Abstract
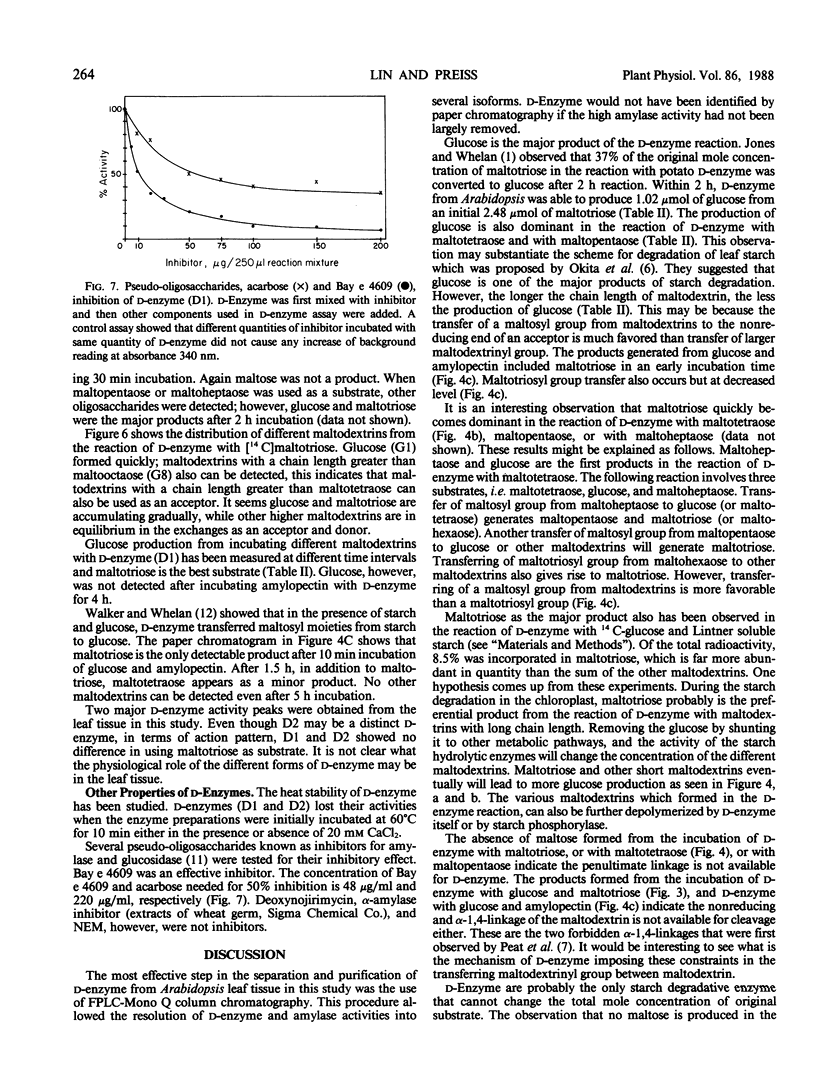
Two major forms of d-enzyme (4-α-glucanotransferase, EC 2.4.1.25) were successfully separated from most of the amylase activity using FPLC-Mono Q column chromatography. Transfer of a maltosyl group was observed upon the incubation of d-enzyme with maltotriose and d-[U-14 C]glucose. About 4.5% of the radioactivity was transferred to maltotriose in 2 hours. End product analysis showed the accumulation of glucose and maltopentaose from maltotriose within the first 10 minutes of the reaction. Several other maltodextrins were also observed with longer incubation times, although maltose was never produced. A quantitative measurement of maltodextrin production from the reaction of [14 C]maltotriose with d-enzyme showed that the quantity of maltotriose decreased from 100% to 31% after 3 hours incubation, while glucose, maltotetraose, maltopentaose, maltohexaose, maltoheptaose, maltooctaose, and higher maltodextrins increased in amount. Glucose is the major product throughout the course of the reaction of d-enzyme with maltotriose. Maltotriose, in addition to glucose, are the major products in the reaction of d-enzyme with maltodextrins with a chain length greater than maltotriose. This study confirms the existence of a transglycosylase that disproportionates maltotriose and higher maltodextrins by transferring maltosyl or maltodextrinyl groups between maltodextrins resulting in the production of glucose and different maltodextrins, but not maltose, in leaf tissue with enzymic properties very similar to the previously reported d-enzyme in potato.
Full text
PDF
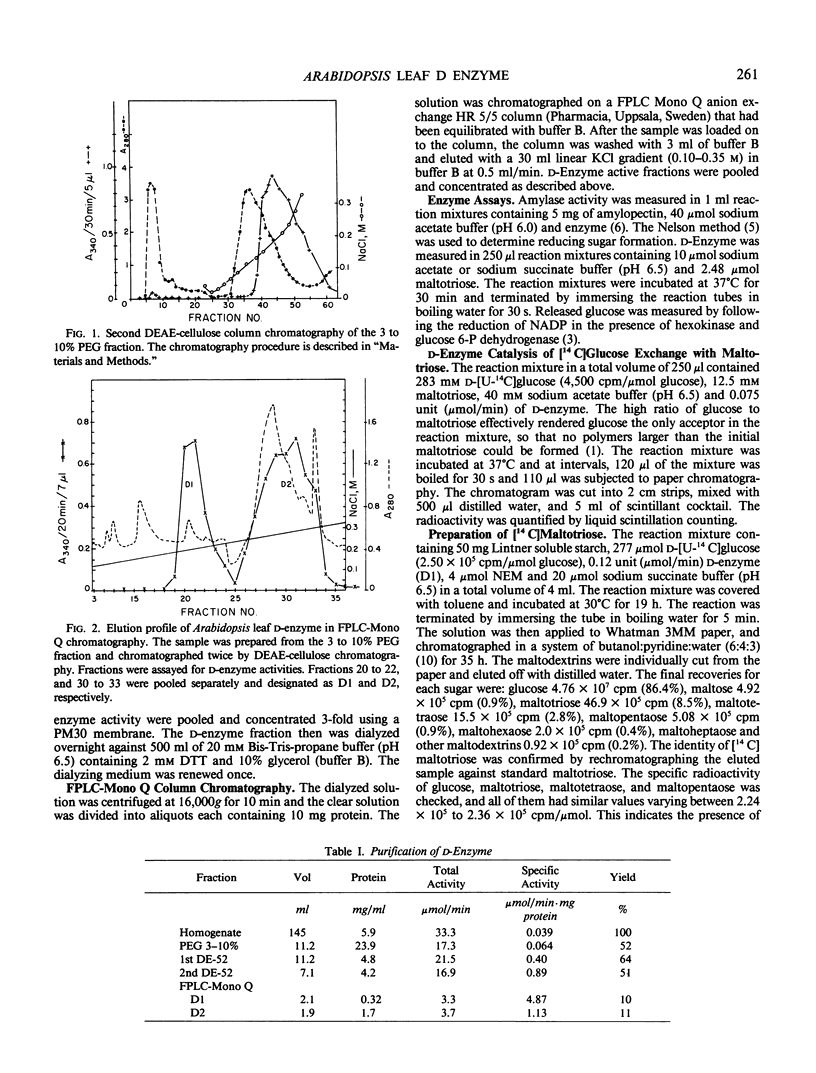
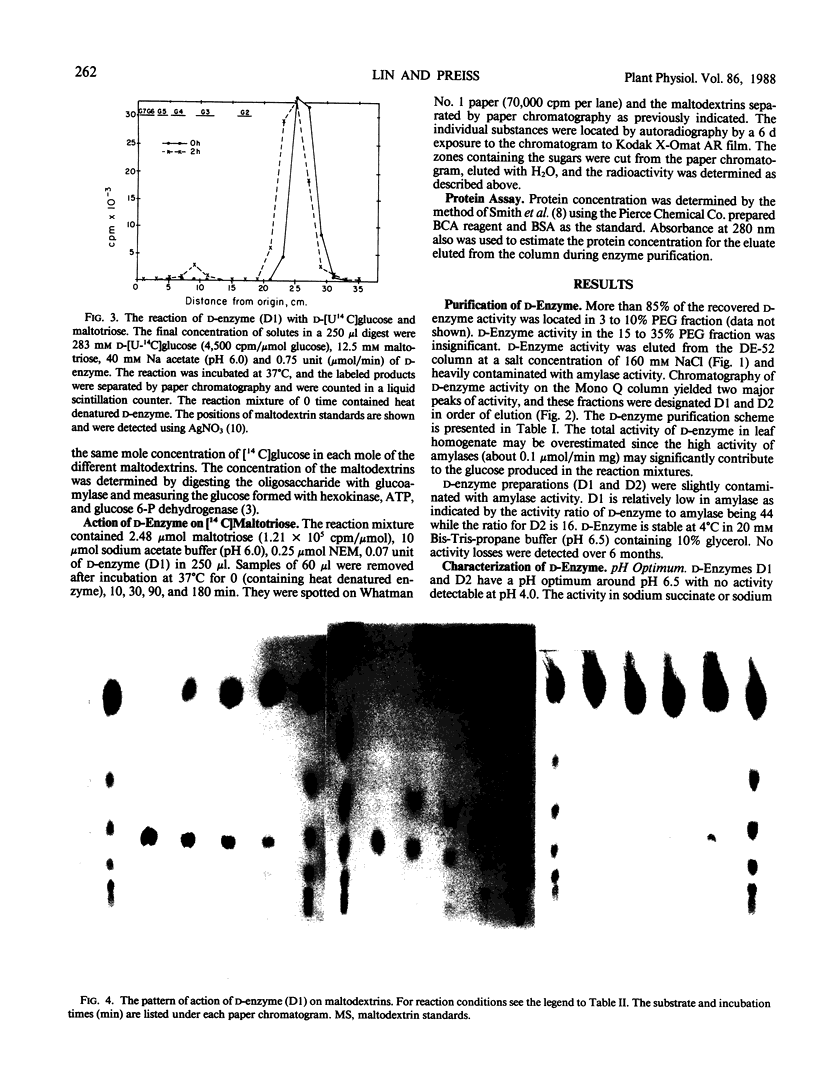
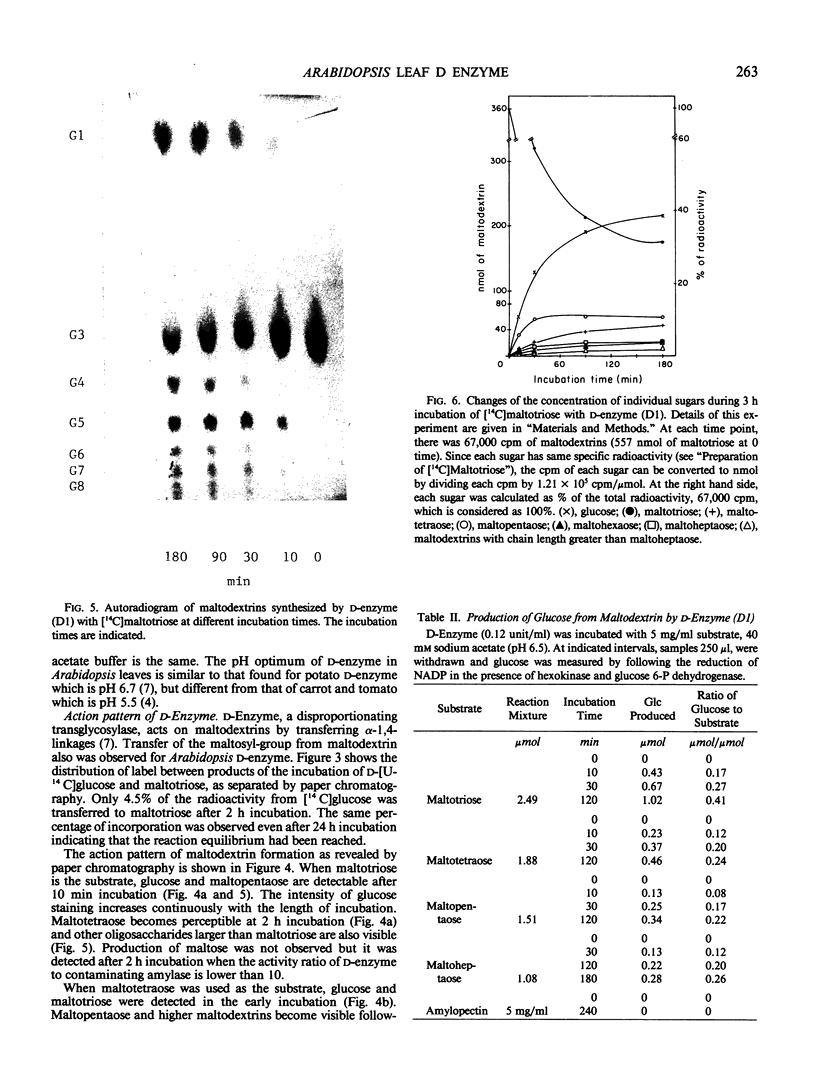

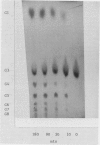
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Levi C., Preiss J. Amylopectin degradation in pea chloroplast extracts. Plant Physiol. 1978 Feb;61(2):218–220. doi: 10.1104/pp.61.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Greenberg E., Kuhn D. N., Preiss J. Subcellular localization of the starch degradative and biosynthetic enzymes of spinach leaves. Plant Physiol. 1979 Aug;64(2):187–192. doi: 10.1104/pp.64.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- WALKER G. J., WHELAN W. J. The mechanism of carbohydrase action. 4. The mechanism of D-enzyme action. Biochem J. 1957 Dec;67(4):548–551. doi: 10.1042/bj0670548. [DOI] [PMC free article] [PubMed] [Google Scholar]