Abstract
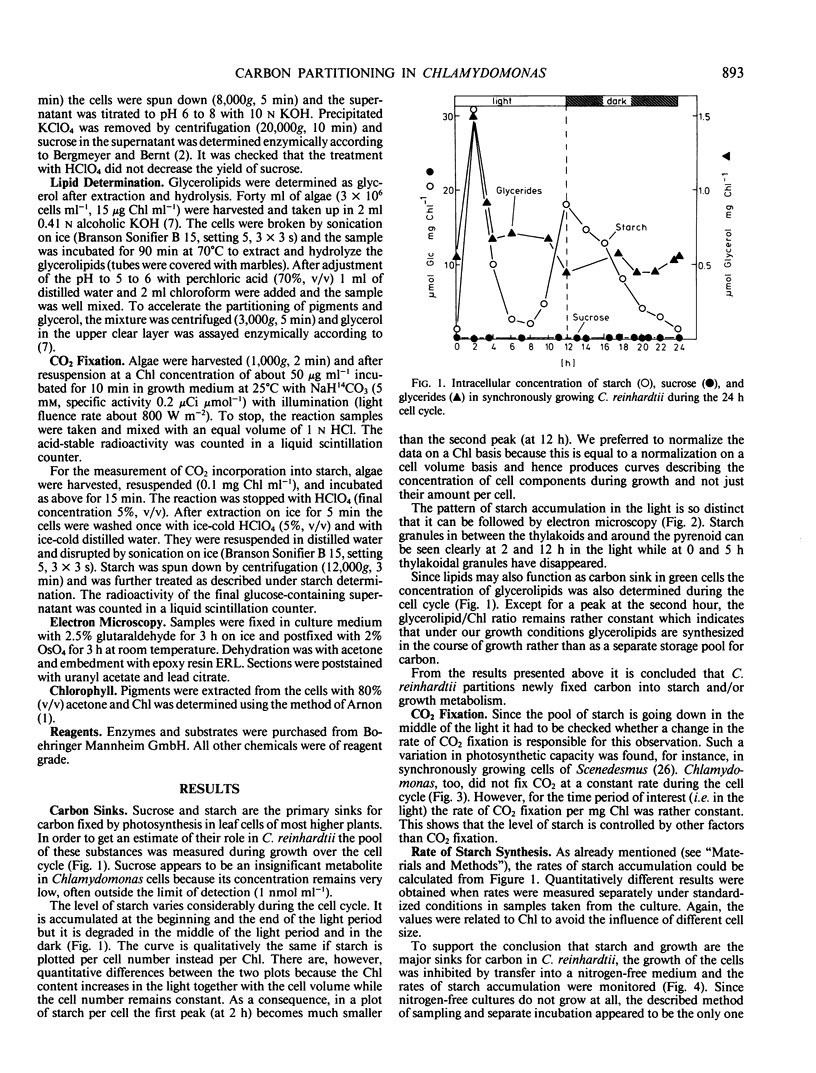
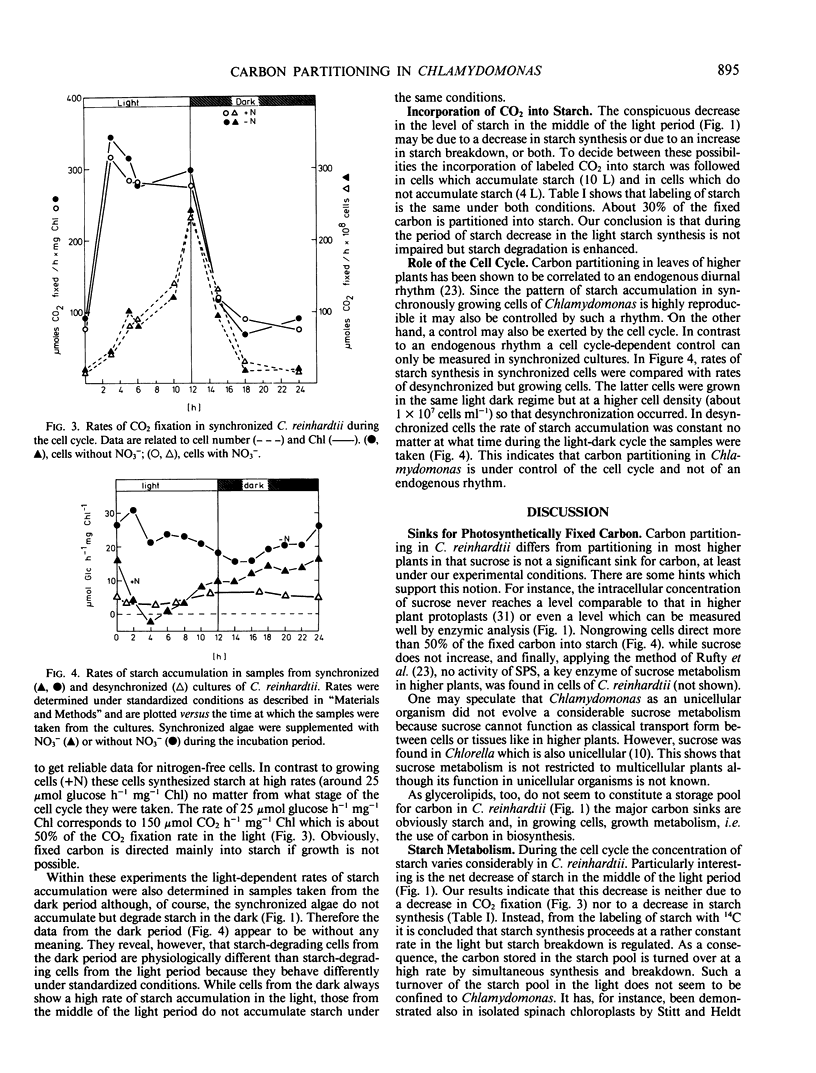
Using enzymic and isotope techniques the intracellular partitioning of newly fixed carbon was studied in synchronized cells of Chlamydomonas reinhardtii. Starch and growth metabolism, i.e. the use of carbon in biosynthesis, were found to be the major sinks for photosynthetically fixed carbon in the alga. Sucrose does not accumulate in significant quantities. The amount of carbon partitioned either into starch or growth varies during the 12 hour light/12 hour dark cell cycle. Starch is accumulated at the beginning and at the end of the light period while a net breakdown is observed in the middle of the light period and in the dark. In contrast, nonsynchronized cells accumulate starch all the time in the light which suggests that carbon partitioning is controlled by the cell cycle. Labeled bicarbonate is incorporated into starch even at times when the total intracellular level of starch is decreasing. This indicates a turnover of the starch pool in the light with synthesis and degradation occurring simultaneously and at different rates.
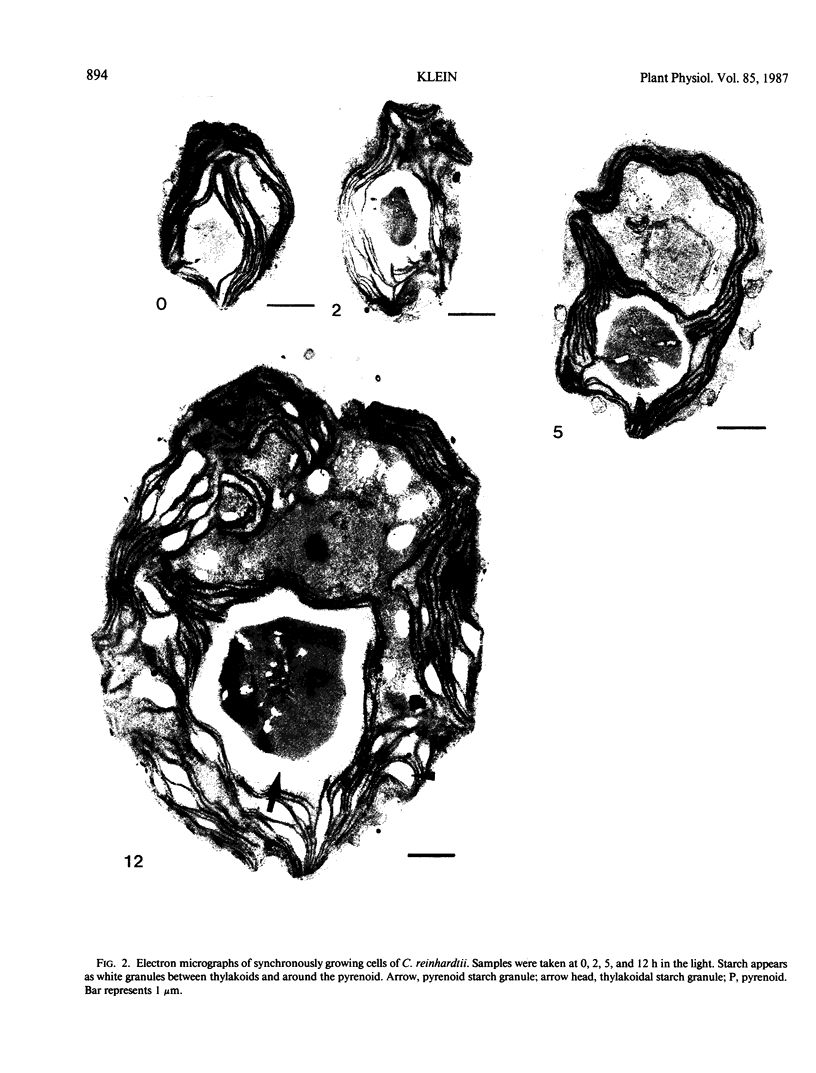
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterton N. J., Silvius J. E. Photosynthate Partitioning into Starch in Soybean Leaves: I. Effects of Photoperiod versus Photosynthetic Period Duration. Plant Physiol. 1979 Nov;64(5):749–753. doi: 10.1104/pp.64.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D., Parnas H. An optimal policy for the metabolism of storage materials in unicellular algae. J Theor Biol. 1976 Jan;56(1):1–18. doi: 10.1016/s0022-5193(76)80043-4. [DOI] [PubMed] [Google Scholar]
- Duynstee E. E., Schmidt R. R. Total starch and amylose levels during synchronous growth of Chlorella pyrenoidosa. Arch Biochem Biophys. 1967 Mar;119(1):382–386. doi: 10.1016/0003-9861(67)90469-9. [DOI] [PubMed] [Google Scholar]
- Huber S. C. Role of sucrose-phosphate synthase in partitioning of carbon in leaves. Plant Physiol. 1983 Apr;71(4):818–821. doi: 10.1104/pp.71.4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr P. S., Rufty T. W., Huber S. C. Endogenous Rhythms in Photosynthesis, Sucrose Phosphate Synthase Activity, and Stomatal Resistance in Leaves of Soybean (Glycine max [L.] Merr.). Plant Physiol. 1985 Feb;77(2):275–280. doi: 10.1104/pp.77.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U., Chen C., Gibbs M., Platt-Aloia K. A. Cellular Fractionation of Chlamydomonas reinhardii with Emphasis on the Isolation of the Chloroplast. Plant Physiol. 1983 Jun;72(2):481–487. doi: 10.1104/pp.72.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I., Siekevitz P., Palade G. E. Biogenesis of chloroplast membranes. II. Plastid differentiation during greening of a dark-grown algal mutant (Chlamydomonas reinhardi). J Cell Biol. 1967 Dec;35(3):553–584. doi: 10.1083/jcb.35.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt S. G. Ammonia regulation of carbon metabolism in photosynthesizing leaf discs. Plant Physiol. 1977 Nov;60(5):739–742. doi: 10.1104/pp.60.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T. W., Kerr P. S., Huber S. C. Characterization of diurnal changes in activities of enzymes involved in sucrose biosynthesis. Plant Physiol. 1983 Oct;73(2):428–433. doi: 10.1104/pp.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanwal G. G., Greenberg E., Hardie J., Cameron E. C., Preiss J. Regulation of starch biosynthesis in plant leaves: activation and inhibition of ADPglucose pyrophosphorylase. Plant Physiol. 1968 Mar;43(3):417–427. doi: 10.1104/pp.43.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanwal G. G., Preiss J. Biosynthesis of starch in Chlorella pyrenoidosa. II. Regulation of ATP: alpha-D-glucose 1-phosphate adenyl transferase (ADP-glucose pyrophosphorylase) by inorganic phosphate and 3-phosphoglycerate. Arch Biochem Biophys. 1967 Mar;119(1):454–469. doi: 10.1016/0003-9861(67)90477-8. [DOI] [PubMed] [Google Scholar]
- Steer B. T. Diurnal variations in photosynthetic products and nitrogen metabolism in expanding leaves. Plant Physiol. 1973 Apr;51(4):744–748. doi: 10.1104/pp.51.4.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Cseke C., Buchanan B. B. Regulation of fructose 2,6-bisphosphate concentration in spinach leaves. Eur J Biochem. 1984 Aug 15;143(1):89–93. doi: 10.1111/j.1432-1033.1984.tb08345.x. [DOI] [PubMed] [Google Scholar]
- Stitt M., Kürzel B., Heldt H. W. Control of Photosynthetic Sucrose Synthesis by Fructose 2,6-Bisphosphate : II. Partitioning between Sucrose and Starch. Plant Physiol. 1984 Jul;75(3):554–560. doi: 10.1104/pp.75.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Wirtz W., Heldt H. W. Regulation of Sucrose Synthesis by Cytoplasmic Fructosebisphosphatase and Sucrose Phosphate Synthase during Photosynthesis in Varying Light and Carbon Dioxide. Plant Physiol. 1983 Jul;72(3):767–774. doi: 10.1104/pp.72.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]