Abstract
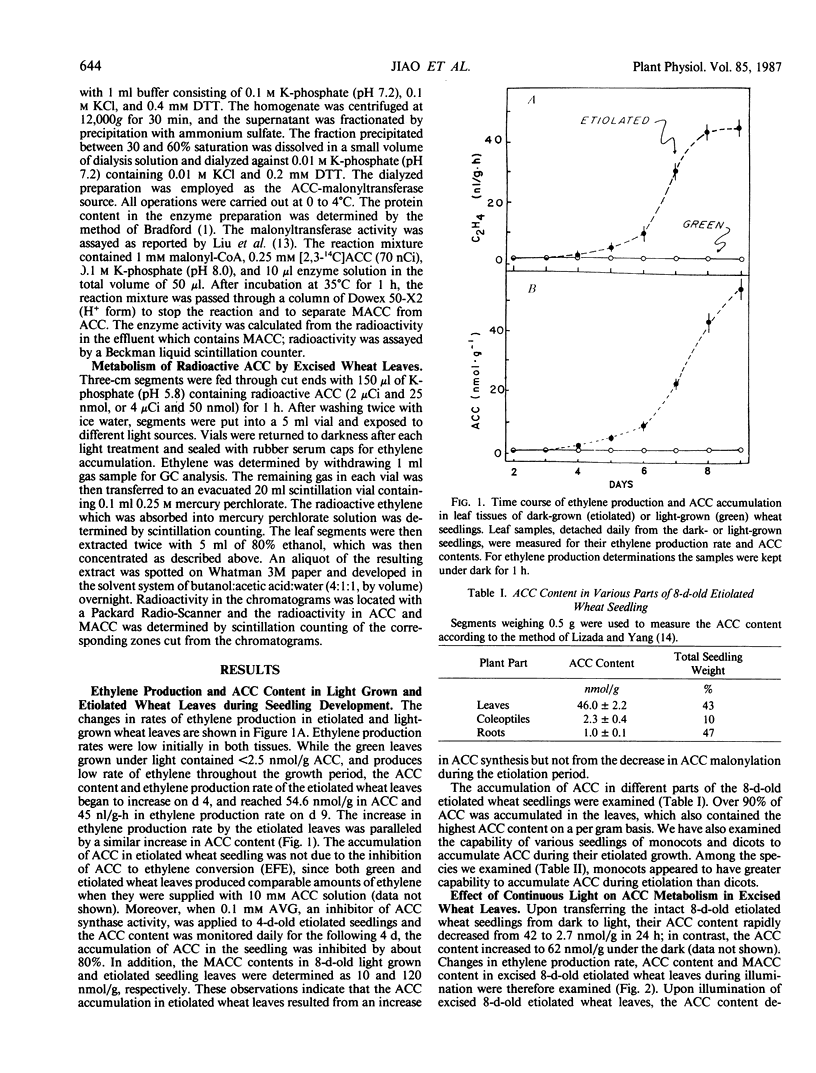
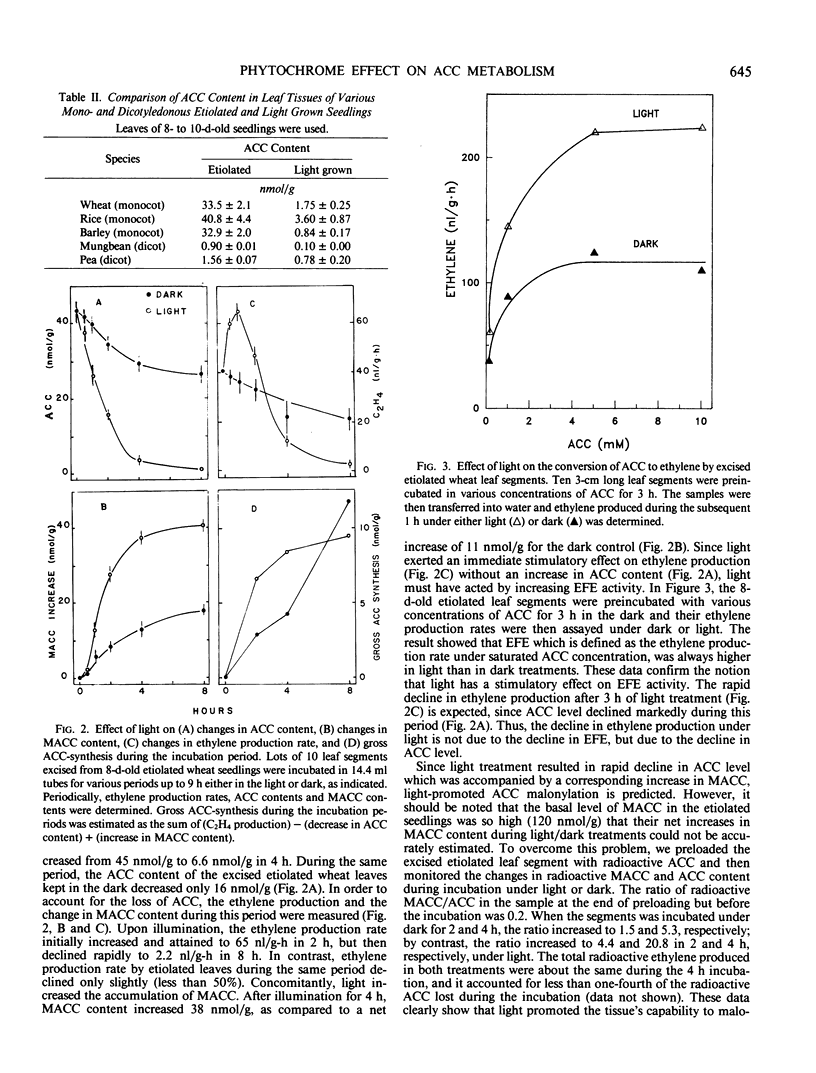
While light-grown wheat leaves produced ethylene at a low rate of <0.1 nanomoles per gram per hour and contained 1-aminocyclopropane-1-carboxylic acid (ACC) at low levels of <2.5 nanomoles per gram, etiolated wheat leaves produced ethylene at a rate of 2 nanomoles per gram per hour and accumulated concentrations of ACC at levels of 40 nanomoles per gram. Upon illumination of 8-day-old etiolated wheat seedlings with white light, the ethylene production rate increased initially, due to the activation of ethylene-forming activity, but subsequently declined to a low level (0.1 nanomoles per gram per hour) at the end of the 6-hour illumination. This light-induced decline in ethylene production rate resulted from a decline (more than 35 nanomoles per gram) in ACC level, which was accompanied by a corresponding increase in 1-(malonylamino)cyclopropane-1-carboxylic acid content. These data indicate that illumination promoted ACC malonylation, resulting in reduced ACC level and consequently reduced ethylene production. However, light did not cause any significant increase in the extractable ACC-malonyltransferase activity. The effect of continuous white light on promotion of ACC malonylation was also observed in intermittent white light or red light. A far-red light treatment following red light partially reversed the red light effect, indicating that phytochrome participates in the promotion of ACC malonylation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Goeschl J. D., Pratt H. K., Bonner B. A. An effect of light on the production of ethylene and the growth of the plumular portion of etiolated pea seedlings. Plant Physiol. 1967 Aug;42(8):1077–1080. doi: 10.1104/pp.42.8.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinski B., Boesel I., Horton R. F. Light Stimulation of Ethylene Release from Leaves of Gomphrena globosa L. Plant Physiol. 1983 Mar;71(3):588–593. doi: 10.1104/pp.71.3.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaseki H., Pjon C. J., Furuya M. Phytochrome Action in Oryza sativa L: IV. Red and Far Red Reversible Effect on the Production of Ethylene in Excised Coleoptiles. Plant Physiol. 1971 Sep;48(3):241–244. doi: 10.1104/pp.48.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B. G., Burg S. P. Relation of Phytochrome-enhanced Geotropic Sensitivity to Ethylene Production. Plant Physiol. 1972 Jul;50(1):132–135. doi: 10.1104/pp.50.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Su L. Y., Yang S. F. Ethylene Promotes the Capability To Malonylate 1-Aminocyclopropane-1-carboxylic Acid and d-Amino Acids in Preclimacteric Tomato Fruits. Plant Physiol. 1985 Apr;77(4):891–895. doi: 10.1104/pp.77.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Su L. Y., Yang S. F. Stereoselectivity of 1-aminocyclopropanecarboxylate malonyltransferase toward stereoisomers of 1-amino-2-ethylcyclopropanecarboxylic acid. Arch Biochem Biophys. 1984 Dec;235(2):319–325. doi: 10.1016/0003-9861(84)90204-2. [DOI] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Philosoph-Hadas S., Aharoni N., Yang S. F. Carbon dioxide enhances the development of the ethylene forming enzyme in tobacco leaf discs. Plant Physiol. 1986 Dec;82(4):925–929. doi: 10.1104/pp.82.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein B. Auxin and red light in the control of hypocotyl hook opening in beans. Plant Physiol. 1971 Aug;48(2):187–192. doi: 10.1104/pp.48.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samimy C. Effect of light on ethylene production and hypocotyl growth of soybean seedlings. Plant Physiol. 1978 May;61(5):772–774. doi: 10.1104/pp.61.5.772. [DOI] [PMC free article] [PubMed] [Google Scholar]


