Abstract
Monoclonal antibodies were raised against fusicoccin. The toxin, linked to bovine serum albumin through its t-pentenyl moiety, served as immunogen. Hybridomas secreting anti-fusicoccin antibodies were screened by radioimmunoassay employing a novel radioactive derivative, [3H]-nor-fusicoccin-alcohol of high specific activity (1.5 × 1014Bq/mole). The two monoclonal antibodies reported here are of high apparent affinity for fusicoccin (0.71 × 10−9 molar and 1.85 × 10−9 molar). This is comparable to the apparent affinity of rabbit antiserum raised against the same type of conjugate (9.3 × 10−9 molar). A method for the single step purification of the monoclonal antibodies from ascites fluid is reported. A solid-phase immunoassay, using alkaline phosphatase as enzyme, exhibits a measuring range from 0.1 to 1.5 picomoles (about 70 picograms to 1 nanogram) of fusicoccin. The displacement of [3H]-nor-fusicoccin-alcohol from the antibodies by compounds structurally related to fusicoccin exhibits similar selectivity as a microsomal binding assay with the same tracer as radiolabeled probe.
Full text
PDF

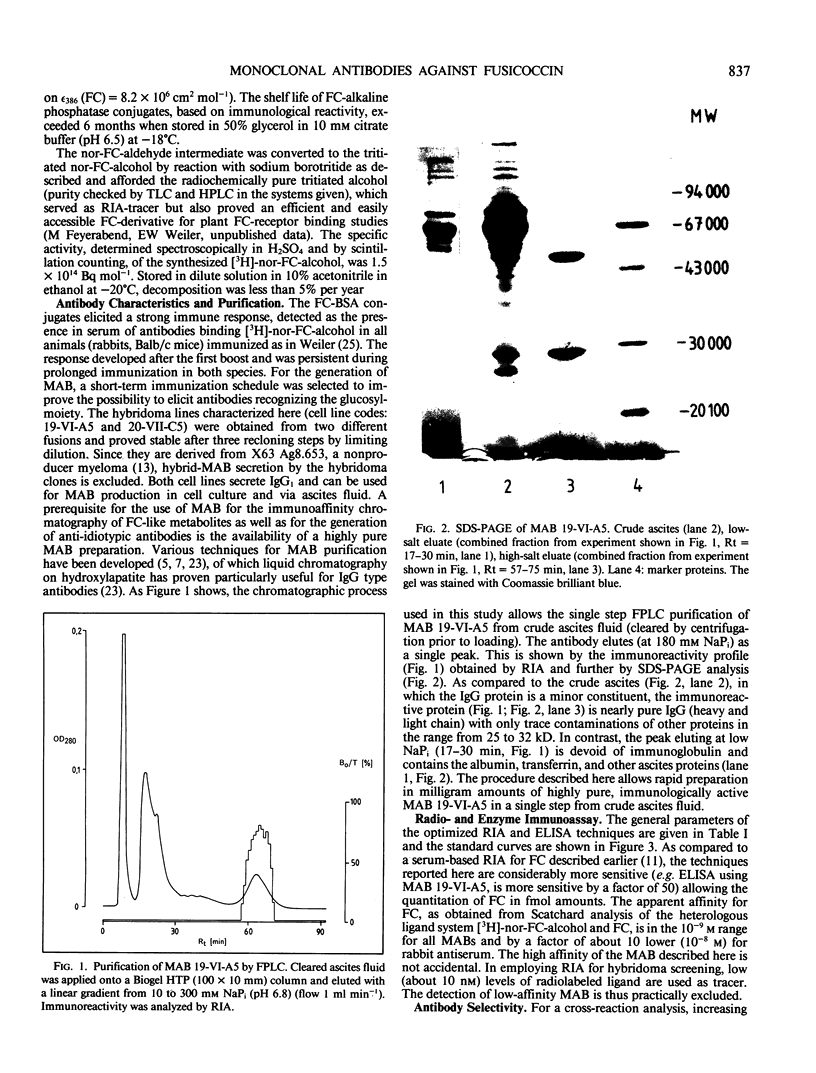
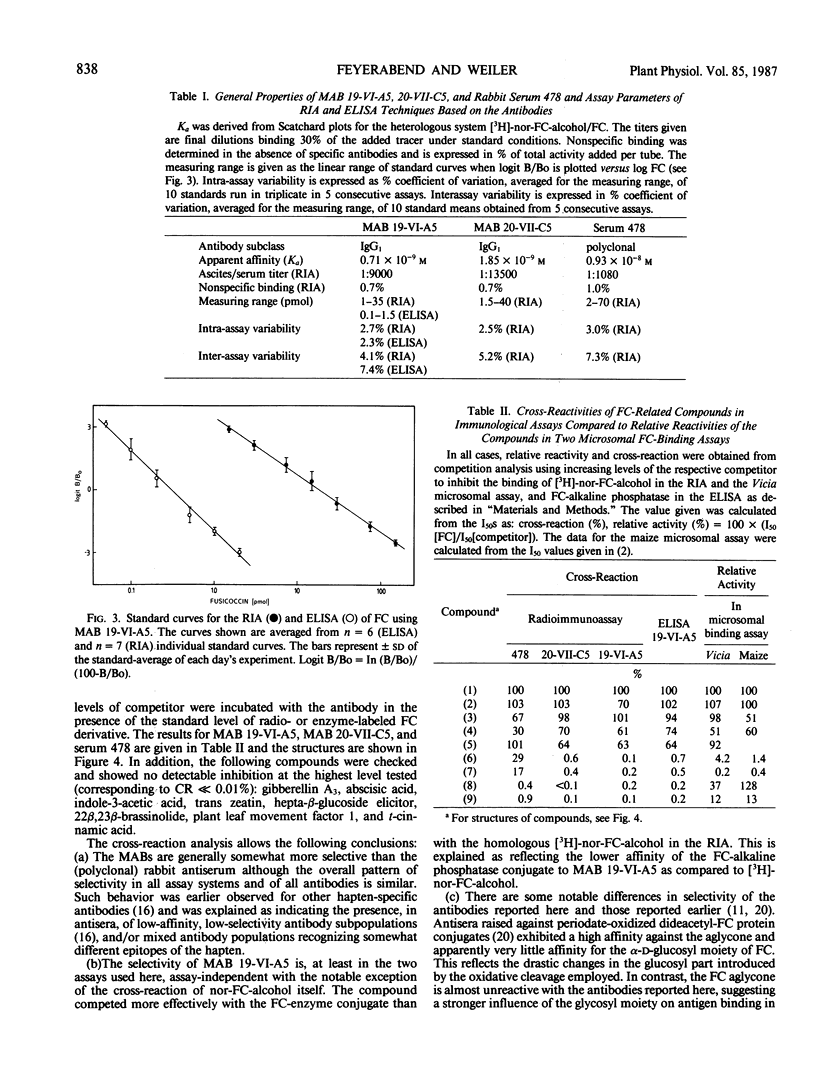
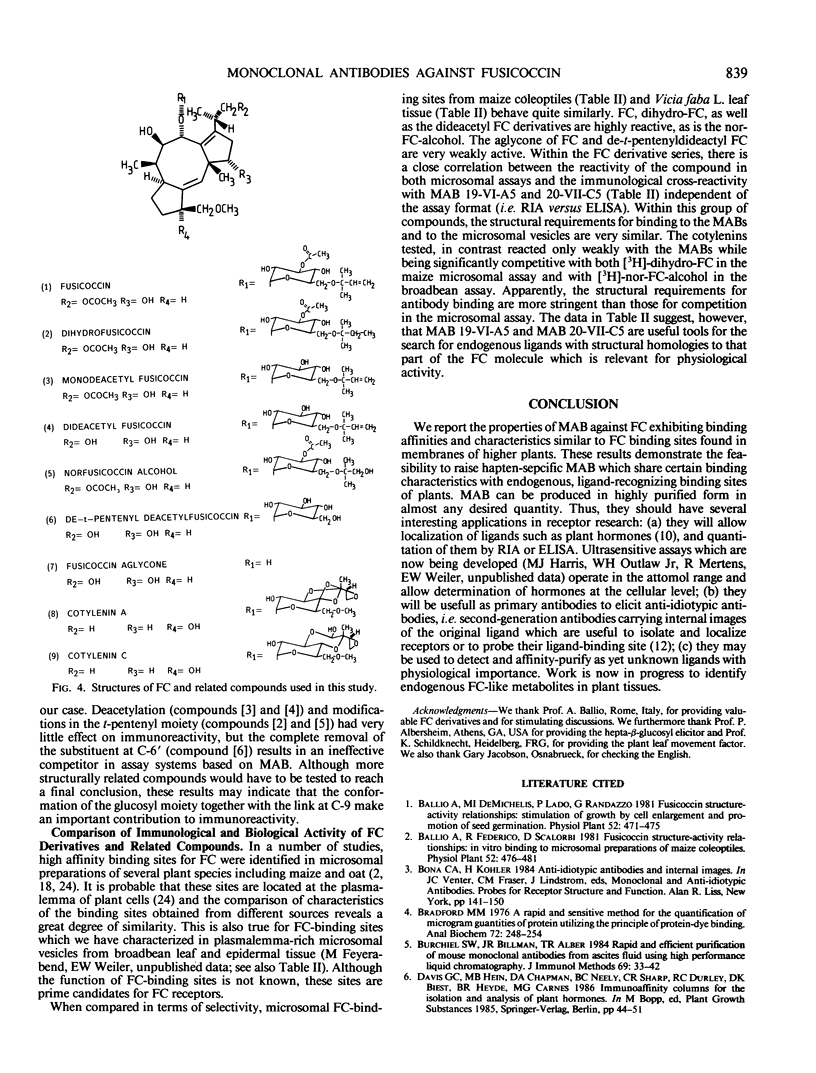

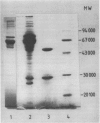
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burchiel S. W., Billman J. R., Alber T. R. Rapid and efficient purification of mouse monoclonal antibodies from ascites fluid using high performance liquid chromatography. J Immunol Methods. 1984 Apr 13;69(1):33–42. doi: 10.1016/0022-1759(84)90274-6. [DOI] [PubMed] [Google Scholar]
- Deschamps J. R., Hildreth J. E., Derr D., August J. T. A high-performance liquid chromatographic procedure for the purification of mouse monoclonal antibodies. Anal Biochem. 1985 Jun;147(2):451–454. doi: 10.1016/0003-2697(85)90296-9. [DOI] [PubMed] [Google Scholar]
- Eberle J., Arnscheidt A., Klix D., Weiler E. W. Monoclonal Antibodies to Plant Growth Regulators: III. Zeatinriboside and Dihydrozeatinriboside. Plant Physiol. 1986 Jun;81(2):516–521. doi: 10.1104/pp.81.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Rasi-Caldogno F., Pugliarello M. C. Fusicoccin stimulates the H+-ATPase of plasmalemma in isolated membrane vesicles from radish. Biochem Biophys Res Commun. 1985 Nov 27;133(1):280–285. doi: 10.1016/0006-291x(85)91872-8. [DOI] [PubMed] [Google Scholar]
- Stanker L. H., Vanderlaan M., Juarez-Salinas H. One-step purification of mouse monoclonal antibodies from ascites fluid by hydroxylapatite chromatography. J Immunol Methods. 1985 Jan 21;76(1):157–169. doi: 10.1016/0022-1759(85)90488-0. [DOI] [PubMed] [Google Scholar]
- Stout R. G., Cleland R. E. Partial characterization of fusicoccin binding to receptor sites on oat root membranes. Plant Physiol. 1980 Sep;66(3):353–359. doi: 10.1104/pp.66.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]