Abstract
Dupuytren disease is a progressive, benign fibroproliferative disorder of the hands that can lead to debilitating hand contractures. Once symptomatic, treatment involves either surgical intervention, specifically fasciectomy or percutaneous needle aponeurotomy, or enzymatic degradation with clostridial collagenase. Currently, collagenase is the only pharmacotherapy that has been approved for the treatment of Dupuytren contracture. There is a need for a pharmacotherapeutic that can be administered to limit disease progression and prevent recurrence after treatment. Targeting the underlying fibrotic pathophysiology is critical. We propose a novel target to be considered in Dupuytren disease—cell communication network factor 2/connective tissue growth factor—an established mediator of musculoskeletal tissue fibrosis.
Key words: CCN2 protein, Connective tissue growth factor, Dupuytren contracture, Fibrosis, TGF-beta
Research into fibroproliferative disorders continues to expand our knowledge of the underlying mechanisms behind disorders such as idiopathic pulmonary fibrosis, Dupuytren disease (DD), scleroderma, and Peyronie disease. As a result, potential therapeutics for targeting these diseases are being identified. Although newer pharmacotherapies are being developed, there has also been interest in repurposing medications already approved by the US Food and Drug Administration for the treatment of fibrotic disorders.1, 2, 3 Despite these efforts, the mainstay of treatment for symptomatic Dupuytren contracture is largely surgical.
The most common nonsurgical treatment is local collagenase injection for enzymatic degradation. To date, collagenase treatment is the only Food and Drug Administration–approved pharmacotherapy in DD. There is a need for additional pharmacotherapeutic options that target the underlying fibrotic response, thereby halting early disease progression, reversing fibrosis once the disease has progressed, and, in doing so, potentially avoiding the risks of invasive treatments.
Pathophysiology and Disease Progression
The fibroproliferation underlying DD affects the palmar fascia of the hand and digits. The clinical course can lead to symptomatic contractures, ultimately resulting in functionally limiting declines in range of motion and diminished quality of life.4 The natural history of DD was initially described, by Luck5, to occur in three distinct stages. Stage I (proliferative phase) is characterized by increased fibroblast activity and nodule formation within the palmar fascia. The predominant and active cell during this phase is the myofibroblast. Stage II (involutional phase) involves notable nodular thickening and an increase in type III collagen production, which orients along lines of tension within the palm. Joint contracture may begin during this stage. Stage III (residual phase) is characterized by a marked disappearance of myofibroblasts and a change in the predominant type of collagen from type III to type I (Fig. 1).6,7
Figure 1.
Pathophysiology of Dupuytren contracture. Dupuytren disease progression exists on histological, cellular, and clinical levels. Resting tissue of the unaffected hand contains quiescent fibroblasts. Upon tissue injury or stress, fibroblasts are activated, proliferate, and differentiate into mature myofibroblasts (proliferative stage). With continued transforming growth factor (TGF)-β stimulation and Dupuytren risk factors, disease progresses. Nodule formation occurs as myofibroblasts differentiate and produce extracellular matrix (ECM), with collagen III:I ratio predominating (involutional stage). The ECM collagen ratio changes to I:III, increased collagen crosslinking occurs, and cellularity decreases as cords form and contraction ensues (residual stage) (Reproduced with permission from Lambi et al. Pharmacotherapies in Dupuytren disease: current and novel strategies. J Hand Surg Am. 20237).
The specific mechanisms and triggers that lead to DD development have not yet been fully elucidated. Disease progression varies between individuals and can be influenced by risk factors, such as alcohol intake, smoking, manual labor, metabolic factors (eg, diabetes), anticonvulsant drugs, and genetic predisposition.6,8, 9, 10, 11, 12, 13, 14, 15 However, it is well-established that the primary cell type responsible for DD progression is the myofibroblast. Derived from fibroblasts, the myofibroblast is characterized by the coexpression of high levels of platelet-derived growth factor and alpha-smooth muscle actin.16 The contractures seen clinically most likely occur on a cellular level through a contractile apparatus of the myofibroblast that involves bundles of actin microfilaments and contractile proteins (eg, nonmuscle myosin). These interact with extracellular matrix proteins and adjacent myofibroblasts through transmembrane integrin proteins at a cell surface adhesion complex—the fibronexus.17, 18, 19, 20, 21
Extensive research has been performed to better understand the modulators of fibroblasts and myofibroblasts in DD development. Transforming growth factor-β (TGF-β) signaling has been implicated as critical in DD development.22 All three mammalian isoforms of TGF-β (TGF-β1, TGF-β2, and TGF-β3) have been identified in DD disease nodules, palmar fascia, and cord tissue.23,24 Fibroblasts and myofibroblasts in all three histologic stages of DD progression have upregulated TGF-β signaling.23, 24, 25, 26 Exogenous application of TGF-β to either normal or DD fibroblasts leads to increased alpha-smooth muscle actin expression and differentiation of a quiescent fibroblast to a contracting myofibroblast.6,26, 27, 28 In culture, DD fibroblasts are induced to contract with TGF-β treatment.29 Furthermore, when TGF-β signaling is blocked in DD cells in vitro, decreases in alpha-smooth muscle actin and collagen gene expression are seen.27 Therefore, the ability to block the profibrotic effects of TGF-β signaling in DD has immense research and clinical potential.
A Novel Target in Dupuytren Disease—The Cell Communication Network Factor 2 Protein
Transforming growth factor-β is a master regulator of tissue growth, from normal healing to abnormal fibrosis. Most TGF-β responses involve stimulation of the cell communication network factor 2 (CCN2), previously known as connective tissue growth factor. CCN2 is the second named member of the CCN protein family.30 This 38 kDa protein is secreted by a number of cell types, including fibroblasts, and resides in the extracellular matrix, where it confers structural stability and retains biological activity, thus earning the designation as a matricellular protein.30 Cell communication network factor 2 is known to have a role in embryological development of the skeleton, face, palate, and lungs as well as wound healing, fibrotic disorders, and tumorigenesis.31, 32, 33, 34, 35, 36, 37, 38 Cell communication network factor 2 is required for TGF-β–mediated fibrosis.39 Not only does CCN2 work in concert with TGF-β and other matricellular components to elicit a fibrotic response in tissues but also its production alone can generate fibrosis in models typically resistant to this process.40 Because of its key downstream role in TGF-β signaling, CCN2 has emerged as a promising target in a host of fibrotic disorders, such as scleroderma and pulmonary, cardiac, hepatic, and renal fibroses.41
Targeting Cell Communication Network Factor 2 with Pamrevlumab
Pamrevlumab, initially described as FG-3019, is a fully humanized monoclonal antibody against CCN2 (FibroGen Inc). Its mechanism specifically involves targeting the von Willebrand factor C domain of the CCN2 protein.42 This is the domain known to interact with TGF-β.43 Pamrevlumab has been granted US Food and Drug Administration Fast Track and Orphan Drug Designation for use in pancreatic carcinoma, muscular dystrophy, and idiopathic pulmonary fibrosis and has entered into phase 3 clinical trials.36 Results of these trials thus far have shown strong potential in decreasing tissue fibrosis, with functional improvements.44,45 When administered intravenously at a dose of 30 mg/kg every 3 weeks, human safety trials exhibited low rates of adverse events.36 Thus, these trials support that FG-3019 is safe and well-tolerated in the patient population, although any long-term adverse events will require further monitoring.
Although the primary published results of anti-CCN2 therapy in humans, to date, relate to its utility in treating idiopathic pulmonary fibrosis, it has also been studied in a variety of pathological conditions in rodent models.41 Outside of solid organ and tumorigenesis models, it has been shown to reduce skin fibrosis and improve muscle function with fibrosis reduction in a model of Duchenne muscular dystrophy.46,47 Our group developed an operant rat model of overuse injuries, in which noted changes include functionally limiting fibrosis of musculoskeletal tissues.34,48, 49, 50, 51, 52, 53, 54, 55 As fibroproliferation occurs with deposition of extracellular matrix proteins, such as type I collagen, we have shown that CCN2 production concomitantly increases.48,56,57 We have administered treatment with Pamrevlumab in this model with remarkable success. In our model, CCN2 blockade is not only able to prevent the development of fibrosis but also reverse it once established (Figs. 2 and 3). These reductions in dermal, muscle, and neural collagen deposition and fibrosis lead to improvements in functional declines, including increased grip strength and median nerve conduction velocity and reduced cold temperature sensitivity.34,48,56,57
Figure 2.
Reduction of nerve and dermal fibrosis after FG-3019 treatment. Masson’s trichrome staining around the median nerve branches and in upper dermis of forepaw digits. A Masson’s trichrome staining, with collagen stained blue around the median nerve (N) at wrist level in longitudinal sections. B Masson’s trichrome staining in the upper dermis. Arrows indicate regions containing nerves within dermal papillae. C Quantification of blue-stained collagen in paraneural regions (ie, areas surrounding nerve) at wrist level. D Quantification of blue-stained collagen in the upper dermis. aa: P < .01, compared with matched food-restricted control (FRC) group; b: P < .05 and bb: P < .01, compared with untreated high repetition high force (HRHF) rats (Barbe et al. Blocking CCN2 reduces progression of sensorimotor declines and fibrosis in a rat model of chronic repetitive overuse. J Orthop Res. 2019;37:200448).
Figure 3.
Reduction of type I collagen in skeletal muscle after FG-3019 treatment. Representative cell communication network factor 2 (CCN2; red) and collagen type I (green) immunoexpression in the flexor digitorum muscles of each group. Representative images of mid-forearm cross-sections are shown. A and B High repetition high force (HRHF)–untreated and HRHF-rest/immunoglobulin (Ig) G muscles showing areas with dense endomyseal deposition of CCN2 and collagen type I around individual myofibers and in band-like formations. Asterisks indicate the band-like formations. Arrows indicate examples of myofibers surrounded by endomyseal CCN2 or collagen type I. C The HRHF-rest/FG-3019 muscles in the same flexor muscle region showed clear reductions in CCN2 and collagen type I deposition. D Food-restricted control (FRC) rat muscles in the same flexor muscle region showed little to no endomyseal CCN2 or collagen type I deposition. M, muscle. Scale bar = 50 microns (Reproduced with permission from Barbe et al. Blocking CTGF/CCN2 reduces established skeletal muscle fibrosis in a rat model of overuse injury. FASEB J. 2020;34:656057).
A Role for Pamrevlumab in Dupuytren Disease?
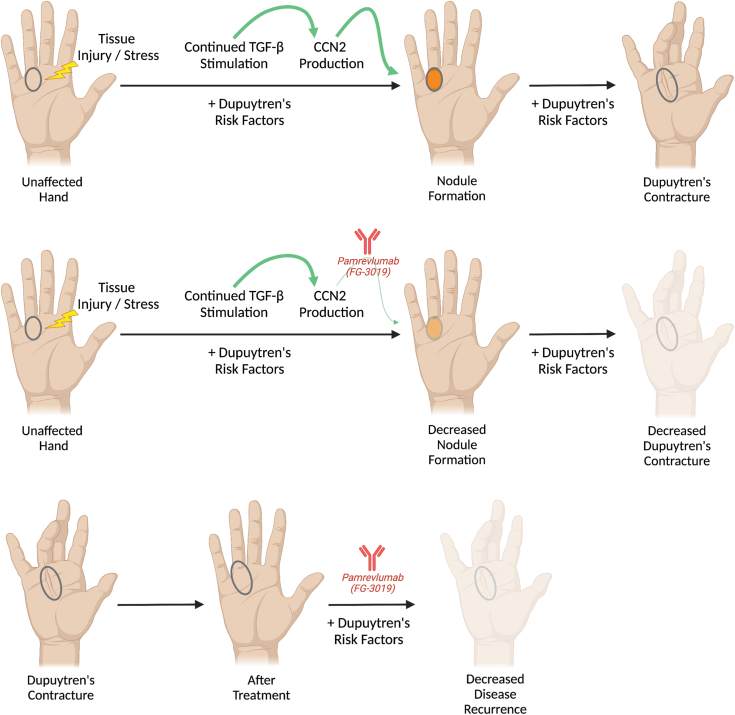
It has been shown that CCN2 expression is increased in DD fibroblasts.28 Cell communication network factor 2 functions as a key player in TGF-β–mediated fibrosis. Cell communication network factor 2 also functions downstream of other factors, including tumor necrosis factor.41 Taking into account that both the inhibition of TGF-β signaling as well as tumor necrosis factor signaling result in a decreased DD development in vitro, one could envision that blockade of CCN2 downstream of both pathways in this disease would have a similar or greater effect. Therefore, we propose that anti-CCN2 therapy with Pamrevlumab constitutes a potential target in the treatment of DD (Fig. 4). To date, no studies have looked at the potential of blocking CCN2 for DD treatment and prevention of recurrence and no studies have evaluated the effect of Pamrevlumab administered as a local injection in any fibrotic tissue model (eg, DD nodules).
Figure 4.
A role for Pamrevlumab in Dupuytren disease treatment. Following tissue injury or stress, nodule formation can occur in the presence of Dupuytren risk factors and continued transforming growth factor (TGF)-β stimulation and cell communication network factor 2 (CCN2) production. This can progress to contracture (top row). Treatment with Pamrevlumab (FG-3019) prevents the profibrotic role of TGF-β through inhibition of its downstream mediator, CCN2. This could lead to decreased nodule formation and less contracture development (middle row). After treatment with established modalities, adjunct therapy with Pamrevlumab could prevent disease recurrence in susceptible individuals (bottom row).
Several steps must be taken to determine the efficacy of Pamrevlumab as a potential antifibrotic agent for DD. First, a local injectable formulation of Pamrevlumab must be developed. This would allow focused administration at the site of disease while minimizing any potential systemic side effects. The drug is currently only developed for intravenous administration. However, there is interest in developing a formulation for local administration (personal communication with FibroGen Inc research team), and precedence already exists for this strategy with other systemically administered antifibrotic pharmacotherapies, such as pirfenidone.58 We anticipate the dosing would be significantly lower than what is being used for current systemic therapy in clinical applications. Second, the timing of Pamrevlumab treatment would need to be determined. It may be that utility exists for injection at both early disease occurrence (eg, nodule development), to prevent progression, as well as following treatment, to prevent recurrence. For a targeted, streamlined approach, one could first choose those who have undergone treatment for symptomatic contracture. Local injection would occur at the time of definitive treatment (eg, fasciectomy, needle aponeurotomy, and manipulation), which is similar to other proposed adjuvants, such as fat grafting,59 as well as at distinct time points after treatment to prevent recurrence, such as at 6 weeks and 3 months, which is consistent with published data on corticosteroid injection.60 Third, we anticipate that the potential side effect profile would be acceptable. Clinical trials thus far have shown that systemic administration of Pamrevlumab is well-tolerated.44 We surmise that since local injection dosing would be lower than systemic dosing and that only a fraction of this is likely to be systemically absorbed, local application is unlikely to have an undesirable risk profile.
Conclusion
Despite our notable advances in understanding the mechanisms underlying fibrosis, DD remains a challenging clinical entity to treat. Invasive procedures have historically been the mainstay of treatment. More recently, enzymatic digestion with collagenase has become a staple for over a decade. However, there is still a large void in the hand surgeon’s armamentarium for an adjunct therapy that can either stop disease progression in 30% to 50% of patients in early stages, at risk, or prevent disease recurrence following treatment with the current approaches. We have previously underscored the need to take an antifibrogenic approach in finding pharmacotherapies for Dupuytren treatment.7 In this article, we highlighted the role of TGF-β signaling in DD and owing to its critical role in TGF-β–induced fibrosis, proposed CCN2 as a new potential high-yield target. The monoclonal antibody Pamrevlumab has already shown strong efficacy as systemic therapy in the treatment of fibrotic disorders. Our group has demonstrated a clear antifibrotic role in blocking CCN2 in an in vivo model of musculoskeletal and dermal tissue fibrosis. These results provide a strong rationale for future feasibility studies and subsequent clinical trials using Pamrevlumab in patients with DD.
Acknowledgments
All authors contributed to the preparation of the manuscript, including design and figure preparation. Figures 1 and 4 were created with BioRender.com. This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Grant 5R01AR056019-13 to senior author (M.F.B.).
Footnotes
Declaration of interests: No benefits in any form have been received or will be received related directly to this article.
References
- 1.Karatzas E., Kakouri A.C., Kolios G., Delis A., Spyrou G.M. Fibrotic expression profile analysis reveals repurposed drugs with potential anti-fibrotic mode of action. PLOS ONE. 2021;16 doi: 10.1371/journal.pone.0249687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenbloom J., Mendoza F.A., Jimenez S.A. Strategies for anti-fibrotic therapies. Biochim Biophys Acta. 2013;1832:1088–1103. doi: 10.1016/j.bbadis.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Tai Y., Woods E.L., Dally J., et al. Myofibroblasts: function, formation, and scope of molecular therapies for skin fibrosis. Biomolecules. 2021;11:1095. doi: 10.3390/biom11081095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shih B., Bayat A. Scientific understanding and clinical management of Dupuytren disease. Nat Rev Rheumatol. 2010;6:715–726. doi: 10.1038/nrrheum.2010.180. [DOI] [PubMed] [Google Scholar]
- 5.Luck J.V. Dupuytren’s contracture; a new concept of the pathogenesis correlated with surgical management. J Bone Joint Surg Am. 1959;41-A:635–664. [PubMed] [Google Scholar]
- 6.Zhang A.Y., Kargel J.S. The basic science of Dupuytren disease. Hand Clin. 2018;34:301–305. doi: 10.1016/j.hcl.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Lambi AG, Popoff SN, Benhaim P, Barbe MF. Pharmacotherapies in Dupuytren’s disease: current and novel strategies. J Hand Surg Am. Published online March 17, 2023. https://doi.org/10.1016/j.jhsa.2023.02.003 [DOI] [PMC free article] [PubMed]
- 8.Godtfredsen N.S., Lucht H., Prescott E., Sørensen T.I., Grønbaek M. A prospective study linked both alcohol and tobacco to Dupuytren’s disease. J Clin Epidemiol. 2004;57:858–863. doi: 10.1016/j.jclinepi.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Burke F.D., Proud G., Lawson I.J., McGeoch K.L., Miles J.N. An assessment of the effects of exposure to vibration, smoking, alcohol and diabetes on the prevalence of Dupuytren’s disease in 97,537 miners. J Hand Surg Eur Vol. 2007;32:400–406. doi: 10.1016/J.JHSE.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Burge P., Hoy G., Regan P., Milne R. Smoking, alcohol and the risk of Dupuytren’s contracture. J Bone Joint Surg Br. 1997;79:206–210. doi: 10.1302/0301-620x.79b2.6990. [DOI] [PubMed] [Google Scholar]
- 11.Liss G.M., Stock S.R. Can Dupuytren’s contracture be work-related?: review of the evidence. Am J Ind Med. 1996;29:521–532. doi: 10.1002/(SICI)1097-0274(199605)29:5<521::AID-AJIM12>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Gudmundsson K.G., Arngrímsson R., Sigfússon N., Björnsson A., Jónsson T. Epidemiology of Dupuytren’s disease: clinical, serological, and social assessment. The Reykjavik study. J Clin Epidemiol. 2000;53:291–296. doi: 10.1016/s0895-4356(99)00145-6. [DOI] [PubMed] [Google Scholar]
- 13.Noble J., Heathcote J.G., Cohen H. Diabetes mellitus in the aetiology of Dupuytren’s disease. J Bone Joint Surg Br. 1984;66:322–325. doi: 10.1302/0301-620X.66B3.6725338. [DOI] [PubMed] [Google Scholar]
- 14.Rydberg M., Zimmerman M., Löfgren J.P., et al. Metabolic factors and the risk of Dupuytren’s disease: data from 30,000 individuals followed for over 20 years. Sci Rep. 2021;11 doi: 10.1038/s41598-021-94025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dibenedetti D.B., Nguyen D., Zografos L., Ziemiecki R., Zhou X. Prevalence, incidence, and treatments of Dupuytren’s disease in the United States: results from a population-based study. Hand (N Y) 2011;6:149–158. doi: 10.1007/s11552-010-9306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 17.Tomasek J.J., Schultz R.J., Haaksma C.J. Extracellular matrix-cytoskeletal connections at the surface of the specialized contractile fibroblast (myofibroblast) in Dupuytren disease. J Bone Joint Surg Am. 1987;69:1400–1407. [PubMed] [Google Scholar]
- 18.Tomasek J.J., Gabbiani G., Hinz B., Chaponnier C., Brown R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 19.Singer, Kawka D.W., Kazazis D.M., Clark R.A. In vivo co-distribution of fibronectin and actin fibers in granulation tissue: immunofluorescence and electron microscope studies of the fibronexus at the myofibroblast surface. J Cell Biol. 1984;98:2091–2106. doi: 10.1083/jcb.98.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burridge K., Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 21.Dugina V., Fontao L., Chaponnier C., Vasiliev J., Gabbiani G. Focal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factors. J Cell Sci. 2001;114:3285–3296. doi: 10.1242/jcs.114.18.3285. [DOI] [PubMed] [Google Scholar]
- 22.Krause C., Kloen P. Concurrent inhibition of TGF-beta and mitogen driven signaling cascades in Dupuytren’s disease—non-surgical treatment strategies from a signaling point of view. Med Hypotheses. 2012;78:385–388. doi: 10.1016/j.mehy.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 23.Berndt A., Kosmehl H., Mandel U., et al. TGF beta and bFGF synthesis and localization in Dupuytren’s disease (nodular palmar fibromatosis) relative to cellular activity, myofibroblast phenotype and oncofetal variants of fibronectin. Histochem J. 1995;27:1014–1020. [PubMed] [Google Scholar]
- 24.Baird K.S., Crossan J.F., Ralston S.H. Abnormal growth factor and cytokine expression in Dupuytren’s contracture. J Clin Pathol. 1993;46:425–428. doi: 10.1136/jcp.46.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badalamente M.A., Sampson S.P., Hurst L.C., Dowd A., Miyasaka K. The role of transforming growth factor beta in Dupuytren’s disease. J Hand Surg Am. 1996;21:210–215. doi: 10.1016/S0363-5023(96)80102-X. [DOI] [PubMed] [Google Scholar]
- 26.Bisson M.A., McGrouther D.A., Mudera V., Grobbelaar A.O. The different characteristics of Dupuytren’s disease fibroblasts derived from either nodule or cord: expression of alpha-smooth muscle actin and the response to stimulation by TGF-beta1. J Hand Surg Br. 2003;28:351–356. doi: 10.1016/s0266-7681(03)00135-9. [DOI] [PubMed] [Google Scholar]
- 27.Verjee L.S., Verhoekx J.S.N., Chan J.K.K., et al. Unraveling the signaling pathways promoting fibrosis in Dupuytren’s disease reveals TNF as a therapeutic target. Proc Natl Acad Sci U S A. 2013;110:E928–E937. doi: 10.1073/pnas.1301100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krause C., Kloen P., Ten Dijke P. Elevated transforming growth factor beta and mitogen-activated protein kinase pathways mediate fibrotic traits of Dupuytren’s disease fibroblasts. Fibrogenesis Tissue Repair. 2011;4:14. doi: 10.1186/1755-1536-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tse R., Howard J., Wu Y., Gan B.S. Enhanced Dupuytren’s disease fibroblast populated collagen lattice contraction is independent of endogenous active TGF-beta2. BMC Musculoskelet Disord. 2004;5:41. doi: 10.1186/1471-2474-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brigstock D.R. The connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocr Rev. 1999;20:189–206. doi: 10.1210/edrv.20.2.0360. [DOI] [PubMed] [Google Scholar]
- 31.Tarr J.T., Visser T.G., Moon J.E., et al. The pivotal role of CCN2 in mammalian palatogenesis. J Cell Commun Signal. 2017;11:25–37. doi: 10.1007/s12079-016-0360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarr J.T., Lambi A.G., Bradley J.P., Barbe M.F., Popoff S.N. Development of normal and cleft palate: a central role for connective tissue growth factor (CTGF)/CCN2. J Dev Biol. 2018;6:18. doi: 10.3390/jdb6030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arnott J.A., Lambi A.G., Mundy C., et al. The role of connective tissue growth factor (CTGF/CCN2) in skeletogenesis. Crit Rev Eukaryot Gene Expr. 2011;21:43–69. doi: 10.1615/critreveukargeneexpr.v21.i1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbe M.F., Amin M., Gingery A., Lambi A.G., Popoff S.N. Blocking CCN2 preferentially inhibits osteoclastogenesis induced by repetitive high force bone loading. Connect Tissue Res. 2021;62:115–132. doi: 10.1080/03008207.2020.1788546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambi A.G., Pankratz T.L., Mundy C., et al. The skeletal site-specific role of connective tissue growth factor in prenatal osteogenesis. Dev Dyn. 2012;241:1944–1959. doi: 10.1002/dvdy.23888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leask A. Breathe, breathe in the air: the anti-CCN2 antibody pamrevlumab (FG-3019) completes a successful phase II clinical trial for idiopathic pulmonary fibrosis. J Cell Commun Signal. 2019;13:441–442. doi: 10.1007/s12079-019-00542-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipson K.E., Wong C., Teng Y., Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5:S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakai N., Nakamura M., Lipson K.E., et al. Inhibition of CTGF ameliorates peritoneal fibrosis through suppression of fibroblast and myofibroblast accumulation and angiogenesis. Sci Rep. 2017;7:5392. doi: 10.1038/s41598-017-05624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mori T., Kawara S., Shinozaki M., et al. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: a mouse fibrosis model. J Cell Physiol. 1999;181:153–159. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 40.Bonniaud P., Martin G., Margetts P.J., et al. Connective tissue growth factor is crucial to inducing a profibrotic environment in “fibrosis-resistant” BALB/c mouse lungs. Am J Respir Cell Mol Biol. 2004;31:510–516. doi: 10.1165/rcmb.2004-0158OC. [DOI] [PubMed] [Google Scholar]
- 41.Ramazani Y., Knops N., Elmonem M.A., et al. Connective tissue growth factor (CTGF) from basics to clinics. Matrix Biol. 2018;68-69:44–66. doi: 10.1016/j.matbio.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Brenner M.C., Krzyzanski W., Chou J.Z., et al. FG-3019, a human monoclonal antibody recognizing connective tissue growth factor, is subject to target-mediated drug disposition. Pharm Res. 2016;33:1833–1849. doi: 10.1007/s11095-016-1918-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jun J.I., Lau L.F. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945–963. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richeldi L., Pérez E.R.F., Costabel U., et al. Pamrevlumab, an anti-connective tissue growth factor therapy, for idiopathic pulmonary fibrosis (PRAISE): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2020;8:25–33. doi: 10.1016/S2213-2600(19)30262-0. [DOI] [PubMed] [Google Scholar]
- 45.Sgalla G., Franciosa C., Simonetti J., Richeldi L. Pamrevlumab for the treatment of idiopathic pulmonary fibrosis. Expert Opin Investig Drugs. 2020;29:771–777. doi: 10.1080/13543784.2020.1773790. [DOI] [PubMed] [Google Scholar]
- 46.Makino K., Makino T., Stawski L., Lipson K.E., Leask A., Trojanowska M. Anti-connective tissue growth factor (CTGF/CCN2) monoclonal antibody attenuates skin fibrosis in mice models of systemic sclerosis. Arthritis Res Ther. 2017;19:134. doi: 10.1186/s13075-017-1356-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morales M.G., Gutierrez J., Cabello-Verrugio C., et al. Reducing CTGF/CCN2 slows down mdx muscle dystrophy and improves cell therapy. Hum Mol Genet. 2013;22:4938–4951. doi: 10.1093/hmg/ddt352. [DOI] [PubMed] [Google Scholar]
- 48.Barbe M.F., Hilliard B.A., Delany S.P., et al. Blocking CCN2 reduces progression of sensorimotor declines and fibrosis in a rat model of chronic repetitive overuse. J Orthop Res. 2019;37:2004–2018. doi: 10.1002/jor.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leask A. Slow train coming: an anti-CCN2 strategy reverses a model of chronic overuse muscle fibrosis. J Cell Commun Signal. 2020;14:349–350. doi: 10.1007/s12079-020-00568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barbe M.F., Massicotte V.S., Assari S., et al. Prolonged high force high repetition pulling induces osteocyte apoptosis and trabecular bone loss in distal radius, while low force high repetition pulling induces bone anabolism. Bone. 2018;110:267–283. doi: 10.1016/j.bone.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barbe M.F., Gallagher S., Massicotte V.S., Tytell M., Popoff S.N., Barr-Gillespie A.E. The interaction of force and repetition on musculoskeletal and neural tissue responses and sensorimotor behavior in a rat model of work-related musculoskeletal disorders. BMC Musculoskelet Disord. 2013;14:303. doi: 10.1186/1471-2474-14-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barr A.E., Safadi F.F., Gorzelany I., Amin M., Popoff S.N., Barbe M.F. Repetitive, negligible force reaching in rats induces pathological overloading of upper extremity bones. J Bone Miner Res. 2003;18:2023–2032. doi: 10.1359/jbmr.2003.18.11.2023. [DOI] [PubMed] [Google Scholar]
- 53.Driban J.B., Barr A.E., Amin M., Sitler M.R., Barbe M.F. Joint inflammation and early degeneration induced by high-force reaching are attenuated by ibuprofen in an animal model of work-related musculoskeletal disorder. J Biomed Biotechnol. 2011;2011 doi: 10.1155/2011/691412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fedorczyk J.M., Barr A.E., Rani S., et al. Exposure-dependent increases in IL-1beta, substance P, CTGF, and tendinosis in flexor digitorum tendons with upper extremity repetitive strain injury. J Orthop Res. 2010;28:298–307. doi: 10.1002/jor.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rani S., Barbe M.F., Barr A.E., Litivn J. Role of TNF alpha and PLF in bone remodeling in a rat model of repetitive reaching and grasping. J Cell Physiol. 2010;225:152–167. doi: 10.1002/jcp.22208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barbe M.F., Hilliard B.A., Amin M., et al. Blocking CTGF/CCN2 reverses neural fibrosis and sensorimotor declines in a rat model of overuse-induced median mononeuropathy. J Orthop Res. 2020;38:2396–2408. doi: 10.1002/jor.24709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barbe M.F., Hilliard B.A., Amin M., et al. Blocking CTGF/CCN2 reduces established skeletal muscle fibrosis in a rat model of overuse injury. FASEB J. 2020;34:6554–6569. doi: 10.1096/fj.202000240RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Panigrahi S., Barry A., Multner S., et al. Pirfenidone as a potential antifibrotic injectable for Dupuytren’s disease. Pharm Dev Technol. 2022;27:242–250. doi: 10.1080/10837450.2022.2038201. [DOI] [PubMed] [Google Scholar]
- 59.Murphy A., Lalonde D.H., Eaton C., et al. Minimally invasive options in Dupuytren’s contracture: aponeurotomy, enzymes, stretching, and fat grafting. Plast Reconstr Surg. 2014;134:822e–829e. doi: 10.1097/PRS.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 60.McMillan C., Binhammer P. Steroid injection and needle aponeurotomy for Dupuytren contracture: a randomized, controlled study. J Hand Surg Am. 2012;37:1307–1312. doi: 10.1016/j.jhsa.2012.04.026. [DOI] [PubMed] [Google Scholar]