Abstract
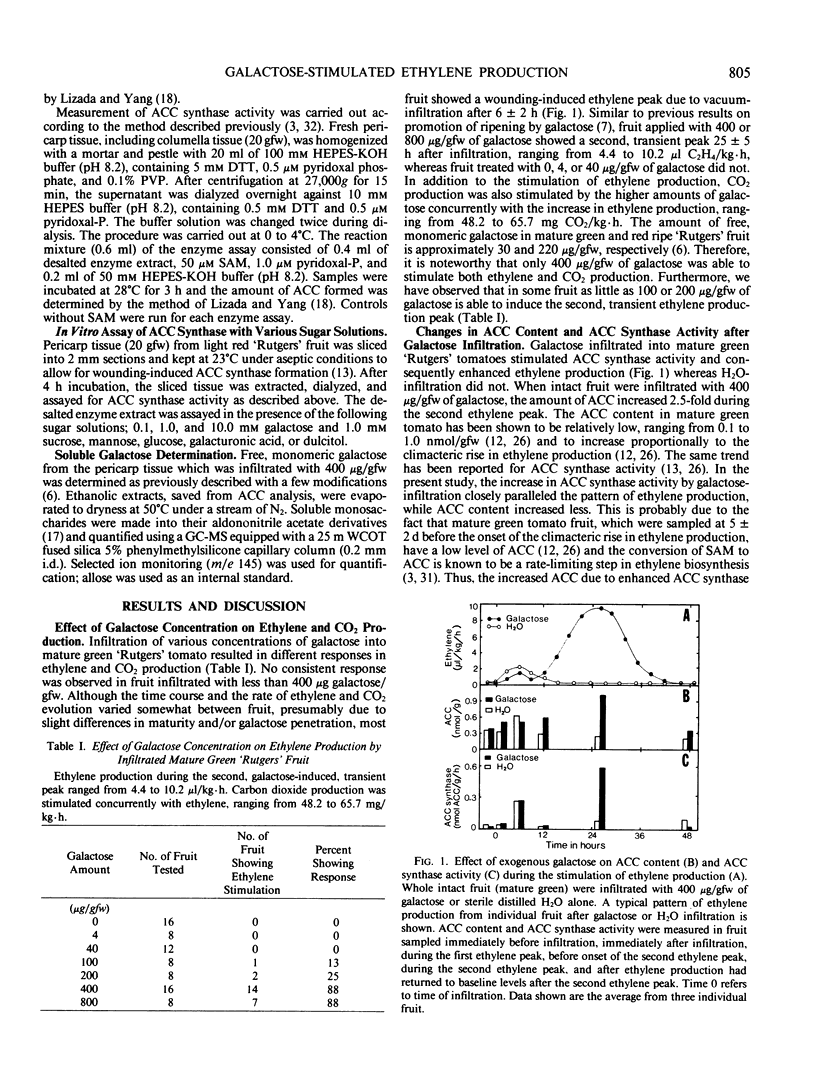
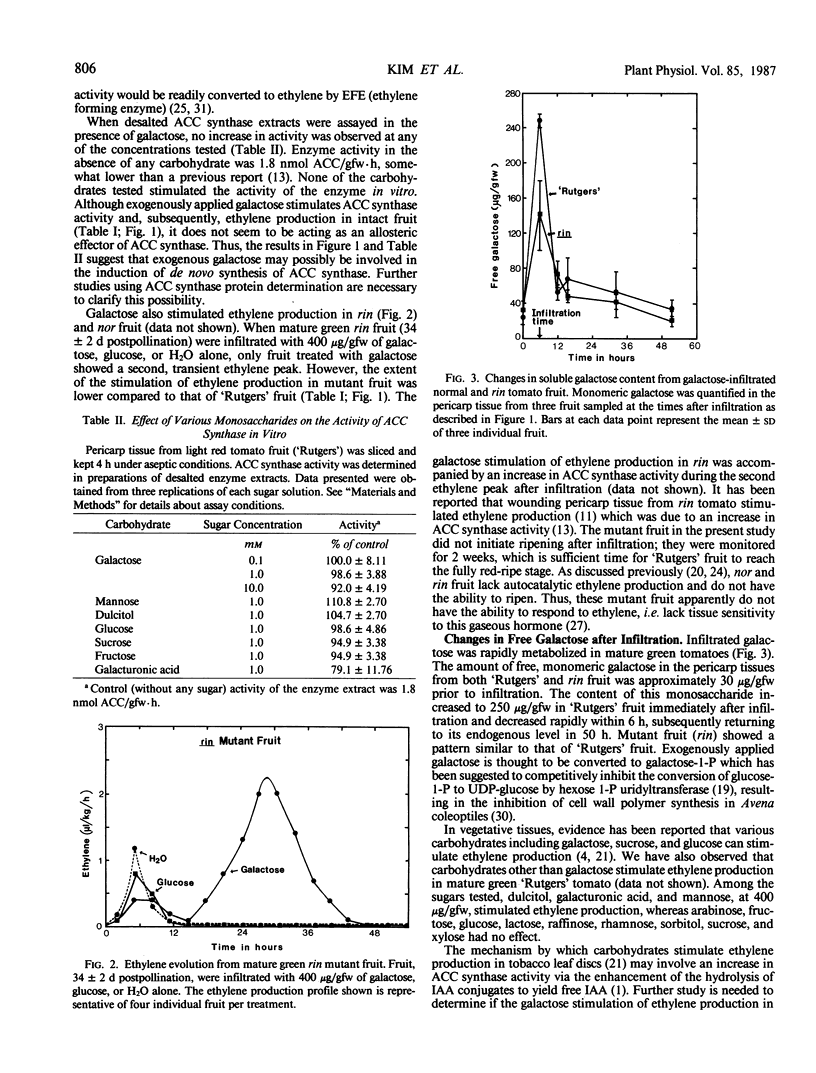
We have characterized the stimulation of ethylene production by galactose in tomatoes (Lycopersicon esculentum Mill.). The effect of concentration was studied by infiltrating 0, 4, 40, 100, 200, 400, or 800 micrograms galactose for each gram of fresh fruit weight into mature green `Rutgers' fruit. Both 400 and 800 micrograms per gram fresh weight consistently stimulated a transient increase in ethylene approximately 25 hours after infiltration; the lower concentrations did not. Carbon dioxide evolution of fruit infiltrated with 400 to 800 micrograms per gram fresh weight was greater than that of lower concentrations. The ripening mutants, rin and nor, also showed the transient increase in ethylene and elevated CO2 evolution by 400 micrograms per gram fresh weight galactose. 1-Aminocyclopropane-1-carboxylic acid (ACC) content and ACC-synthase activity increased concurrently with ethylene production. However, galactose did not stimulate ACC-synthase activity in vitro. The infiltrated galactose in pericarp tissue was rapidly metabolized, decreasing to endogenous levels within 50 hours. Infiltrated galacturonic acid, dulcitol, and mannose stimulated transient increases in ethylene production similar to that of galactose. The following sugars produced no response: sucrose, fructose, glucose, rhamnose, arabinose, xylose, raffinose, lactose, and sorbitol.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gross K. C. Promotion of ethylene evolution and ripening of tomato fruit by galactose. Plant Physiol. 1985 Sep;79(1):306–307. doi: 10.1104/pp.79.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross K. C., Wallner S. J. Degradation of Cell Wall Polysaccharides during Tomato Fruit Ripening. Plant Physiol. 1979 Jan;63(1):117–120. doi: 10.1104/pp.63.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herner R. C., Sink K. C. Ethylene Production and Respiratory Behavior of the rin Tomato Mutant. Plant Physiol. 1973 Jul;52(1):38–42. doi: 10.1104/pp.52.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackey G. D., Gross K. C., Wallner S. J. Loss of tomato cell wall galactan may involve reduced rate of synthesis. Plant Physiol. 1980 Sep;66(3):532–533. doi: 10.1104/pp.66.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrfeld J. Differential gas-liquid chromatography method for determination of uronic acids in carbohydrate mixtures. Anal Biochem. 1981 Aug;115(2):410–418. doi: 10.1016/0003-2697(81)90026-9. [DOI] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Meir S., Philosoph-Hadas S., Epstein E., Aharoni N. Carbohydrates stimulate ethylene production in tobacco leaf discs : I. Interaction with auxin and the relation to auxin metabolism. Plant Physiol. 1985 May;78(1):131–138. doi: 10.1104/pp.78.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philosoph-Hadas S., Meir S., Aharoni N. Carbohydrates Stimulate Ethylene Production in Tobacco Leaf Discs : II. Sites of Stimulation in the Ethylene Biosynthesis Pathway. Plant Physiol. 1985 May;78(1):139–143. doi: 10.1104/pp.78.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey R. beta-Galactosidases in Ripening Tomatoes. Plant Physiol. 1983 Jan;71(1):132–135. doi: 10.1104/pp.71.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitrit Y., Riov J., Blumenfeld A. Regulation of Ethylene Biosynthesis in Avocado Fruit during Ripening. Plant Physiol. 1986 May;81(1):130–135. doi: 10.1104/pp.81.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. 1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis. Arch Biochem Biophys. 1979 Nov;198(1):280–286. doi: 10.1016/0003-9861(79)90420-x. [DOI] [PubMed] [Google Scholar]


