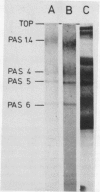
Abstract
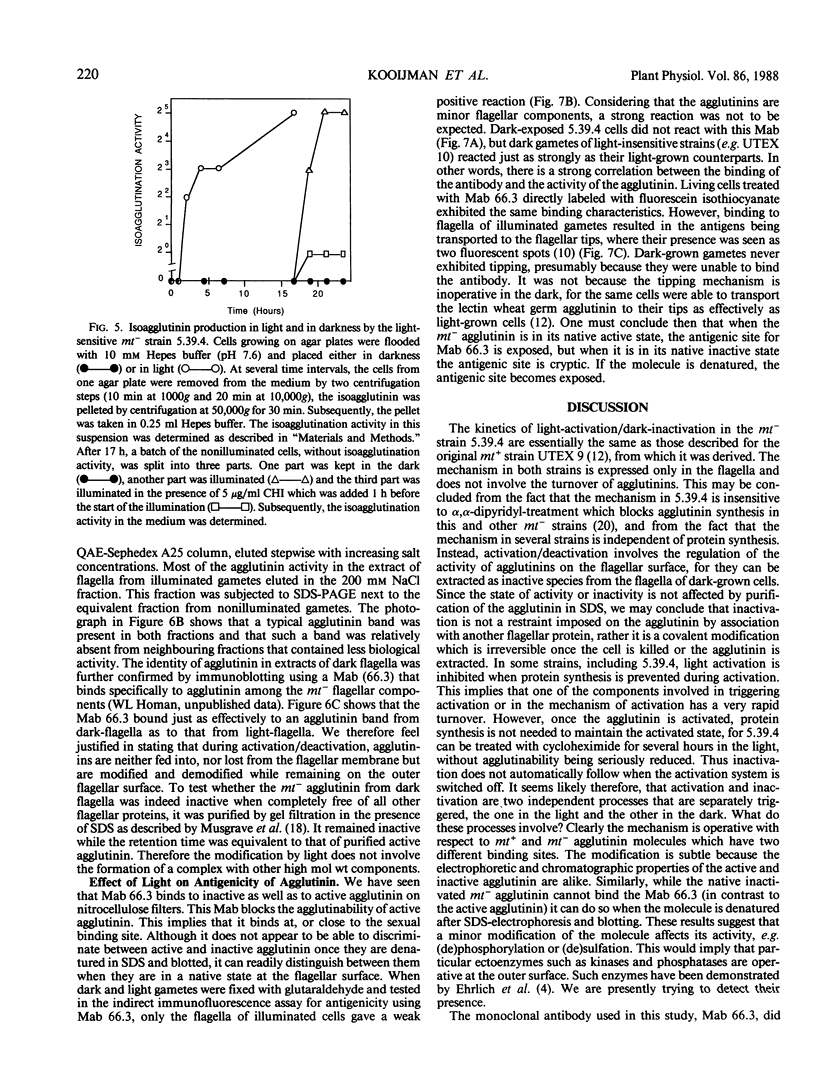
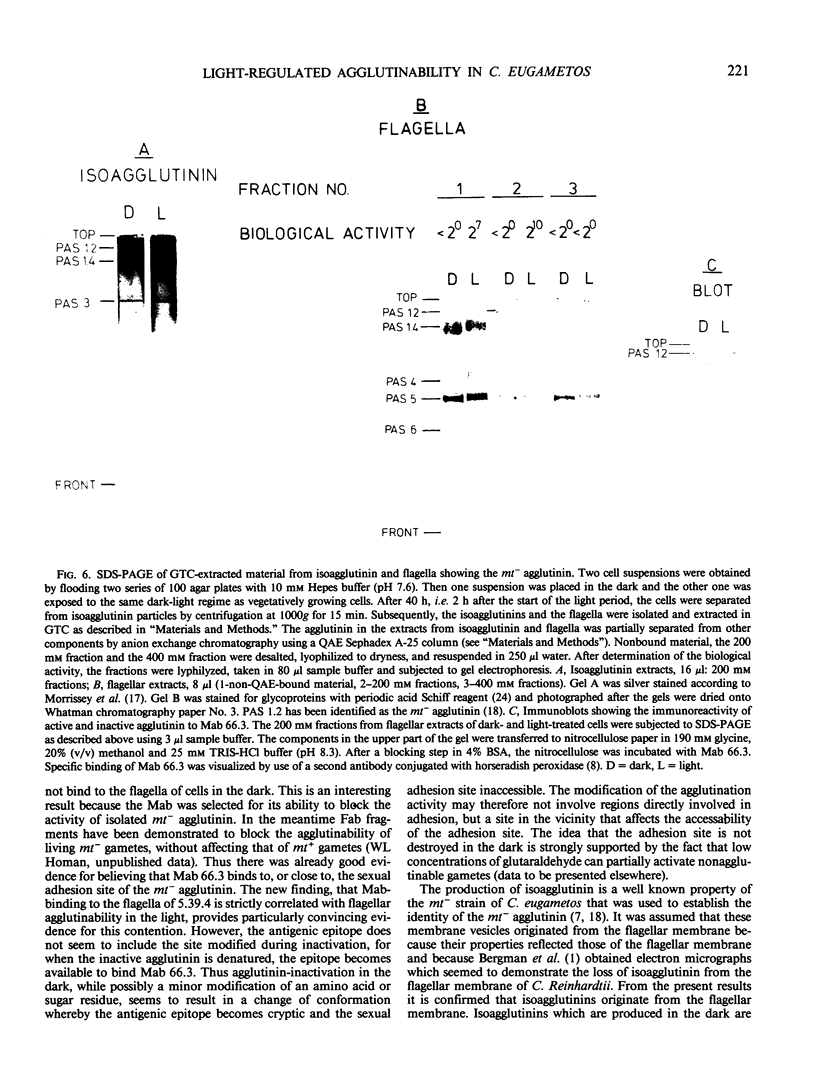
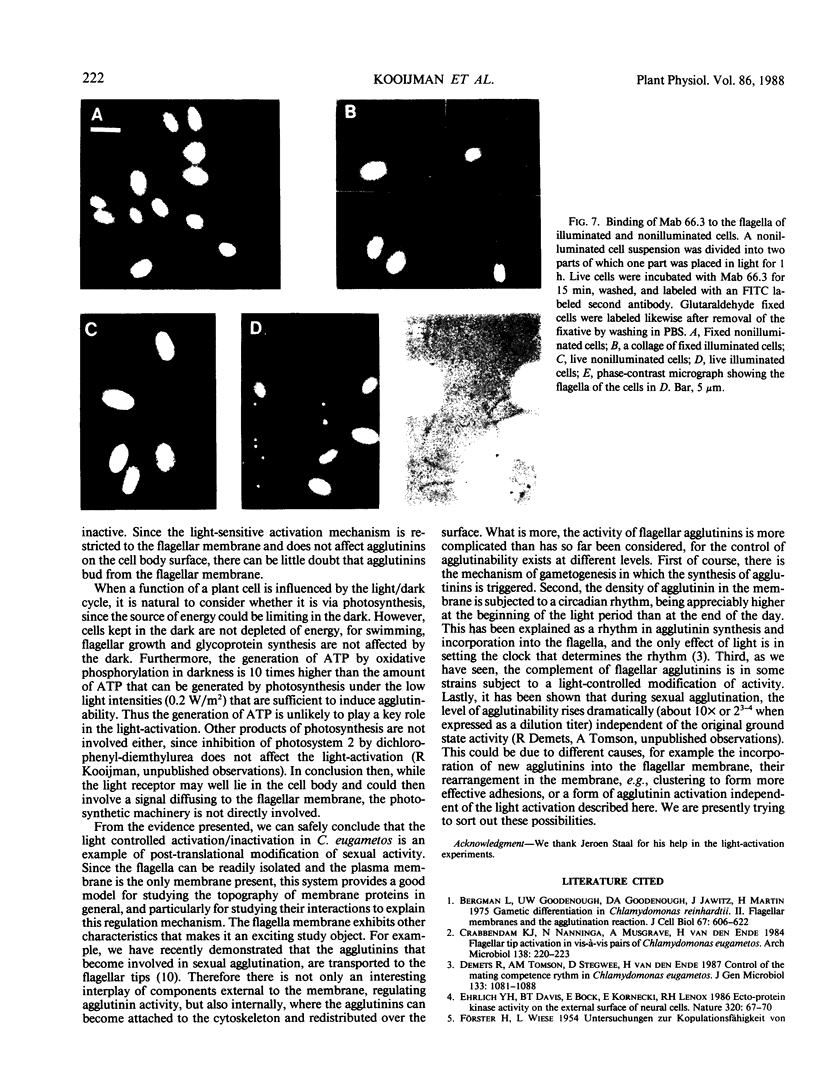
The effect of light on the sexual competence of a light-sensitive mating type minus strain (mt−) of Chlamydomonas eugametos obtained by crossing a light-sensitive mating type plus strain (mt+) with a light-insensitive mt− strain is described. As previously demonstrated for the mt+ parent, this study of one of the mt− offspring shows that (a) a light-sensitive mechanism affects flagellar agglutinability in a rapid process that does not require protein synthesis; (b) only the activity of the flagellar agglutinins (glycoproteins responsible for agglutination) is susceptible to light while agglutinins on the cell body surface are not affected by light. We further demonstrate that (a) membrane vesicles naturally released from nonagglutinable dark gametes remain inactive. Extracts of these vesicles also remain inactive even though they contain agglutinin-like components; (b) inactive mt− agglutinin is present in extracts of flagella from nonagglutinable dark gametes by comparison of its chromatographic, electrophoretic, and immunogenic properties with those of active agglutinin. When purified of all other flagellar proteins, it remains inactive; (c) a monoclonal antibody directed against the sexual agglutination site of the mt− agglutintin discriminates between active and inactive agglutinins when present in a native state on the flagellar surface, but is unable to discriminate between them when they are denatured in sodium dodecyl sulfate-electrophoresis gels and blotted onto nitrocellulose. Taken collectively these observations suggest that light activation involves the chemical modification of the agglutinins in situ on the flagellar surface.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergman K., Goodenough U. W., Goodenough D. A., Jawitz J., Martin H. Gametic differentiation in Chlamydomonas reinhardtii. II. Flagellar membranes and the agglutination reaction. J Cell Biol. 1975 Dec;67(3):606–622. doi: 10.1083/jcb.67.3.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich Y. H., Davis T. B., Bock E., Kornecki E., Lenox R. H. Ecto-protein kinase activity on the external surface of neural cells. Nature. 1986 Mar 6;320(6057):67–70. doi: 10.1038/320067a0. [DOI] [PubMed] [Google Scholar]
- Goodenough U. W., Jurivich D. Tipping and mating-structure activation induced in Chlamydomonas gametes by flagellar membrane antisera. J Cell Biol. 1978 Dec;79(3):680–693. doi: 10.1083/jcb.79.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klis F. M., Samson M. R., Touw E., Musgrave A., van den Ende H. Sexual Agglutination in the Unicellular Green Alga Chlamydomonas eugametos: Identification and Properties of the Mating Type plus Agglutination Factor. Plant Physiol. 1985 Nov;79(3):740–745. doi: 10.1104/pp.79.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McLEAN R. J., Laurendi C. J., Brown R. M., Jr The relationship of gamone to the mating reaction in Chlamydomonas moewusii. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2610–2613. doi: 10.1073/pnas.71.7.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesland D. A. Mating in Chlamydomonas eugametos. A scanning electron microscopical study. Arch Microbiol. 1976 Aug;109(1-2):31–35. doi: 10.1007/BF00425109. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Witman G. B., Carlson K., Berliner J., Rosenbaum J. L. Chlamydomonas flagella. I. Isolation and electrophoretic analysis of microtubules, matrix, membranes, and mastigonemes. J Cell Biol. 1972 Sep;54(3):507–539. doi: 10.1083/jcb.54.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]