Abstract
β-thalassemia is an inherited anemia characterized by ineffective erythropoiesis. Blood transfusions are required for survival in transfusion-dependent b-thalassemia and are also occasionally needed in patients with non-transfusion-dependent β-thalassemia. Patients with transfusion-dependent b-thalassemia often have elevated transferrin saturation (TSAT) and non-transferrin-bound iron (NTBI) levels, which can lead to organ iron overload, oxidative stress, and vascular damage. Vamifeport is an oral ferroportin inhibitor that was previously shown to ameliorate anemia, ineffective erythropoiesis, and dysregulated iron homeostasis in the Hbbth3/+ mouse model of b-thalassemia, under non-transfused conditions. Our study aimed to assess the effects of oral vamifeport on iron-related parameters (including plasma NTBI levels) and ineffective erythropoiesis following blood transfusions in Hbbth3/+ mice. A single dose of vamifeport prevented the transient transfusion-mediated NTBI increase in Hbbth3/+ mice. Compared with vehicle treatment, vamifeport significantly increased hemoglobin levels and red blood cell counts in transfused mice. Vamifeport treatment also significantly improved ineffective erythropoiesis in the spleens of Hbbth3/+ mice, with additive effects observed when treatment was combined with repeated transfusions. Vamifeport corrected leukocyte counts and significantly improved iron-related parameters (serum transferrin, TSAT and erythropoietin levels) versus vehicle treatment in Hbbth3/+ mice, irrespective of transfusion status. In summary, vamifeport prevented transfusion-mediated NTBI formation in Hbbth3/+ mice. When given alone or combined with blood transfusions, vamifeport also ameliorated anemia, ineffective erythropoiesis, and dysregulated iron homeostasis. Administering vamifeport together with repeated blood transfusions additively ameliorated anemia and ineffective erythropoiesis in this mouse model, providing preclinical proof-of-con-cept for the efficacy of combining vamifeport with blood transfusions in b-thalassemia.
Introduction
β-thalassemia is an inherited anemia caused by mutations in the b-globin gene of hemoglobin, which decrease the synthesis of b-globin chains, resulting in an imbalance in α- and b-globin chains and an accumulation of insoluble α-globin aggregates on the membranes of red blood cells (RBC).1-3 These α-globin aggregates contain free heme and iron, which can cause the formation of reactive oxygen species that reduce the lifespan of RBC and result in anemia and tissue hypoxia. In order to compensate for this, erythropoiesis is stimulated in patients with β-thalassemia, which leads to the increased proliferation but decreased differentiation of erythroid precursors (so-called ineffective erythropoiesis).1,2,4-6 As levels of the ferroportin-blocking hormone hepcidin are suppressed by erythropoiesis, iron deficiency, and hypoxia, β-thalassemia is also associated with organ iron overload due to increased dietary iron absorption via ferroportin-mediated iron export from enterocytes, as well as iron efflux from spleen macrophages recycling damaged erythrocytes.1,3,7,8
Thalassemia is often classified based on the transfusion requirement of the patient. Those with non-transfusiondependent thalassemia usually receive only occasional blood transfusions,9 while patients with the most severe form, transfusion-dependent β-thalassemia, require regular blood transfusions for survival, which can lead to secondary iron overload that requires iron chelation therapy.10 Moreover, these patients often show elevated transferrin saturation (TSAT) and non-transferrin-bound iron (NTBI), triggered by ferroportin-mediated iron release from macrophage recycling of damaged RBC, which can lead to oxidative stress, vascular injury and organ iron overload.8,11 In addition, the accelerated recycling of RBC due to blood transfusions results in a high serum iron concentration and an increase in NTBI that correlates with the occurrence of heart disease.12
One promising therapeutic approach to correcting the dysregulated iron metabolism seen in patients with β-thalassemia is to target the hepcidin-ferroportin axis,13,14 for example by substituting for the reduced endogenous hepcidin levels by administering a synthetic small-molecule ferroportin inhibitor. Vamifeport (CSL Vifor, St. Gallen, Switzerland), also known as VIT-2763, is an oral ferroportin inhibitor that is currently being investigated as a potential treatment option for patients with β-thalassemia and sickle cell disease.8,15 Limiting iron availability with vamifeport ameliorated anemia, ineffective erythropoiesis, and dysregulated iron homeostasis in the Hbbth3/+ mouse model of β-thalassemia, under non-transfused conditions.1 This mouse model closely resembles the pathophysiology of patients with β-thalassemia intermedia.16 It is characterized by ineffective erythropoiesis, splenomegaly, and secondary iron overload and has been used to test the efficacy of other candidate drugs for β-thalassemia.17 The aim of the present study was to determine the effect of vamifeport on plasma NTBI levels and ineffective erythropoiesis following a single transfusion or repeated blood transfusions in the Hbbth3/+ mouse model of β-thalassemia.
Methods
Ethics statement
Experiments complied with Swiss law and associated animal experimentation guidelines were approved by the responsible authority (Veterinary Department, Canton Zurich; License Number ZH108/2017). Studies complied with the CSL Vifor Code of Conduct and the principles of 3R (Replace, Reduce, Refine). Experimental mice were scored daily for body weight and physical condition.
Animal model
Hbbth3/+ mice16 were bred in the CSL Vifor animal facility, which employs 12-hour dark/12-hour light cycles. These non-blinded studies utilized 12–14-week-old Hbbth3/+ male and female mice (7-8 per group). Mice were housed 3–5 per cage and provided with standard chow (containing 250 ppm iron) and water ad libitum. Experiments were conducted in the experimental room; animals were taken to the holding room after each procedure. In the single-transfusion studies, mice received single-dose vamifeport (120 mg/kg; 10 mL/kg), or vehicle (0.5% methylcellulose in Milli-Q purified water), by oral gavage. Thirty minutes post dose, mice received a 200 μL tail vein transfusion of blood from preterminally anesthetized sex-matched wild-type mice, ubiquitously expressing green fluorescent protein (GFP).18 Animals were terminally anesthetized using isoflurane 3 or 6 hours post transfusion and peripheral blood was collected. Two studies was conducted, one in males, one in females, and data was pooled.
In the repeated-transfusion studies, transfusions were administered on days 1, 9, 16, and 22 or 23. Hbbth3/+ mice received once-daily oral vamifeport 120 mg/kg or vehicle (on transfusion days, dosing was 30 minutes before transfusion). Animals were terminally anesthetized approximately 24 hours after the fourth transfusion. Non-transfused, vehicle-treated Hbbth3/+ mice or wild-type littermates acted as controls.
Iron-related parameters
Serum NTBI concentrations were measured19 in all studies; the remaining parameters were assessed after repeated transfusion. Serum iron (Abbott) and serum transferrin (Abcam) were analyzed using commercial kits according to manufacturer’s instructions. TSAT levels were calculated according to the formula:
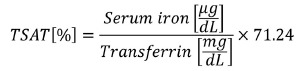 |
Liver Hamp1 and spleen erythroferrone (Erfe) messenger RNA (mRNA) levels were determined by quantitative polymerase chain reaction using PrimePCR™ SYBR® Green Assays (BIO-RAD). Plasma hepcidin levels were determined using enzyme-linked immunosorbent assay (Intrinsic Lifesciences). Organ iron levels were determined as previously described.1
Hematological parameters and erythropoiesis
All hematological parameters were analyzed using a ProCyte analyzer (Idexx Bioresearch). Spleen erythroid cells were analyzed by flow cytometry.1 Distinct stages of RBC precursors were identified based on TER119, CD44, and CD71 expression levels, and using the forward scatter to measure cell size. Donor-derived GFP-positive RBC were excluded from the analysis.
Membrane α-globin aggregates in erythroid cells
At least 450 μL of blood were needed for the preparation of membrane cytoskeletons and so blood samples were pooled from two mice from the same treatment group in these analyses, meaning that each data point was obtained from two biological replicates. Harvested membrane cytoskeletons were snap frozen and analyzed using TAU polyacrylamide gel electrophoresis.1
Statistical analysis
Assessment of parameters over time used two-way analysis of variance (ANOVA), with repeated measures for time course effects. Where significant effects were observed, post hoc tests were performed using Dunnett’s adjustment for multiple comparisons, where each group was compared with vehicle-treated non-transfused or vehicletreated transfused controls. Endpoint parameters were analyzed using one-way ANOVA with Dunnett’s multiple comparison test. All analyses were performed using GraphPad Prism, Version 8.4.2 (San Diego, CA).
Results
Vamifeport reduced plasma non-transferrin-bound iron levels afer a single blood transfusion in Hbbth3/+ mice
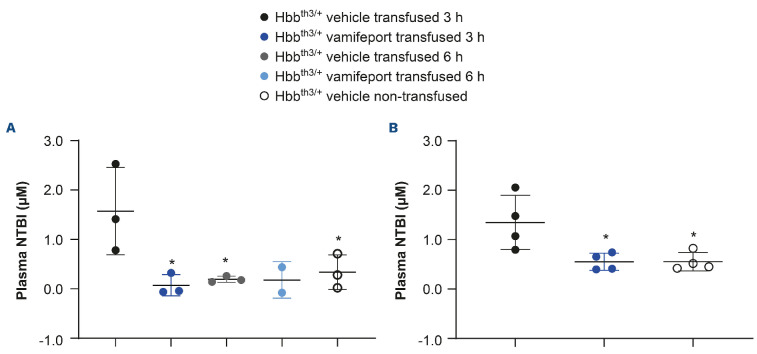
A single blood transfusion resulted in significantly higher plasma NTBI levels (mean [standard deviation] 1.35 [±0.55] μM) compared with those observed in non-transfused Hbbth3/+ mice (mean [standard deviation] 0.55 [±0.18] μM) (Figure 1). NTBI levels were transiently elevated 3 hours after blood transfusion in vehicle-treated transfused mice. A single oral dose of vamifeport prevented the plasma NTBI increase seen in transfused vehicle-treated Hbbth3/+ mice at 3 hours post transfusion, with levels remaining similar to those observed in non-transfused controls, demonstrating the potential of vamifeport to sequester iron recycled from transfused RBC. Non-transfused, vehicle-treated Hbbth3/+ mice had low NTBI levels (mean [standard deviation] 0.55 [±0.185] μM). NTBI returned to baseline levels by 6 hours in vehicle-treated transfused mice, suggesting that damaged RBC are cleared from the circulation and their heme iron recycled by macrophages in the first few hours after transfusion in this mouse model. Similar results were observed for donor blood collected 1 day or 15 days before transfusion, suggesting that prolonging the storage of blood before transfusion does not appear to increase the levels of NTBI. In vehicle-treated mice after repeated blood transfusions, no significant effect was observed on plasma NTBI levels, supporting an acute increase in NTBI post transfusion and reflecting the late time point of blood sampling (24 hours post blood transfusion). As anticipated, vamifeport alone or combined with repeated transfusions had no significant effects on chronic NTBI levels (Online Supplementary Figure S1).
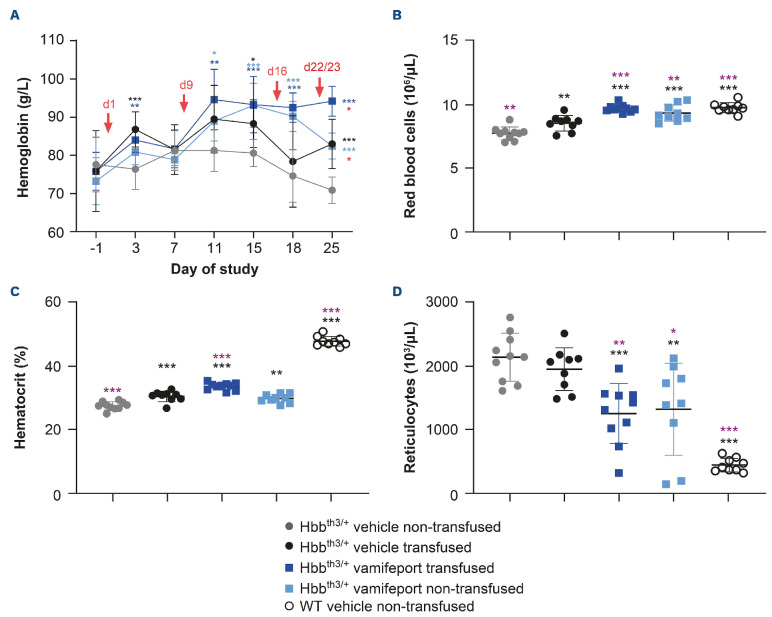
Vamifeport improved hematological parameters in Hbbth3/+ mice receiving repeated blood transfusions
Hbbth3/+ mice have lower levels of hemoglobin than their wild-type counterparts. Two days after the first blood transfusion, significantly higher hemoglobin levels were observed in Hbbth3/+ mice compared with non-transfused controls, both in the presence or absence of vamifeport (Figure 2). Vamifeport alone increased hemoglobin levels starting from study day 11 in Hbbth3/+ mice. Hemoglobin levels were significantly higher on study day 25 in the vamifeporttreated transfused group compared with the vamifeporttreated non-transfused group. Non-transfused, vehicletreated Hbbth3/+ mice exhibited significantly lower RBC counts and hematocrit levels and significantly higher reticulocyte counts than their wild-type counterparts. After repeated blood transfusions, RBC counts and hematocrit levels significantly increased in Hbbth3/+ mice, but levels remained lower than those seen in wild-type mice. Repeated blood transfusions had no significant impact on reticulocyte counts in this mouse model. Vamifeport treatment significantly increased RBC counts in Hbbth3/+ mice, independent of blood transfusion status. Vamifeport treatment also significantly increased hematocrit levels and decreased reticulocyte counts, independent of blood transfusion status. Furthermore, the transfusionmediated rise in hematocrit was additively increased when transfusion was combined with vamifeport treatment.
Figure 1.
Non-transferrin-bound iron levels in Hbbth3/+ mice afer vamifeport treatment alone or in combination with a single blood transfusion. Representative scatter plots of individual values (with mean and standard deviation) showing the effects of transfusion, vehicle, and vamifeport treatment. Donor blood was collected (A) 1 day or (B) 15 days before the blood transfusion. Analysis was performed by using one-way analysis of variance with Dunnett’s multiple comparison test. Significant differences compared with the Hbbth3/+ vehicle transfused group are indicated as: *P<0.05, **P<0.01, and ***P<0.001. NTBI: non-transferrinbound iron.
Figure 2.
Hematological parameters in Hbbth3/+ and wild-type mice afer vamifeport treatment alone or in combination with repeated blood transfusions. (A) Effects of transfusion, vehicle, and vamifeport treatment on hemoglobin levels. Timing of blood transfusions are indicated by red arrows. The remaining figures are representative scatter plots of individual values (with mean and standard deviation) showing the effects of transfusion, vehicle, and vamifeport treatment at the end of the study on: (B) red blood cell counts, (C) hematocrit levels, (D) reticulocyte counts. WT: wild-type. Parameters over time were analyzed by using two-way analysis of variance (ANOVA), with repeated measures for time course effects (A) and end point parameters by using one-way analysis of variance (B, C and D) with Dunnett’s multiple comparison test. Significant differences compared with the Hbbth3/+ vehicle non-transfused group (black) and vehicle-transfused group (purple) are indicated as: *P<0.05, **P<0.01, and ***P<0.001. In part (A), significant differences between the Hbbth3/+ vehicle non-transfused and the vehicle-transfused, vamifeport-treated transfused, and vamifeport-treated non-transfused groups are indicated in black, dark blue, and light blue, respectively. Significant differences between the vamifeport-treated transfused group compared with the vamifeport-treated non-transfused group are indicated in red (Wilcoxon matched pairs signed-rank test).
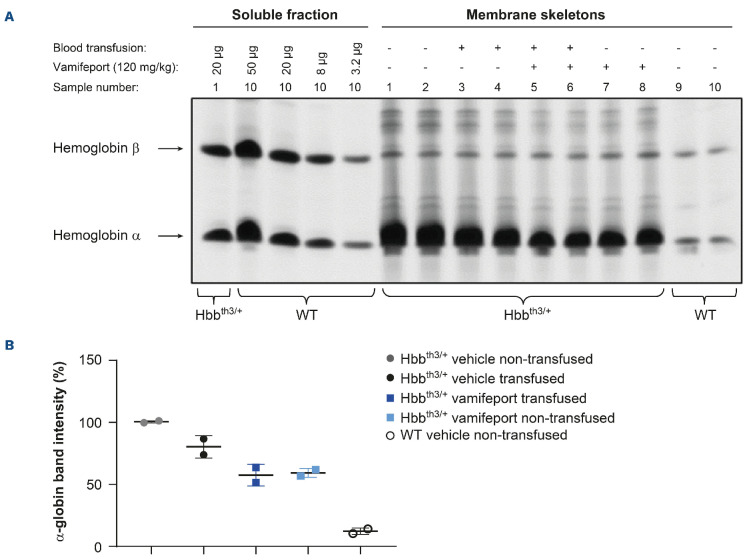
Vamifeport reduced the formation of α-globin aggregates in the membranes of red blood cells in Hbbth3/+ mice receiving repeated blood transfusions
The imbalanced synthesis of α- and b-globin chains of hemoglobin in Hbbth3/+ mice compared with wild-type mice leads to the formation of α-globin aggregates in RBC membranes.16 Vamifeport, alone or in combination with repeated blood transfusions, decreased α-globin aggregates in the RBC of Hbbth3/+ mice (Figure 3). Repeated blood transfusions alone caused a less pronounced reduction of α-globin aggregates in RBC compared with vamifeport plus repeated transfusion.
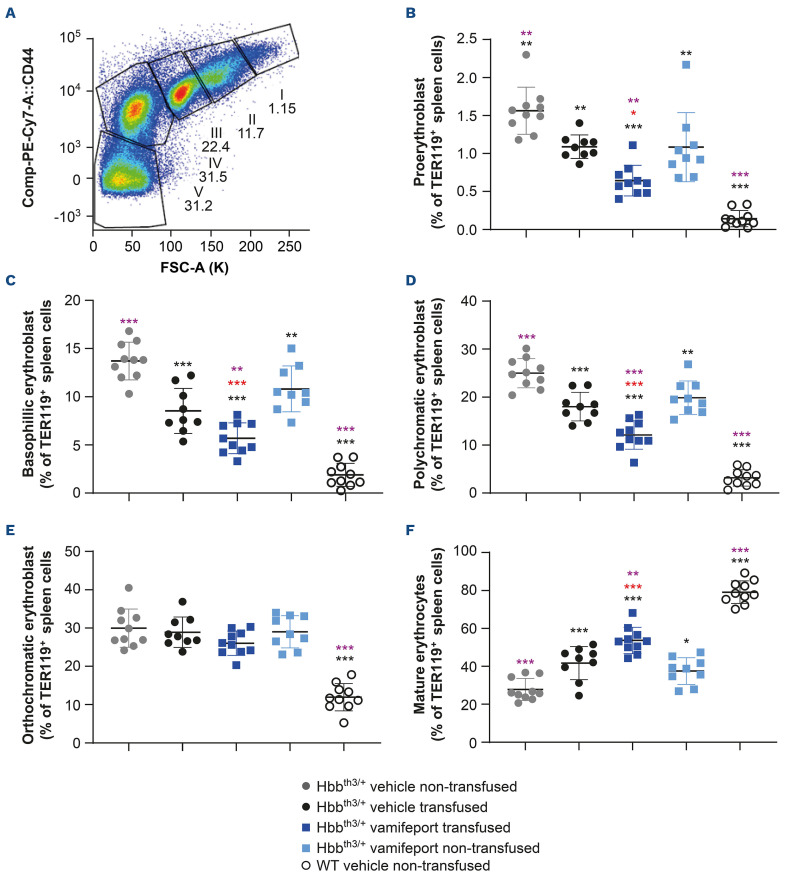
Vamifeport combined with repeated blood transfusions additively improved ineffective erythropoiesis in Hbbth3/+ mice
Non-transfused, vehicle-treated Hbbth3/+ mice exhibited ineffective erythropoiesis with increased proliferation of immature erythroid cells (proerythroblasts, basophilic erythroblasts, polychromatic erythroblasts, and orthochromatic erythroblasts) and decreased differentiation of erythroid progenitors to mature erythrocytes in the spleen, compared with their wild-type counterparts (Figure 4). Treatment with either vamifeport, or repeated blood transfusions alone, significantly improved ineffective erythropoiesis in the spleens of recipient Hbbth3/+ mice. Combining vamifeport with blood transfusions resulted in a lower proportion of immature erythroid cells and a higher percentage of mature erythrocytes, compared with either treatment alone, suggesting additive effects of vamifeport and repeated blood transfusions on erythropoiesis (donor-derived RBC were excluded from the erythropoiesis analysis).
Hbbth3/+ mice have increased spleen weight due to ineffective erythropoiesis in their spleens. In agreement with the improvement in ineffective erythropoiesis, repeated transfusions significantly reduced splenomegaly; splenomegaly was further reduced with vamifeport alone or combined with transfusion (Online Supplementary Figure S2).
Figure 3.
α-globin aggregates in red blood cell membranes of Hbbth3/+ mice afer vamifeport treatment alone or in combination with repeated blood transfusions. (A) TAU gel electrophoresis of membrane-bound globins in red blood cells from Hbbth3/+ and wild-type (WT) mice. Soluble α and b hemoglobin from wild-type red blood cells are shown for reference. (B) Quantification of the signal intensity of the TAU gel α bands by densitometry represented as a scatter plot of individual values (with mean and standard deviation).
Figure 4.
Erythropoiesis in the spleens of Hbbth3/+ mice afer vamifeport treatment alone or in combination with repeated blood transfusions. (A) Gating strategy used to identify erythroid progenitors in the spleen by flow cytometry: gate I, proerythroblast; gate II, basophilic erythroblast; gate III, polychromatic erythroblast; gate IV, orthochromatic erythroblast; gate V, mature erythrocytes. Green fluorescent protein-positive (GFP+) transfused donor cells are excluded from the analysis. The remaining figures are representative scatter plots of individual values (with mean and standard deviation) showing the effects of transfusion, vehicle, and vamifeport treatment at the end of the study on levels of: (B) proerythroblasts, (C) basophilic erythroblasts, (D) polychromatic erythroblasts, (E) orthochromatic erythroblasts, and (F) mature erythrocytes. WT: wild-type. Analysis was performed by using one-way analysis of variance with Dunnett’s multiple comparison test. Significant differences compared with the Hbbth3/+ vehicle non-transfused group (black) and vehicle-transfused group (purple) are indicated as: *P<0.05, **P<0.01, and ***P<0.001. Significant differences between the vamifeport-treated transfused group compared with the vamifeport-treated non-transfused group are indicated in red (unpaired t test).
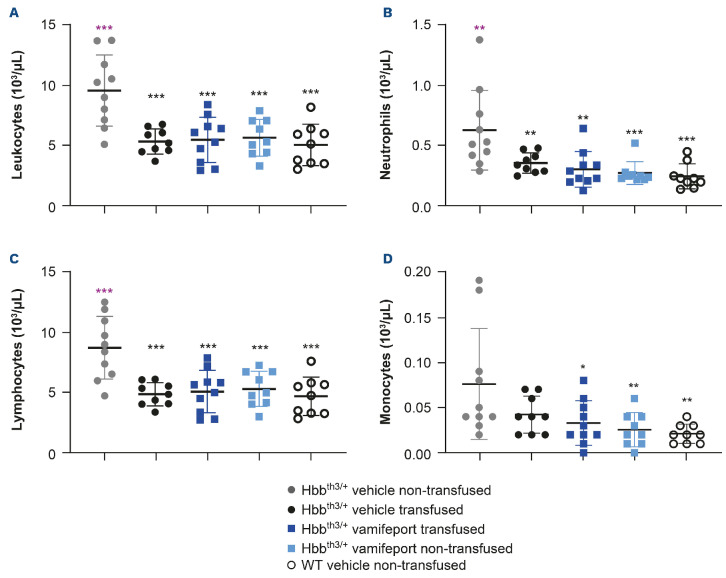
Vamifeport corrected leukocyte counts in Hbbth3/+ mice receiving repeated blood transfusions
Non-transfused, vehicle-treated Hbbth3/+ mice had significantly elevated overall leukocyte counts, including neutrophil, lymphocyte, and monocyte counts compared with their wild-type counterparts (Figure 5). Repeated blood transfusions significantly reduced overall leukocyte, neutrophil, and lymphocyte counts, but did not significantly impact monocyte numbers in Hbbth3/+ mice. Vamifeport, alone or in combination with repeated blood transfusions, significantly reduced overall leukocyte, neutrophil, and lymphocyte counts, and also significantly reduced monocyte counts in Hbbth3/+ mice. Levels of all these cells were similar to those observed in wild-type mice, demonstrating that iron restriction by vamifeport not only corrects ineffective erythropoiesis, but also leads to amelioration of abnormal myelopoiesis (both recipient and donor blood cells were included in these analyses).
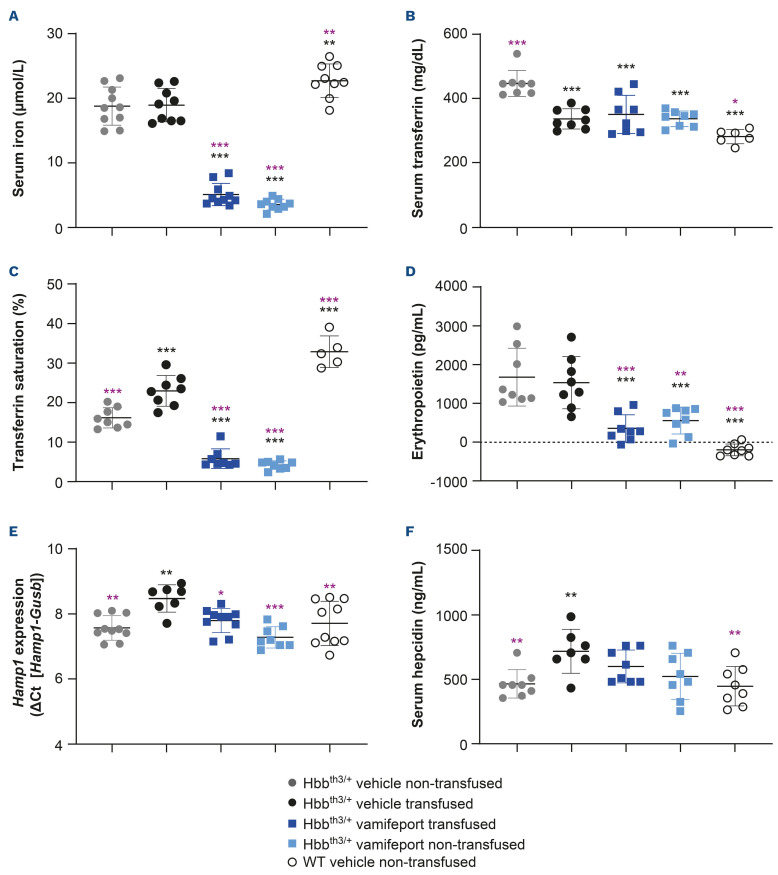
Vamifeport improved iron-related parameters in Hbbth3/+ mice receiving repeated blood transfusions
Hbbth3/+ mice had significantly lower serum iron and TSAT levels and significantly higher serum transferrin and erythropoietin levels than their wild-type counterparts (Figure 6). Repeated blood transfusions had no significant effect on serum iron or plasma erythropoietin levels but significantly decreased serum transferrin levels and significantly increased TSAT, liver Hamp expression, and plasma hepcidin levels. Vamifeport, alone or in combination with transfusions, significantly decreased serum iron, serum transferrin and TSAT levels in Hbbth3/+ mice when compared with vehicle-treated animals, reflecting the ability of vamifeport to inhibit ferroportin-mediated iron efflux into the plasma. This iron-lowering effect was transient, with the effect reversed 4–6 hours post dose, reflecting the pharmacodynamic activity of vamifeport inhibiting ferroportin. Vamifeport also significantly decreased plasma erythropoietin levels, both alone and when combined with repeated transfusions. Non-transfused, vehicle-treated Hbbth3/+ mice had similar liver Hamp1 mRNA levels and serum hepcidin levels as compared with wild-type mice. As expected, repeated blood transfusions significantly increased liver Hamp1 mRNA levels and serum hepcidin levels, compared with non-transfused, vehicle-treated controls. Vamifeport alone or combined with transfusion significantly decreased liver Hamp1 expression compared with vehicle-treated transfused Hbbth3/+ mice. Serum hepcidin levels in transfused Hbbth3/+ mice also reduced following vamifeport treatment, but this did not reach statistical significance.
Figure 5.
Leukocyte, neutrophil, lymphocyte, and monocyte counts in Hbbth3/+ mice afer vamifeport treatment alone or in combination with repeated blood transfusions. Representative scatter plots of individual values (with mean and standard deviation) showing the effects of transfusion, vehicle, and vamifeport treatment at the end of the study on counts of: (A) leukocytes, (B) neutrophils, (C) lymphocytes, (D) monocytes. WT: wild-type. Analysis was performed by using one-way analysis of variance with Dunnett’s multiple comparison test. Significant differences compared with the Hbbth3/+ vehicle non-transfused group (black) are indicated as: *P<0.05, **P<0.01, and ***P<0.001. There were no significant differences between any other treatment group compared with the vehicle-transfused group, for any of these parameters.
Figure 6.
Iron-related parameters in Hbbth3/+ mice afer vamifeport treatment alone or in combination with repeated blood transfusions. Representative scatter plots of individual values (with mean and standard deviation) showing the effects of transfusion, vehicle, and vamifeport treatment at the end of the study on: (A) serum iron, (B) transferrin, (C) transferrin saturation, (D) erythropoietin, (E) Hamp1 mRNA, (F) serum hepcidin. WT: wild-type. Analysis was performed by using one-way analysis of variance with Dunnett’s multiple comparison test. Significant differences compared with the Hbbth3/+ vehicle nontransfused group (black) and vehicle-transfused group (purple) are indicated as: *P<0.05, **P<0.01, and ***P<0.001.
Non-transfused, vehicle-treated Hbbth3/+ mice had elevated liver and kidney iron concentrations compared with their wild-type counterparts; heart iron concentration was significantly lower in Hbbth3/+ mice compared with wild-type mice (Online Supplementary Figure S3). Repeated blood transfusions had no effects on iron concentrations in liver or heart, but increased kidney iron levels in Hbbth3/+ mice, likely due to iron derived from transfused RBC.11 In transfused or non-transfused Hbbth3/+ mice, vamifeport had no significant effects compared with vehicle treatment on liver and heart iron content, suggesting that vamifeport does not remove existing iron, but rather prevents further iron accumulation in cells from these tissues.1 In contrast, vamifeport significantly reduced spleen iron content in non-transfused Hbbth3/+ mice (Online Supplementary Figure S2). Although transfusion alone did not affect spleen iron content, it did significantly increase spleen iron concentration, which might be due to iron originating from transfused RBC. Vamifeport alone significantly increased spleen iron concentration and combining vamifeport with transfusion resulted in an additive effect. This additive effect most likely reflects iron derived from recycled RBC. Hbbth3/+ mice have significantly increased spleen Erfe expression compared with wild-type control mice. Repeated transfusions had no effect on spleen Erfe expression, but when transfusion was combined with vamifeport treatment, Erfe expression was significantly reduced. Vamifeport alone had no effect on spleen Erfe expression in this experimental setting in Hbbth3/+ mice.
Discussion
After a single blood transfusion, vamifeport prevented the formation of blood transfusion-mediated NTBI in the plasma of Hbbth3/+ mice, suggesting that the iron restriction resulting from ferroportin inhibition prevents the formation of potentially harmful reactive plasma iron species. In the present study, plasma NTBI levels returned to baseline levels at 6 hours post blood transfusion; one possible explanation for this is that blood transfusion induces hepcidin (Hamp1) expression, which prevents the formation of NTBI. Another possible explanation for this is that NTBI is rapidly cleared from plasma by parenchymal tissues, as has previously been demonstrated in rodent models.20 We also assessed the longer-term effects of vamifeport after repeated blood transfusions; these studies did not use the same time points as the single blood transfusion study (3 hours and 6 hours post transfusion) due to ethical concerns with repeated blood sampling in mice so soon after tail vein blood transfusion. NTBI did not accumulate in Hbbth3/+ mice after four repeated blood transfusions, hence we did not observe any effect on NTBI levels with vamifeport treatment, most likely due to the timing of the blood sampling post transfusion (24 hours). These observations are of interest as patients with transfusion-dependent β-thalassemia receiving regular transfusions and iron chelation therapy have been shown to have elevated NTBI levels.12 Those with heart disease reportedly also had significantly higher NTBI levels than those without,12 suggesting a potential link between transfusional iron overload and heart disease. Our findings in this mouse model suggest that vamifeport might prevent such adverse effects by sequestrating NTBI in macro-phages, thereby interrupting a vicious cycle in β-thalassemia.1
Vamifeport ameliorated anemia and improved ineffective erythropoiesis and dysregulated iron homeostasis in Hbbth3/+ mice, either when administered alone or in combination with repeated blood transfusions. Importantly, when given along with blood transfusions, vamifeport additively ameliorated ineffective erythropoiesis in Hbbth3/+ mice. The effects of vamifeport on erythropoiesis were also associated with a significant reduction in splenomegaly. These results are consistent with our previous observations in Hbbth3/+ mice under non-transfused conditions.1 β-thalassemia is associated with several abnormalities of the innate immune system. The Hbbth3/+ mice show substantially elevated leukocyte counts compared with wild-type mice, as we have previously reported.21 Furthermore, vamifeport normalizes the myeloid spleen composition by reducing the proportion of immature myeloid cells and inflammatory monocytes.1 It has also been shown that the proportion of immature myeloid cells is significantly increased in Hbbth3/+ mice compared with wild-type mice,22 suggesting that abnormal myeloid cell maturation could contribute to the increased levels of circulating granulocytes seen in Hbbth3/+ mice. We have recently demonstrated that vamifeport reduces systemic and vascular inflammation in Townes mice (a sickle cell disease model) as shown by lower levels of Cxcl1, RANTES and soluble P-selectin levels.15 Vamifeport treatment also reduced peripheral neutrophil counts in this model. Furthermore, plasma iron has been shown to control neutrophil production and function, and neutrophil production is much more iron-demanding than the generation of other white blood cell types.23 Hence, we hypothesize that iron restriction by vamifeport-induced hypoferremia in Hbbth3/+ mice, could be a possible mechanism for the observed normalization of white blood cell counts during vamifeport treatment.
In our study, vamifeport also reduced the transfusion-mediated increase in hepcidin (Hamp1) expression, which led to an associated reduction in serum hepcidin levels. Although hepcidin levels increase following transfusions in patients with transfusion-dependent β-thalassemia,7 hepcidin levels may decrease during the intervals between blood transfusions, which could result in the formation of NTBI via ferroportin. It is possible that the ferroportin inhibitor vamifeport might prevent these noxious effects by two mechanisms: i) sequestration of iron from recycled RBC in macrophages; ii) prevention of increased intestinal iron absorption during the intervals between blood transfusions. Based on the data from the present study, it seems most likely that vamifeport inhibits ferroportin on macrophages recycling iron from engulfed RBC, and thereby prevents the generation of plasma NTBI.
The effects of other candidate drugs for β-thalassemia have been investigated in Hbbth3/+ mice, supporting the validity of this model. In β-thalassemia, increased erythropoietin production leads to persistent phosphorylation of JAK2, activated STAT5, and increased erythroid progenitor levels in the spleen, which result in splenomegaly and contribute to ineffective erythropoiesis.6 In Hbbth3/+ mice, administration of JAK2 inhibitors has been shown to decrease the number of erythroid nucleated cells in the spleen and to reduce splenomegaly, but was also associated with a slight reduction in hemoglobin levels.17 Coadministration of JAK2 inhibitors with blood transfusion further reduced splenomegaly in both Hbbth3/+ mice and mice requiring chronic transfusions (Hbbth3/th3).17 However, co-administration did not significantly affect hemoglobin or circulating RBC levels. Combining vamifeport with blood transfusions in the current study produced additive effects on erythropoiesis, with reduced proportions of immature erythroid cells and increased proportions of mature erythrocytes observed, and a reduction in splenomegaly, compared with either treatment alone. In contrast to findings with JAK2 inhibitors, however, vamifeport significantly increased hemoglobin levels when given alone or combined with transfusion. The effects of apo-transferrin on NTBI levels have also been assessed in the Hbbth3/+ mouse model.24 The rapid reduction in NTBI levels observed after vamifeport treatment in the present study are in line with data reported after the administration of apo-transferrin. The effects of the SMAD2/3 signaling inhibitor RAP-536 (the mouse version of luspatercept) have been investigated in a different mouse model of β-thalassemia intermedia (Hbbth1/th1).25,26 In this model, RAP-536 had similar effects to those reported here for vamifeport: it reduced α-globin aggregates in RBC, promoted differentiation of late-stage erythroid precursors, reduced anemia, and improved iron overload, splenomegaly, and erythrocyte life span.25,26
Due to the very low levels of hemoglobin and RBC production and their short lifespan, it is not possible to fully assess the effects of some candidate thalassemia treatments in the Hbbth3/th3 mouse chronic transfusion model. Casu et al., therefore, developed the C57-FLCth1/th2 mouse model, which more closely resembles the phenotype of human transfusion-dependent thalassemia.27 In the C57-FLCth1/th2 model, minihepcidins alone improved RBC life-span, ineffective erythropoiesis, and splenomegaly. When combined with chronic RBC transfusion, ineffective erythropoiesis and splenomegaly were further improved and iron overload was reversed in this mouse model. As similar results were observed in the present study, this may provide some validation for the repeated-transfusion model used here. In contrast to our results in the Hbbth3/+ model, however, minihepcidins did not improve anemia but did decrease liver iron content when combined with transfusions in the C57-FLCth1/th2 mouse model.27 As mentioned previously, Hbbth3/+ mice are a well-established disease model of β-thalassemia intermedia.16 Currently, the published disease models for transfusion-dependent thalassemia are complex and difficult to access. Although Hbbth3/+ mice do not require blood transfusions for survival, they are anemic and have reduced RBC counts, which makes this model suitable for studying the effects of vamifeport combined with transfusions. Furthermore, some patients with thalassemia intermedia receive sporadic blood transfusions if their blood parameters and physical condition deteriorate. Although this and previously published studies17 demonstrated that it is possible to establish a model for repeated blood transfusions in Hbbth3/+ mice, the system presented here is not a model for transfusion-dependent β-thalassemia. Due to its positive effects on ineffective erythropoiesis, splenomegaly, and anemia in Hbbth3/+ mice, it would be of interest to also assess the effects of vamifeport in the C57-FLCth1/th2 mouse model of transfusion-dependent thalassemia.
The pharmacodynamic effects of vamifeport have also recently been evaluated in phase I and II clinical trials. Vamifeport reduced serum iron and TSAT levels in healthy volunteers28 and more recently has been shown to reduce serum iron and TSAT levels in patients with non-transfusion-dependent β-thalassemia.29 In addition to β-thalassemia, vamifeport has been shown to correct ineffective erythropoiesis and to ameliorate abnormal myelopoiesis in a mouse model of myelodysplastic syndrome.30 Moreover, vamifeport prevented further liver iron overload in a mouse model of hereditary hemochromatosis,31 improved hemodynamics in a mouse model of sickle cell disease,15 and corrected hematocrit and RBC levels in a mouse model of polycythemia vera.32,33 The clinical relevance of these observations in rodent disease models now needs to be assessed in controlled clinical trials. In summary, in the present study in Hbbth3/+ mice, vamifeport prevented the formation of transfusion-mediated NTBI and ameliorated anemia, ineffective erythropoiesis, and dysregulated iron homeostasis, either when given alone or when combined with repeated blood transfusions. Importantly, administering vamifeport together with blood transfusions additively ameliorated anemia and ineffective erythropoiesis in this mouse model. The clinical utility of combination treatment with vamifeport and transfusions in patients with β-thalassemia receiving blood transfusions remains to be investigated in controlled clinical trials.
Supplementary Material
Acknowledgments
The authors thank the following colleagues from CSL Vifor for their valuable contributions: Stefan Reim and the chemical development team for providing vamifeport; and Maria Wilhelm, Anna-Lena Steck, Martin Khoeiklang, and the analytical team for tissue iron analyses. Special thanks to the animal facility head Marco Franchini and animal caretaker Martin Haenggi. Medical writing support, including development of a draft outline and subsequent drafts in consultation with the authors, collating author comments, copyediting, fact checking, and referencing, was provided by Dawn Batty, PhD, at Aspire Scientific Limited (Bollington, UK).
Funding Statement
Funding: This study was funded by CSL Vifor (St. Gallen, Switzerland). Funding for medical writing support for this article was provided by CSL Vifor.
References
- 1.Manolova V, Nyffenegger N, Flace A, et al. Oral ferroportin inhibitor ameliorates ineffective erythropoiesis in a model of β-thalassemia. J Clin Invest. 2019;130(1):491-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaing TH, Chang TY, Chen SH, Lin CW, Wen YC, Chiu CC. Molecular genetics of β-thalassemia: a narrative review. Medicine (Baltimore). 2021;100(45):e27522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taher AT, Weatherall DJ, Cappellini MD. Thalassaemia. Lancet. 2018;391(10116):155-167. [DOI] [PubMed] [Google Scholar]
- 4.Fibach E, Rachmilewitz EA. Pathophysiology and treatment of patients with beta-thalassemia - an update. F1000Res. 2017;6:2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khandros E, Kwiatkowski JL. Beta thalassemia: monitoring and new treatment approaches. Hematol Oncol Clin North Am. 2019;33(3):339-353. [DOI] [PubMed] [Google Scholar]
- 6.Ginzburg Y, Rivella S. β-thalassemia: a model for elucidating the dynamic regulation of ineffective erythropoiesis and iron metabolism. Blood. 2011;118(16):4321-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasricha SR, Frazer DM, Bowden DK, Anderson GJ. Transfusion suppresses erythropoiesis and increases hepcidin in adult patients with β-thalassemia major: a longitudinal study. Blood. 2013;122(1):124-133. [DOI] [PubMed] [Google Scholar]
- 8.Porter J, Taher A, Viprakasit V, et al. Oral ferroportin inhibitor vamifeport for improving iron homeostasis and erythropoiesis in β-thalassemia: current evidence and future clinical development. Expert Rev Hematol. 2021;14(7):633-644. [DOI] [PubMed] [Google Scholar]
- 9.Musallam KM, Rivella S, Vichinsky E, Rachmilewitz EA. Non-transfusion-dependent thalassemias. Haematologica. 2013;98(6):833-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olivieri NF. The beta-thalassemias. N Engl J Med. 1999;341(2):99-109. [DOI] [PubMed] [Google Scholar]
- 11.Baek JH, Shin HKH, Gao Y, Buehler PW. Ferroportin inhibition attenuates plasma iron, oxidant stress, and renal injury following red blood cell transfusion in guinea pigs. Transfusion. 2020;60(3):513-523. [DOI] [PubMed] [Google Scholar]
- 12.Piga A, Longo F, Duca L, et al. High nontransferrin bound iron levels and heart disease in thalassemia major. Am J Hematol. 2009;84(1):29-33. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt PJ, Fleming MD. Modulation of hepcidin as therapy for primary and secondary iron overload disorders: preclinical models and approaches. Hematol Oncol Clin North Am. 2014;28(2):387-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginzburg YZ. Hepcidin-ferroportin axis in health and disease. Vitam Horm. 2019;110:17-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyffenegger N, Zennadi R, Kalleda N, et al. The oral ferroportin inhibitor vamifeport improved hemodynamics in a mouse model of sickle cell disease. Blood. 2022;140(7):769-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang B, Kirby S, Lewis J, Detloff PJ, Maeda N, Smithies O. A mouse model for beta 0-thalassemia. Proc Natl Acad Sci U S A. 1995;92(25):11608-11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casu C, Presti VL, Oikonomidou PR, et al. Short-term administration of JAK2 inhibitors reduces splenomegaly in mouse models of β-thalassemia intermedia and major. Haematologica. 2018;103(2):e46-e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huo Y, McConnell SC, Ryan TM. Preclinical transfusiondependent humanized mouse model of beta thalassemia major. Blood. 2009;113(19):4763-4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkitkasemwong S, Wang CY, Knutson MD. Measurement of transferrin- and non-transferrin-bound iron uptake by mouse tissues. Bio Protoc. 2016;6(17):e1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craven CM, Alexander J, Eldridge M, Kushner JP, Bernstein S, Kaplan J. Tissue distribution and clearance kinetics of non-transferrin-bound iron in the hypotransferrinemic mouse: a rodent model for hemochromatosis. Proc Natl Acad Sci U S A. 1987;84(10):3457-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nyffenegger N, Flace A, Doucerain C, Dürrenberger F, Manolova V. The oral ferroportin inhibitor VIT-2763 improves erythropoiesis without interfering with iron chelation therapy in a mouse model of β-thalassemia. Int J Mol Sci. 2021;22(2):873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siwaponanan P, Siegers JY, Ghazali R, et al. Reduced PU.1 expression underlies aberrant neutrophil maturation and function in β-thalassemia mice and patients. Blood. 2017;129(23):3087-3099. [DOI] [PubMed] [Google Scholar]
- 23.Frost JN, Wideman SK, Preston AE, et al. Plasma iron controls neutrophil production and function. Sci Adv. 2022;8(40):eabq5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gelderman MP, Baek JH, Yalamanoglu A, et al. Reversal of hemochromatosis by apotransferrin in non-transfused and transfused Hbbth3/+ (heterozygous B1/B2 globin gene deletion) mice. Haematologica. 2015;100(5):611-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suragani RN, Cawley SM, Li R, et al. Modified activin receptor IIB ligand trap mitigates ineffective erythropoiesis and disease complications in murine β-thalassemia. Blood. 2014;123(25):3864-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suragani RN, Cadena SM, Cawley SM, et al. Transforming growth factor-β superfamily ligand trap ACE-536 corrects anemia by promoting late-stage erythropoiesis. Nat Med. 2014;20(4):408-414. [DOI] [PubMed] [Google Scholar]
- 27.Casu C, Chessa R, Liu A, et al. Minihepcidins improve ineffective erythropoiesis and splenomegaly in a new mouse model of adult β-thalassemia major. Haematologica. 2020;105(7):1835-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richard F, van Lier JJ, Roubert B, Haboubi T, Göhring UM, Dürrenberger F. Oral ferroportin inhibitor VIT-2763: first-inhuman, phase 1 study in healthy volunteers. Am J Hematol. 2020;95(1):68-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taher A, Kourakli-Symeonidis A, Tantiworawit A, Wong P, Szecsödy P. Safety and preliminary pharmacodynamic effects of the ferroportin inhibitor vamifeport (VIT-2763) in patients with non-transfusion-dependent beta-thalassemia (NTDT): results from a phase 2A study. HemaSphere. 2022;6(Suppl 3):350. [Google Scholar]
- 30.Vance SZ, Antypiuk A, Sharma S, Dürrenberger F, Manolova V, Vinchi F. Alteration of iron homeostasis through genetic and pharmacologic modulation of ferroportin modifies MDS pathophysiology in a preclinical mouse model. HemaSphere. 2022;6(Suppl 3):356-357. [Google Scholar]
- 31.Nyffenegger N, Flace A, Varol A, et al. Oral ferroportin inhibitor prevents iron overload in the HFE C282Y mouse model of hereditary hemochromatosis. European Iron Club Annual Meeting: Oral Sessions and Posters. 2018; February:54. [Google Scholar]
- 32.Kubovcakova L, Manolova V, Nyffenegger N, et al. Efficacy of oral ferroportin inhibitor in a mouse model of polycythemia vera. European Iron Club Annual Meeting: Oral Sessions and Posters. 2018; February:144. [Google Scholar]
- 33.Stetka J, Usart M, Kubovcakova L, et al. Iron is a modifier of the phenotypes of JAK2-mutant myeloproliferative neoplasms. Blood. 2023;141(17):2127-2140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.