Abstract
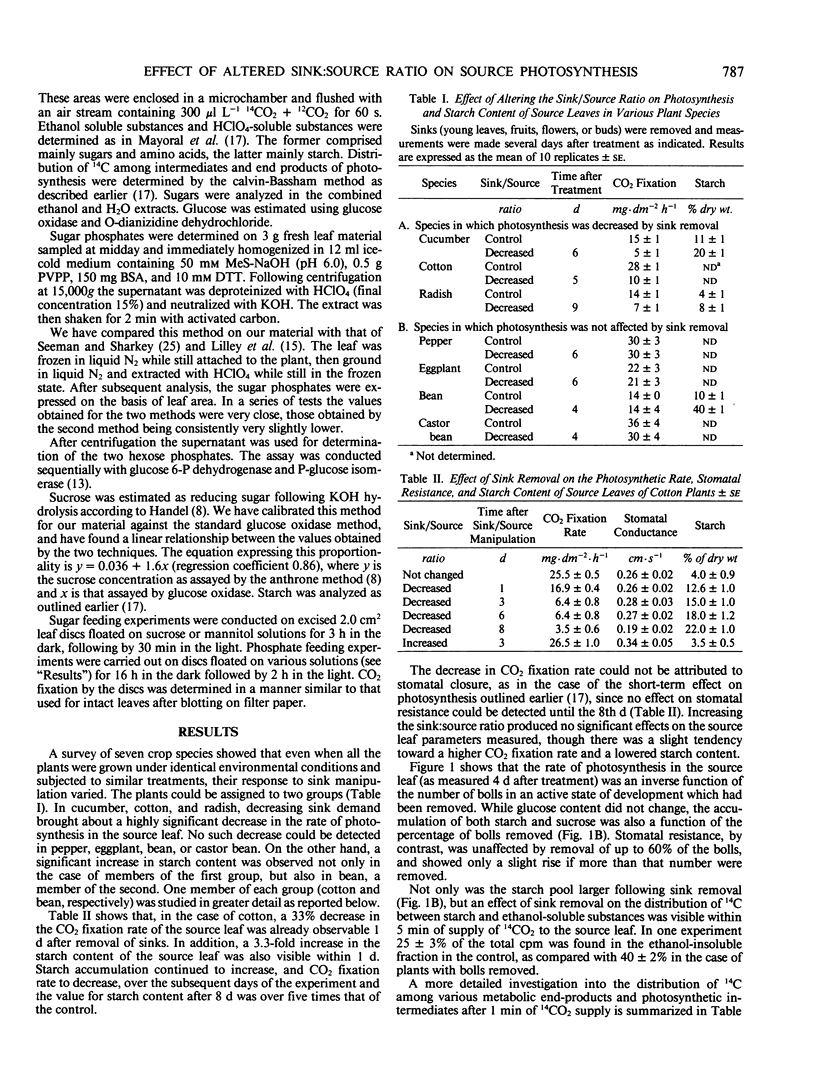
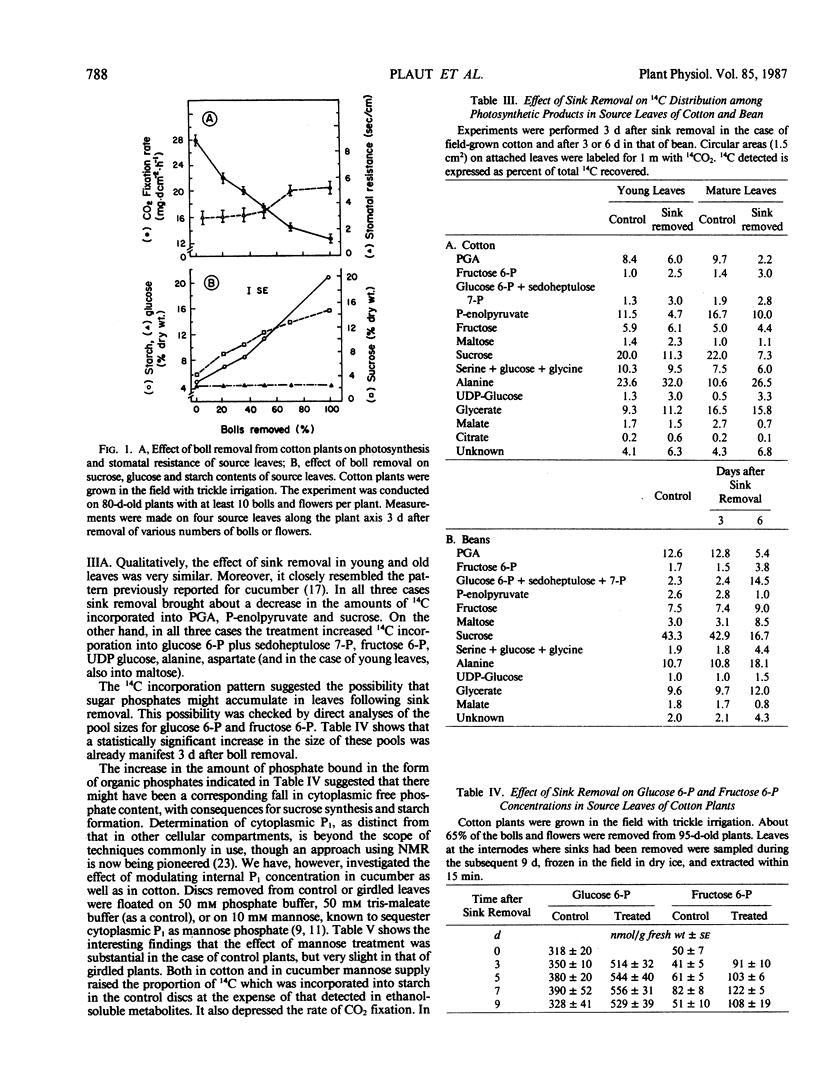
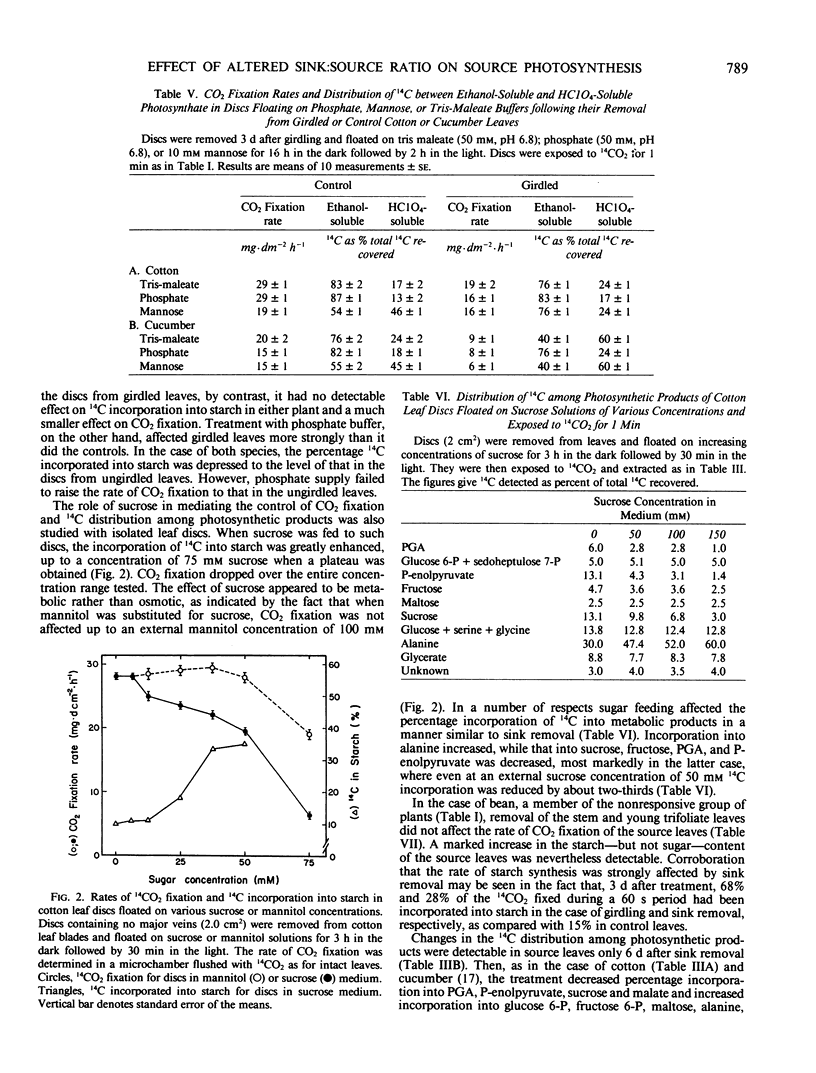
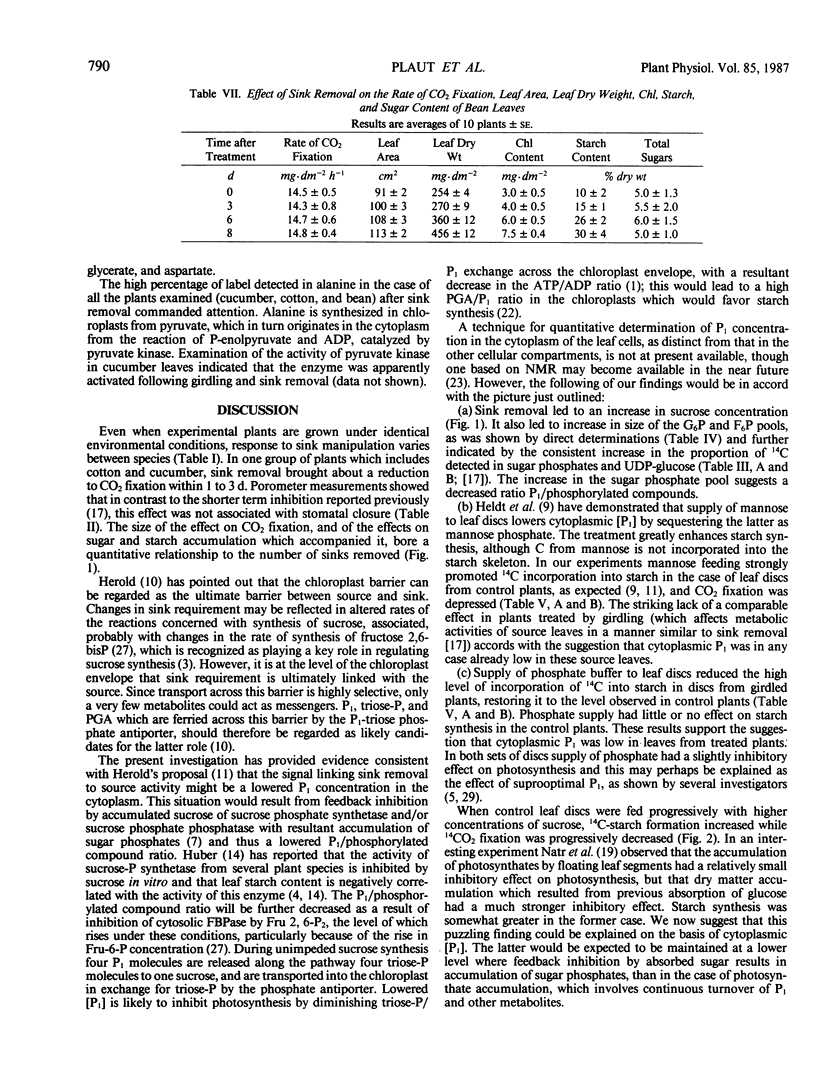
When seven crop species were grown under identical environmental conditions, decreased sink:source ratio led to a decreased photosynthetic rate within 1 to 3 days in Cucumis sativus L., Gossypium hirsutum L., and Raphanus sativus L., but not in Capsicum annuum L., Solanum melongena L., Phaseolus vulgaris L., or Ricinus communis L. The decrease was not associated with stomatal closure. In cotton and cucumber, sink removal led to an increase in starch and sugar content, in glucose 6-phosphate and fructose 6-phosphate pools, and in the proportion of 14C detected in sugar phosphates and UDPglucose following 14CO2 supply. When mannose was supplied to leaf discs to sequester cytoplasmic inorganic phosphate, promotion of starch synthesis, and inhibition of CO2 fixation, were observed in control discs, but not in discs from treated plants. Phosphate buffer reduced starch synthesis in the latter, but not the former discs. The findings suggest that sink removal led to a decreased ratio inorganic phosphate:phosphorylated compounds. In beans 14C in sugar phosphates increased following sink removal, but without sucrose accumulation, suggesting tighter feedback control of sugar level. Starch accumulated to higher levels than in the other plants, but CO2 fixation rate was constant for several days.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carmi A., Koller D. Regulation of Photosynthetic Activity in the Primary Leaves of Bean (Phaseolus vulgaris L.) by Materials Moving in the Water-conducting System. Plant Physiol. 1979 Aug;64(2):285–288. doi: 10.1104/pp.64.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cséke C., Weeden N. F., Buchanan B. B., Uyeda K. A special fructose bisphosphate functions as a cytoplasmic regulatory metabolite in green leaves. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4322–4326. doi: 10.1073/pnas.79.14.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehlert D. C., Huber S. C. Regulation of Spinach Leaf Sucrose Phosphate Synthase by Glucose-6-Phosphate, Inorganic Phosphate, and pH. Plant Physiol. 1983 Dec;73(4):989–994. doi: 10.1104/pp.73.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügge U. I., Freisl M., Heldt H. W. Balance between Metabolite Accumulation and Transport in Relation to Photosynthesis by Isolated Spinach Chloroplasts. Plant Physiol. 1980 Apr;65(4):574–577. doi: 10.1104/pp.65.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Chon C. J., Maronde D. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol. 1977 Jun;59(6):1146–1155. doi: 10.1104/pp.59.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M., Stitt M., Mader G., Heldt H. W. Rapid fractionation of wheat leaf protoplasts using membrane filtration : the determination of metabolite levels in the chloroplasts, cytosol, and mitochondria. Plant Physiol. 1982 Oct;70(4):965–970. doi: 10.1104/pp.70.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayoral M. L., Plaut Z., Reinhold L. Effect of translocation-hindering procedures on source leaf photosynthesis in cucumber. Plant Physiol. 1985 Mar;77(3):712–717. doi: 10.1104/pp.77.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafziger E. D., Koller H. R. Influence of Leaf Starch Concentration on CO(2) Assimilation in Soybean. Plant Physiol. 1976 Apr;57(4):560–563. doi: 10.1104/pp.57.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T. W., Huber S. C. Changes in Starch Formation and Activities of Sucrose Phosphate Synthase and Cytoplasmic Fructose-1,6-bisphosphatase in Response to Source-Sink Alterations. Plant Physiol. 1983 Jun;72(2):474–480. doi: 10.1104/pp.72.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann J. R., Sharkey T. D. Salinity and Nitrogen Effects on Photosynthesis, Ribulose-1,5-Bisphosphate Carboxylase and Metabolite Pool Sizes in Phaseolus vulgaris L. Plant Physiol. 1986 Oct;82(2):555–560. doi: 10.1104/pp.82.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D. A., Wu M. X., Black C. C. Pyrophosphate and fructose 2,6-bisphosphate effects on glycolysis in pea seed extracts. Plant Physiol. 1984 Oct;76(2):316–320. doi: 10.1104/pp.76.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Herzog B., Heldt H. W. Control of Photosynthetic Sucrose Synthesis by Fructose 2,6-Bisphosphate : I. Coordination of CO(2) Fixation and Sucrose Synthesis. Plant Physiol. 1984 Jul;75(3):548–553. doi: 10.1104/pp.75.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Goto Y., Akazawa T. Pyruvate Kinase Activity of Wheat Plants Grown under Potassium Deficient Conditions. Plant Physiol. 1968 May;43(5):730–734. doi: 10.1104/pp.43.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Handel E. Direct microdetermination of sucrose. Anal Biochem. 1968 Feb;22(2):280–283. doi: 10.1016/0003-2697(68)90317-5. [DOI] [PubMed] [Google Scholar]


