Abstract
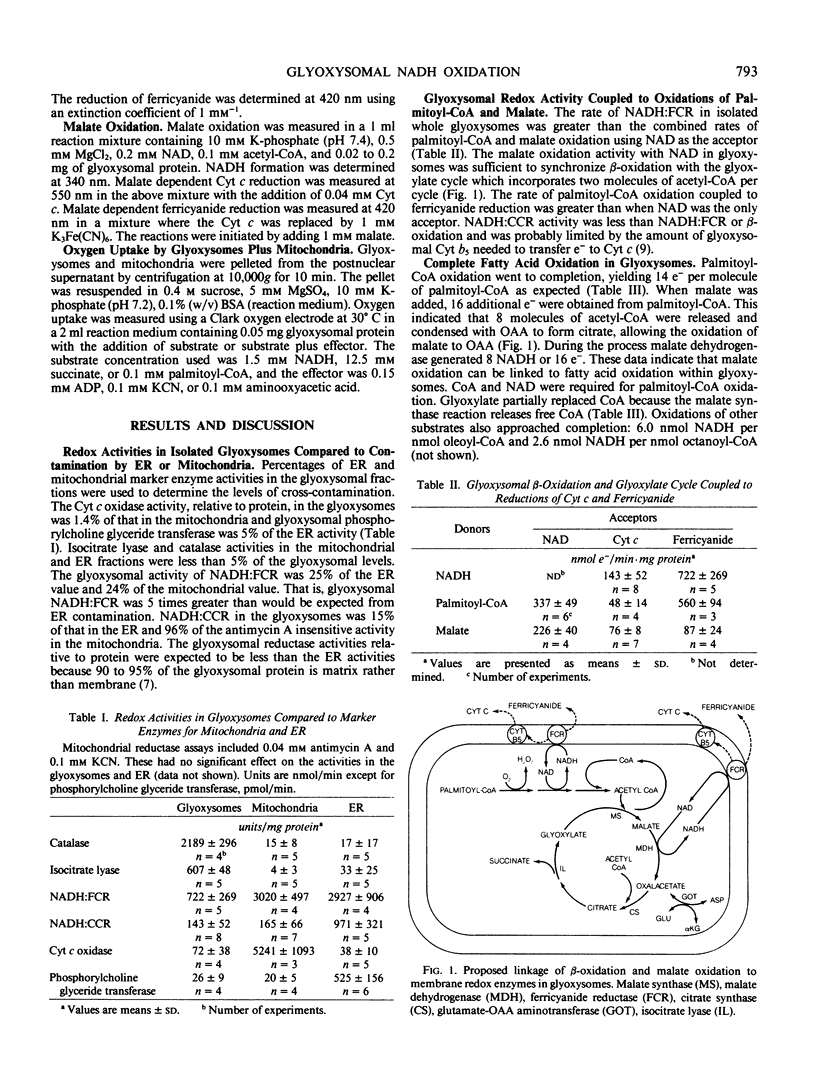
Glyoxysomes isolated from castor bean (Ricinus communis L., var Hale) endosperm had NADH:ferricyanide reductase and NADH:cytochrome c reductase activities averaging 720 and 140 nanomole electrons/per minute per milligram glyoxysomal protein, respectively. These redox activities were greater than could be attributed to contamination of the glyoxysomal fractions in which 1.4% of the protein was mitochondrial and 5% endoplasmic reticulum. The NADH:ferricyanide reductase activity in the glyoxysomes was greater than the palmitoyl-coenzyme A (CoA) oxidation activity which generated NADH at a rate of 340 nanomole electrons per minute per milligram glyoxysomal protein. Palmitoyl-CoA oxidation could be coupled to ferricyanide or cytochrome c reduction. Complete oxidation of palmitoyl-CoA, yielding 14 nanomole electrons/per nanomole palmitoyl-CoA, was demonstrated with the acceptors, NAL, cytochrome c, and ferricyanide. Malate was also oxidized by glyoxysomes, if acetyl-CoA, ferricyanide, or cytochrome c was present. Glyoxysomal NADH:ferricyanide reductase activity has the capacity to support the combined rates of NADH generation by β-oxidation and the glyoxylate cycle.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Beta oxidation in glyoxysomes from castor bean endosperm. J Biol Chem. 1969 Jul 10;244(13):3514–3520. [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Donaldson R. P. Nicotinamide cofactors (NAD and NADP) in glyoxysomes, mitochondria, and plastids isolated from castor bean endosperm. Arch Biochem Biophys. 1982 Apr 15;215(1):274–279. doi: 10.1016/0003-9861(82)90305-8. [DOI] [PubMed] [Google Scholar]
- Donaldson R. P. Organelle Membranes from Germinating Castor Bean Endosperm: II. ENZYMES, CYTOCHROMES, AND PERMEABILITY OF THE GLYOXYSOME MEMBRANE. Plant Physiol. 1981 Jan;67(1):21–25. doi: 10.1104/pp.67.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B. P., Beevers H. Developmental studies on glyoxysomes in Ricinus endosperm. J Cell Biol. 1970 Jan;44(1):94–102. doi: 10.1083/jcb.44.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks D. B., Donaldson R. P. Electron transport in glyoxysomal membranes. Arch Biochem Biophys. 1982 Apr 15;215(1):280–288. doi: 10.1016/0003-9861(82)90306-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Beevers H. Intracellular distribution of enzymes of the cytidine diphosphate choline pathway in castor bean endosperm. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2429–2432. doi: 10.1073/pnas.69.9.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler I. J., Beevers H. Oxidation of NADH in Glyoxysomes by a Malate-Aspartate Shuttle. Plant Physiol. 1980 Oct;66(4):555–560. doi: 10.1104/pp.66.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin B. J., Beevers H. Isolation of intact plastids from a range of plant tissues. Plant Physiol. 1974 Jun;53(6):870–874. doi: 10.1104/pp.53.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparace S. A., Moore T. S. Phospholipid metabolism in plant mitochondria: submitochondrial sites of synthesis. Plant Physiol. 1979 May;63(5):963–972. doi: 10.1104/pp.63.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Just W. W., Mannaerts G. P. Permeability of the peroxisomal membrane to cofactors of beta-oxidation. Evidence for the presence of a pore-forming protein. J Biol Chem. 1987 Mar 25;262(9):4310–4318. [PubMed] [Google Scholar]
- Wakefield L. M., Cass A. E., Radda G. K. Functional coupling between enzymes of the chromaffin granule membrane. J Biol Chem. 1986 Jul 25;261(21):9739–9745. [PubMed] [Google Scholar]
- Yu C., Huang A. H. Conversion of serine to glycerate in intact spinach leaf peroxisomes: role of malate dehydrogenase. Arch Biochem Biophys. 1986 Feb 15;245(1):125–133. doi: 10.1016/0003-9861(86)90196-7. [DOI] [PubMed] [Google Scholar]


