Structured Abstract
INTRODUCTION:
We examined whether sex modifies the association between APOE ε2 and cognitive decline in two independent samples.
METHODS:
We used observational data from cognitively unimpaired non-Hispanic White (NHW) and non-Hispanic Black (NHB) adults. Linear mixed models examined interactive associations of APOE genotype (ε2 or ε4 carrier vs. ε3/ε3) and sex on cognitive decline in NHW and NHB participants separately.
RESULTS:
In both Sample 1 (N=9,766) and Sample 2 (N=915), sex modified the association between APOE ε2 and cognitive decline in NHW participants. Specifically, relative to APOE ε3/ε3, APOE ε2 protected against cognitive decline in men but not women. Among APOE ε2 carriers, men had slower decline than women. Among APOE ε3/ε3 carriers, cognitive trajectories did not differ between sexes. There were no sex-specific associations of APOE ε2 with cognition in NHB participants (N=2,010).
DISCUSSION:
In NHW adults, APOE ε2 may protect men but not women against cognitive decline.
Keywords: Alzheimer’s disease, APOE, cognitive decline, sex differences, race/ethnicity
1. Background
Women have a greater lifetime risk of developing Alzheimer’s disease (AD) dementia than men.1 While some studies observe that women’s increased risk is related to longer survival,2,3 other studies report that sex/gender disparities exist beyond what can be explained by female longevity alone.4 Mounting evidence suggests that biological mechanisms underpin sex differences in AD risk and progression.5-10
The APOE gene encodes a protein that facilitates lipid transport in the brain.11 APOE ε3 is the most common allele12 and is neutral in relation to risk for AD dementia.11 APOE ε4 is associated with a higher risk of AD dementia13 (mostly in non-Hispanic White populations14), whereas APOE ε2 is associated with a lower risk of AD dementia.15 Studies suggest that there are sex differences in the effects of APOE ε4 on AD risk, such that women with APOE ε4 are disproportionately vulnerable to cognitive impairment16 and AD15 compared to their counterpart men.
Although a less robust literature, APOE ε2 may also have sex-specific effects on AD risk. The few reports on sex-specific effects of APOE ε2 have been in the context of studies focused on APOE ε4 sex differences. One study found that in men but not women, APOE ε2 was associated with reduced risk of progression from normal cognition to mild cognitive impairment (MCI) or AD dementia.16 By contrast, a meta-analysis found that in cognitively unimpaired older adults, APOE ε2/ε3 decreased the risk of AD dementia more strongly in women than in men.17 That same meta-analysis reported the opposite pattern for APOE ε2 homozygosity (<30/sex), such that APOE ε2/ε2 was protective against AD dementia in men but not in women.17 Other studies examining sex-specific effects of APOE ε2 on cognition have also yielded mixed results, with some showing greater protection for women and others showing greater protection for men.18-20 These studies had small numbers of APOE ε2 carriers, and were cross-sectional in design or had limited longitudinal follow-up.18-20
Allele frequencies can vary widely between populations of different ancestral backgrounds (i.e., population stratification), which can lead to unreliable associations between genetic factors and phenotypic outcomes.21-23 There is evidence that APOE ε4 confers differential risk for AD across races. While APOE ε4 is more common among Black (vs. White) populations, the association of APOE ε4 with risk for cognitive decline and AD dementia may be attenuated in Black adults.24-26
In the present study, we carried out an in-depth investigation of sex differences in associations between APOE ε2 and longitudinal cognition. We first examined sex differences using pooled data from cognitively unimpaired adults participating in either the National Alzheimer’s Coordinating Center (NACC) or Rush Alzheimer’s Disease Center cohort studies (Sample 1). To control for population stratification21-23 and potentially differing effects of APOE across racial/ethnic groups,24-26 we performed analyses separately in non-Hispanic White (NHW) and non-Hispanic Black (NHB) participants. On finding sex-specific effects in NHW participants, we then sought to replicate these findings in an independent sample of participants from Alzheimer’s Disease Neuroimaging Initiative (ADNI) and Pre-symptomatic Evaluation of Experimental or Novel Treatments for Alzheimer Disease (Prevent-AD) (Sample 2).
2. Methods
2.1. Participants
Data were obtained from four independent sources: 1) NACC; 2) Rush Alzheimer’s Disease Center cohort studies: Religious Orders Study (ROS), Memory Aging Project (MAP), and Minority Aging Research Study (MARS); 3) ADNI; and 4) Prevent-AD. Sample 1 consisted of data from NACC and ROS/MAP/MARS. Sample 2 consisted of data from ADNI and Prevent-AD. Research procedures were approved by the relevant ethics committees and participants provided written informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for cohort studies.
Since APOE ε2 protects against cognitive decline,27 we restricted our sample to participants classified as cognitively unimpaired at baseline. This allowed us to maintain a representative proportion of ε2 carriers and to examine early cognitive changes with respect to APOE genotype. We also required that participants were ≥50 years old at baseline and had at least one follow-up cognitive assessment. In NACC, cognitively unimpaired is defined as a Clinical Dementia Rating (CDR) global score of 0.28 In ROS/MAP/MARS, cognitively unimpaired is defined as the absence of MCI or dementia.29,30 In ADNI and Prevent-AD, cognitively unimpaired is defined according to several criteria, one of which is a CDR global score of 0.31,32 In the present study, we only included participants who self-identified as NHW or NHB, since these were the largest racial/ethnic groups across data sources. Further details on the sample selection process are described in Figure S1 in the supplemental material.
2.2. Cognition
All four data sources (i.e., NACC, ROS/MAP/MARS, ADNI, Prevent-AD) assess cognition approximately annually. For each data source, we created a comparable cognitive composite that was weighted towards episodic memory (see supplemental material for specific tests). To calculate the composite, we z-transformed raw test scores using the mean and standard deviation of the baseline study samples, and then computed the average of the standardized scores.
2.3. Genotype
We used publicly available APOE genotype data to classify participants as ε2, ε3/ε3, or ε4 carriers. The samples had relatively few APOE ε2 homozygotes (N=56 in NACC; N=13 in ROS/MAP/MARS, N=1 in ADNI, N=0 in Prevent-AD), and therefore participants with one versus two copies of ε2 were not examined separately. APOE ε3 homozygotes were the reference group. APOE ε2/ε4 carriers were excluded due to the opposing effects of ε2 and ε4 alleles on AD risk.27 All samples met Hardy-Weinberg Equilibrium expectations.33
2.4. Statistical Analysis
Analyses were conducted in R (v.4.1.2). We used t-tests and X2 tests to assess differences in demographic and baseline characteristics between men and women. We used linear mixed models to examine the interactive effects of APOE allele (ε2 and ε4 vs. reference ε3/ε3), sex (reference female), and time (years from baseline) on longitudinal cognition separately in NHW and NHB participants. Where possible sex-specific APOE ε2 effects were observed, we then performed sex- and genotype-stratified analyses. Sex-stratified analyses examined the two-way interaction between APOE allele and time on cognition, allowing us to compare cognitive trajectories of APOE ε2 vs. ε3/ε3 carriers among men and women separately. Genotype-stratified analyses examined the two-way interaction between sex and time on cognition, allowing us to compare cognitive trajectories of men and women APOE ε2 carriers as well as men and women APOE ε3/ε3 carriers. All models included random intercepts and slopes. As in a previous study,34 including an additional quadratic term for time (to account for accelerated decline with aging) resulted in better model fit compared to models without this term (p<.05). Therefore, all models included this term.
We first examined sex differences in associations between APOE ε2 and cognitive decline in NHW and NHB participants from Sample 1. On observing sex-specific effects in NHW participants, we sought to replicate these effects in an independent sample of NHW participants (Sample 2). In exploratory analyses, we examined whether the sex-specific effects of APOE ε2 on longitudinal cognition were more pronounced at older ages. To do so, we repeated the main analyses after restricting the baseline age according to four cut-off values: age ≥65, ≥70, ≥75, and ≥80 years. Finally, to contextualize the sex-specific APOE ε2 findings, we compared them against sex-specific APOE ε4 findings.15,16
2.4.1. Covariates
In all analyses, we adjusted for data source (i.e., NACC vs. ROS/MAP/MARS or ADNI vs. Prevent-AD), baseline age, years of education, and their interactions with time. To account for practice effects on neuropsychological tests, we included a term for the square root of the number of previous study visits. This method assumes the largest improvement in performance after the first testing session, with diminishing returns on subsequent sessions.35 If this covariate was not significant, it was removed from the models. Because vascular risk factors are associated with cognitive decline,36,37 we also adjusted for baseline vascular risk and its interaction with time. Vascular risk was quantified using a summary score38 that includes the presence/absence of up to five conditions (diabetes, hypertension, high cholesterol, stroke, and heart conditions; see supplemental material for details). Finally, to determine whether length of follow-up impacted the results, we re-ran all models after adjusting for total number of visits. When follow-up visits were explicitly modelled, the estimates for the effects of interest were essentially unchanged. For simplicity, we report the results without including terms for visit number.
3. Results
3.1. Demographic characteristics
Table 1 summarizes the demographic characteristics for NHW and NHB participants in Sample 1 and NHW participants in Sample 2 (Tables S1 and S2 summarize demographic data for each data source separately). In Sample 1 (NACC and ROS/MAP/MARS), 9,766 NHW and 2,010 NHB participants met inclusion criteria. In Sample 2 (ADNI and Prevent-AD), 915 NHW participants met inclusion criteria. With respect to NHW participants, Sample 1 was slightly older than Sample 2 (73.0 years vs. 70.1 years), had a higher proportion of women (65.0% vs 59.1%), a slightly higher proportion of APOE ε2 carriers (12.9% vs. 11.8%), and greater number of study visits (median of 6 vs. 5 visits).
Table 1.
Baseline demographic and clinical characteristics by racial/ethnic group and cohort.
| Non-Hispanic Black participants in Sample 1 (NACC & ROS/MAP/MARS) | |||
|---|---|---|---|
| Variables | Total sample (n = 2,010) |
Women (n = 1,583, 78.8%) |
Men (n = 427, 21.2%) |
| Age in years, mean (SD) | 71.3 (7.59) | 71.4 (7.57) | 71.0 (7.67) |
| Education in years, mean (SD) | 14.9 (3.10) | 14.9 (3.02) | 14.9 (3.41) |
| APOE ε2 carriers, n (%) | 336 (16.7) | 263 (16.6) | 73 (17.1) |
| ε2/ε3, n (%) | 316 (15.7) | 248 (15.7) | 68 (15.9) |
| ε2/ε2, n (%) | 20 (1.00) | 15 (0.95) | 5 (1.17) |
| APOE ε4 carriers, n (%) | 662 (32.9) | 506 (32.0) | 156 (36.5) |
| ε3/ε4, n (%) | 595 (29.6) | 454 (28.7) | 141 (33.0) |
| ε4/ε4, n (%) | 67 (3.33) | 52 (3.28) | 15 (3.51) |
| APOE ε3/ε3 carriers, n (%) | 1,012 (50.3) | 814 (51.4) | 198 (46.4) |
| Total number of visits, median (SD) | 5 (3.96) | 6 (4.02)* | 5 (3.72)* |
| ε2 carriers, median (SD) | 6 (4.11) | 6 (4.20) | 6 (3.79) |
| ε4 carriers, median (SD) | 5 (3.70) | 5 (3.80) | 5 (3.33) |
| ε3/3 carriers, median (SD) | 6 (4.05) | 6 (4.07) | 5 (3.92) |
| Vascular risk score (range 0-1), mean (SD) | 0.36 (0.21) | 0.36 (0.21) | 0.36 (0.22) |
| Non-Hispanic White participants in Sample 1 (NACC & ROS/MAP) | |||
| Variables | Total sample (n = 9,766) |
Women (n = 6,344, 65.0%) |
Men (n = 3,422, 35.0%) |
| Age in years, mean (SD) | 73.0 (9.00) | 73.0 (9.14) | 72.9 (8.75) |
| Education in years, mean (SD) | 16.3 (2.83) | 16.0 (2.75)* | 16.9 (2.90)* |
| APOE ε2 carriers, n (%) | 1,260 (12.9) | 840 (13.2) | 420 (12.3) |
| ε2/ε3, n (%) | 1,211 (12.4) | 814 (12.8) | 397 (11.6) |
| ε2/ε2, n (%) | 49 (0.50) | 26 (0.41) | 23 (0.67) |
| APOE ε4 carriers, n (%) | 2,622 (26.8) | 1,670 (26.3) | 952 (27.8) |
| ε3/ε4, n (%) | 2,362 (24.2) | 1,508 (23.8) | 854 (25.0) |
| ε4/ε4, n (%) | 260 (2.66) | 162 (2.55) | 98 (2.86) |
| APOE ε3/ε3 carriers, n (%) | 5,884 (60.2) | 3,834 (60.4) | 2,050 (59.9) |
| Total number of visits, median (SD) | 6 (4.41) | 6 (4.48)* | 5 (4.27)* |
| ε2 carriers, median (SD) | 6 (4.53) | 6 (4.49) | 6 (4.61) |
| ε4 carriers, median (SD) | 5 (4.18) | 6 (4.26)* | 5 (4.02)* |
| ε3/3 carriers, median (SD) | 6 (4.48) | 6 (4.56)* | 5 (4.30)* |
| Vascular risk score (range 0-1), mean (SD) | 0.26 (0.21) | 0.25 (0.20)* | 0.28 (0.21)* |
| Non-Hispanic White participants in Sample 2 (ADNI & Prevent-AD) | |||
| Variables | Total sample (n = 915) |
Women (n = 542, 59.1%) |
Men (n = 373, 40.8%) |
| Age in years, mean (SD) | 70.1 (7.35) | 68.8 (7.17)* | 71.9 (7.24)* |
| Education in years, mean (SD) | 16.2 (2.92) | 15.7 (2.96)* | 16.9 (2.71)* |
| APOE ε2 carriers, n (%) | 108 (11.8) | 55 (10.1) | 53 (14.2) |
| ε2/ε3, n (%) | 107 (11.7) | 55 (10.1) | 52 (13.9) |
| ε2/ε2, n (%) | 1 (0.11) | 0 (0) | 1 (0.27) |
| APOE ε4 carriers, n (%) | 287 (31.4) | 176 (32.5) | 111 (29.8) |
| ε3/ε4, n (%) | 263 (28.7) | 160 (29.5) | 103 (27.6) |
| ε4/ε4, n (%) | 24 (2.62) | 16 (2.95) | 8 (2.14) |
| APOE ε3/ε3 carriers, n (%) | 520 (56.8) | 311 (57.3) | 209 (56.0) |
| Total number of visits, median (SD) | 5 (2.86) | 5 (2.70) | 5 (3.07) |
| ε2 carriers, median (SD) | 5 (2.61) | 5 (2.25) | 5 (2.96) |
| ε4 carriers, median (SD) | 5 (2.70) | 5 (2.60) | 5 (2.86) |
| ε3/3 carriers, median (SD) | 5 (2.99) | 5 (2.83) | 5 (3.21) |
| Vascular risk score (range 0-1), mean (SD) | 0.37 (0.19) | 0.37 (0.20) | 0.37 (0.18) |
p < .05. P-values represent results of independent samples t-tests and chi-square tests comparing men vs. women.
NACC: National Alzheimer’s Coordinating Center
ROS: Religious Orders Study
MAP: Memory Aging Project
MARS: Minority Aging Research Study
ADNI: Alzheimer’s Disease Neuroimaging Initiative
Prevent-AD: Pre-symptomatic Evaluation of Experimental or Novel Treatments for Alzheimer Disease
SD: standard deviation
3.2. Sex-specific associations of APOE ε2 with cognitive decline in NHB participants
In Sample 1, the interaction between sex, APOE ε2, and time on cognitive decline was not significant in NHB participants (β= −0.011, 95% CI: −0.153–0.131, p=.88; Table S3; Figure 1). The lower-order two-way interaction between sex and APOE ε2 was also not significant (β=0.056, 95% CI: −0.188 – 0.301, p=.65; Table S3), suggesting that there are no sex-specific associations of APOE ε2 with longitudinal cognition or with cognition collapsed across all time-points. We next tested the two-way interaction between APOE ε2 (vs. ε3/ε3) and time on cognitive decline (adjusting for sex). In this analysis, APOE ε2 carriers did not exhibit significantly slower cognitive decline relative to ε3/ε3 carriers (β=0.046, 95% CI: −0.012–0.104, p=.12; Table S3; Figure S2). Similar findings were observed in sex-stratified analyses (Table S3). With respect to sex-specific effects of APOE ε4, we observed a non-significant interaction between male sex, APOE ε4, and time in NHB participants (β=0.103, 95% CI: −0.017–0.223, p=.09; Table S3; Figure S3). However, sex- and genotype-stratified analyses showed that women with APOE ε4 exhibited faster cognitive decline relative to both women carrying ε3/ε3 and men carrying ε4 (Table S3).
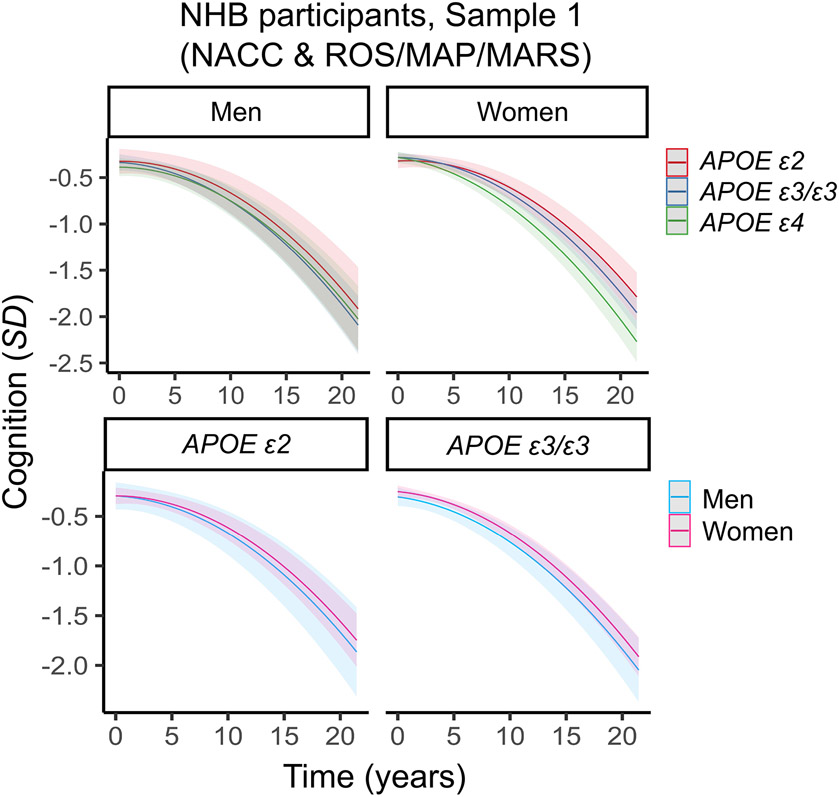
Figure 1. Three-way interaction between sex, APOE and time on cognitive decline in non-Hispanic Black (NHB) participants in Sample 1 (NACC & ROS/MAP/MARS).
Plots depict marginal effects, showing change in cognition (standardized score) over time, stratified by sex and genotype (APOE ε4 plot not shown). There were no significant sex differences in associations between APOE ε2 and global cognitive decline. The models are adjusted for baseline age, years of education, and vascular risk, and their interactions with time. Shaded regions represent 95% confidence intervals.
3.3. Sex-specific associations of APOE ε2 with cognitive decline in NHW participants
In NHW participants from Sample 1, there was a significant interaction between sex, APOE ε2, and time (Table 2; Table S4, Figure 2). In sex-stratified analyses, men with APOE ε2 exhibited slower cognitive decline than men with APOE ε3/ε3 (Table 2; Table S4). By contrast, cognitive trajectories did not differ between women with APOE ε2 versus ε3/ε3 (Table 2; Table S4). In genotype-stratified analyses, cognitive trajectories differed by sex among APOE ε2 carriers, but not among APOE ε3/ε3 carriers. Specifically, among APOE ε2 carriers, men exhibited slower decline relative to women, whereas rates of decline were similar between men and women carrying APOE ε3/ε3 (Table 2; Table S4).
Table 2.
Sex-specific associations between APOE ε2 (vs APOE ε3/ε3) and longitudinal cognition in non-Hispanic White participants.
| Analyses |
Sample 1 (NACC
&
ROS/MAP) |
NACC | ROS/MAP |
Sample 2 (ADNI
&
Prevent-AD) |
||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Three-way interaction: | ||||||||
| Sex × APOE ε2 (vs. ε3/ε3) × time | 0.097 (0.023 – 0.172) | .01 | 0.081 (0.010 – 0.152) | .02 | 0.127 (−0.069 – 0.323) | .20 | 0.195 (0.006 – 0.385) | .04 |
| Sex-stratified two-way interactions: | ||||||||
| APOE ε2 (vs. ε3/ε3) × time in men | 0.096 (0.037 – 0.155) | .001 | 0.074 (0.020 – 0.128) | .008 | 0.149 (−0.022 – 0.319) | .09 | 0.093 (−0.056 – 0.243) | .22 |
| APOE ε2 (vs. ε3/ε3) × time in women | −0.001 (−0.044 – 0.043) | .97 | −0.008 (−0.051 – 0.035) | .71 | 0.012 (0.089 – 0.114) | .81 | −0.104 (−0.228 – 0.020) | .10 |
| Genotype-stratified two-way interaction: | ||||||||
| Male sex × time in APOE ε2 carriers | 0.120 (0.051 – 0.190) | .001 | 0.095 (0.028 – 0.161) | .005 | 0.191 (0.012 – 0.371) | .04 | 0.160 (−0.002 – 0.321) | .05 |
| Male sex × time in APOE ε3/ε3 carriers | −0.000 (−0.031 – 0.030) | .99 | −0.005 (−0.033 – 0.024) | .75 | 0.038 (−0.044 – 0.121) | .36 | −0.009 (−0.091 – 0.074) | .84 |
NACC: National Alzheimer’s Coordinating Center
ROS: Religious Orders Study
MAP: Memory Aging Project
ADNI: Alzheimer’s Disease Neuroimaging Initiative
Prevent-AD: Pre-symptomatic Evaluation of Experimental or Novel Treatments for Alzheimer Disease
CI: confidence interval
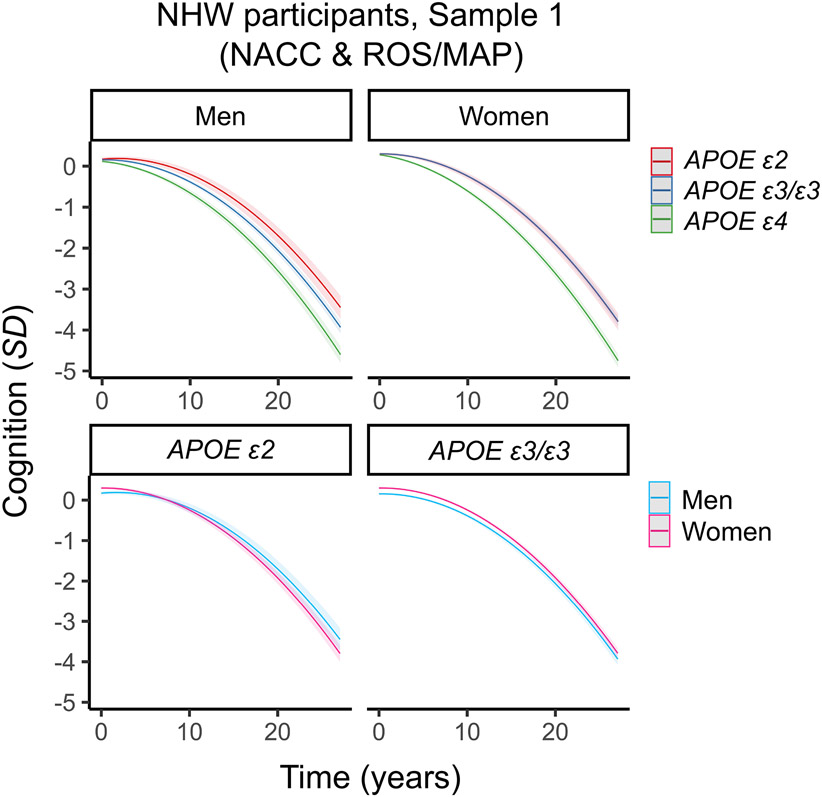
Figure 2. Three-way interaction between sex, APOE and time on cognitive decline in non-Hispanic White (NHW) participants in Sample 1 (NACC & ROS/MAP).
Plots depict marginal effects, showing change in cognition (standardized score) over time, stratified by sex and genotype (APOE ε4 plot not shown). In sex-stratified analyses, men carrying APOE ε2 were more protected against decline than men carrying APOE ε3/ε3. In women, APOE ε2 was no more protective than APOE ε3/ε3. In genotype-stratified analyses, men carrying APOE ε2 were more protected against decline than women carrying APOE ε2. By contrast, rates of decline did not differ between men and women APOE ε3/ε3 carriers. The models are adjusted for baseline age, years of education, and vascular risk, and their interactions with time. Shaded regions represent 95% confidence intervals.
Given the relatively large number of participants in NACC (N=7,931, N women=4,980, 62.8%) and ROS/MAP (N=1,835, N women=1,364, 74.3%), we examined whether the pattern of results was present in each data source separately. In NACC, there was a significant interaction between male sex, APOE ε2, and time on cognitive decline (Table 2; Table S5, Figure S4, Figure S5). In ROS/MAP, the same three-way interaction was not significant (Table 2; Table S6, Figure S4, Figure S5). However, sex- and genotype-stratified analyses revealed a similar pattern of findings in both data sources (Table 2, Table S5, Table S6). Sex-stratified analyses showed that men with APOE ε2 had a pattern of slower cognitive decline than men with APOE ε3/ε3, although the interaction was not statistically significant in ROS/MAP (β=0.149, 95% CI: −0.022 – 0.319, p=.09; Table 2, Table S6). In both cohorts, women with APOE ε2 did not have slower decline than women carrying APOE ε3/ε3. In genotype-stratified analyses, men with APOE ε2 had significantly slower decline than women with APOE ε2. Similarly, men and women APOE ε3/ε3 carriers did not exhibit different cognitive trajectories.
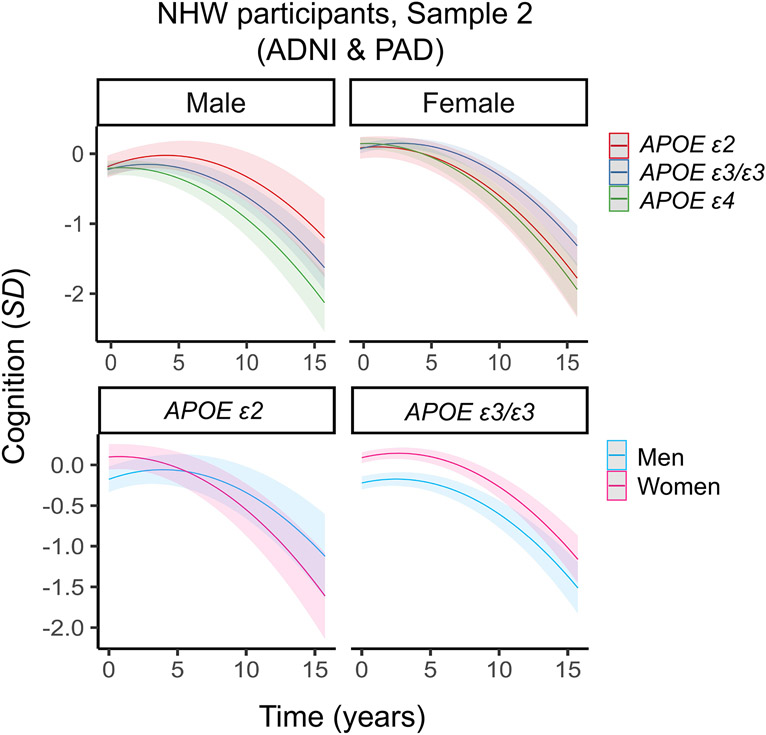
Next, we sought to replicate the main sex-specific findings in an independent sample of NHW participants from ADNI and Prevent-AD (Sample 2). We again observed a significant interaction between male sex, APOE ε2 and time (Table 2; Table S7, Figure 3), with Sample 2 showing a larger effect than Sample 1 (as demonstrated by a larger standardized beta coefficient). Next, we performed sex-stratified analyses. In men, APOE ε2 carriers had a non-significant pattern of slower decline than ε3/ε3 carriers. While this finding did not reach statistical significance, the effect size was similar to that reported in Sample 1. Surprisingly, women with APOE ε2 had a non-significant pattern of faster decline compared to women with APOE ε3/ε3 (Table 2; Table S7). In genotype-stratified analyses, men with APOE ε2 exhibited significantly slower decline than women with ε2, whereas the rates of decline did not differ between men and women with APOE ε3/ε3 (Table 2; Table S7). The effect sizes in these genotype-stratified analyses were equivalent to or larger than those observed in Sample 1.
Figure 3. Three-way interaction between sex, APOE and time on cognitive decline in non-Hispanic White (NHW) participants in Sample 2 (ADNI & Prevent-AD).
Plots depict marginal effects showing change in cognition (standardized score) over time, stratified by sex and genotype (APOE ε4 plot not shown). Sex-stratified analyses were not significant. In genotype-stratified analyses, men carrying APOE ε2 were more protected against decline than women carrying APOE ε2, whereas the rates of decline did not differ between men and women carrying APOE ε3/ε3. The models are adjusted for baseline age, years of education, and vascular risk, and their interactions with time. Shaded regions represent 95% confidence intervals.
In exploratory analyses, we examined whether the sex-specific effect of APOE ε2 on cognitive decline differed across increasing baseline age cut-offs (age ≥65, ≥70, ≥75, and ≥80 years). In Sample 1, we observed that the magnitude of the 3-way interaction term increased in a positive direction as baseline age increased (Table S8). In Sample 2, we observed a similar increase in magnitude among participants aged 50 through 70 (Table S9). However, the magnitude of the interaction term began to decrease again above the age of 75. This is likely due to the considerably smaller sample sizes at these older ages (Table S9). Together, these findings suggest that male-specific APOE ε2 protection may become more pronounced in older age.
3.4. Sex-specific associations of APOE ε4 with cognitive decline in NHW participants
To contextualize the APOE ε2 findings in NHW participants in Sample 1 and Sample 2, we sought to replicate previously reported sex differences in associations between APOE ε4 and cognitive decline. In Sample 1, there was a significant interaction between male sex, APOE ε4, and time on cognition in NHW participants (β=0.064, 95% CI: 0.007–0.120, p=.03; Table S4; Figure 2). Sex-stratified analyses demonstrated that APOE ε4 (vs. APOE ε3/ε3) was more strongly associated with cognitive decline in women (β=−0.192, 95% CI: −0.227 – −0.158, p<.001; Table S4) than men (β=−0.127, 95% CI: −0.171 – −0.083, p<.001; Table S4). A genotype-stratified analysis showed that men with APOE ε4 declined more slowly than women with APOE ε4 (β=0.053, 95% CI: .002 – 0.104, p=.04; Table S4; Figure S6). These same findings were not observed in Sample 2, as the interaction between male sex, APOE ε4, and time on longitudinal cognition was not significant (β=0.041, 95% CI: −0.095–0.177, p=.56; Table S7; Figure 2; Figure S7).
4. Discussion
Across two independent samples of cognitively unimpaired NHW participants (Sample 1: NACC and ROS/MAP, Sample 2: ADNI and Prevent-AD), we found that men with APOE ε2 were more protected against cognitive decline compared to both men with APOE ε3/ε3 and women with APOE ε2. Notably, no sex differences were observed among APOE ε3/ε3 carriers. Analyses performed separately in NACC and ROS/MAP showed the same pattern of male-specific protection in APOE ε2 carriers. In both Sample 1 and Sample 2, the magnitude of the sex-specific effect of APOE ε2 on cognitive decline was generally more pronounced at older ages when risk for AD is higher.39 The replication of these findings in cognitively unimpaired NHW adults across NACC, ROS/MAP and ADNI/Prevent-AD provide compelling evidence that APOE ε2 protects men but not women against cognitive decline. In contrast, we observed no sex-specific associations in NHB participants, and APOE ε2 was not significantly associated with attenuated cognitive decline (relative to ε3/ε3) in men or women.
The biological mechanisms driving the observed sex differences in the NHW participants are unclear. One possibility may relate to sex hormones, which regulate ApoE protein synthesis.40 Estrogen upregulates ApoE synthesis,40,41 and like other metabolic and neurological systems,42,43 estrogen-mediated APOE processes may become disrupted around menopause when estrogen levels decline. If so, APOE ε2 protection against AD pathology and its downstream cognitive effects may be reduced in postmenopausal women. Additional research is needed to elucidate the biological mechanisms underlying the sex-specific effects of APOE ε2.
The finding of sex-specific associations between APOE ε2 and cognitive decline complements evidence that women (vs. men) carrying APOE ε4 are at disproportionately higher risk for AD.15,16,44,45 We replicated this finding in NHW and NHB from Sample 1 (but not Sample 2), observing that women with APOE ε4 had faster rates of cognitive decline than their counterpart men. Interestingly, in NHW participants from Sample 1, the effect size of the three-way interaction of APOE ε2, sex, and time on cognitive decline (β=0.097) was greater than that of the equivalent interaction for APOE ε4 (β=0.064). This suggests that sex-specific protective effects of APOE ε2 may represent an important yet overlooked contribution to sex disparities in cognitive and AD outcomes.
It is not clear why we did not observe significant associations between APOE ε2 and attenuated cognitive decline in NHB participants of either sex. Previous research demonstrates that pathological drivers of cognitive decline may differ across races.46,47 It is possible that in NHB participants, associations of APOE ε2 with cognition (including potential sex-specific associations) are obscured by more salient predictors of cognitive decline. Alternatively, APOE ε2 protection against AD may be weaker or non-existent in NHB persons. This idea is consistent with previous research in Black samples,48,49 and broader evidence that APOE genotypes differentially impact cognition across racial and ethnic groups.19,24-26,50
Despite observing no significant associations of APOE ε2 with attenuated cognitive decline across both sexes in NHB participants, APOE ε2 was more prevalent in NHB compared to NHW participants. This difference aligns with existing reports of racial/ethnic differences in APOE carriage19,24,51 and is consistent with broader observations that allele frequencies vary across populations of different ancestral backgrounds.21 Future work should seek to further clarify sex-specific APOE effects in diverse cohorts.
In the NHW participants, there were some notable differences in the effect sizes across data sources. Specifically, the effect size of the three-way interaction of APOE ε2, sex, and time was larger in ROS/MAP and Sample 2 (ADNI and Prevent-AD) compared to NACC. Similarly, the effect size of the two-way interaction between APOE ε2 and time in men was larger in ROS/MAP and Sample 2 (ADNI and Prevent-AD) compared to NACC. The reasons for these differences remain unclear but may relate to selection bias. For example, ROS/MAP participants are generally older than NACC participants (77 vs. 72 years old) and have more follow-up data (10 vs 5 visits). Given our findings that sex-specific APOE ε2 effects become more salient at older ages, we might expect larger effects in older samples, particularly if they have more follow-up data. Additionally, all participants in Prevent-AD have either a parent or multiple siblings with AD.32 Therefore, the larger effects observed in Sample 2 may suggest that sex-specific APOE effects are more pronounced in a sample enriched with familial AD risk.
The major strength of this study is the replication of sex-specific findings across two independent samples of pooled data (as well as NACC & ROS/MAP). This is particularly notable given different sampling procedures, demographic characteristics, cognitive tests, and follow-up times across the studies. The present study has several limitations. First, study participants are generally well-educated, which may limit the generalizability of our findings. Second, since whole genome sequencing or equivalent data were not available for many study participants, we were unable to adjust our analyses for genetic principal components (to account for possible population admixture). This approach is ideal, as there may be multiple genetic subpopulations in our samples. Third, in Sample 2, there were too few NHB participants (N=52) to perform a replication analysis. It will be important for future research to replicate and extend the present findings to other diverse groups. Fourth, while we verified that the NHW and NHB samples aligned with Hardy-Weinberg Equilibrium expectations, the recorded APOE genotypes may contain miscalls, which may bias effect estimates, particularly in smaller APOE genotype stratified samples. Fifth, a challenge to studying sex differences in AD is that women are more likely than men to survive to older ages.3 When a gene, such as APOE, has pleiotropic effects on risk for mortality and AD,52 this survival bias can cause spurious associations. Finally, given the rarity of APOE ε2 homozygosity, we were unable to investigate sex differences in allelic dose effects.
5. Conclusion
Our results clarify the longstanding view that APOE ε2 protects against AD.11,27,50,53,54 Among NHW adults, APOE ε2 protects men but not women against cognitive decline. These findings have important implications for understanding the biological drivers of sex differences in AD risk, which is crucial for developing sex-specific strategies to prevent and treat AD dementia. Large and diverse samples are needed to replicate the present findings and to further clarify the sex-specific effects of APOE ε2 on risk for AD.
Supplementary Material
Acknowledgements
MEW receives support from the Canadian Institutes of Health Research (Canada Graduate Scholarships – Master’s). LYX gratefully acknowledges financial support from the Canadian Institutes of Health Research (Doctoral Research Award: Canadian Graduate Scholarships; 202111FBD-476226). RFB is supported by a K99/R00 award from NIA (R00AG061238-03), an Alzheimer’s Association Research Fellowship (AARF-20-675646) and philanthropic support. WS gratefully acknowledges financial support from the Canadian Institute of Health Research (Team Grant); The Natural Sciences and Engineering Research Council of Canada (RGPIN-2017-06962); The Alzheimer's Association & Brain Canada (AARG501466); Weston Brain Institute & Alzheimer's Research UK, Alzheimer's Association and Michael J. Fox Foundation (BAND3). The work was supported in part through funding from the Canada Research Chairs Program. ASPL receives support from R01AG071638, R01AG052488 and RF1AG070436. EN receives support from T32AG000247. RL receives support from K99AG065501. KBC receives support from R01AG072475. CS receives support from the Texas Alzheimer’s Research and Care Consortium (2020-58-81- CR) and R01 AG059727, UF1 NS125513, and P30 AG066546. LLB receives support from R01AG022018 and P30AG010161/P30AG072975. JAS receives support from P30AG010161/P30AG072975, R01AG015819, R01AG17917, R01AG022018. JP receives support from RF1AG054617. JSR receives support from the Harquail Centre for Neuromodulation, the Dr. Sandra Black Centre for Brain Resilience & Recovery, and Canadian Institutes of Health Research (173253, 438475), and the Alzheimer’s Society of Canada.
The NACC database is funded by NIA/NIH Grant U24 AG072122. NACC data are contributed by the NIA-funded ADCs: P50 AG005131 (PI James Brewer, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG005138 (PI Mary Sano, PhD), P50 AG005142 (PI Helena Chui, MD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005681 (PI John Morris, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG008051 (PI Thomas Wisniewski, MD), P50 AG008702 (PI Scott Small, MD), P30 AG010124 (PI John Trojanowski, MD, PhD), P30 AG010129 (PI Charles DeCarli, MD), P30 AG010133 (PI Andrew Saykin, PsyD), P30 AG010161 (PI David Bennett, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG013846 (PI Neil Kowall, MD), P30 AG013854 (PI Robert Vassar, PhD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P30 AG019610 (PI Eric Reiman, MD), P50 AG023501 (PI Bruce Miller, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P30 AG028383 (PI Linda Van Eldik, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P30 AG035982 (PI Russell Swerdlow, MD), P50 AG047266 (PI Todd Golde, MD, PhD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG049638 (PI Suzanne Craft, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG066546 (PI Sudha Seshadri, MD), P20 AG068024 (PI Erik Roberson, MD, PhD), P20 AG068053 (PI Marwan Sabbagh, MD), P20 AG068077 (PI Gary Rosenberg, MD), P20 AG068082 (PI Angela Jefferson, PhD), P30 AG072958 (PI Heather Whitson, MD), P30 AG072959 (PI James Leverenz, MD).
We thank the study participants and staff of the Rush Alzheimer’s Disease Center. This work was supported by NIA grants P30 AG010161 (ROS; PI David Bennett, MD), R01AG17917 (MAP; PI David Bennett, MD) and R01AG22018 (MARS; PI Lisa Barnes, PhD).
Footnotes
Conflicts of Interest
ASPL sat on a paid advisory board for Eisai within the past 12 months. LLB was named Deputy Editor of Alzheimer’s & Dementia in 2023. JAS serves on the Scientific Advisory Boards for AVID radiopharmaceuticals (subsidiary of Lilly), Alnylam Pharmaceuticals, Apellis Pharmaceuticals, Takeda Pharmaceuticals, and the National Hockey League. MEW, LYX, YYW, RFB, WS, MM, EN, RLJ, KBC, RGK, KDOC, PP, KMG, CLS, APB, SV, JP, AMB, SEB and JSR have no conflicts of interests to disclose. Author disclosures are available in the supporting information.
Consent Statement
All human participants in the included cohort studies provided informed consent.
6. References
- 1.Chêne G, Beiser A, Au R, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement J Alzheimers Assoc. 2015;11(3):310–320. doi: 10.1016/j.jalz.2013.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seshadri S, Wolf PA, Beiser A, et al. Lifetime risk of dementia and Alzheimer’s disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49(6):1498–1504. doi: 10.1212/wnl.49.6.1498 [DOI] [PubMed] [Google Scholar]
- 3.Shaw C, Hayes-Larson E, Glymour MM, et al. Evaluation of selective survival and sex/gender differences in dementia incidence using a simulation model. JAMA Netw Open. 2021;4(3):e211001. doi: 10.1001/jamanetworkopen.2021.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nebel RA, Aggarwal NT, Barnes LL, et al. Understanding the impact of sex and gender in Alzheimer’s disease: A call to action. Alzheimers Dement. 2018;14(9):1171–1183. doi: 10.1016/j.jalz.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckley RF, Mormino EC, Amariglio RE, et al. Sex, amyloid, and APOEε4 and risk of cognitive decline in preclinical Alzheimer’s disease: Findings from three well-characterized cohorts. Alzheimers Dement J Alzheimers Assoc. 2018;14(9):1193–1203. doi: 10.1016/j.jalz.2018.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards L, La Joie R, Iaccarino L, et al. Multimodal neuroimaging of sex differences in cognitively impaired patients on the Alzheimer’s continuum: Greater tau-PET retention in females. Neurobiol Aging. 2021;105:86–98. doi: 10.1016/j.neurobiolaging.2021.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liesinger AM, Graff-Radford NR, Duara R, et al. Sex and age interact to determine clinicopathologic differences in Alzheimer’s disease. Acta Neuropathol (Berl). 2018;136(6):873–885. doi: 10.1007/s00401-018-1908-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oveisgharan S, Arvanitakis Z, Yu L, Farfel J, Schneider JA, Bennett DA. Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathol (Berl). 2018;136(6):887–900. doi: 10.1007/s00401-018-1920-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palta P, Rippon B, Tahmi M, et al. Sex differences in in vivo tau neuropathology in a multiethnic sample of late middle-aged adults. Neurobiol Aging. 2021;103:109–116. doi: 10.1016/j.neurobiolaging.2021.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snyder HM, Asthana S, Bain L, et al. Sex biology contributions to vulnerability to Alzheimer’s disease: A think tank convened by the Women’s Alzheimer’s Research Initiative. Alzheimers Dement. 2016;12(11):1186–1196. doi: 10.1016/j.jalz.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms, and therapy. Nat Rev Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh PP, Singh M, Mastana SS. APOE distribution in world populations with new data from India and the UK. Ann Hum Biol. 2006;33(3):279–308. doi: 10.1080/03014460600594513 [DOI] [PubMed] [Google Scholar]
- 13.Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nat Genet. 2007;39(1):17–23. doi: 10.1038/ng1934 [DOI] [PubMed] [Google Scholar]
- 14.Rubin L, Ingram LA, Resciniti NV, et al. Genetic Risk Factors for Alzheimer’s disease in racial/ethnic minority populations in the U.S.: A scoping review. Front Public Health. 2021;9:784958. doi: 10.3389/fpubh.2021.784958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrer LA. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–1356. doi: 10.1001/jama.278.16.1349 [DOI] [PubMed] [Google Scholar]
- 16.Altmann A, Tian L, Henderson VW, Greicius MD, Alzheimer’s Disease Neuroimaging Initiative Investigators. Sex modifies the APOE-related risk of developing Alzheimer disease: Sex and APOE -Related AD Risk. Ann Neurol. 2014;75(4):563–573. doi: 10.1002/ana.24135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neu SC, Pa J, Kukull W, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer’s disease. JAMA Neurol. 2017;74(10):1178–1189. doi: 10.1001/jamaneurol.2017.2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamonja-Vicente N, Dacosta-Aguayo R, López-Olóriz J, et al. Sex-specific protective effects of APOE ε2 on cognitive performance. J Gerontol Ser A. 2021;76(1):41–49. doi: 10.1093/gerona/glaa247 [DOI] [PubMed] [Google Scholar]
- 19.Beydoun MA, Weiss J, Beydoun HA, et al. Race, APOE genotypes, and cognitive decline among middle-aged urban adults. Alzheimers Res Ther. 2021;13(1):120. doi: 10.1186/s13195-021-00855-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu C, Kivipelto M, Agüero-Torres H, Winblad B, Fratiglioni L. Risk and protective effects of the APOE gene towards Alzheimer’s disease in the Kungsholmen project: variation by age and sex. J Neurol Neurosurg Psychiatry. 2004;75(6):828–833. doi: 10.1136/jnnp.2003.021493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hellwege J, Keaton J, Giri A, Gao X, Velez Edwards DR, Edwards TL. Population stratification in genetic association studies. Curr Protoc Hum Genet. 2017;95:1.22.1–1.22.23. doi: 10.1002/cphg.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardon LR, Palmer LJ. Population stratification and spurious allelic association. Lancet Lond Engl. 2003;361(9357):598–604. doi: 10.1016/S0140-6736(03)12520-2 [DOI] [PubMed] [Google Scholar]
- 23.Liu XQ, Paterson AD, John EM, Knight JA. The role of self-defined race/ethnicity in population structure control. Ann Hum Genet. 2006;70(Pt 4):496–505. doi: 10.1111/j.1469-1809.2005.00255.x [DOI] [PubMed] [Google Scholar]
- 24.Qin W, Li W, Wang Q, et al. Race-related association between APOE genotype and Alzheimer’s disease: A systematic review and meta-analysis. J Alzheimers Dis. 2021;83(2):897–906. doi: 10.3233/JAD-210549 [DOI] [PubMed] [Google Scholar]
- 25.Weuve J, Barnes LL, Mendes de Leon CF, et al. Cognitive aging in Black and White Americans: Cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiol Camb Mass. 2018;29(1):151–159. doi: 10.1097/EDE.0000000000000747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griswold AJ, Celis K, Bussies PL, et al. Increased APOE ε4 expression is associated with the difference in Alzheimer’s disease risk from diverse ancestral backgrounds. Alzheimers Dement. 2021;17(7):1179–1188. doi: 10.1002/alz.12287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suri S, Heise V, Trachtenberg AJ, Mackay CE. The forgotten APOE allele: A review of the evidence and suggested mechanisms for the protective effect of APOE ε2. Neurosci Biobehav Rev. 2013;37(10 Pt 2):2878–2886. doi: 10.1016/j.neubiorev.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 28.National Alzheimer’s Coordinating Center. NACC Uniform Data Set initial visit packet version 3.0. Published online 2015.
- 29.Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27(3):169–176. doi: 10.1159/000096129 [DOI] [PubMed] [Google Scholar]
- 30.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59(2):198–205. doi: 10.1212/wnl.59.2.198 [DOI] [PubMed] [Google Scholar]
- 31.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI). Neurology. 2010;74(3):201–209. doi: 10.1212/WNL.0b013e3181cb3e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tremblay-Mercier J, Madjar C, Das S, et al. Open science datasets from PREVENT-AD, a longitudinal cohort of pre-symptomatic Alzheimer’s disease. NeuroImage Clin. 2021;31:102733. doi: 10.1016/j.nicl.2021.102733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Namipashaki A, Razaghi-Moghadam Z, Ansari-Pour N. The essentiality of reporting Hardy-Weinberg Equilibrium calculations in population-based genetic association studies. Cell J. 2015;17(2):187–192. doi: 10.22074/cellj.2016.3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckley RF, Scott MR, Jacobs HIL, et al. Sex mediates relationships between regional tau pathology and cognitive decline. Ann Neurol. 2020;88(5):921–932. doi: 10.1002/ana.25878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vivot A, Power MC, Glymour MM, et al. Jump, hop, or skip: Modeling practice effects in studies of determinants of cognitive change in older adults. Am J Epidemiol. 2016;183(4):302–314. doi: 10.1093/aje/kwv212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rabin JS, Schultz AP, Hedden T, et al. Interactive associations of vascular risk and β-amyloid burden with cognitive decline in clinically normal elderly individuals: Findings from the Harvard Aging Brain Study. JAMA Neurol. 2018;75(9):1124–1131. doi: 10.1001/jamaneurol.2018.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabin JS, Nichols E, La Joie R, et al. Cerebral amyloid angiopathy interacts with neuritic amyloid plaques to promote tau and cognitive decline. Brain. Published online June 27, 2022:awac178. doi: 10.1093/brain/awac178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riedel BC, Thompson PM, Brinton RD. Age, APOE and sex: Triad of risk of Alzheimer’s disease. J Steroid Biochem Mol Biol. 2016;160:134–147. doi: 10.1016/j.jsbmb.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gamache J, Yun Y, Chiba-Falek O. Sex-dependent effect of APOE on Alzheimer’s disease and other age-related neurodegenerative disorders. Dis Model Mech. 2020;13(8):dmm045211. doi: 10.1242/dmm.045211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ratnakumar A, Zimmerman SE, Jordan BA, Mar JC. Estrogen activates Alzheimer’s disease genes. Alzheimers Dement Transl Res Clin Interv. 2019;5:906–917. doi: 10.1016/j.trci.2019.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E. Perimenopause as a neurological transition state. Nat Rev Endocrinol. 2015;11(7):393–405. doi: 10.1038/nrendo.2015.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosconi L, Berti V, Quinn C, et al. Sex differences in Alzheimer risk. Neurology. 2017;89(13):1382–1390. doi: 10.1212/WNL.0000000000004425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hohman TJ, Dumitrescu L, Barnes LL, et al. Sex-specific association of Apolipoprotein E with cerebrospinal fluid levels of tau. JAMA Neurol. 2018;75(8):989–998. doi: 10.1001/jamaneurol.2018.0821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fleisher A, Grundman M, Jack CR Jr, et al. Sex, Apolipoprotein E ε4 status, and hippocampal volume in Mild Cognitive Impairment. Arch Neurol. 2005;62(6):953–957. doi: 10.1001/archneur.62.6.953 [DOI] [PubMed] [Google Scholar]
- 46.Barnes LL, Leurgans S, Aggarwal NT, et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology. 2015;85(6):528–534. doi: 10.1212/WNL.0000000000001834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris JC, Schindler SE, McCue LM, et al. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol. 2019;76(3):264–273. doi: 10.1001/jamaneurol.2018.4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deters KD, Napolioni V, Sperling RA, et al. Amyloid PET imaging in self-identified non-Hispanic Black participants of the Anti-Amyloid in Asymptomatic Alzheimer’s Disease (A4) Study. Neurology. 2021;96(11):e1491–e1500. doi: 10.1212/WNL.0000000000011599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blue EE, Horimoto ARVR, Mukherjee S, Wijsman EM, Thornton TA. Local ancestry at APOE modifies Alzheimer’s disease risk in Caribbean Hispanics. Alzheimers Dement J Alzheimers Assoc. 2019;15(12):1524–1532. doi: 10.1016/j.jalz.2019.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blair CK, Folsom AR, Knopman DS, et al. APOE genotype and cognitive decline in a middle-aged cohort. Neurology. 2005;64(2):268–276. doi: 10.1212/01.WNL.0000149643.91367.8A [DOI] [PubMed] [Google Scholar]
- 51.Rajan KB, Barnes LL, Wilson RS, Weuve J, McAninch EA, Evans DA. Apolipoprotein E genotypes, age, race, and cognitive decline in a population sample. J Am Geriatr Soc. 2019;67(4):734–740. doi: 10.1111/jgs.15727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eissman JM, Dumitrescu L, Mahoney ER, et al. Sex differences in the genetic architecture of cognitive resilience to Alzheimer’s disease. Brain J Neurol. 2022;145(7):2541–2554. doi: 10.1093/brain/awac177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martins C a. R, Oulhaj A, de Jager CA, Williams JH. APOE alleles predict the rate of cognitive decline in Alzheimer disease: A nonlinear model. Neurology. 2005;65(12):1888–1893. doi: 10.1212/01.wnl.0000188871.74093.12 [DOI] [PubMed] [Google Scholar]
- 54.Wilson RS. The apolipoprotein E epsilon2 allele and decline in episodic memory. J Neurol Neurosurg Psychiatry. 2002;73(6):672–677. doi: 10.1136/jnnp.73.6.672 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.