Abstract
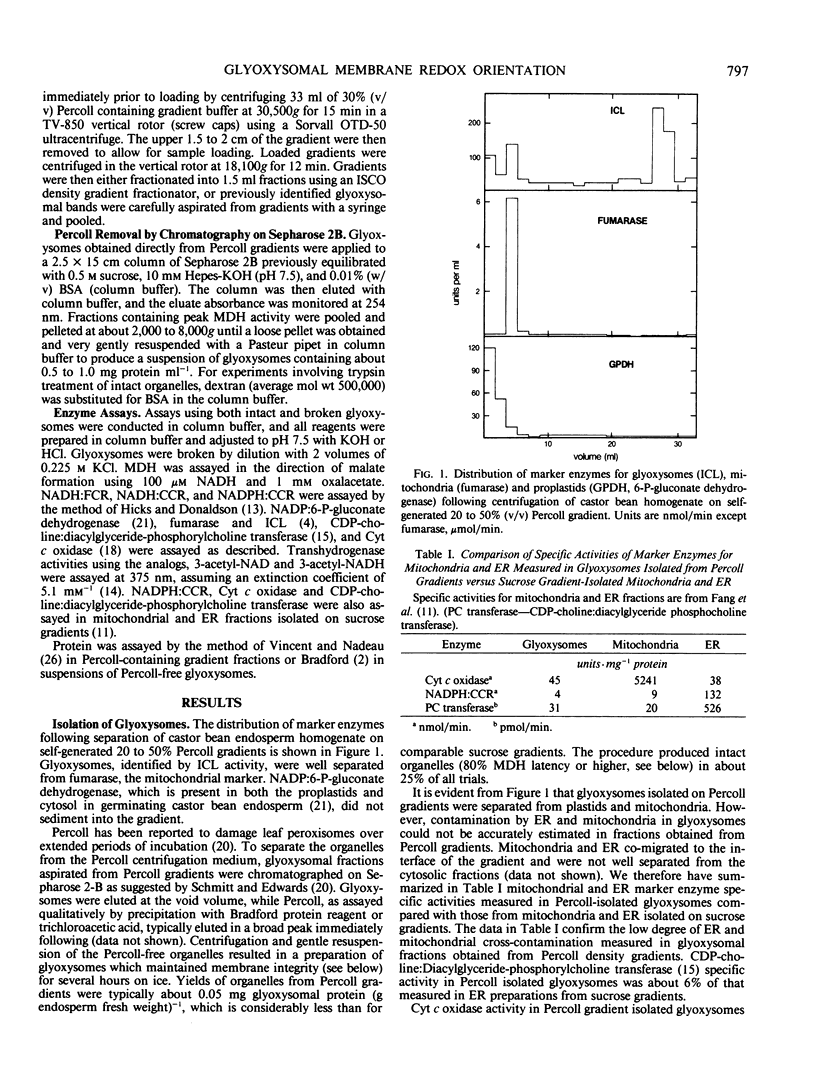
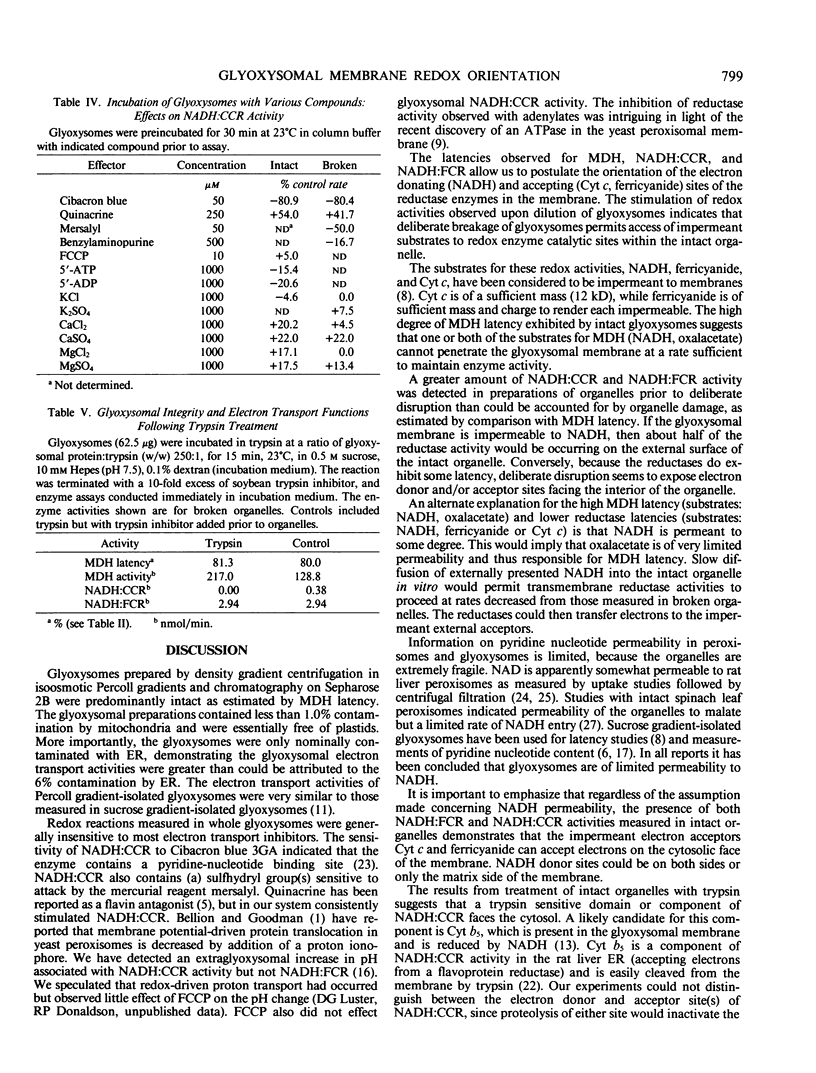
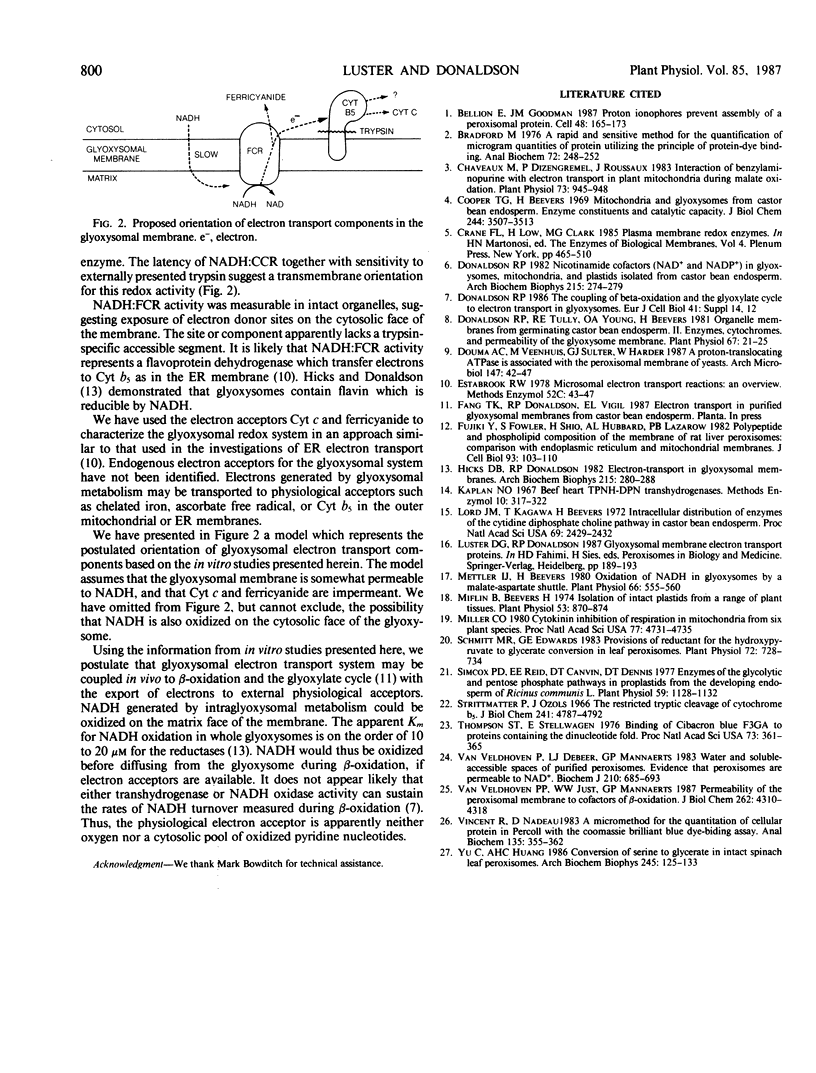
Intact glyoxysomes were isolated from castor bean endosperm on isometric Percoll gradients. The matrix enzyme, malate dehydrogenase, was 80% latent in the intact glyoxysomes. NADH:ferricyanide and NADH:cytochrome c reductase activities were measured in intact and deliberately broken organelles. The latencies of these redox activities were found to be about half the malate dehydrogenase latency. Incubation of intact organelles with trypsin eliminated NADH:cytochrome c reductase activity, but did not affect NADH:ferricyanide reductase activity. NADH oxidase and transhydrogenase activities were negligible in isolated glyoxysomes. Mersalyl and Cibacron blue 3GA were potent inhibitors of NADH:cytochrome c reductase. Quinacrine, Ca2+ and Mg2+ stimulated NADH:cytochrome c reductase activity in intact glyoxysomes. The data suggest that some electron donor sites are on the matrix side and some electron acceptor sites are on the cytosolic side of the membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellion E., Goodman J. M. Proton ionophores prevent assembly of a peroxisomal protein. Cell. 1987 Jan 16;48(1):165–173. doi: 10.1016/0092-8674(87)90367-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chauveau M., Dizengremel P., Roussaux J. Interaction of Benzylaminopurine with Electron Transport in Plant Mitochondria during Malate Oxidation. Plant Physiol. 1983 Dec;73(4):945–948. doi: 10.1104/pp.73.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Donaldson R. P. Nicotinamide cofactors (NAD and NADP) in glyoxysomes, mitochondria, and plastids isolated from castor bean endosperm. Arch Biochem Biophys. 1982 Apr 15;215(1):274–279. doi: 10.1016/0003-9861(82)90305-8. [DOI] [PubMed] [Google Scholar]
- Donaldson R. P. Organelle Membranes from Germinating Castor Bean Endosperm: II. ENZYMES, CYTOCHROMES, AND PERMEABILITY OF THE GLYOXYSOME MEMBRANE. Plant Physiol. 1981 Jan;67(1):21–25. doi: 10.1104/pp.67.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douma A. C., Veenhuis M., Sulter G. J., Harder W. A proton-translocating adenosine triphosphatase is associated with the peroxisomal membrane of yeasts. Arch Microbiol. 1987 Feb;147(1):42–47. doi: 10.1007/BF00492903. [DOI] [PubMed] [Google Scholar]
- Estabrook R. W. Microsomal electron-transport reactions: an overview. Methods Enzymol. 1978;52:43–47. doi: 10.1016/s0076-6879(78)52004-1. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Fowler S., Shio H., Hubbard A. L., Lazarow P. B. Polypeptide and phospholipid composition of the membrane of rat liver peroxisomes: comparison with endoplasmic reticulum and mitochondrial membranes. J Cell Biol. 1982 Apr;93(1):103–110. doi: 10.1083/jcb.93.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks D. B., Donaldson R. P. Electron transport in glyoxysomal membranes. Arch Biochem Biophys. 1982 Apr 15;215(1):280–288. doi: 10.1016/0003-9861(82)90306-x. [DOI] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Beevers H. Intracellular distribution of enzymes of the cytidine diphosphate choline pathway in castor bean endosperm. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2429–2432. doi: 10.1073/pnas.69.9.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler I. J., Beevers H. Oxidation of NADH in Glyoxysomes by a Malate-Aspartate Shuttle. Plant Physiol. 1980 Oct;66(4):555–560. doi: 10.1104/pp.66.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin B. J., Beevers H. Isolation of intact plastids from a range of plant tissues. Plant Physiol. 1974 Jun;53(6):870–874. doi: 10.1104/pp.53.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. O. Cytokinin inhibition of respiration in mitochondria from six plant species. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4731–4735. doi: 10.1073/pnas.77.8.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. R., Edwards G. E. Provisions of reductant for the hydroxypyruvate to glycerate conversion in leaf peroxisomes : a critical evaluation of the proposed malate/aspartate shuttle. Plant Physiol. 1983 Jul;72(3):728–734. doi: 10.1104/pp.72.3.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcox P. D., Reid E. E., Canvin D. T., Dennis D. T. Enzymes of the Glycolytic and Pentose Phosphate Pathways in Proplastids from the Developing Endosperm of Ricinus communis L. Plant Physiol. 1977 Jun;59(6):1128–1132. doi: 10.1104/pp.59.6.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter P., Ozols J. The restricted tryptic cleavage of cytochrome b5. J Biol Chem. 1966 Oct 25;241(20):4787–4792. [PubMed] [Google Scholar]
- Thompson S. T., Stellwagen E. Binding of Cibacron blue F3GA to proteins containing the dinucleotide fold. Proc Natl Acad Sci U S A. 1976 Feb;73(2):361–365. doi: 10.1073/pnas.73.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Just W. W., Mannaerts G. P. Permeability of the peroxisomal membrane to cofactors of beta-oxidation. Evidence for the presence of a pore-forming protein. J Biol Chem. 1987 Mar 25;262(9):4310–4318. [PubMed] [Google Scholar]
- Van Veldhoven P., Debeer L. J., Mannaerts G. P. Water- and solute-accessible spaces of purified peroxisomes. Evidence that peroxisomes are permeable to NAD+. Biochem J. 1983 Mar 15;210(3):685–693. doi: 10.1042/bj2100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent R., Nadeau D. A micromethod for the quantitation of cellular proteins in Percoll with the Coomassie brilliant blue dye-binding assay. Anal Biochem. 1983 Dec;135(2):355–362. doi: 10.1016/0003-2697(83)90696-6. [DOI] [PubMed] [Google Scholar]
- Yu C., Huang A. H. Conversion of serine to glycerate in intact spinach leaf peroxisomes: role of malate dehydrogenase. Arch Biochem Biophys. 1986 Feb 15;245(1):125–133. doi: 10.1016/0003-9861(86)90196-7. [DOI] [PubMed] [Google Scholar]


