Abstract
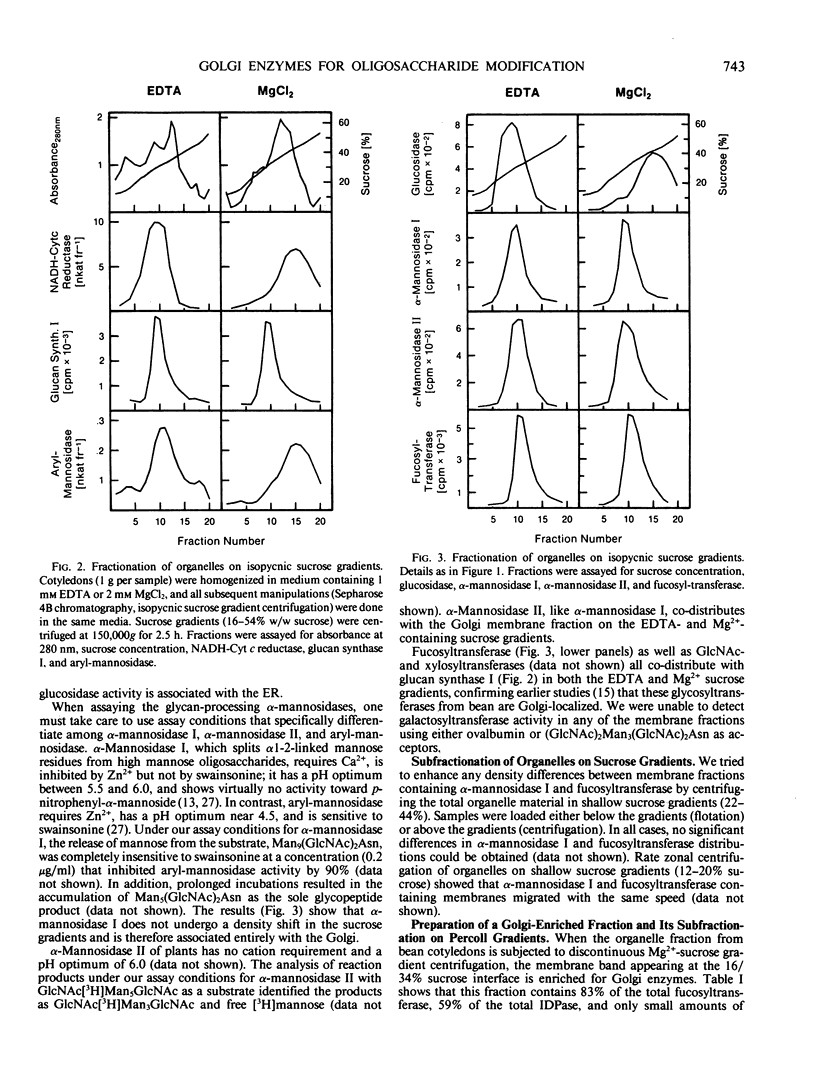
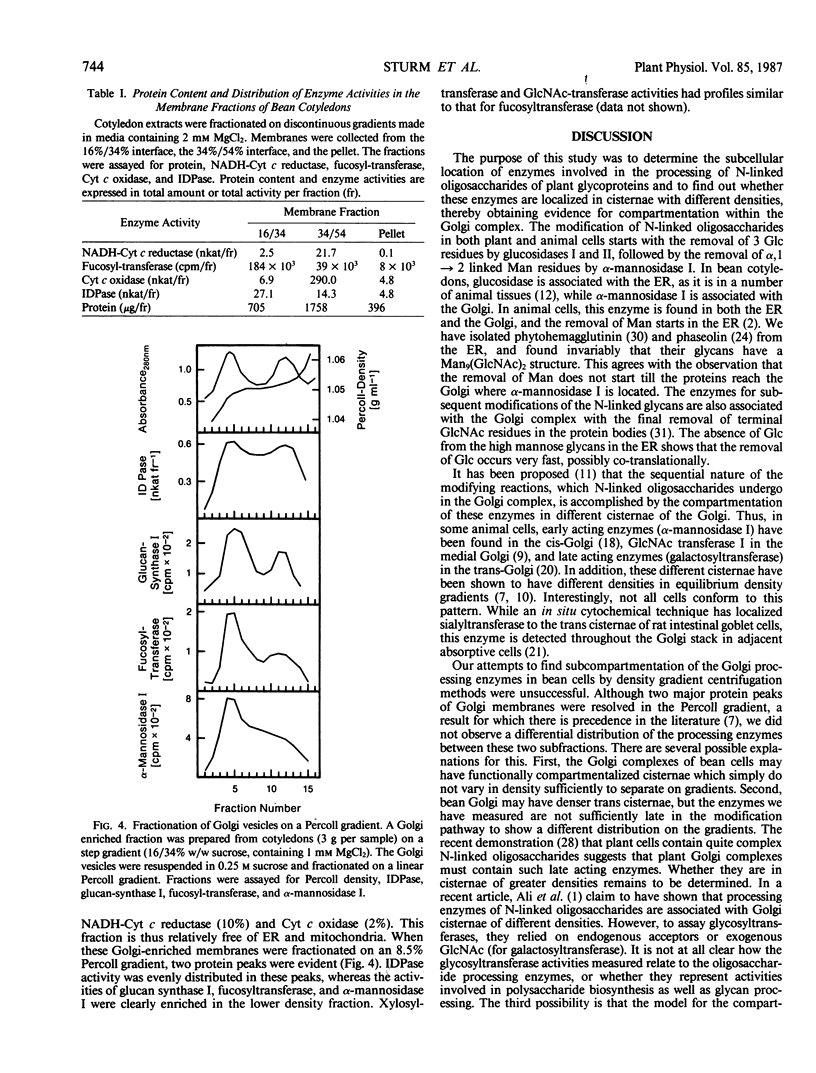
Using isopycnic sucrose gradients, we have ascertained the subcellular location of several enzymes involved in the processing of the N-linked oligosaccharides of glycoproteins in developing cotyledons of the common bean, Phaseolus vulgaris. All are localized in the endoplasmic reticulum (ER) or Golgi complex as determined by co-sedimentation with the ER marker, NADH-cytochrome c reductase, or the Golgi marker, glucan synthase I. Glucosidase activity, which removes glucose residues from Glc3Man9(GlcNAc)2, was found exclusively in the ER. All other processing enzymes, which act subsequent to the glucose trimming steps, are associated with the Golgi. These include mannosidase I (removes 1-2 mannose residues from Man6-9[GlcNAc]2), mannosidase II (removes mannose residues from GlcNAcMan5[GlcNAc]2), and fucosyltransferase (transfers a fucose residue to the Asn-linked GlcNAc of appropriate glycans). We have previously reported the localization of two other glycan modifying enzymes (GlcNAc-transferase and xylosyltransferase activities) in the Golgi complex. Attempts at subfractionation of the Golgi fraction on shallow sucrose gradients yielded similar patterns of distribution for all the Golgi processing enzymes. Subfractionation on Percoll gradients resulted in two peaks of the Golgi marker enzyme inosine diphosphatase, whereas the glycan processing enzymes were all enriched in the peak of lower density. These results do not lend support to the hypothesis that N-linked oligosaccharide processing enzymes are associated with Golgi cisternae of different densities.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali M. S., Mitsui T., Akazawa T. Golgi-specific localization of transglycosylases engaged in glycoprotein biosynthesis in suspension-cultured cells of sycamore (Acer pseudoplatanus L.). Arch Biochem Biophys. 1986 Dec;251(2):421–431. doi: 10.1016/0003-9861(86)90348-6. [DOI] [PubMed] [Google Scholar]
- Bischoff J., Kornfeld R. Evidence for an alpha-mannosidase in endoplasmic reticulum of rat liver. J Biol Chem. 1983 Jul 10;258(13):7907–7910. [PubMed] [Google Scholar]
- Chrispeels M. J. UDP-GlcNAc:Glycoprotein GlcNAc-Transferase is Located in the Golgi Apparatus of Developing Bean Cotyledons. Plant Physiol. 1985 Aug;78(4):835–838. doi: 10.1104/pp.78.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauwalder M., Whaley W. G., Kephart J. E. Phosphatases and differentiation of the Golgi apparatus. J Cell Sci. 1969 Mar;4(2):455–497. doi: 10.1242/jcs.4.2.455. [DOI] [PubMed] [Google Scholar]
- Deutscher S. L., Creek K. E., Merion M., Hirschberg C. B. Subfractionation of rat liver Golgi apparatus: separation of enzyme activities involved in the biosynthesis of the phosphomannosyl recognition marker in lysosomal enzymes. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3938–3942. doi: 10.1073/pnas.80.13.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson R. P., Tolbert N. E., Schnarrenberger C. A comparison of microbody membranes with microsomes and mitochondria from plant and animal tissue. Arch Biochem Biophys. 1972 Sep;152(1):199–215. doi: 10.1016/0003-9861(72)90208-1. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Brands R., Rothman J. E. Attachment of terminal N-acetylglucosamine to asparagine-linked oligosaccharides occurs in central cisternae of the Golgi stack. Cell. 1985 Feb;40(2):463–472. doi: 10.1016/0092-8674(85)90161-8. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Fries E., Urbani L. J., Rothman J. E. Early and late functions associated with the Golgi apparatus reside in distinct compartments. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7453–7457. doi: 10.1073/pnas.78.12.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W. G., Rothman J. E. Compartmentation of asparagine-linked oligosaccharide processing in the Golgi apparatus. J Cell Biol. 1983 Jul;97(1):270–275. doi: 10.1083/jcb.97.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elting J. J., Chen W. W., Lennarz W. J. Characterization of a glucosidase involved in an initial step in the processing of oligosaccharide chains. J Biol Chem. 1980 Mar 25;255(6):2325–2331. [PubMed] [Google Scholar]
- Forsee W. T. Characterization of microsomal and cytosolic alpha-1,2-mannosidases from mung bean hypocotyls. Arch Biochem Biophys. 1985 Oct;242(1):48–57. doi: 10.1016/0003-9861(85)90478-3. [DOI] [PubMed] [Google Scholar]
- Gardiner M., Chrispeels M. J. Involvement of the Golgi Apparatus in the Synthesis and Secretion of Hydroxyproline-rich Cell Wall Glycoproteins. Plant Physiol. 1975 Mar;55(3):536–541. doi: 10.1104/pp.55.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Chrispeels M. J. Substrate Specificities of N-Acetylglucosaminyl-, Fucosyl-, and Xylosyltransferases that Modify Glycoproteins in the Golgi Apparatus of Bean Cotyledons. Plant Physiol. 1987 Aug;84(4):1301–1308. doi: 10.1104/pp.84.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Nawa Y., Asahi T. Rapid Development of Mitochondria in Pea Cotyledons during the Early Stage of Germination. Plant Physiol. 1971 Dec;48(6):671–674. doi: 10.1104/pp.48.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann R., Waheed A., Hasilik A., von Figura K. Synthesis of phosphorylated recognition marker in lysosomal enzymes is located in the cis part of Golgi apparatus. J Biol Chem. 1982 May 25;257(10):5323–5325. [PubMed] [Google Scholar]
- Ray P. M. Auxin-binding Sites of Maize Coleoptiles Are Localized on Membranes of the Endoplasmic Reticulum. Plant Physiol. 1977 Apr;59(4):594–599. doi: 10.1104/pp.59.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Berger E. G. Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J Cell Biol. 1982 Apr;93(1):223–229. doi: 10.1083/jcb.93.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Taatjes D. J., Weinstein J., Paulson J. C., Greenwell P., Watkins W. M. Differential subcompartmentation of terminal glycosylation in the Golgi apparatus of intestinal absorptive and goblet cells. J Biol Chem. 1986 Oct 25;261(30):14307–14312. [PubMed] [Google Scholar]
- Rothman J. E., Fries E. Transport of newly synthesized vesicular stomatitis viral glycoprotein to purified Golgi membranes. J Cell Biol. 1981 Apr;89(1):162–168. doi: 10.1083/jcb.89.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter H., Narasimhan S., Gleeson P., Vella G. Glycosyltransferases involved in elongation of N-glycosidically linked oligosaccharides of the complex or N-acetyllactosamine type. Methods Enzymol. 1983;98:98–134. doi: 10.1016/0076-6879(83)98143-0. [DOI] [PubMed] [Google Scholar]
- Szumilo T., Elbein A. D. A simple and reliable assay for glycoprotein-processing glycosidases. Anal Biochem. 1985 Nov 15;151(1):32–40. doi: 10.1016/0003-2697(85)90049-1. [DOI] [PubMed] [Google Scholar]
- Szumilo T., Kaushal G. P., Elbein A. D. Purification and properties of glucosidase I from mung bean seedlings. Arch Biochem Biophys. 1986 Jun;247(2):261–271. doi: 10.1016/0003-9861(86)90583-7. [DOI] [PubMed] [Google Scholar]
- Szumilo T., Kaushal G. P., Hori H., Elbein A. D. Purification and Properties of a Glycoprotein Processing alpha-Mannosidase from Mung Bean Seedlings. Plant Physiol. 1986 Jun;81(2):383–389. doi: 10.1104/pp.81.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurl S., Witke W., Buhrow I., Schäfer W. Quinones from archaebacteria, II. Different types of quinones from sulphur-dependent archaebacteria. Biol Chem Hoppe Seyler. 1986 Mar;367(3):191–197. doi: 10.1515/bchm3.1986.367.1.191. [DOI] [PubMed] [Google Scholar]
- Van Der Wilden W., Chrispeels M. J. Characterization of the Isozymes of alpha-Mannosidase Located in the Cell Wall, Protein Bodies, and Endoplasmic Reticulum of Phaseolus vulgaris Cotyledons. Plant Physiol. 1983 Jan;71(1):82–87. doi: 10.1104/pp.71.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale A., Ceriotti A., Bollini R., Chrispeels M. J. Biosynthesis and processing of phytohemagglutinin in developing bean cotyledons. Eur J Biochem. 1984 May 15;141(1):97–104. doi: 10.1111/j.1432-1033.1984.tb08162.x. [DOI] [PubMed] [Google Scholar]
- Vitale A., Chrispeels M. J. Transient N-acetylglucosamine in the biosynthesis of phytohemagglutinin: attachment in the Golgi apparatus and removal in protein bodies. J Cell Biol. 1984 Jul;99(1 Pt 1):133–140. doi: 10.1083/jcb.99.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]


