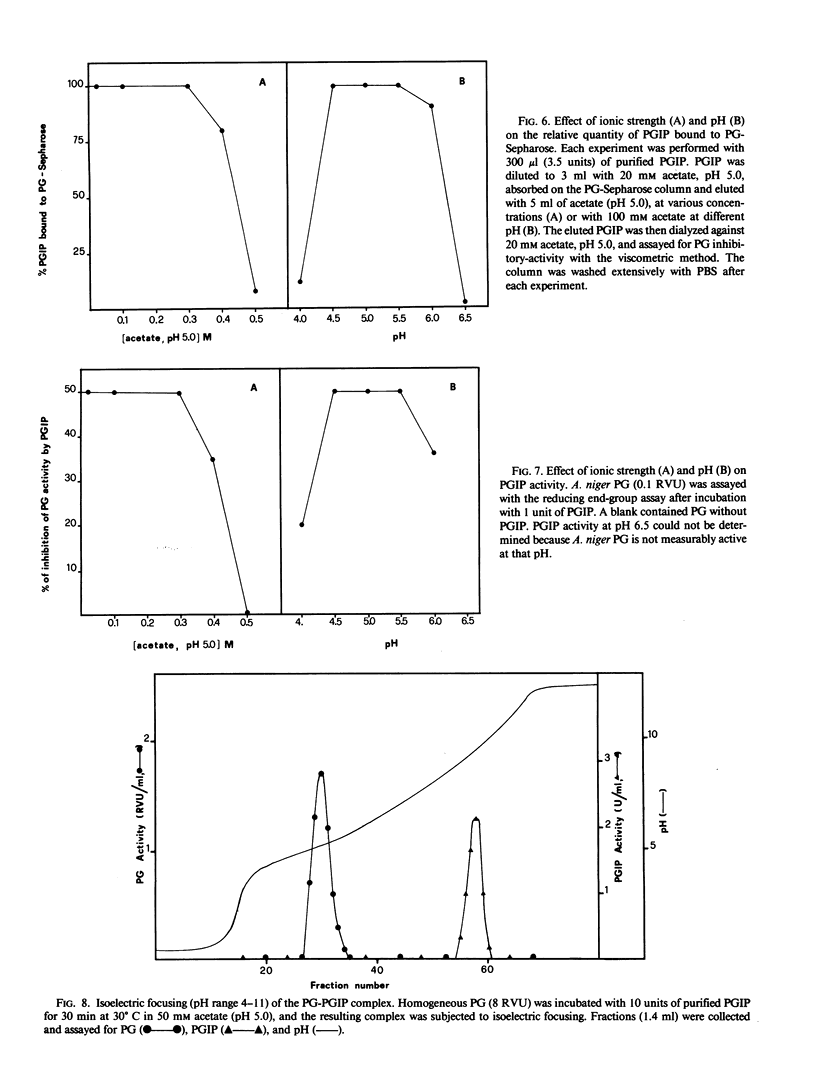
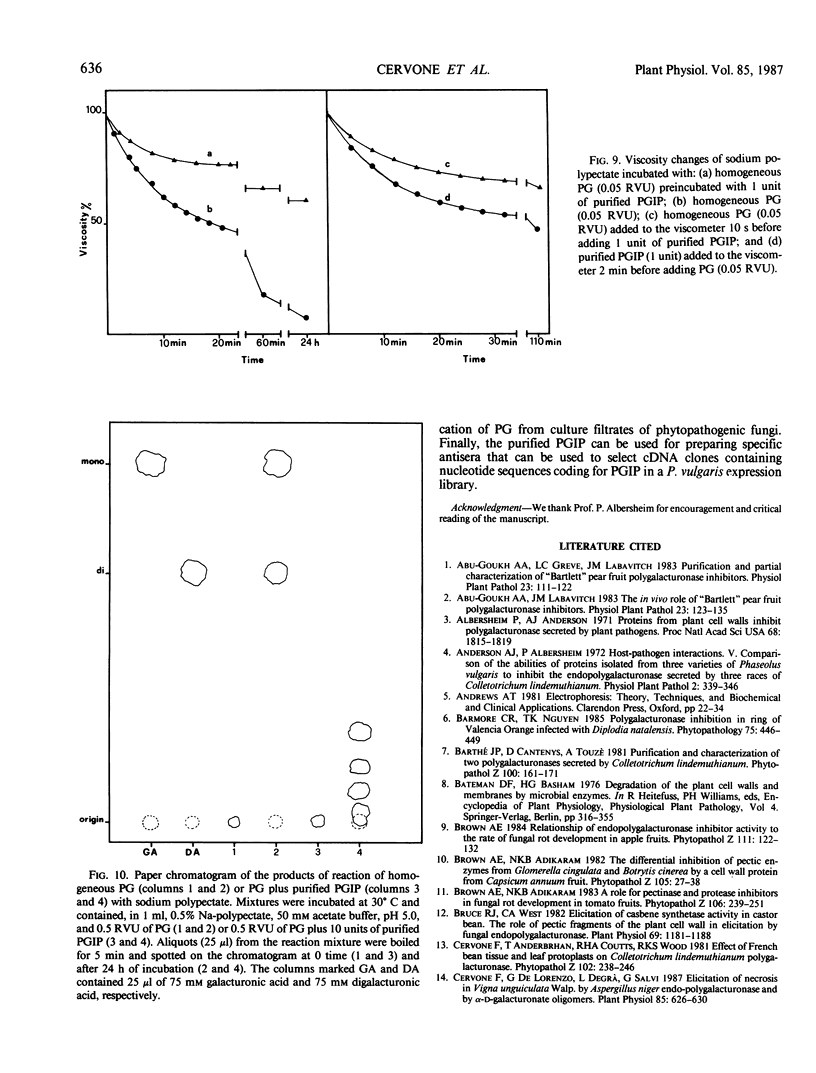
Abstract
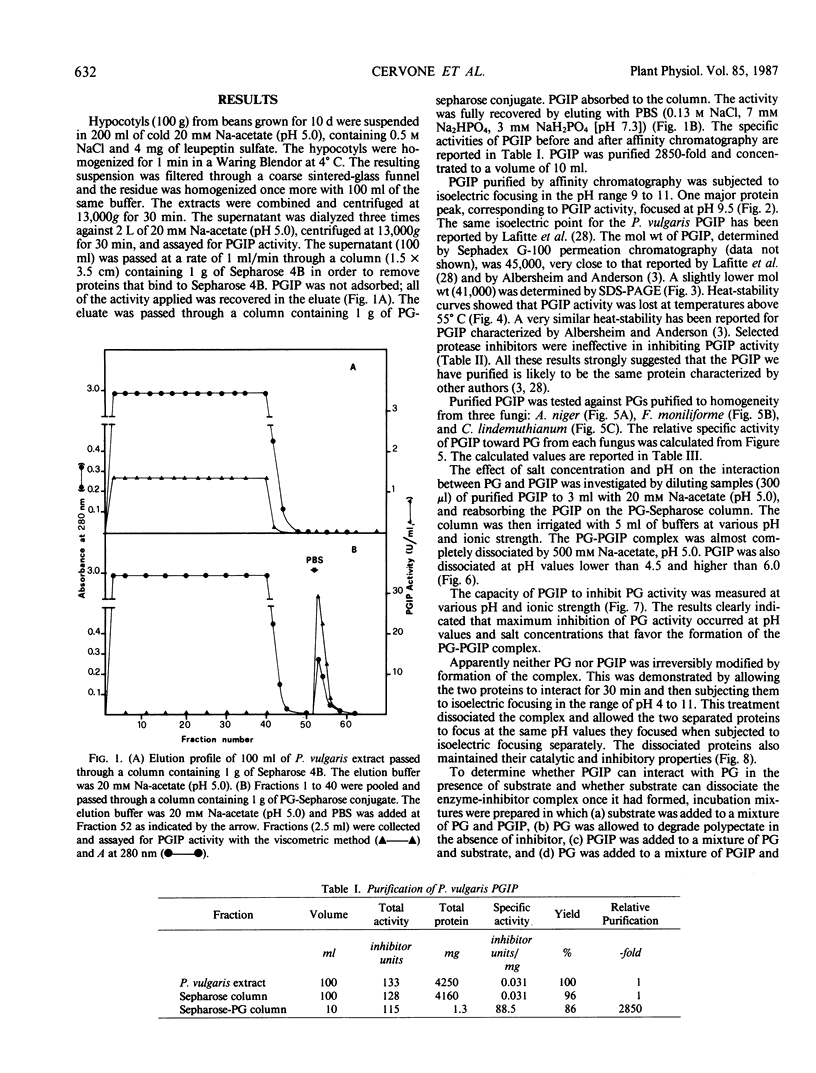
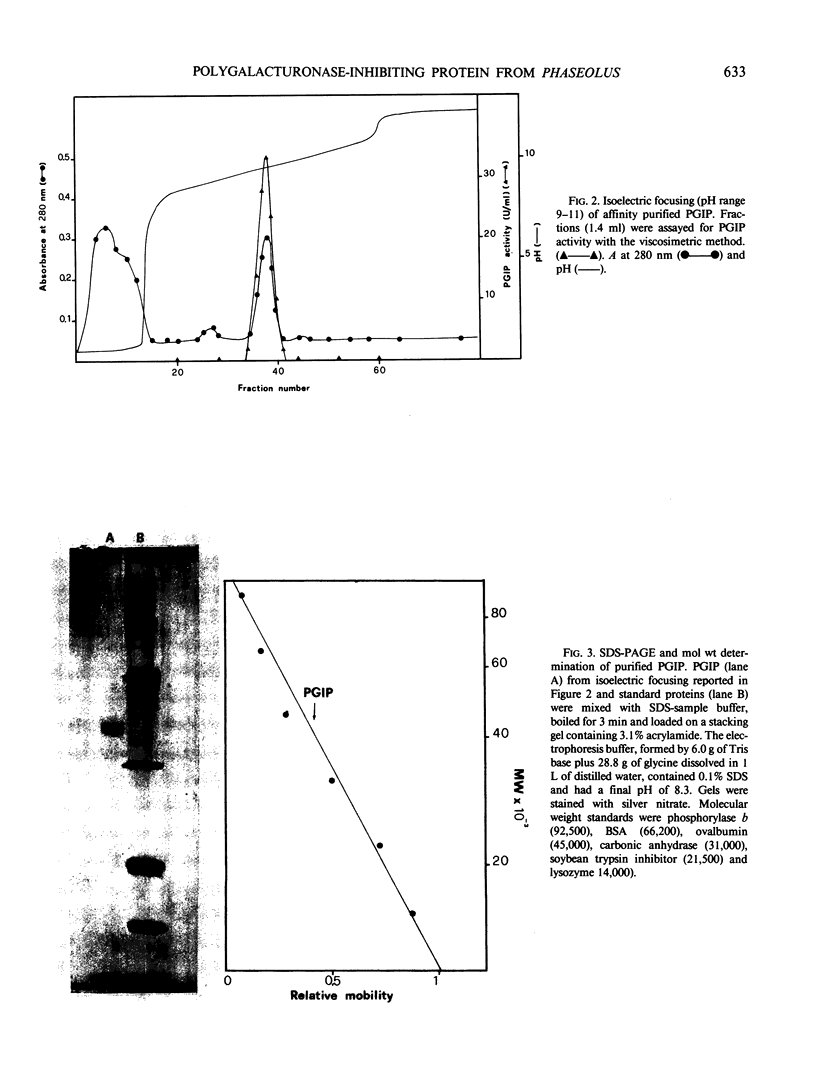
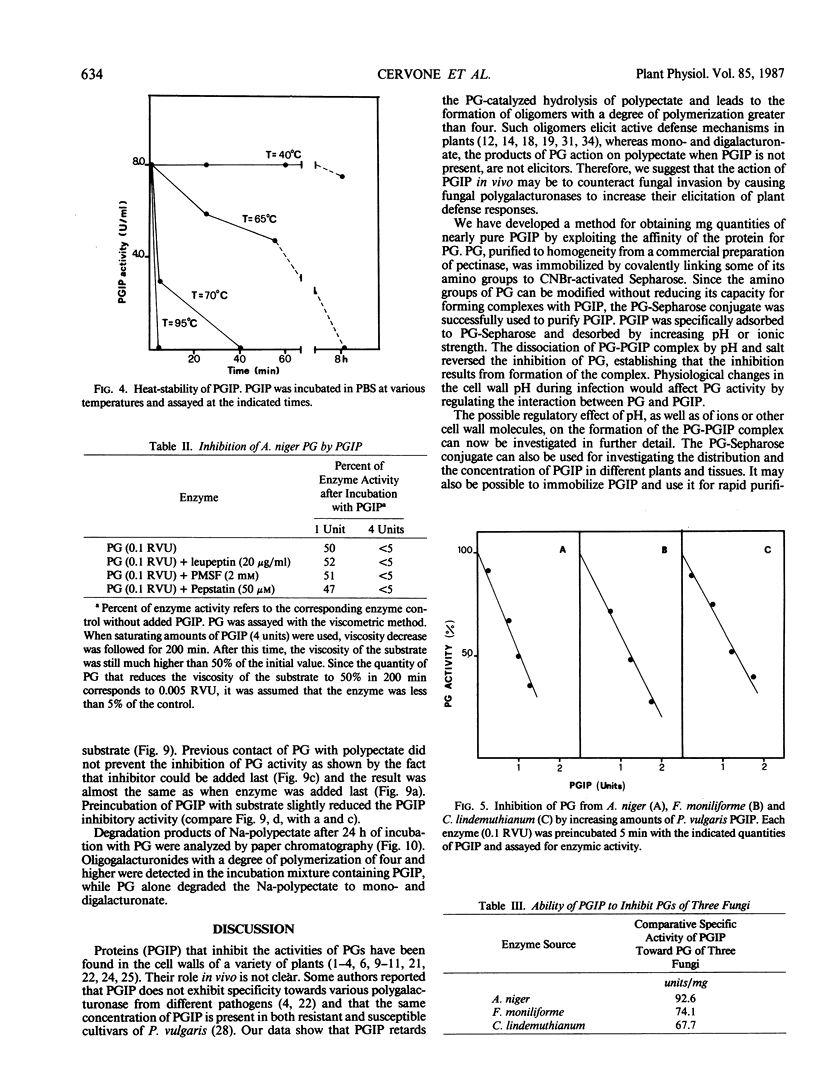
Homogeneous endo-polygalacturonase (PG) was covalently bound to cyanogen-bromide-activated Sepharose, and the resulting PG-Sepharose conjugate was utilized to purify, by affinity chromatography, a protein from Phaseolus vulgaris hypocotyls that binds to and inhibits PG. Isoelectric focusing of the purified PG-inhibiting protein (PGIP) showed a major protein band that coincided with PG-inhibiting activity. PGIP formed a complex with PG at pH 5.0 and at low salt concentrations. The complex dissociated in 0.5 m Na-acetate and pH values lower than 4.5 or higher than 6.0. Formation of the PG-PGIP complex resulted in complete inhibition of PG activity. PG activity was restored upon dissociation of the complex. The protein exhibited inhibitory activity toward PGs from Colletotrichum lindemuthianum, Fusarium moniliforme and Aspergillus niger. The possible role of PGIP in regulating the activity of fungal PG's and their ability to elicit plant defense reactions are discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albersheim P., Anderson A. J. Proteins from plant cell walls inhibit polygalacturonases secreted by plant pathogens. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1815–1819. doi: 10.1073/pnas.68.8.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce R. J., West C. A. Elicitation of Casbene Synthetase Activity in Castor Bean : THE ROLE OF PECTIC FRAGMENTS OF THE PLANT CELL WALL IN ELICITATION BY A FUNGAL ENDOPOLYGALACTURONASE. Plant Physiol. 1982 May;69(5):1181–1188. doi: 10.1104/pp.69.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervone F., De Lorenzo G., Degrà L., Salvi G. Elicitation of Necrosis in Vigna unguiculata Walp. by Homogeneous Aspergillus niger Endo-Polygalacturonase and by alpha-d-Galacturonate Oligomers. Plant Physiol. 1987 Nov;85(3):626–630. doi: 10.1104/pp.85.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K. R., Darvill A. G., Albersheim P., Dell A. Host-Pathogen Interactions : XXIX. Oligogalacturonides Released from Sodium Polypectate by Endopolygalacturonic Acid Lyase Are Elicitors of Phytoalexins in Soybean. Plant Physiol. 1986 Feb;80(2):568–577. doi: 10.1104/pp.80.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. L., Anderson A. J., Albersheim P. Host-Pathogen Interactions: VI. A Single Plant Protein Efficiently Inhibits Endopolygalacturonases Secreted by Colletotrichum Lindemuthianum and Aspergillus Niger. Plant Physiol. 1973 Mar;51(3):489–491. doi: 10.1104/pp.51.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr A. L., Albersheim P. Polysaccharide-degrading Enzymes are Unable to Attack Plant Cell Walls without Prior Action by a "Wall-modifying Enzyme". Plant Physiol. 1970 Jul;46(1):69–80. doi: 10.1104/pp.46.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasuno S., Starr M. P. Polygalacturonase of Erwinia carotovora. J Biol Chem. 1966 Nov 25;241(22):5298–5306. [PubMed] [Google Scholar]
- Nothnagel E. A., McNeil M., Albersheim P., Dell A. Host-Pathogen Interactions : XXII. A Galacturonic Acid Oligosaccharide from Plant Cell Walls Elicits Phytoalexins. Plant Physiol. 1983 Apr;71(4):916–926. doi: 10.1104/pp.71.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Walker-Simmons M., Hadwiger L., Ryan C. A. Chitosans and pectic polysaccharides both induce the accumulation of the antifungal phytoalexin pisatin in pea pods and antinutrient proteinase inhibitors in tomato leaves. Biochem Biophys Res Commun. 1983 Jan 14;110(1):194–199. doi: 10.1016/0006-291x(83)91279-2. [DOI] [PubMed] [Google Scholar]