Abstract
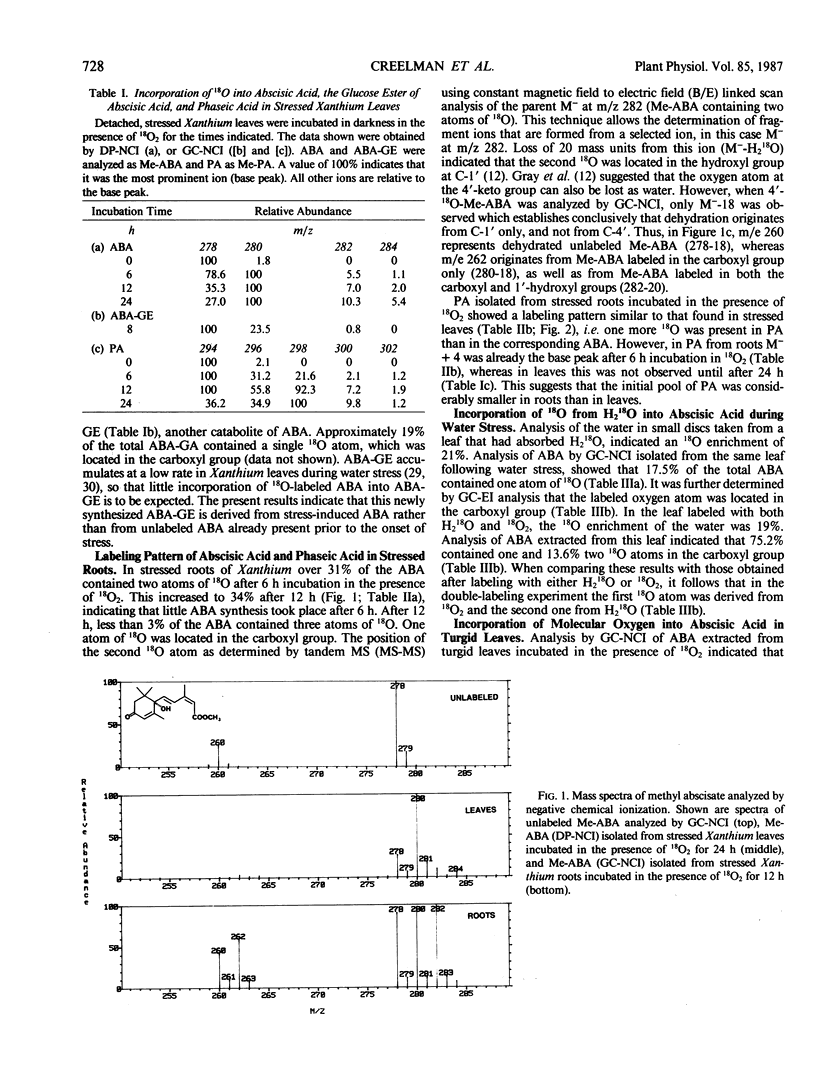
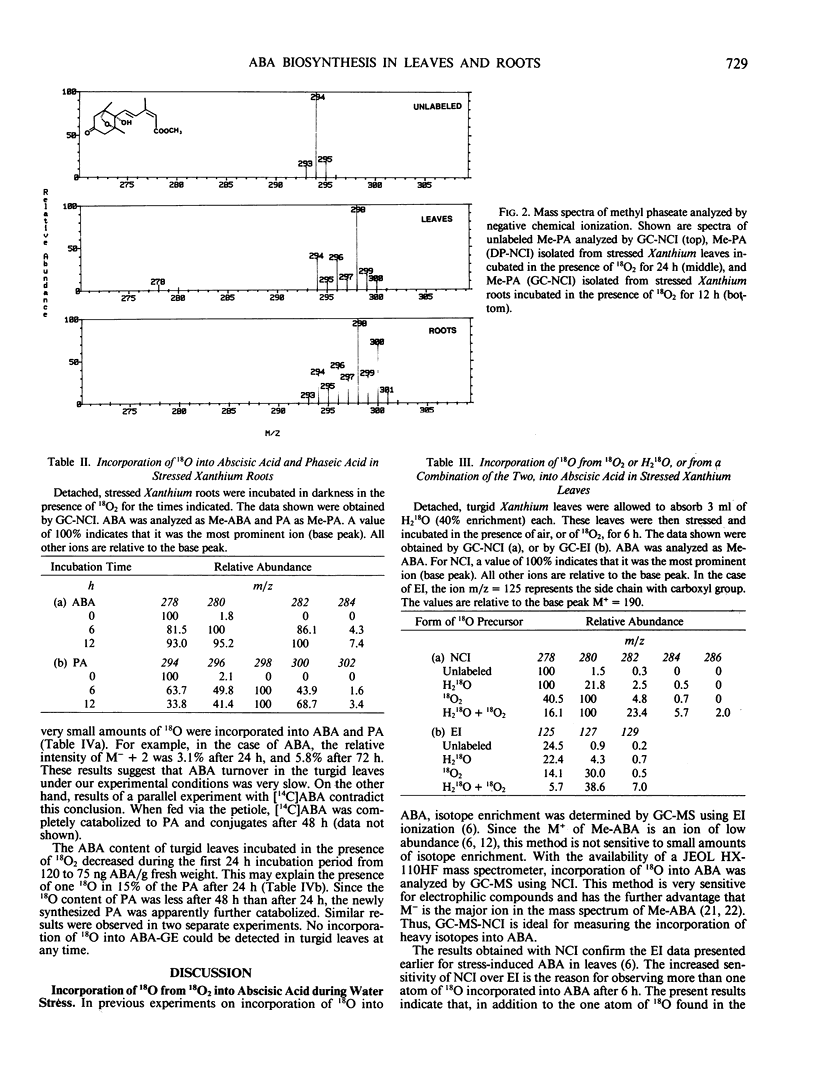
Research on the biosynthesis of abscisic acid (ABA) has focused primarily on two pathways: (a) the direct pathway from farnesyl pyrophosphate, and (b) the indirect pathway involving a carotenoid precursor. We have investigated which biosynthetic pathway is operating in turgid and stressed Xanthium leaves, and in stressed Xanthium roots using long-term incubations in 18O2. It was found that in stressed leaves three atoms of 18O from 18O2 are incorporated into the ABA molecule, and that the amount of 18O incorporated increases with time. One 18O atom is incorporated rapidly into the carboxyl group of ABA, whereas the other two atoms are very slowly incorporated into the ring oxygens. The fourth oxygen atom in the carboxyl group of ABA is derived from water. ABA from stressed roots of Xanthium incubated in 18O2 shows a labeling pattern similar to that of ABA in stressed leaves, but with incorporation of more 18O into the tertiary hydroxyl group at C-1′ after 6 and 12 hours than found in ABA from stressed leaves. It is proposed that the precursors to stress-induced ABA are xanthophylls, and that a xanthophyll lacking an oxygen function at C-6 (carotenoid numbering scheme) plays a crucial role in ABA biosynthesis in Xanthium roots. In turgid Xanthium leaves, 18O is incorporated into ABA to a much lesser extent than it is in stressed leaves, whereas exogenously applied 14C-ABA is completely catabolized within 48 hours. This suggests that ABA in turgid leaves is either (a) made via a biosynthetic pathway which is different from the one in stressed leaves, or (b) has a half-life on the order of days as compared with a half-life of 15.5 hours in water-stressed Xanthium leaves. Phaseic acid showed a labeling pattern similar to that of ABA, but with an additional 18O incorporated during 8′-hydroxylation of ABA to phaseic acid.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cornish K., Zeevaart J. A. Abscisic Acid Accumulation by Roots of Xanthium strumarium L. and Lycopersicon esculentum Mill. in Relation to Water Stress. Plant Physiol. 1985 Nov;79(3):653–658. doi: 10.1104/pp.79.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K., Zeevaart J. A. Abscisic Acid Metabolism in Relation to Water Stress and Leaf Age in Xanthium strumarium. Plant Physiol. 1984 Dec;76(4):1029–1035. doi: 10.1104/pp.76.4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman R. A., Zeevaart J. A. Incorporation of oxygen into abscisic Acid and phaseic Acid from molecular oxygen. Plant Physiol. 1984 May;75(1):166–169. doi: 10.1104/pp.75.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong F., Smith J. D., Koehler D. E. Early Events in Maize Seed Development : 1-Methyl-3-phenyl-5-(3-[trifluoromethyl]phenyl)-4-(1H)-Pyridinone Induction of Vivipary. Plant Physiol. 1983 Dec;73(4):899–901. doi: 10.1104/pp.73.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble P. E., Mullet J. E. Inhibition of carotenoid accumulation and abscisic acid biosynthesis in fluridone-treated dark-grown barley. Eur J Biochem. 1986 Oct 1;160(1):117–121. doi: 10.1111/j.1432-1033.1986.tb09947.x. [DOI] [PubMed] [Google Scholar]
- Harrison M. A., Walton D. C. Abscisic Acid Metabolism in Water-stressed Bean Leaves. Plant Physiol. 1975 Aug;56(2):250–254. doi: 10.1104/pp.56.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Smith J. D. Graviresponsiveness and abscisic-acid content of roots of carotenoid-deficient mutants of Zea mays L. Planta. 1985;164:126–128. [PubMed] [Google Scholar]
- Moore R., Smith J. D. Growth, graviresponsiveness and abscisic-acid content of Zea mays seedlings treated with fluridone. Planta. 1984;162:342–344. [PubMed] [Google Scholar]
- Yamamoto H. Y., Chichester C. O. Dark incorporation of 18-O2 into antheraxanthin by bean leaf. Biochim Biophys Acta. 1965 Sep 27;109(1):303–305. doi: 10.1016/0926-6585(65)90115-9. [DOI] [PubMed] [Google Scholar]
- Zeevaart J. A. Changes in the Levels of Abscisic Acid and Its Metabolites in Excised Leaf Blades of Xanthium strumarium during and after Water Stress. Plant Physiol. 1980 Oct;66(4):672–678. doi: 10.1104/pp.66.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A. Metabolism of Abscisic Acid and Its Regulation in Xanthium Leaves during and after Water Stress. Plant Physiol. 1983 Mar;71(3):477–481. doi: 10.1104/pp.71.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]


