Abstract
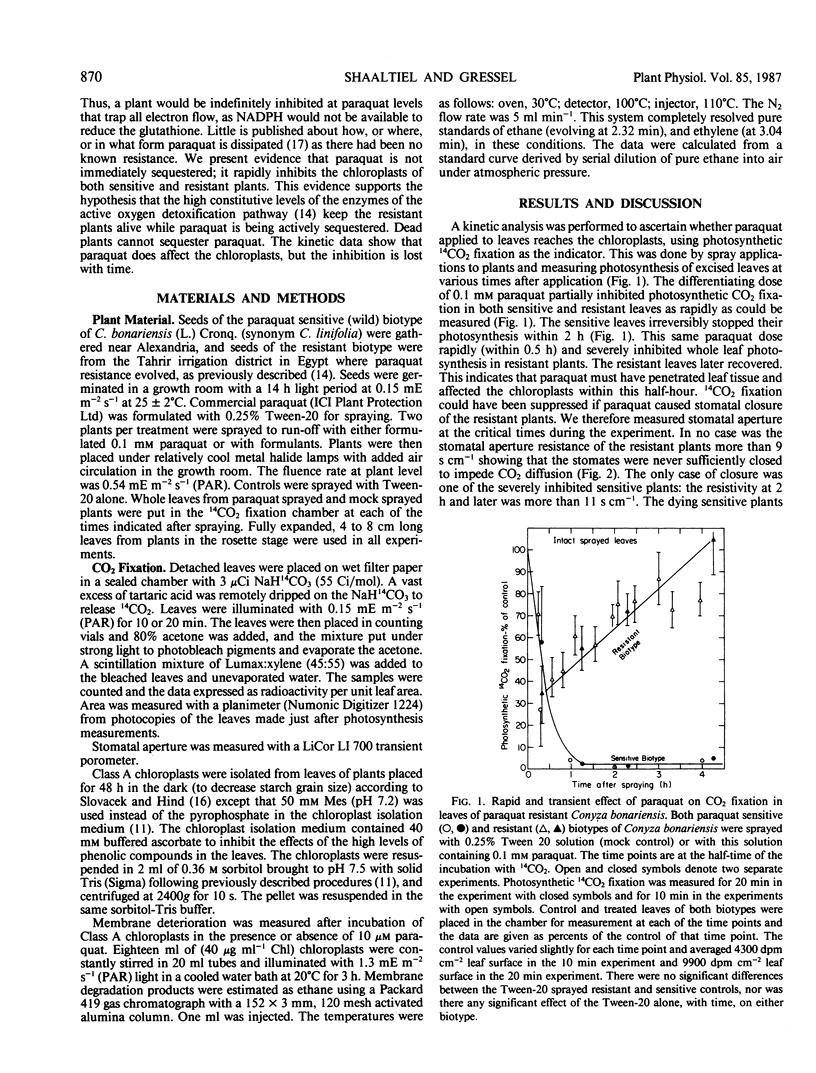
Paraquat resistance has been claimed to be due to a sequestration of the herbicide before it reaches chloroplasts. This is based on the sensitivity of photosystem I in isolated thylakoids to paraquat, and autoradiographic analyses showing label from paraquat near veins 4 hours after treatment of a resistant biotype. Conversely, the enzymes of the superoxide detoxification pathway were found to be at constitutively elevated levels in intact class A chloroplasts of the resistant biotype of Conyza bonariensis (L.) Cronq. Evidence is presented here that physiologically active levels of paraquat rapidly inhibit chloroplast function in both the resistant and sensitive biotype, before the first sequestration was visualized. This inhibition is transient (completed in 2 hours) in the resistant biotype and irreversible in the sensitive type. Intact class A chloroplasts of the resistant biotype with or without paraquat are less susceptible to photoinduced membrane damage than the sensitive biotype without paraquat, as measured by ethane evolution. These data support a hypothesis that the ability to prevent superoxide damage keeps the resistant biotype viable while paraquat or its metabolites are being sequestered.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke J. J., Gamble P. E., Hatfield J. L., Quisenberry J. E. Plant morphological and biochemical responses to field water deficits: I. Responses of glutathione reductase activity and paraquat sensitivity. Plant Physiol. 1985 Oct;79(2):415–419. doi: 10.1104/pp.79.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elroy-Stein O., Bernstein Y., Groner Y. Overproduction of human Cu/Zn-superoxide dismutase in transfected cells: extenuation of paraquat-mediated cytotoxicity and enhancement of lipid peroxidation. EMBO J. 1986 Mar;5(3):615–622. doi: 10.1002/j.1460-2075.1986.tb04255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst E. P., Nakatani H. Y., Dodge A. D., Penner D., Arntzen C. J. Paraquat resistance in conyza. Plant Physiol. 1985 Apr;77(4):984–989. doi: 10.1104/pp.77.4.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groden D., Beck E. H2O2 destruction by ascorbate-dependent systems from chloroplasts. Biochim Biophys Acta. 1979 Jun 5;546(3):426–435. doi: 10.1016/0005-2728(79)90078-1. [DOI] [PubMed] [Google Scholar]
- Nakatani H. Y., Barber J. An improved method for isolating chloroplasts retaining their outer membranes. Biochim Biophys Acta. 1977 Sep 14;461(3):500–512. [PubMed] [Google Scholar]
- Schaedle M. Chloroplast glutathione reductase. Plant Physiol. 1977 May;59(5):1011–1012. doi: 10.1104/pp.59.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovacek R. E., Hind G. Influence of antimycin a and uncouplers on anaerobic photosynthesis in isolated chloroplasts. Plant Physiol. 1977 Oct;60(4):538–542. doi: 10.1104/pp.60.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]


