Abstract
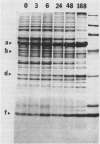
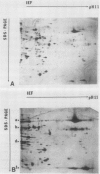
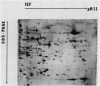
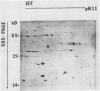
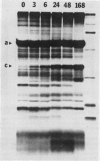
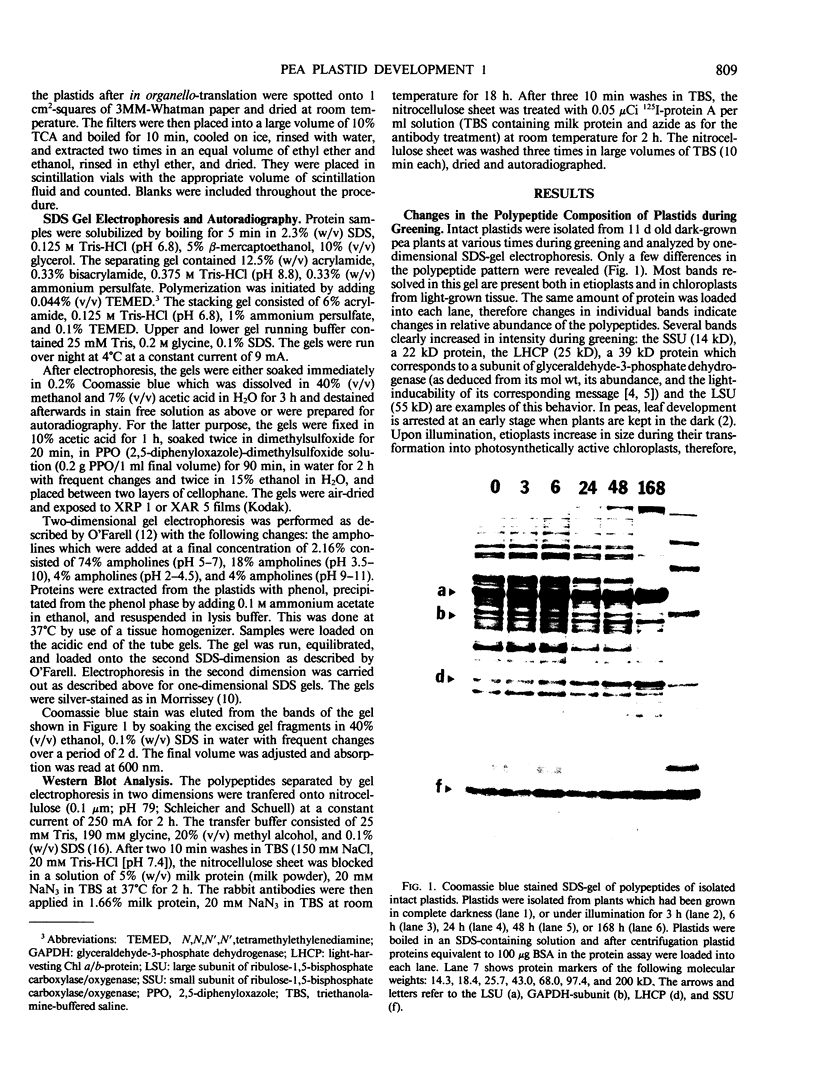
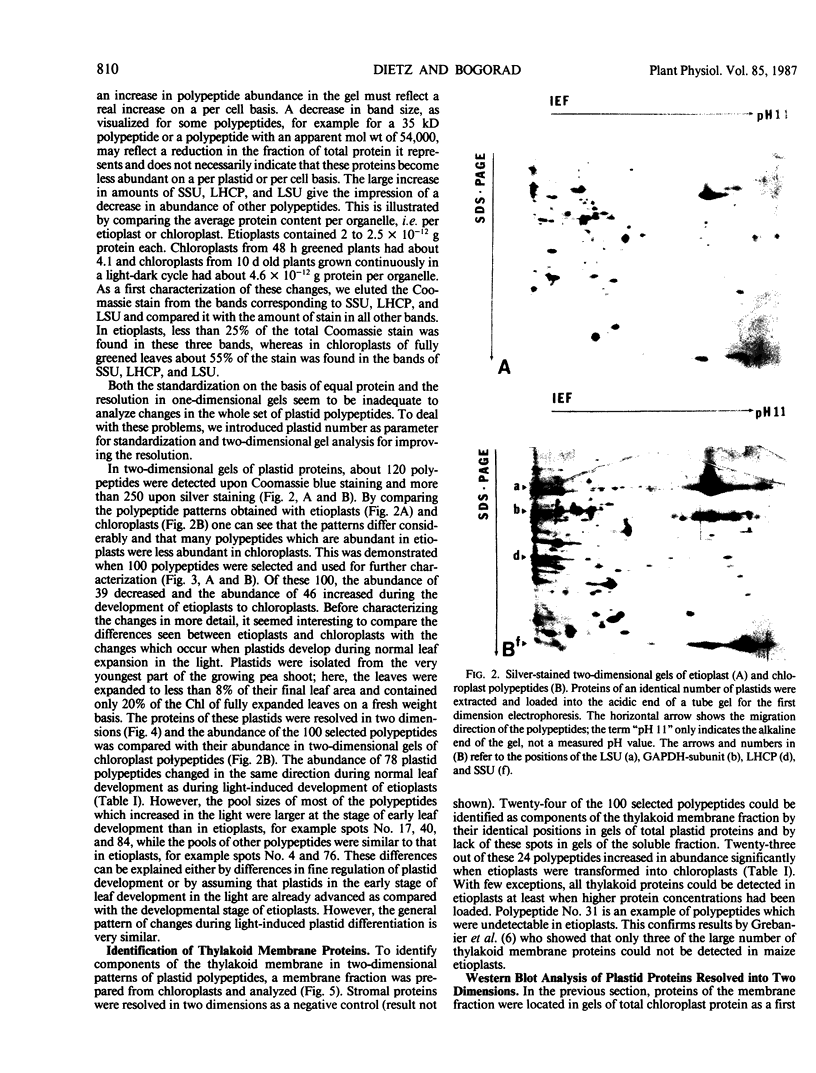
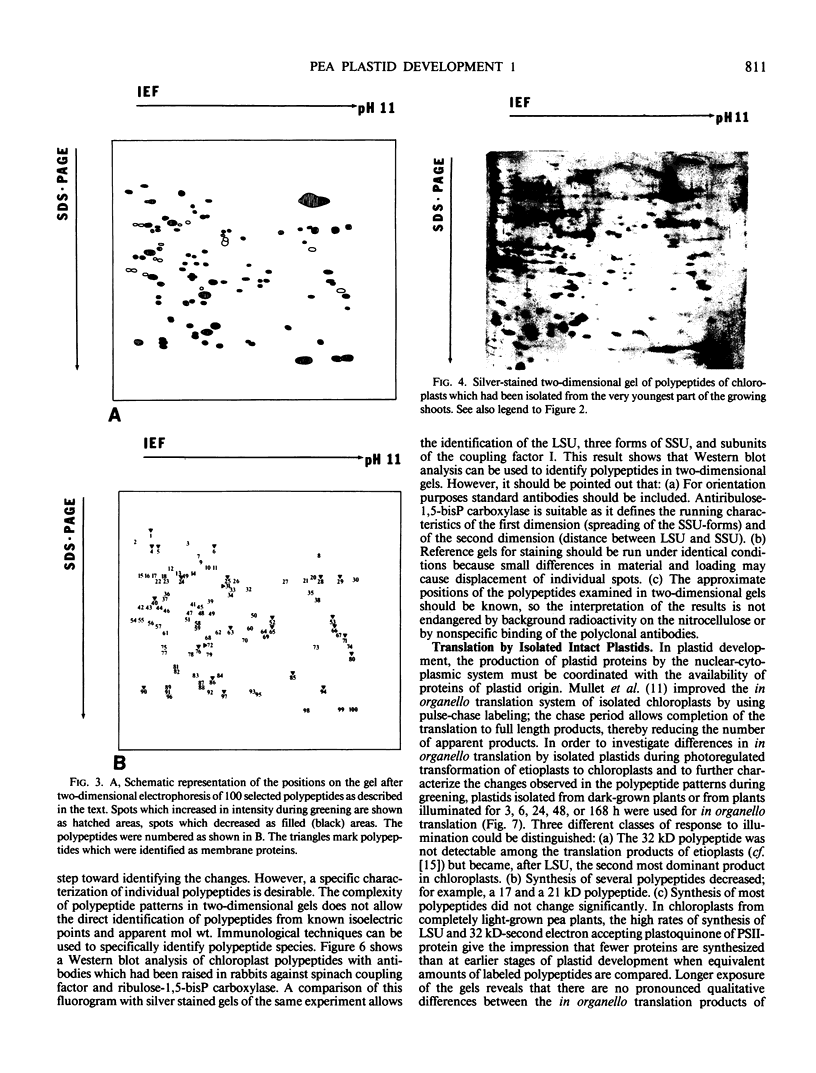
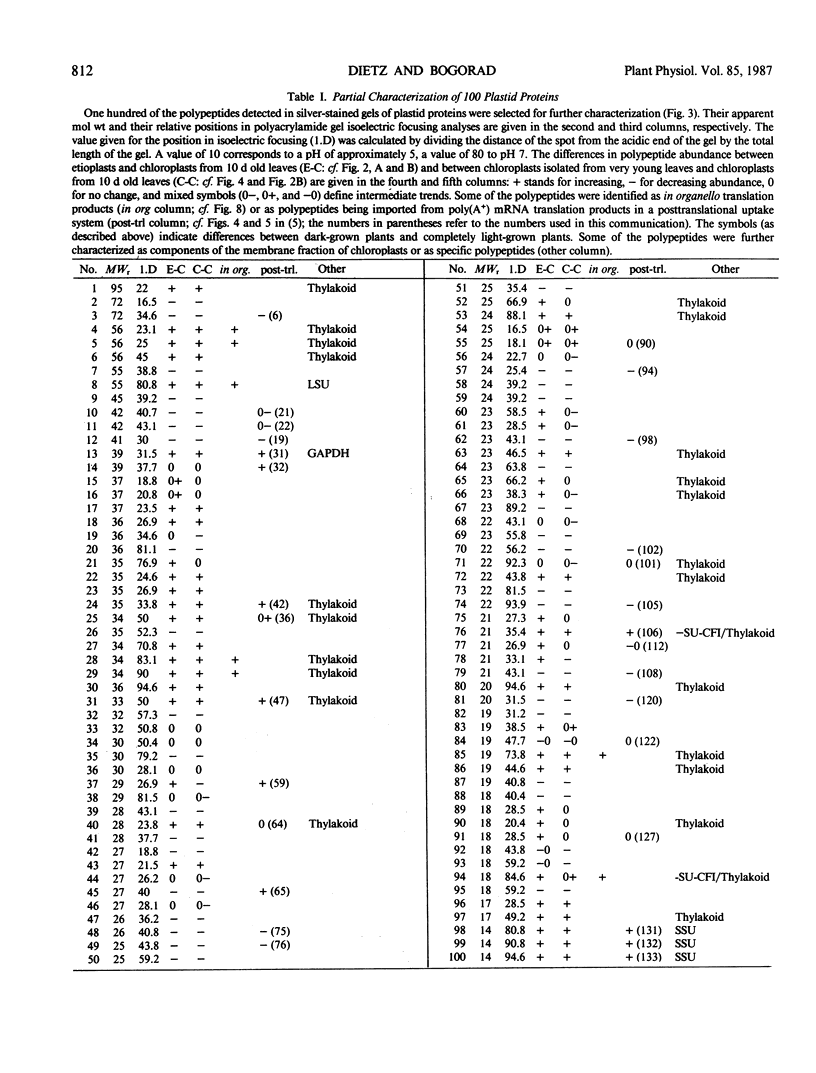
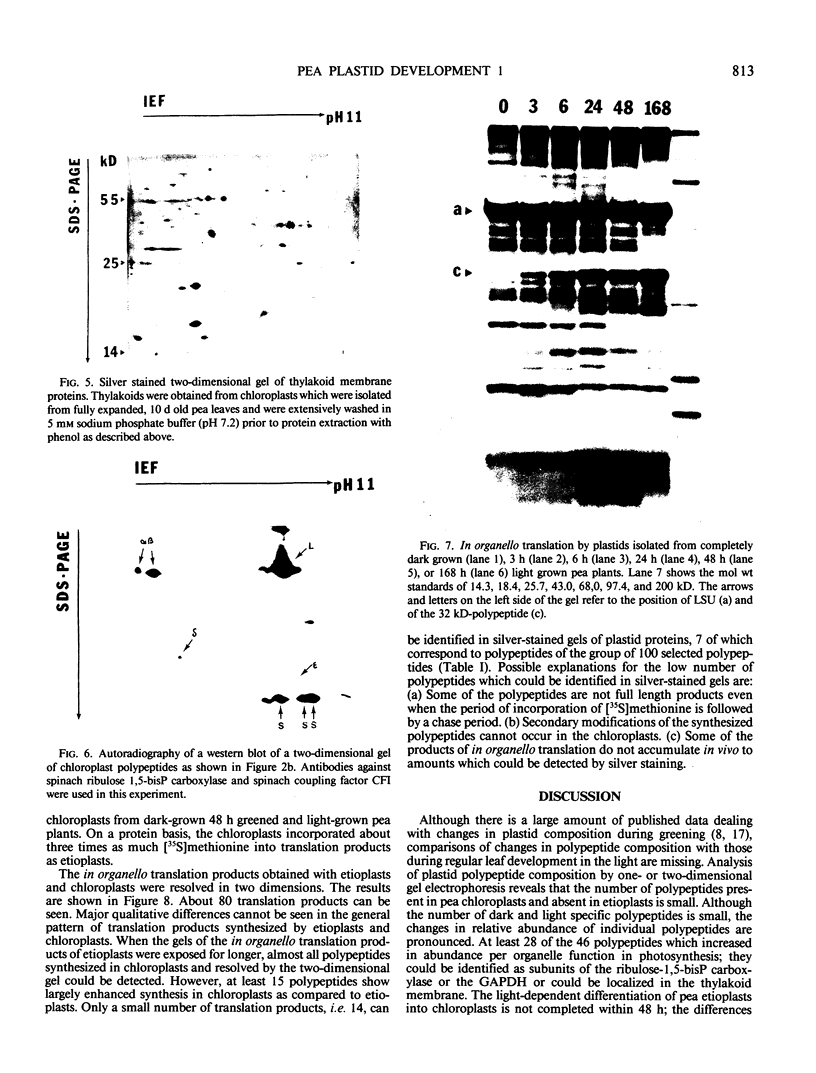
Changes in plastid polypeptide composition during greening of etiolated peas were investigated by two-dimensional gel electrophoresis. One hundred of the more than 250 polypeptides which could be detected upon silver staining were followed during plastid development. Thirty-nine polypeptides decreased in abundance on a per organelle basis. Twentythree of the 46 polypeptides which increased in abundance upon greening could be identified as proteins of the thylakoid membrane. The changes in proteins observed during greening of etiolated leaves corresponded largely to those observed during normal leaf expansion. The origin of some of the polypeptides was traced back by comparing the two-dimensional gels of plastid proteins with in organello translation products and with polypeptides which had been synthesized in vitro from poly(A+) mRNA preparations and posttranslationally imported by chloroplasts. Some polypeptides were specifically identified in two-dimensional gels by Western blot analysis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J. O., Nikolau B. J., Carr J. P., Klessig D. F. Translational regulation of light-induced ribulose 1,5-bisphosphate carboxylase gene expression in amaranth. Mol Cell Biol. 1986 Jul;6(7):2347–2353. doi: 10.1128/mcb.6.7.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerff R., Kloppstech K. Structural diversity and differential light control of mRNAs coding for angiosperm glyceraldehyde-3-phosphate dehydrogenases. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7624–7628. doi: 10.1073/pnas.79.24.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K. J., Bogorad L. Plastid Development in Pisum sativum Leaves during Greening : II. Post-Translational Uptake by Plastids as an Indicator System to Monitor Changes in Translatable mRNA for Nuclear-Encoded Plastid Polypeptides. Plant Physiol. 1987 Nov;85(3):816–822. doi: 10.1104/pp.85.3.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. Stoichiometry of reduction and phosphorylation during illumination of intact chloroplasts. Biochim Biophys Acta. 1973 Apr 27;305(1):140–152. doi: 10.1016/0005-2728(73)90239-9. [DOI] [PubMed] [Google Scholar]
- Kreuz K., Dehesh K., Apel K. The light-dependent accumulation of the P700 chlorophyll a protein of the photosystem I reaction center in barley. Evidence for translational control. Eur J Biochem. 1986 Sep 15;159(3):459–467. doi: 10.1111/j.1432-1033.1986.tb09908.x. [DOI] [PubMed] [Google Scholar]
- Mullet J. E., Klein R. R., Grossman A. R. Optimization of protein synthesis in isolated higher plant chloroplasts. Identification of paused translation intermediates. Eur J Biochem. 1986 Mar 3;155(2):331–338. doi: 10.1111/j.1432-1033.1986.tb09495.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Siddell S. G., Ellis R. J. Protein synthesis in chloroplasts. Characteristics and products of protein synthesis in vitro in etioplasts and developing chloroplasts from pea leaves. Biochem J. 1975 Mar;146(3):675–685. doi: 10.1042/bj1460675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman D. C., Coudron C. A. Identification of Traumatin, a Wound Hormone, as 12-Oxo-trans-10-dodecenoic Acid. Plant Physiol. 1979 Mar;63(3):536–541. doi: 10.1104/pp.63.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]