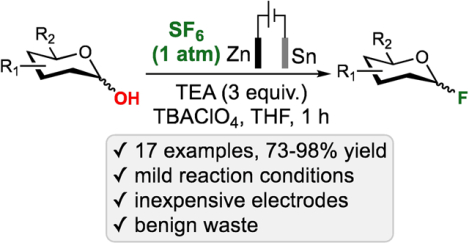
Abstract
This manuscript describes electrochemical synthesis of 17 different glycosyl fluorides in 73–98% yield on up to 5 g scale that relies on using SF6 as an inexpensive and safe fluorinating agent. Subsequently carried cyclic voltammetry and related mechanistic studies suggest that the generated through the cathodic reduction of SF6 active fluorinating species are transient under these reductive conditions, and that the sulfur and fluoride by-products are effectively scavenged by Zn(II) to generate benign salts.
Keywords: Electrochemical, Fluorination, Sulfur(VI) Hexafluoride, Glycosyl Fluorides, Reduction
Graphical Abstract
INTRODUCTION
With the increasing awareness about the impacts of the atmospheric greenhouse gasses on the climate changes, many recent efforts of the scientific community have focused on reducing the amount of greenhouse gas emission. Among the various problems associated with this topic that modern science and engineering face, the utilization of sulfur(VI) hexafluoride is one of the significant challenges.1 Sulfur (VI) hexafluoride (SF6), is a very stable and nontoxic gas that is produced on an industrial scale and used worldwide as a dielectric insulator.2 Due to its chemical stability and high susceptibility to infrared light excitation, SF6 is considered to be one of the strongest greenhouse gasses that is regulated by the Kyoto protocol.3 Several recent studies have examined the possibility of degrading or converting SF6; however, a feasible protocol is yet to emerge from these studies. The inertness of this gas presents a significant challenge as the majority of the examples describing SF6 activation involve extreme reaction conditions (very high temperatures, pressures or UV radiation <190 nm) that are not desirable for the modern organic processes.4 In addition, the decomposition of SF6 results in fluorine and sulfur-containing waste, disposal of which is often a challenge. Therefore, the processes that do not simply decompose SF6, but also take advantage of SF6 as a chemical entity are highly desired.5–10
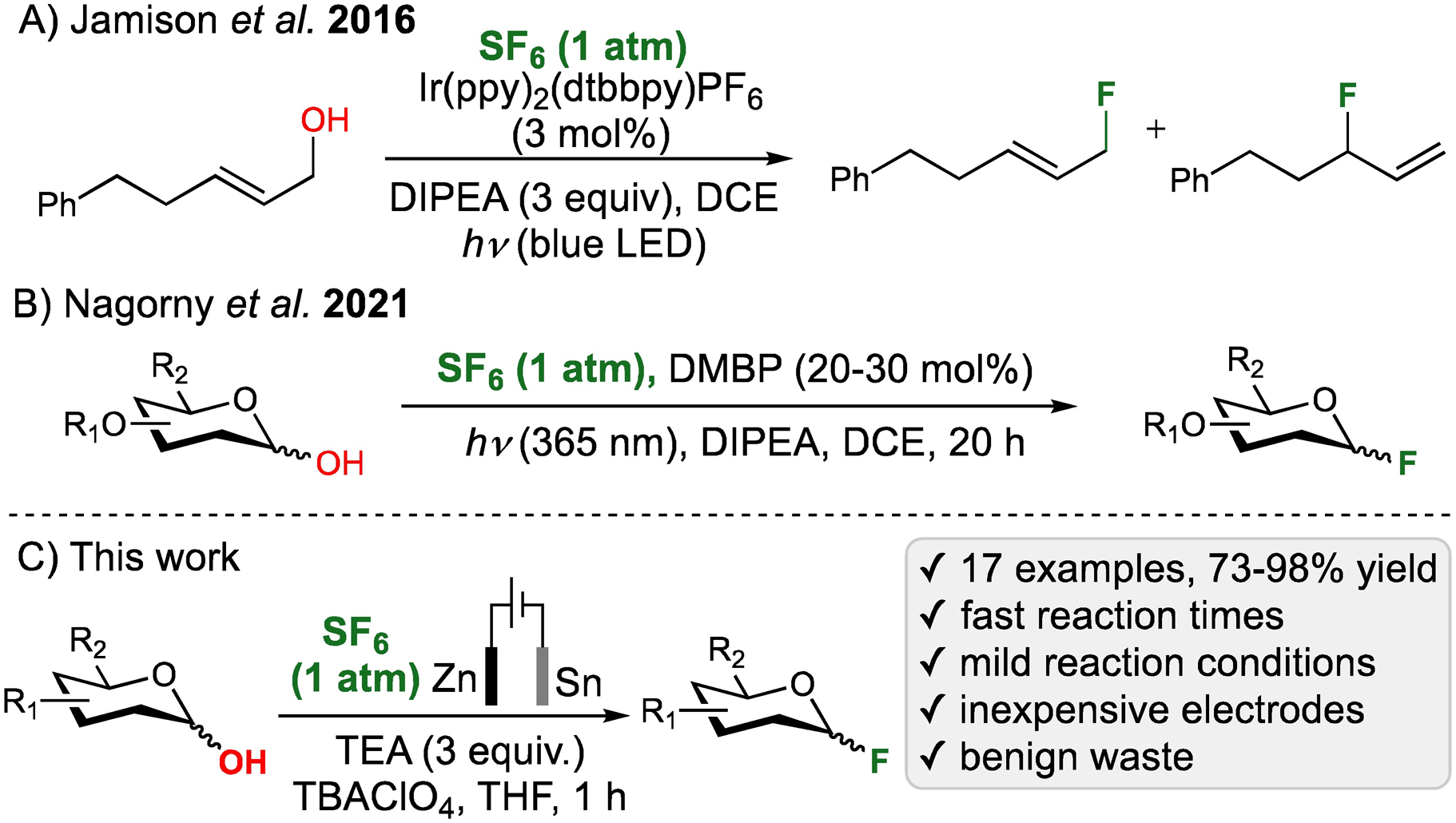
One of the practical uses of SF6 as a chemical reagent was recently reported by Jamison and McTeague who demonstrated that photoredox activation of SF6 with Ir(III)-based catalysts and blue LED light may result in active fluorinating species that mediate deoxyfluorination of allylic alcohols (Scheme 1A).6 The idea of exploring SF6 to generate stoichiometric fluorinating reagents for deoxyfluorination was subsequently explored by the Rueping group7a and Braun and Kemnitz.7b In addition, several different studies including the reduction of SF6 with phosphide anions by Speed and coworkers5i and reductive deconstruction of SF6 by Crimmin and coworkers5j have been reported. In parallel to the aforementioned studies, the Wagenknecht group described several different applications of SF6 for the photochemical pentafluorosulfanylation of alkenes.8
Scheme 1.
Summary of deoxyfluorination with SF6
Our group has long-standing interests in developing new organocatalytic methods and their application for the synthesis of carbohydrate derivatives.10,11 As a part of these efforts, we recently investigated the possibility of developing new approach for the synthesis of glycosyl fluorides.11 Glycosyl fluorides represent a unique class of glycosyl donors, which are stable to a variety of conditions, but could serve as highly active glycosylating agents upon activation with fluorophilic Lewis acids.12 While there are many methods that are available for the synthesis of glycosyl fluorides, the deoxyfluorination-based reactions involving (diethylamino)sulfur(IV) trifluoride (DAST)13 or HF•Py14 are by far the most common and general. The use of these highly corrosive, reactive and toxic reagents for larger scale processes is not ideal, and our group recently explored the possibility of utilizing nontoxic and inexpensive SF6 as a safer alternative. By using 4,4’-dimethoxybenzophenone as an inexpensive photocatalyst and DIPEA as the reducing agent, we achieved a photochemical activation of SF6 with UVA LED source (λmax=365 nm) that resulted in efficient fluorination of 16 protected carbohydrates containing a hydroxyl group at the anomeric position.11 While this represented an efficient and safe method for utilizing SF6 and producing valuable carbohydrate-based building blocks, this transformation had several limitations in comparison to the more traditionally employed DAST-mediated 2-deoxyfluorination reactions. These limitations included significantly extended reaction times, in particular on a larger scale, and, as the result of it, lower conversions. In addition, the reaction required using a large excess of DIEA (10 equiv.) and resulted in multiple side-products including the photocatalyst decomposition products and sulfur-containing waste. These limitations prompted us to search for the alternative new methods to achieve SF6 activation, and this manuscript describes electrochemical deoxyfluorination with SF6 leading to the fast, efficient and scalable formation of glycosyl fluorides.15 The use of zinc sacrificial anode provides an effective mean to scavenge sulfur and fluoride waste to form benign Zn(II) sulfides and fluorides, and this protocol holds promise as an inexpensive, safe and mild method for utilization of SF6 for the fluorination of organic molecules.
RESULTS AND DISCUSSION
Our study commenced with subjecting the armed 2,3,4,6-tetra-O-benzyl-D-galactose 1a to galvanostatic electrolysis in the atmosphere of SF6 using platinum (Pt) anode(+) and cathode(–), DIPEA as the base, tetrabutylammonium perchlorate (TBAClO4) as the electrolyte, and DCE as the solvent (Table 1, entry 1). Gratifyingly, the exposure of 1a to the aforementioned reaction conditions for 1 h resulted in the formation of desired product 2a albeit in only 32% yield. In attempts to optimize these conditions, we evaluated various solvents (cf. Table S1) and observed that using THF resulted in slightly faster reaction with ~70% consumption of the starting material and 35% yield of 2a (Table 1, entry 2). The NMR analysis of the crude reaction indicated the formation of unidentified fluorine-containing debenzylation side-products in addition to 2a. We surmised that the debenzylation reactions happen due to the anodic oxidation of 1a and 2a. Therefore, to minimize these undesired oxidation reactions, the use of sacrificial zinc (Zn) anode, which would get oxidized to produce Zn2+ cations, was evaluated next (Table 1, entry 3).16 Excitingly, this modification dramatically improved the yield of 2a and eliminated the formation of the side-products. Our further optimizations were focused on making this process more practical and identifying a less expensive type of the cathode (entries 4–6 and Table S2). While the use of C(–) instead of Pt(–) cathode led to a significant reduction in the yield (entry 4), we found that Sn(–) cathode provided 2a in 84% yield (entry 5). Further attempts to optimize this yield by using magnesium as sacrificial anode17 was not successful (entry 6), and, therefore, Zn(+) anode and Sn(–) cathode were selected as the optimal electrodes and other reaction parameters were optimized next.
Table 1.
Reaction condition optimization

| ||
| Entry | Conditionsa | Yieldb |
|---|---|---|
| 1 | (+)Pt/(−)Pt, DIEA (5 eq.), DCE, 1 h | 32%c |
| 2 | (+)Pt/(−)Pt, DIEA (5 eq.) THF, 45 min |
35% (α : β = 1:2.1)c |
| 3 | (+)Zn/(−)Pt, DIEA (5 eq.) THF, 30 min |
94% (α : β = 1 : 1.3) |
| 4 | (+)Zn/(−)C, DIEA (5 eq.) THF, 30 min |
22% (α : β = 1 : 1.3) |
| 5 | (+)Zn/(−)Sn, DIEA (5 eq.) THF, 30 min |
84% (α : β = 1 : 1.5) |
| 6 | (+)Mg/(−)Sn, DIEA (5 eq.) THF, 30 min |
66% (α : β = 1 : 1.2) |
| 7d | (+)Zn/(−)Sn, DIEA (3 eq.) THF, 1 h |
93% (α : β = 1 : 1.3) |
| 8d | (+)Zn/(−)Sn, NEt3 (3 eq.) THF, 1 h |
93% (α : β = 1 : 1.6) |
| 9 | (+)Zn/(−)Sn, no base THF, 1 h |
67% (α : β = 1 : 1.2) |
The reactions were carried on 0.1 mmol scale.
Represents isolated yield.
Determined by 19F NMR with trifluorotoluene as internal standard.
TBAClO4 = 0.15M
While our attempts to improve the yield by replacing TBAClO4 with other salts were not successful, other electrolytes such as TBAPF6 were found to be compatible with the reaction conditions (cf. Table S4). It was also observed that the electrolysis time and the nature/quantities of the base could be used to improve the yield of 2a (entries 7–9 and Table S3 Thus, 2a could be produced in 93% yield if the amounts of DIEA and TBAClO4 are reduced to 3 equivalents and 0.15 M, respectively, and the solution is electrolyzed for 1 h instead of 30 min (entry 7). DIEA could be substituted with less expensive triethylamine without the decrease in the yield of 2a (entry 8). In addition, our subsequent studies indicated that triethylamine is preferred over DIEA for the fluorination of acetylated and PMB protected galactose derivatives 1k and 1h (Table S6). Amine base is not an essential component as the reaction progresses to 67% in the absence of triethylamine (Table 1, entry 9). However, the use of the amine base ensures a full progression of the reaction either by preventing the electrode passivation or by promoting the solvation of Zn2+ cation (Figure S1).
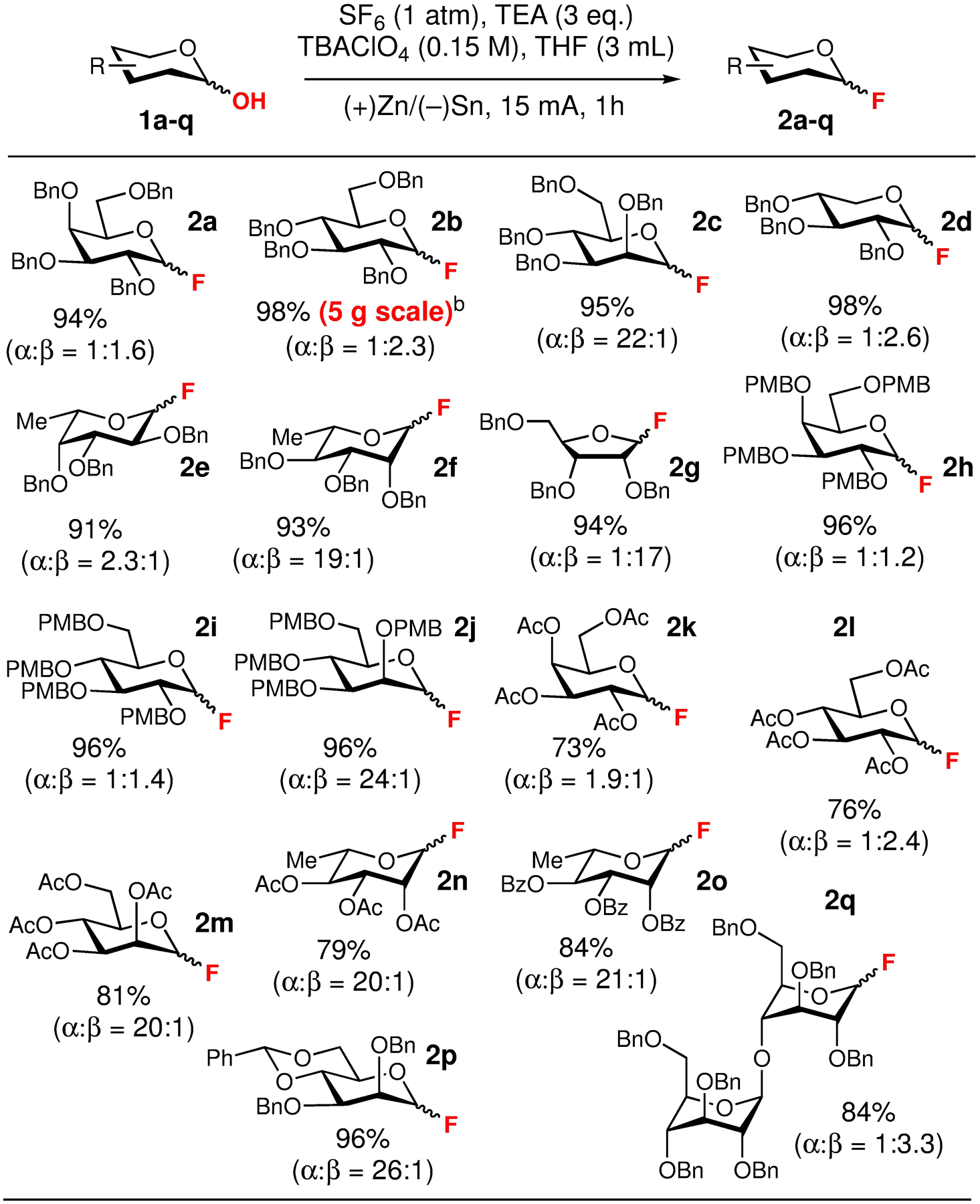
With the optimal conditions for the electrochemical fluorination of 1a in hand, our subsequent studies were focused on evaluating the substrate scope of this reaction (Scheme 2). This evaluation revealed that this method is suitable for a variety of sugars and protecting groups typically employed in carbohydrate synthesis. The deoxyfluorination of perbenzylated D-galactose, D-glucose, D-mannose, D-xylose, L-fucose and L-rhamnose derivatives 1a–1f and D-ribose derivative 1g proceeded in excellent yields (91–98%) and resulted in the corresponding products 2a–2g. While the PMB protecting groups are sensitive to both oxidative and reductive conditions, the deoxyfluorination of PMB-protected D-galactose, D-glucose and D-mannose derivatives 1h–1j proceeded cleanly to provide the corresponding products in 96% yield.
Scheme 2.
Substrate scopea
aThe provided yields and diastereomeric ratios represent the average values over two runs on 0.1 mmol scale. For the specific yields and selectivities obtained for each individual run please refer to the SI. bThis reaction was performed on 9.25 mmol (5.0 g) scale.
The electrochemical fluorination conditions did not affect the acid-sensitive benzylidene acetal moiety present in 1p, and glycosidic linkage present in perbenzylated cellobiose derivative 1q, and the corresponding products were obtained in 96% and 84% yield correspondingly. Finally, the electrochemical fluorination conditions were compatible with disarmed peracetylated and perbenzylated glycosides 1k–1o that were previously found to be significantly less reactive under the photochemical conditions, and the corresponding products 2k–2o were obtained in 73–84% yields. It is important to emphasize that these transformations are amenable to scale up, and we were able to adopt an experimental setup developed by Baran and coworkers 18 and modified it to carry a 5 g scale deoxyfluorination of D-glucose derivative 1b in 98% yield (cf. SI-III-e for additional details).
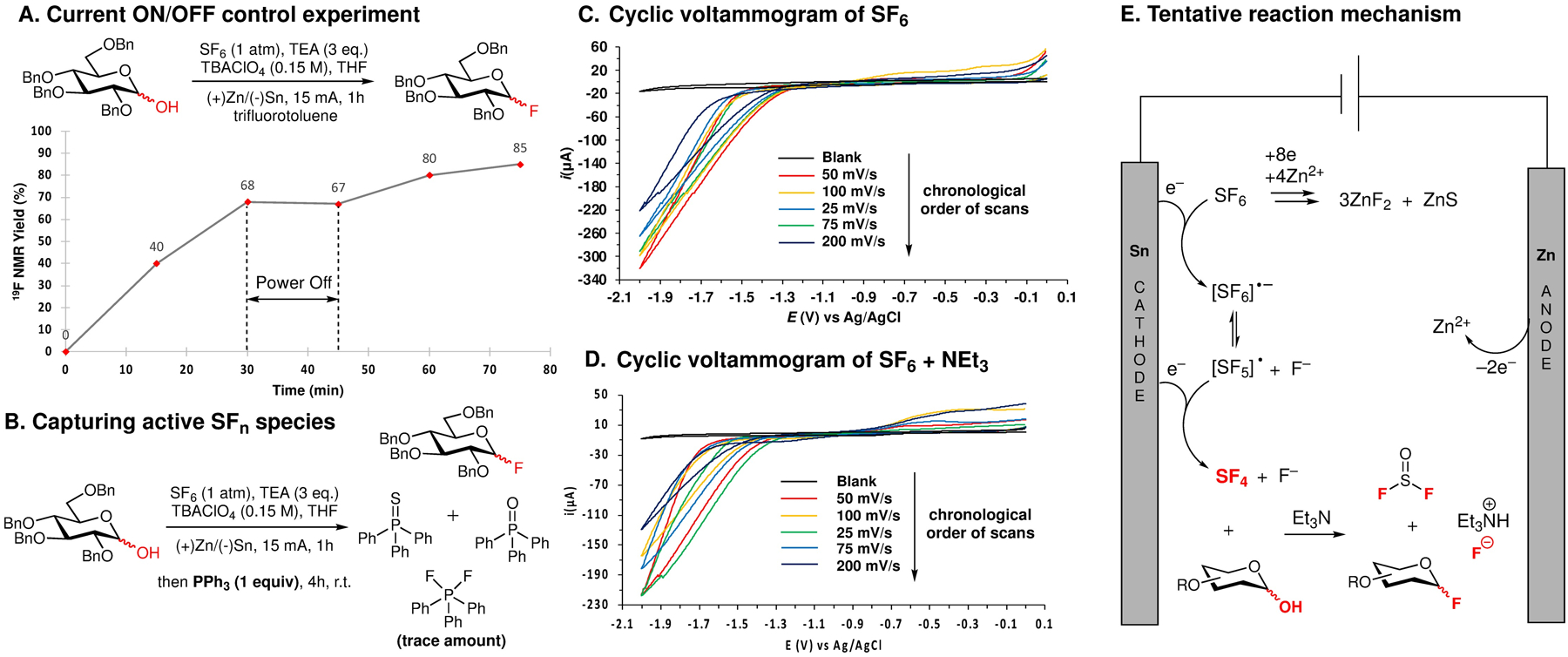
The results summarized in Scheme 2 suggest that the electrochemical fluorination with SF6 proceeds with excellent efficiencies and under mild conditions, which makes it favorably comparable with the other popular deoxyfluorination protocols (cf. Table S8). Therefore, our subsequent studies were focused on understanding the reaction mechanism and showcasing the safety of this protocol by demonstrating that no toxic SFn and SOmFn species are accumulated throughout the reaction (cf. Figure 1). Similar to the photochemical deoxyfluorination with SF6, no active fluorinating species, such as SF4, were detected by the 19F NMR throughout the reaction. A control experiment with Zn(II) fluoride instead of SF6 demonstrated that the presence of salt alone does not lead to the formation of glycosyl fluorides (cf. SI-Vb). The continuous electrolysis was essential for the progression of this reaction, and the formation of 2b from 1b stopped when the current was turned off (Figure 1A). While the photochemical activation of SF6 with DMBP led to significant accumulation of the active fluorination species that reacted with triphenylphosphine to produce difluorotriphenylphosphine, only trace amounts of this compound were detected by 31P and 19F NMR after the treatment of the crude electrochemical fluorination mixture with PPh3 (Figure 1B). This suggests that electrochemical conditions prevent accumulation of the active SFn and SOmFn species by reducing them to Zn(II) fluorides and sulfides. To demonstrate this and to understand the role of the amine base in this process, we carried series of cyclic voltammetry (CV) studies (cf. Figure 1C and 1D, and Figure S6–S12). Based on the observation from chemical experiments, our initial assumption was that the amine base is not directly involved in the catalytic cycle, but it is important for preventing the electrode passivation. We first attempted to identify the half reduction potential of SF6 by adopting the previously published experimental setup (Figure S6).15b The voltammogram showed irreversible reduction of SF6 around –2.4 V (vs Fc+/Fc), which is similar to the previously measured value (–2.17 V vs Fc+/Fc). CV data for the reduction of SF6 with zinc as counter electrode in the absence of Et3N revealed the current attenuation in the chronological order of the scan (Figure 1C). This suggests the electrode passivation via the formation of nonconducting zinc salts on its surface, which leads to the increased internal resistance. As expected, we observed consistent current throughout the scans when the CV experiment was repeated with Pt as counter electrode (Figure S8). Next, we conducted the CV experiment in the presence of triethyl amine for the reduction of SF6 with zinc as counter electrode (Figure 1D). The current was dependent on the scan rate rather than the chronological order of the scan. With the slower scan rate, triethylamine has more time to either promote the solvation of zinc cation or scavenge the deposited zinc salts. This would prevent the electrode passivation and keep the resistance in the cell relatively steady, which enhances the current flow compared to faster scan rate. Since our method is under galvanostatic condition, triethylamine keeps the voltage steady and prevents the undesired reduction reactions. Based on these considerations, the tentative mechanism of this transformation is proposed in Figure 1D. SF6 undergoes cathodic reduction that leads to SF4 or related S(IV)- or S(II)-based active fluorinating agents. The various SFn or SOmFn species resulting from the reduction of SF6 and deoxyfluorination undergo subsequent reduction to S2− and F− anions that undergo binding to Zn2+ cations to form zinc(II) sulfides and fluorides and their complexes with triethylamine.
Figure 1.
Studies focused on understanding the reaction mechanism
CONCLUSION
In conclusion, we have developed a mild and highly efficient protocol for the electrochemical utilization of SF6 leading to the formation of 17 different glycosyl fluorides in 73–98% yield. To demonstrate the utility of this transformation, 5g scale synthesis of 2,3,4,6-O-tetrabenzyl-D-glucopyranosyl fluoride was accomplished in 98% yield. Subsequently carried cyclic voltammetry and other mechanistic studies were consistent with the cathodic reduction of sulfur(VI) fluoride and oxidation of Zn anode. These studies suggest that the generated from SF6 active fluorinating species are transient under these reductive conditions, and that the sulfur and fluoride by-products are effectively scavenged by Zn(II) to generate benign and unreactive salts.
Supplementary Material
Funding Sources
P.N. is thankful for NIH U01GM125274 for supporting this work and ACS PRF grant #66230-NDI for supporting the completion of these studies.
Footnotes
Supporting Information. The Supporting Information and NMR spectra of reaction products are available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1).a) Maiss M; Brenninkmeijer CAM Atmospheric SF6: Trends, Sources, and Prospects. Environ. Sci. Technol 1998, 32, 3077; [Google Scholar]; b) Dervos CT; Vassiliou P Sulfur Hexafluoride (SF6): Global Environmental Effects and Toxic Byproduct Formation. J. Air Waste Manage. Assoc 2000, 50, 137. [DOI] [PubMed] [Google Scholar]
- 2).Christophorou LG; Olthoff JK; Van Brunt RJ Sulfur hexafluoride and the electric power industry. IEEE Electr. Insul. Mag 1997, 5, 20. [Google Scholar]
- 3).Reilly J; Prinn R; Harnisch J; Fitzmaurice J; Jacoby H; Kicklighter D; Melillo J; Stone P; Sokolov A; Wang C Multi-gas assessment of the Kyoto Protocol. Nature 1999, 401, 549. [Google Scholar]
- 4).a) Lee MH; Chang MB; Wu KY Abatement of sulfur hexafluoride emissions from the semiconductor manufacturing process by atmospheric-pressure plasma. J. Air. Waste Manage. Assoc 2004, 54, 960; [DOI] [PubMed] [Google Scholar]; b) Huang L; Gu D; Yang L; Xia L; Zhang R; Hou H Photoreductive Degradation of Sulfur Hexafluoride in the Presence of Styrene. J. Env. Sci 2008, 20, 183; [DOI] [PubMed] [Google Scholar]; c) Zhang J; Zhou JZ; Liu Q; Qian G; Xu ZP Efficient Removal of Sulfur Hexafluoride (SF6) Through Reacting with Recycled Electroplating Sludge. Environ. Sci. Technol 2013, 47, 6493. [DOI] [PubMed] [Google Scholar]
- 5).Selected examples of chemical activation of SF6:; a) Cowen HC; Riding F; Warhurst E The reaction of sulphur hexafluoride with sodium, J. Chem. Soc 1953, 4168; [Google Scholar]; b) Case JR; Nyman F Some Chemical Reactions of Sulphur Hexafluoride. Nature 1962, 193, 473; [Google Scholar]; c) Demitras GC; MacDiarmid AG The Low Temperature Reaction of Sulfur Hexafluoride with Solutions of Sodium. Inorg. Chem 1964, 3, 1198; [Google Scholar]; d) Padma DK; Vasudeva Murthy AR; Becher W; Massonne J Reduction of Sulphur Hexafluoride by Lithium Aluminum Hydride. J. Fluor. Chem 1972, 2, 113; [Google Scholar]; e) Basta R; Harvey BG; Arif AM; Ernst RD Reactions of SF6 with Organotitanium and Organozirconium Complexes: The “Inert” SF6 as a Reactive Fluorinating Agent. J. Am. Chem. Soc 2005, 127, 11924; [DOI] [PubMed] [Google Scholar]; f) Holze P; Horn B; Limberg C; Matlachowski C; Mebs S The Activation of Sulfur Hexafluoride at Highly Reduced Low-Coordinate Nickel Dinitrogen Complexes. Angew. Chem. Int. Ed 2014, 53, 2750; [DOI] [PubMed] [Google Scholar]; g) Zamostna L; Braun T; Braun B S-F and S-C Activation of SF6 and SF5 Derivatives at Rhodium: Conversion of SF6 into H2S. Angew. Chem. Int. Ed 2014, 53, 2745; [DOI] [PubMed] [Google Scholar]; h) Bub F; Muck-Lichtenfeld C; Mehlmann P; Dielmann F Nucleophilic Activation of Sulfur Hexafluoride: Metal‐Free, Selective Degradation by Phosphines. Angew. Chem. Int. Ed 2018, 57, 4951; [DOI] [PubMed] [Google Scholar]; i) Huchenski BSN; Speed AWH Room-temperature Reduction of Sulfur Hexafluoride with Metal Phosphides. Chem. Commun 2021, 57, 7128; [DOI] [PubMed] [Google Scholar]; j) Sheldon DJ; Crimmin MR Complete Deconstruction of SF6 by an Aluminium(i) Compound. Chem. Commun 2021, 57, 7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).McTeague TA; Jamison TF Photoredox Activation of SF6 for Fluorination. Angew. Chem. Int. Ed 2016, 55, 15072. [DOI] [PubMed] [Google Scholar]
- 7).a) Rueping M; Nikolaienko P; Lebedev Y; Adams A Metal-Free Reduction of the Greenhouse Gas Sulfur Hexafluoride, Formation of SF5 Containing Ion Pairs and the Application in Fluorinations. Green Chem. 2017, 19, 2571; [Google Scholar]; b) Tomar P; Braun T; Kemnitz E Photochemical Activation of SF6 by N-heterocyclic Carbenes to Provide a Deoxyfluorinating Reagent. Chem. Commun 2018, 54, 9753. [DOI] [PubMed] [Google Scholar]
- 8).a) Rombach D; Wagenknecht H-A Photoredox Catalytic Activation of Sulfur Hexafluoride for Pentafluorosulfanylation of α-Methyl- and α-PhenylStyrene. ChemCatChem. 2018, 10, 2955; [Google Scholar]; b) Rombach D; Wagenknecht H-A Photoredox Catalytic α-Alkoxypentafluorosulfanylation of α-Methyl- and α-Phenylstyrene Using SF6. Angew. Chem. Int. Ed 2020, 59, 300; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Rombach D; Birenheide B; Wagenknecht H-A Photoredox Catalytic Pentafluorosulfanylative Domino Cyclization of a-Substituted Alkenes to Oxaheterocycles by Using SF6. Chem.-Eur. J 2021, 27, 8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Iakobson G; Posta M; Beier P Reductive Activation of Sulfur Hexafluoride with TEMPOLi: Addition of the Pentafluorosulfanyl Group and TEMPO to Terminal Alkenes. J. Flour. Chem 2018, 213, 51. [Google Scholar]
- 10).a) Sun Z; Winschel GA; Borovika A; Nagorny P Chiral Phosphoric Acid-Catalyzed Enantioselective and Diastereoselective Spiroketalizations. J. Am. Chem. Soc 2012, 134, 8074; [DOI] [PubMed] [Google Scholar]; b) Mensah E; Camasso N; Kaplan W; Nagorny P Chiral Phosphoric Acid-Directed Regioselective Acetalization of Carbohydrate-Derived 1,2-diols. Angew. Chem. Int. Ed 2013, 52, 13939; [DOI] [PubMed] [Google Scholar]; c) Tay J-H; Arguelles AJ; DeMars MD II; Zimmerman PM; Sherman DH; Nagorny P Regiodivergent Glycosylation of 6-deoxy-Erythronolide B and Oleandomycin-derived Macrolides Enabled by Chrial Acid Catalysis” J. Am. Chem. Soc 2017, 139, 8570; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Lee J; Borovika A; Khomutnyk Y; Nagorny P Chem. Commun 2017, 53, 8976; [DOI] [PubMed] [Google Scholar]; e) Wang S; Zhelavskyi O; Lee J; Arguelles AJ; Khomutnyk Y; Mensah EA; Guo H; Hourani R; Zimmerman PM; Nagorny P Studies of Catalyst-controlled Regioselective Acetalization and its Application to Single-pot Synthesis of Differentially Protected Saccharides. J. Am. Chem. Soc 2021, 143, 18592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Kim S; Khomutnyk Y; Bannykh A; Nagorny P Synthesis of Glycosyl Fluorides by Photochemical Fluorination with Sulfur(VI) Hexafluoride. Org. Lett 2021, 23, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).a) Shimizu M; Togo H; Yokoyama M Chemistry of Glycosyl Fluorides. Synthesis. 1998, 6, 799; [Google Scholar]; b) Toshima K Glycosyl Fluorides in Glycosidations. Carb. Res 2000, 327, 15; [DOI] [PubMed] [Google Scholar]; c) Mukaiyama T; Jona, Glycosyl Fluoride: A Superb Glycosyl Donor in Glycosylation. H. Proc. Japan Acad. Ser. B 2002, 78, 73; [Google Scholar]; d) Randall JL; Nicolaou KC Preparation and Reactions of Glycosyl Fluorides in Fluorinated Carbohydrates, Vol. 374(2) (Ed.: Taylor NF) ACS Symposium Series, 1988, 13; [Google Scholar]; e) Williams SJ; Withers SG “Glycosyl fluorides in enzymatic reactions” Carbohydr. Res 2000, 327, 27. [DOI] [PubMed] [Google Scholar]
- 13).a) Rosenbrook Wm. Jr.; Riley DA; Lartey PA A New Method for the Synthesis of Glycosyl Fluorides. Tetrahedron Lett. 1985, 26, 3; [Google Scholar]; b) Posner GH; Haines SR A Convenient, One-step, High-yield Replacement of an Anomeric Hydroxyl Group by a Fluorine Atom Using DAST. Preparation of glycosyl fluorides. Tetrahedron Lett. 1985, 26, 5. [Google Scholar]
- 14).Hayashi M; Hashimoto S; Noyori R Simple Synthesis of Glycosyl Fluorides. Chem. Lett 1984, 13, 1747. [Google Scholar]
- 15).Prior examples of electrochemical decomposition of SF6:; a) Das PS; Adhikari B; Maiti S Fluorination of Polymers by Sulfur Hexafluoride Gas under Electric Discharge. J. Polym. Sci.: Part A,: Polym. Chem 1994, 32, 39; [Google Scholar]; b) Bouvet S; Pegot B; Sengmany S; Le Gall E; Leonel E; Goncalves A-M; Magnier E Controlled Decomposition of SF6 by Electrochemical Reduction. Belstein J. Org. Chem 2020, 16, 2948; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Li Y; Khurram A; Gallant BM A high-capacity lithium-gas battery based on sulfur fluoride conversion. J. Phys. Chem. C 2018, 122, 7128; [Google Scholar]; d) Gao H; Li Y; Guo R; Gallant BM Controlling fluoride-forming reactions for improved rate capability in lithium-perfluorinated gas conversion batteries. Adv. Energy Mater 2019, 1900393; [Google Scholar]; e) Gao H; He M; Guo R; Gallant BM Electrochemical fluoridation of manganese oxide by perfluorinated-gas conversion for lithium-ion cathodes. Batteries & Supercaps 2021, 4, 1771. [Google Scholar]
- 16).Kingston C; Palkowitz MD; Takahira Y; Vantourout JC; Peters BK; Kawamata Y; Baran PS A Survival Guide for the “Electro-curious”. Acc. Chem. Res 2020, 53, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Lu L; Siu JC; Lai Y; Lin S An electroreductive approach to radical silylation via the activation of strong Si-Cl bond. J. Am. Chem. Soc 2020, 142, 21272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Hu P; Peter BK; Malapit CA; Vantourout JC; Wang P; Li J; Mele L; Escheverria P-G; Minteer SD; Baran PS Electroreductive olefin-ketone coupling. J. Am. Chem. Soc 2020, 142, 20979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


