Abstract
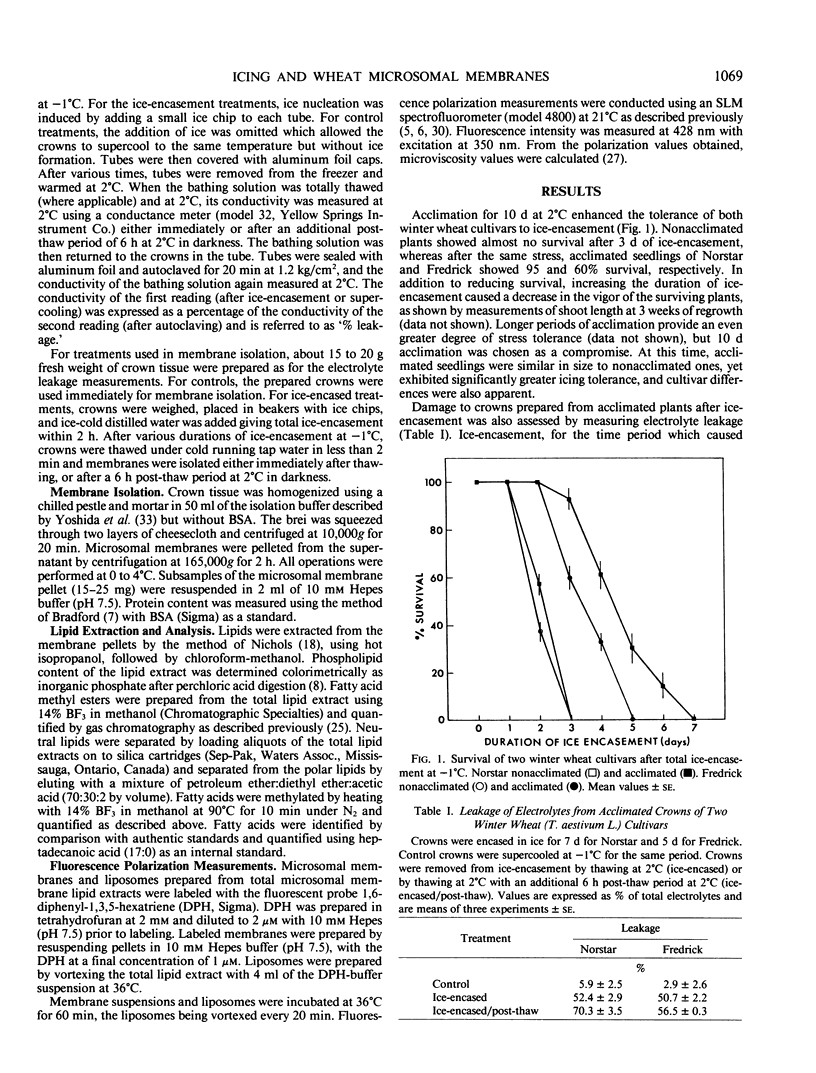
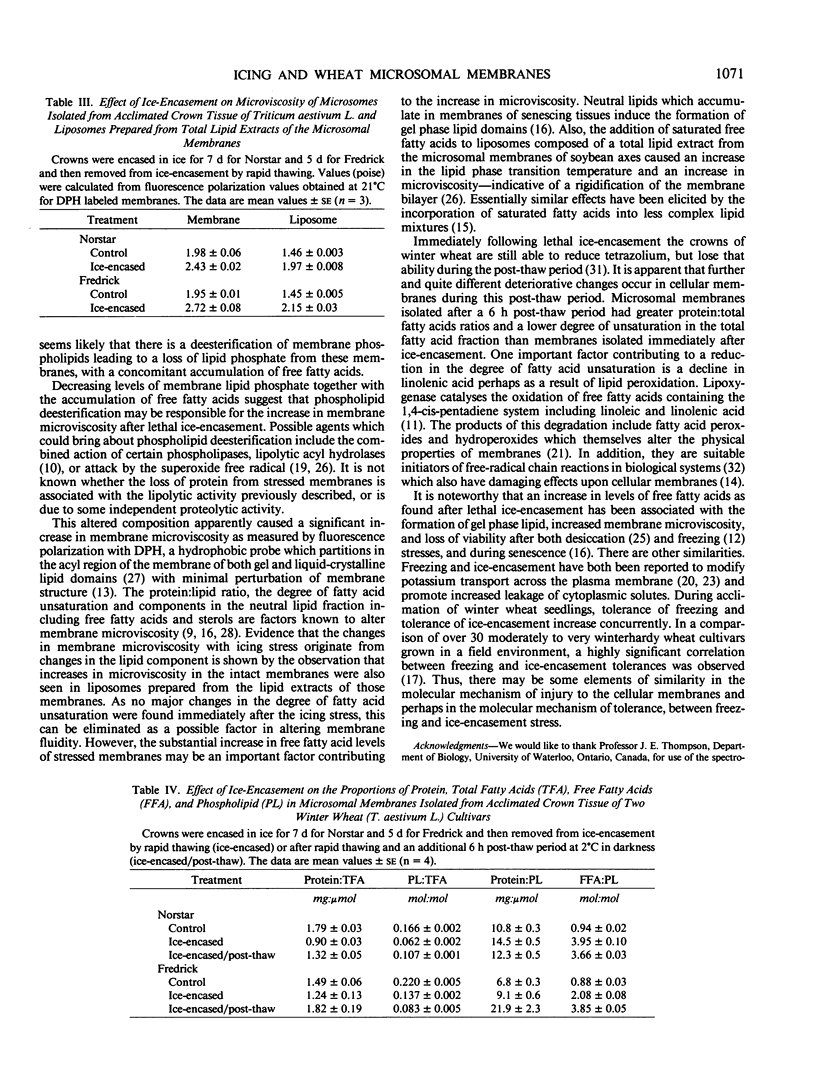
The functional and physical properties of cellular membranes isolated from Triticum aestivum, cvs Norstar and Fredrick, were altered coincident with changes in composition after a lethal ice-encasement stress and further during a 6 hour post-thaw period. Crowns encased in ice for a duration which inhibited regrowth, exhibited enhanced rates of electrolyte leakage. Furthermore, the recovery of total microsomal protein and phospholipid declined, suggesting that some membrane degradation had been induced during the anoxic stress. The microviscosity of microsomes and liposomes prepared from such membranes increased during stress, and this was correlated with a 2- to 4-fold increase in the free fatty acid levels in the microsomal fraction. There was, however, only a relatively minor change in fatty acid unsaturation during the ice-encasement stress. The process continued during a 6 hour aerobic post-thaw treatment, but the pattern was somewhat different. During this phase, the leakage of electrolytes was further increased and the recovery of microsomal protein and phospholipid continued to decline, indicating general degradation; but, in contrast to the anoxic phase, the degree of fatty acid unsaturation declined markedly, indicating lipid peroxidation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews C. J., Pomeroy M. K. Mitochondrial Activity and Ethanol Accumulation in Ice-encased Winter Cereal Seedlings. Plant Physiol. 1977 Jun;59(6):1174–1177. doi: 10.1104/pp.59.6.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews C. J., Pomeroy M. K. Toxicity of Anaerobic Metabolites Accumulating in Winter Wheat Seedlings during Ice Encasement. Plant Physiol. 1979 Jul;64(1):120–125. doi: 10.1104/pp.64.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borochov A., Halevy A. H. Microviscosity of plasmalemmas in rose petals as affected by age and environmental factors. Plant Physiol. 1978 May;61(5):812–815. doi: 10.1104/pp.61.5.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borochov H., Shinitzky M. Vertical displacement of membrane proteins mediated by changes in microviscosity. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4526–4530. doi: 10.1073/pnas.73.12.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Freire E., Biltonen R. L. Fluorescence and calorimetric studies of phase transitions in phosphatidylcholine multilayers: kinetics of the pretransition. Biochemistry. 1978 Oct 17;17(21):4475–4480. doi: 10.1021/bi00614a018. [DOI] [PubMed] [Google Scholar]
- Mabrey S., Sturtevant J. M. Incorporation of saturated fatty acids into phosphatidylcholine bilayers. Biochim Biophys Acta. 1977 Mar 25;486(3):444–450. doi: 10.1016/0005-2760(77)90094-7. [DOI] [PubMed] [Google Scholar]
- McKersie B. D., Thompson J. E. Phase properties of senescing plant membranes: role of the neutral lipids. Biochim Biophys Acta. 1979 Jan 5;550(1):48–58. doi: 10.1016/0005-2736(79)90114-7. [DOI] [PubMed] [Google Scholar]
- Pauls K. P., Thompson J. E. Evidence for the accumulation of peroxidized lipids in membranes of senescing cotyledons. Plant Physiol. 1984 Aug;75(4):1152–1157. doi: 10.1104/pp.75.4.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy M. K., Andrews C. J. Changes in adenine nucleotides and energy charge in isolated winter wheat cells during low temperature stress. Plant Physiol. 1986 Jun;81(2):361–366. doi: 10.1104/pp.81.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy M. K., Pihakaski S. J., Andrews C. J. Membrane properties of isolated winter wheat cells in relation to icing stress. Plant Physiol. 1983 Jun;72(2):535–539. doi: 10.1104/pp.72.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senaratna T., McKersie B. D., Stinson R. H. Association between Membrane Phase Properties and Dehydration Injury in Soybean Axes. Plant Physiol. 1984 Nov;76(3):759–762. doi: 10.1104/pp.76.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta. 1978 Dec 15;515(4):367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Henkart P. Fluidity of cell membranes--current concepts and trends. Int Rev Cytol. 1979;60:121–147. [PubMed] [Google Scholar]
- Stubbs G. W., Litman B. J. Microviscosity of the hydrocarbon region of the bovine retinal rod outer segment disk membrane determined by fluorescent probe measurements. Biochemistry. 1976 Jun 29;15(13):2766–2772. doi: 10.1021/bi00658a009. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Uemura M., Niki T., Sakai A., Gusta L. V. Partition of membrane particles in aqueous two-polymer phase system and its practical use for purification of plasma membranes from plants. Plant Physiol. 1983 May;72(1):105–114. doi: 10.1104/pp.72.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]


