Abstract
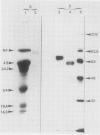
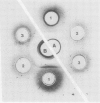
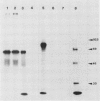
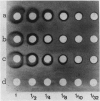
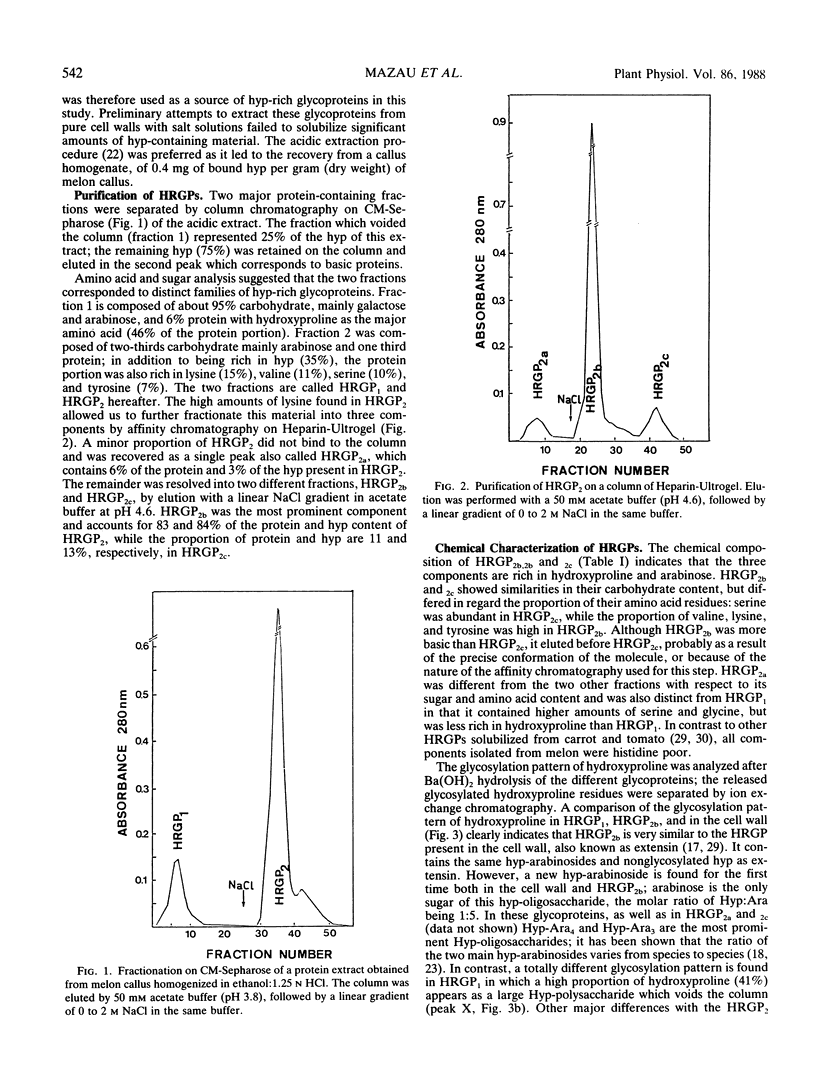
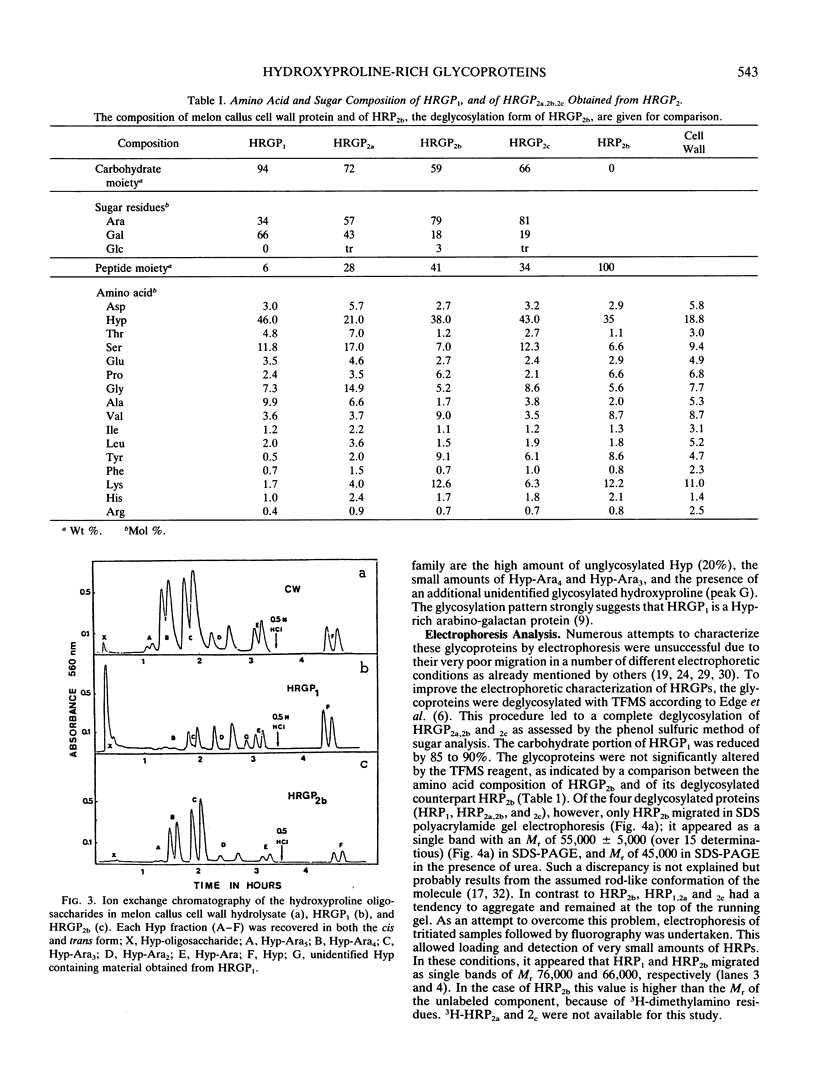
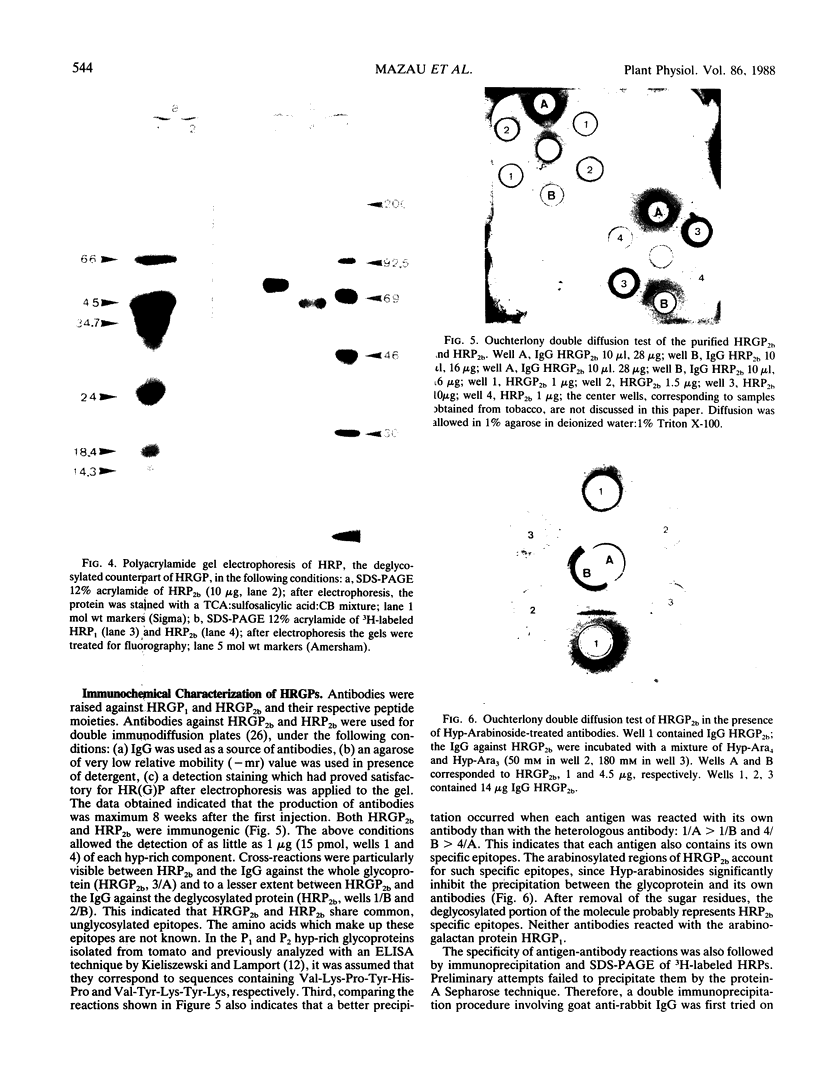
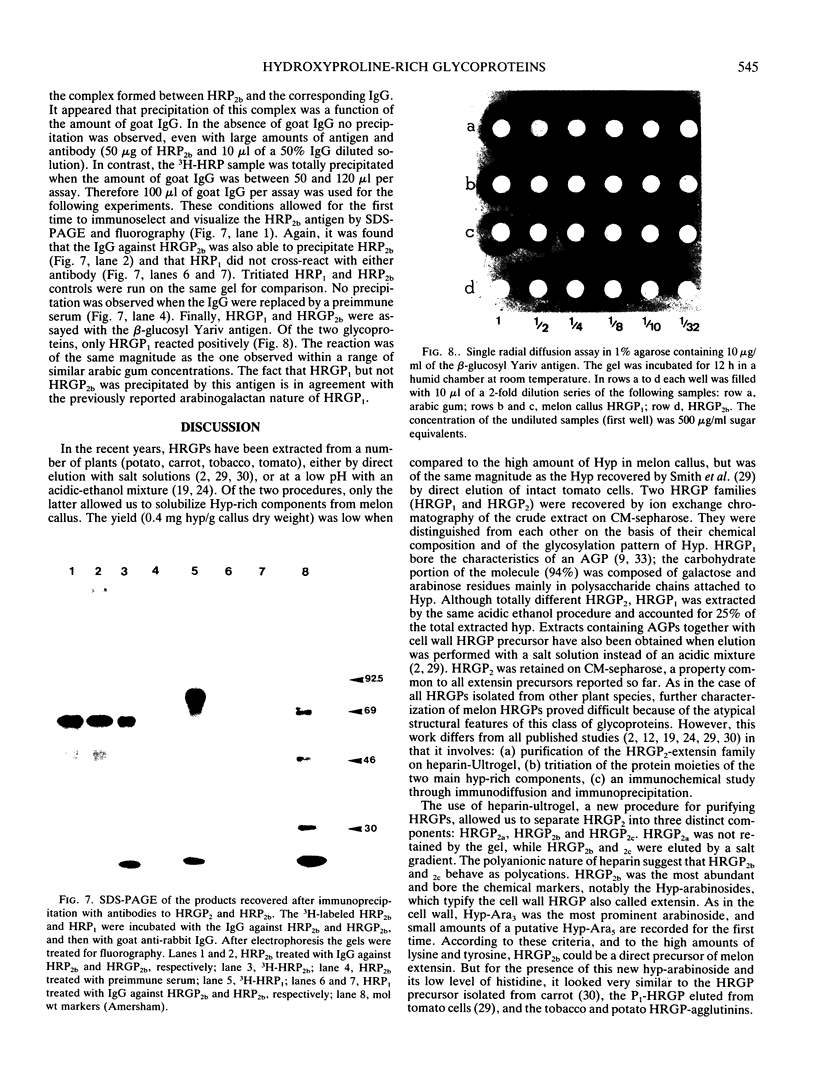
Two different families of hydroxyproline-rich glycoproteins, HRGP1 and HRGP2, have been isolated from melon callus and separated by ion exchange chromatography on CM-sepharose. HRGP1 corresponds to an arabinogalactan protein. The sugar portion of HRGP1 accounts for 94% of the molecule and contains galactose (66%) and arabinose (34%); these residues are present as polysaccharide side chains attached to hydroxyproline. Hydroxyproline is the main amino acid residue (46%) of the protein moiety. The arabinogalactan protein nature of HRGP1 has been checked by its ability to positively react with the β-glucosyl Yariv antigen; the 3H-labeled deglycosylated HRGP1 also called HRP1 migrates upon electrophoresis as a single band of molecular weight 76,000. HRGP2 was fractionated by affinity chromatography on heparin-Ultrogel into three different glycoproteins, HRGP2a,2b and 2c. Two of these glycoproteins behave as polycations (HRGP2b and 2c) and are chemically distinct from HRGP2a. HRGP2b is the most abundant component and contains 41% protein and 50% sugar. Hydroxyproline, lysine, tyrosine, and arabinose are the most prominent residues of their respective moiety. The glycosylation pattern of hydroxyproline indicates that HRGP2b is related to and possibly a precursor of the wall HRGP; as in melon cell wall HRGP, Hyp-Ara3 predominates, and small amounts of a putative Hyp-Ara5 a hitherto unreported hyp-arabinoside, are recorded. The molecular weight of HRP2b, the protein portion of HRGP2b is 55,000 ± 5,000, as estimated after deglycosylation of the molecule with trifluoromethane sulfonic acid. Antibodies have been raised against HRGP2b and HRP2b. Immunodiffusion shows that each antigen (HRGP2b or HRP2b) reacts with its own IgG, and cross-reacts with the heterologous IgG, thereby indicating the presence of common (unglycosylated) and specific (glycosylated and deglycosylated) epitopes. The arabinogalactan protein HRGP1 is not recognized by either antibody and HRGP2b does not react with the Yariv antigen. Immunoprecipitation of 3H-labeled HRP1 and HRP2b in the presence of goat antirabbit IgG, followed by gel electrophoresis, allows to recover HRP2b only. Again, HRP2b is immunoprecipitated by the two antisera.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cassab G. I., Nieto-Sotelo J., Cooper J. B., van Holst G. J., Varner J. E. A developmentally regulated hydroxyproline-rich glycoprotein from the cell walls of soybean seed coats. Plant Physiol. 1985 Mar;77(3):532–535. doi: 10.1104/pp.77.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J. Synthesis and secretion of hydroxyproline containing macromolecules in carrots. I. Kinetic analysis. Plant Physiol. 1969 Aug;44(8):1187–1193. doi: 10.1104/pp.44.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Esquerré-Tugayé M. T., Lafitte C., Mazau D., Toppan A., Touzé A. Cell Surfaces in Plant-Microorganism Interactions: II. Evidence for the Accumulation of Hydroxyproline-rich Glycoproteins in the Cell Wall of Diseased Plants as a Defense Mechanism. Plant Physiol. 1979 Aug;64(2):320–326. doi: 10.1104/pp.64.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyns W., Bossyns D., Peeters B., Rombauts W. Study of a proline-rich polypeptide bound to the prostatic binding protein of rat ventral prostate. J Biol Chem. 1982 Jul 10;257(13):7407–7413. [PubMed] [Google Scholar]
- Kumarasamy R., Symons R. H. The tritium labeling of small amounts of protein for analysis by electrophoresis on sodium dodecyl sulfate--polyacrylamide slab gels. Anal Biochem. 1979 Jun;95(2):359–363. doi: 10.1016/0003-2697(79)90739-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamport D. T., Miller D. H. Hydroxyproline arabinosides in the plant kingdom. Plant Physiol. 1971 Oct;48(4):454–456. doi: 10.1104/pp.48.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach J. E., Cantrell M. A., Sequeira L. Hydroxyproline-rich bacterial agglutinin from potato : extraction, purification, and characterization. Plant Physiol. 1982 Nov;70(5):1353–1358. doi: 10.1104/pp.70.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARINKOVICH V. A. PURIFICATION AND CHARACTERIZATION OF THE HEMAGGLUTININ PRESENT IN POTATOES. J Immunol. 1964 Nov;93:732–741. [PubMed] [Google Scholar]
- Mellon J. E., Helgeson J. P. Interaction of a hydroxyproline-rich glycoprotein from tobacco callus with potential pathogens. Plant Physiol. 1982 Aug;70(2):401–405. doi: 10.1104/pp.70.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenzer J., Bildstein C., Gleason M., Carlson D. M. Properties of proline-rich proteins from parotid glands of isoproterenol-treated rats. J Biol Chem. 1979 Jul 10;254(13):5629–5634. [PubMed] [Google Scholar]
- Roby D., Toppan A., Esquerré-Tugayé M. T. Cell surfaces in plant-microorganism interactions : v. Elicitors of fungal and of plant origin trigger the synthesis of ethylene and of cell wall hydroxyproline-rich glycoprotein in plants. Plant Physiol. 1985 Mar;77(3):700–704. doi: 10.1104/pp.77.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. A. Characterization of Carrot Cell Wall Protein : II. IMMUNOLOGICAL STUDY OF CELL WALL PROTEIN. Plant Physiol. 1981 Oct;68(4):964–968. doi: 10.1104/pp.68.4.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart D. A., Varner J. E. Purification and Characterization of a Salt-extractable Hydroxyproline-rich Glycoprotein from Aerated Carrot Discs. Plant Physiol. 1980 Nov;66(5):787–792. doi: 10.1104/pp.66.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemer M. A., Mason A., Carlson D. M. Cell-free translations of proline-rich protein mRNAs. J Biol Chem. 1982 Sep 25;257(18):11176–11180. [PubMed] [Google Scholar]
- van Holst G. J., Clarke A. E. Quantification of arabinogalactan-protein in plant extracts by single radial gel diffusion. Anal Biochem. 1985 Aug 1;148(2):446–450. doi: 10.1016/0003-2697(85)90251-9. [DOI] [PubMed] [Google Scholar]
- van Holst G. J., Varner J. E. Reinforced Polyproline II Conformation in a Hydroxyproline-Rich Cell Wall Glycoprotein from Carrot Root. Plant Physiol. 1984 Feb;74(2):247–251. doi: 10.1104/pp.74.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]