat-shock induction of ionizing radiation resistance in Saccharomyces cerevisiae. Transient changes in growth cycle distribution and recombinational ability. Radiat Res. 1982 Oct;92(1):182–187. [PubMed] [Google Scholar]
- Petko L., Lindquist S. Hsp26 is not required for growth at high temperatures, nor for thermotolerance, spore development, or germination. Cell. 1986 Jun 20;45(6):885–894. doi: 10.1016/0092-8674(86)90563-5. [DOI] [PubMed] [Google Scholar]
- Plesset J., Palm C., McLaughlin C. S. Induction of heat shock proteins and thermotolerance by ethanol in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1340–1345. doi: 10.1016/0006-291x(82)92147-7. [DOI] [PubMed] [Google Scholar]
- Rochester D. E., Winer J. A., Shah D. M. The structure and expression of maize genes encoding the major heat shock protein, hsp70. EMBO J. 1986 Mar;5(3):451–458. doi: 10.1002/j.1460-2075.1986.tb04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Simmons M., Holländer-Czytko H., Andersen J. K., Ryan C. A. Wound signals in plants: A systemic plant wound signal alters plasma membrane integrity. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3737–3741. doi: 10.1073/pnas.81.12.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widelitz R. B., Magun B. E., Gerner E. W. Effects of cycloheximide on thermotolerance expression, heat shock protein synthesis, and heat shock protein mRNA accumulation in rat fibroblasts. Mol Cell Biol. 1986 Apr;6(4):1088–1094. doi: 10.1128/mcb.6.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C. M., Mascarenhas J. P. High temperature-induced thermotolerance in pollen tubes of tradescantia and heat-shock proteins. Plant Physiol. 1985 Aug;78(4):887–890. doi: 10.1104/pp.78.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]