Abstract
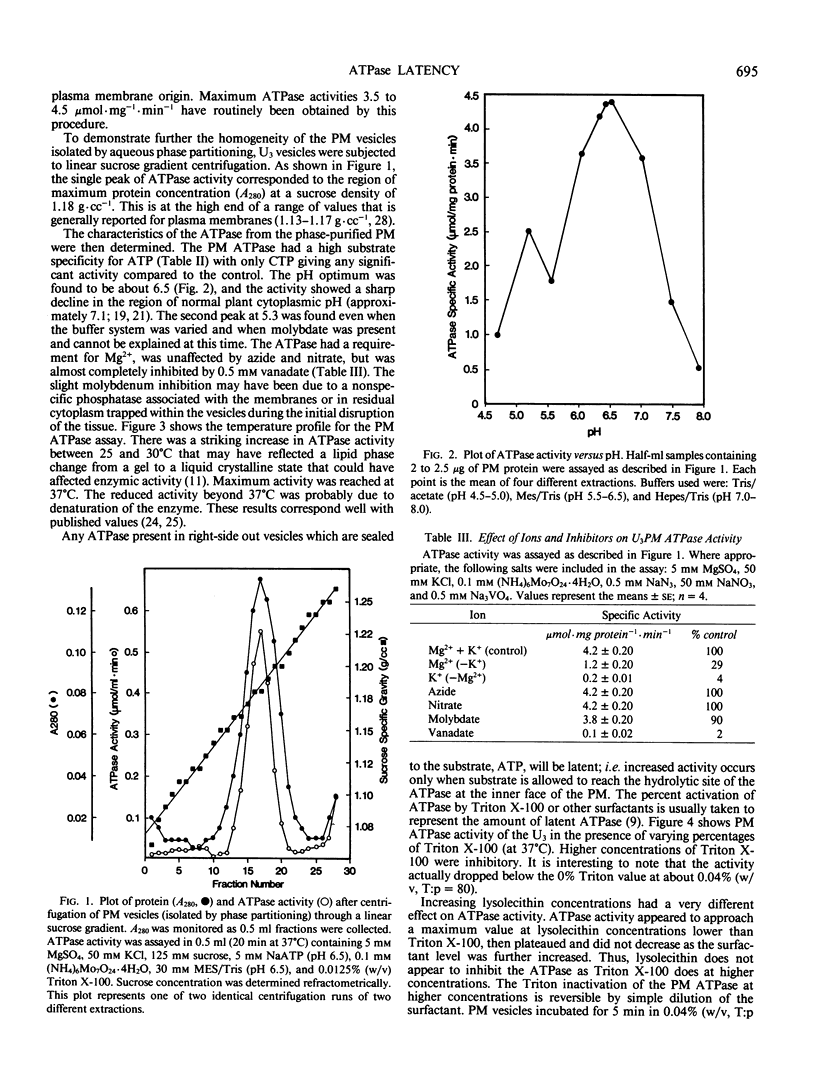
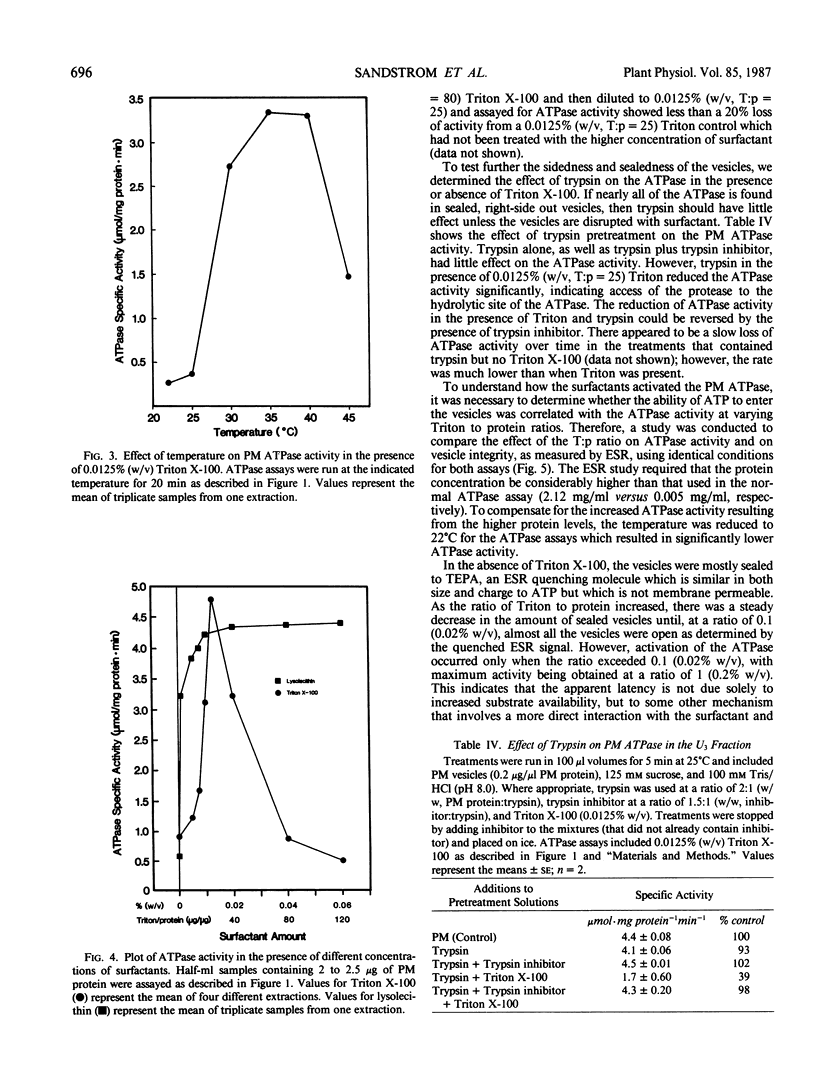
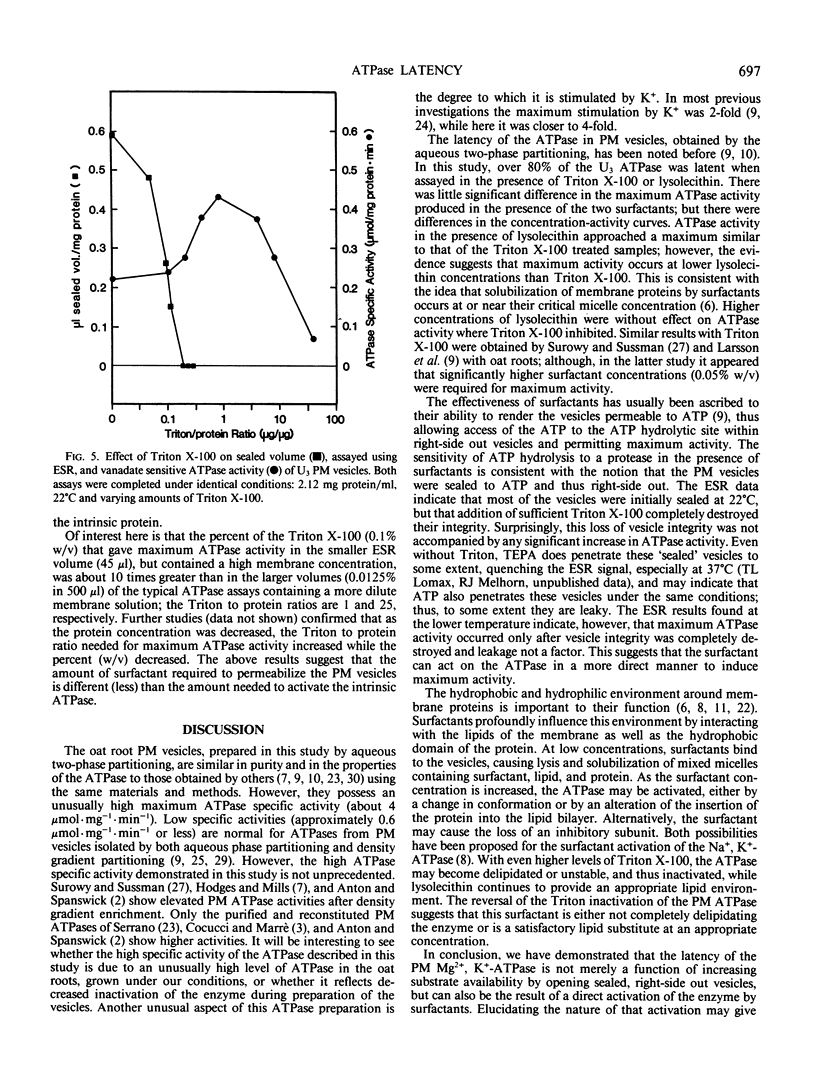
The properties of the plasma membrane H+-ATPase and the cause of its latency have been studied using a highly purified plasma membrane fraction from oat (Avena sativa L., cv Victory) roots, prepared by aqueous two-phase partitioning. The ATPase has a maximum specific activity (at 37°C) in excess of 4 micromoles inorganic phosphate per milligram protein per minute in the presence of nondenaturing surfactants. It is inhibited by more than 90% by vanadate, is specific for ATP, has a pH optimum of 6.5, and is stimulated more than 4-fold by 50 millimolar K+ in the presence of low levels of the nondenaturing surfactants Triton X-100 and lysolecithin. This `latent' activity is usually explained as being a result of the inability of ATP to reach the ATPase in right-side out, sealed vesicles, until they are disrupted by surfactants. Consistent with this idea, trypsin digestion significantly inhibited the ATPase only in the presence of the surfactants. Electron spin resonance spectroscopy volume measurements confirmed that surfactant-free vesicles were mostly sealed to molecules similar to ATP. However, the Triton to protein ratio required to disrupt vesicle integrity completely is 10-fold less than that needed to promote maximum ATPase activity. We propose that plasma membrane ATPase activation is due not solely to vesicle disruption and accessibility of ATP to the ATPase but to the surfactants activating the ATPase by altering the lipid environment in its vicinity or by removing an inhibitory subunit.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthon G. E., Spanswick R. M. Purification and properties of the h-translocating ATPase from the plasma membrane of tomato roots. Plant Physiol. 1986 Aug;81(4):1080–1085. doi: 10.1104/pp.81.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulley J. R. Determination of inorganic phosphate in the presence of detergents or protein. Anal Biochem. 1975 Jul;67(1):91–96. doi: 10.1016/0003-2697(75)90275-4. [DOI] [PubMed] [Google Scholar]
- Gabathuler R., Cleland R. E. Auxin regulation of a proton translocating ATPase in pea root plasma membrane vesicles. Plant Physiol. 1985 Dec;79(4):1080–1085. doi: 10.1104/pp.79.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Jørgensen P. L. Mechanism of the Na+, K+ pump. Protein structure and conformations of the pure (Na+ +K+)-ATPase. Biochim Biophys Acta. 1982 Aug 11;694(1):27–68. doi: 10.1016/0304-4157(82)90013-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandala S., Taiz L. Partial purification of a tonoplast ATPase from corn coleoptiles. Plant Physiol. 1985 Jun;78(2):327–333. doi: 10.1104/pp.78.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Rasi-Caldogno F., De Michelis M. I., Pugliarello M. C., Marrè E. H-pumping driven by the plasma membrane ATPase in membrane vesicles from radish: stimulation by fusicoccin. Plant Physiol. 1986 Sep;82(1):121–125. doi: 10.1104/pp.82.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. Control of plant cell enlargement by hydrogen ions. Curr Top Dev Biol. 1977;11:187–214. doi: 10.1016/s0070-2153(08)60746-2. [DOI] [PubMed] [Google Scholar]
- Sandermann H., Jr Regulation of membrane enzymes by lipids. Biochim Biophys Acta. 1978 Sep 29;515(3):209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Serrano R. Purification of the proton pumping ATPase from plant plasma membranes. Biochem Biophys Res Commun. 1984 Jun 15;121(2):735–740. doi: 10.1016/0006-291x(84)90243-2. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E. Isolation of subcellular organelles of metabolism on isopycnic sucrose gradients. Methods Enzymol. 1974;31:734–746. doi: 10.1016/0076-6879(74)31077-4. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Uemura M., Niki T., Sakai A., Gusta L. V. Partition of membrane particles in aqueous two-polymer phase system and its practical use for purification of plasma membranes from plants. Plant Physiol. 1983 May;72(1):105–114. doi: 10.1104/pp.72.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]


